ABSTRACT
Recombinant hepatitis C virus (HCV) clones propagated in human hepatoma cell cultures yield relatively low infectivity titers. Here, we adapted the JFH1-based Core-NS2 recombinant SA13/JFH1C3405G,A3696G (termed SA13/JFH1orig), of the poorly characterized genotype 5a, to Huh7.5 cells, yielding a virus with greatly improved spread kinetics and an infectivity titer of 6.7 log10 focus-forming units (FFU)/ml. We identified several putative adaptive amino acid changes. In head-to-head infections at fixed multiplicities of infection, one SA13/JFH1orig mutant termed SA13/JFH1Core-NS5B, containing 13 amino acid changes (R114W and V187A [Core]; V235L [E1]; T385P [E2]; L782V [p7]; Y900C [NS2]; N2034D, E2238G, V2252A, L2266P, and I2340T [NS5A]; A2500S and V2841A [NS5B]), displayed fitness comparable to that of the polyclonal high-titer adapted virus. Single-cycle virus production assays in CD81-deficient Huh7-derived cells demonstrated that these changes did not affect replication but increased HCV assembly and specific infectivity as early as 24 h posttransfection. Infectious coculture assays in Huh7.5 cells showed a significant increase in cell-to-cell transmission for SA13/JFH1Core-NS5B viruses as well as viruses with only p7 and nonstructural protein mutations. Interestingly, the E2 hypervariable region 1 (HVR1) mutation T385P caused (i) increased sensitivity to neutralizing patient IgG and human monoclonal antibodies AR3A and AR4A and (ii) increased accessibility of the CD81 binding site without affecting the usage of CD81 and SR-BI. We finally demonstrated that SA13/JFH1orig and SA13/JFH1Core-NS5B, with and without the E2 mutation T385P, displayed similar biophysical properties following iodixanol gradient ultracentrifugation. This study has implications for investigations requiring high virus concentrations, such as studies of HCV particle composition and development of whole-virus vaccine antigens.
IMPORTANCE Hepatitis C virus (HCV) is a major global health care burden, affecting more than 150 million people worldwide. These individuals are at high risk of developing severe end-stage liver diseases. No vaccine exists. While it is possible to produce HCV particles resembling isolates of all HCV genotypes in human hepatoma cells (HCVcc), production efficacy varies. Thus, for several important studies, including vaccine development, in vitro systems enabling high-titer production of diverse HCV strains would be advantageous. Our study offers important functional data on how cell culture-adaptive mutations identified in genotype 5a JFH1-based HCVcc permit high-titer culture by affecting HCV genesis through increasing virus assembly and HCV fitness by enhancing the virus specific infectivity and cell-to-cell transmission ability, without influencing the biophysical particle properties. High-titer HCVcc like the one described in this study may be pivotal in future vaccine-related studies where large quantities of infectious HCV particles are necessary.
INTRODUCTION
Hepatitis C virus (HCV) is an important human pathogen with more than 150 million chronically infected individuals worldwide. These individuals are at high risk of developing severe end-stage liver diseases such as cirrhosis and hepatocellular carcinoma, making HCV the most frequent indication for liver transplantation in the United States and Europe (1, 2). HCV is an enveloped positive-stranded RNA virus classified as a Hepacivirus of the Flaviviridae family. The HCV open reading frame (ORF) encodes a polyprotein of ∼3,000 amino acids (aa), which is cleaved into 10 viral proteins: Core; the envelope glycoproteins E1 and E2; the viroporin p7; and the nonstructural (NS) proteins NS2, NS3, NS4A, NS4B, NS5A, and NS5B (3). HCV is genetically highly heterogeneous with 7 major genotypes and 67 subtypes recognized (4). Whereas HCV genotypes 1 to 3 can be found in most parts of the world and thus have been thoroughly characterized (5), genotype 5a is relatively poorly characterized. Genotype 5a is primarily found in southern Africa, but cases of genotype 5a infection have recently been reported in other parts of the world, including Europe, North America, South America, and the Middle East (6). A prototype strain, SA13, isolated from a South African patient, was previously shown to be infectious in both chimpanzees and human liver-uPA-SCID mice (7, 8). A genotype 5a replicon system was only recently established (9).
The JFH1-based infectious HCV cell culture (HCVcc) system has been of great importance for HCV research since its development in 2005 (10–12). Subsequently, several different types of intra- and intergenotypic JFH1-based recombinant culture systems, as well as full-length cultures of other strains, have been developed (13–17), with Core-NS2 and NS5A infection cultures available for all 7 major HCV genotypes (18–20). Introduction of adaptive mutations has been necessary for efficient propagation of most HCVcc recombinants (18, 19, 21–26), except JFH1-based 5′ untranslated region (UTR)-NS2 or Core-NS2 genotype 2 recombinants (11, 18, 21, 23, 27, 28). Although these systems have advanced HCV research, they produce insufficient amounts of virus particles for morphological or vaccine studies, highlighting the need for improved culture systems. Continuous passage of HCVcc in Huh7-derived hepatoma cells results in the emergence of viral quasispecies with adaptive mutations, as reported almost exclusively for genotype 2a HCVcc (29–38). Such mutations may enhance interactions between genotype-specific HCV proteins (e.g., Core-NS2) and the JFH1 replicase and 5′ and 3′ UTRs, as well as interactions between HCV proteins and hepatoma cell-specific host factors. Thus, cell culture adaptation could be employed to enhance one or several steps of the viral life cycle, thereby increasing viral genesis and/or fitness.
HCV entry into the hepatocyte is a complex process involving several attachment factors and coreceptors, such as CD81 and scavenger receptor class B type I (SR-BI) (39). HCV has also been reported to be capable of direct transfer between adjacent hepatocytes, an event termed cell-to-cell transmission (40, 41). Cell-to-cell transmission is considered to be more rapid and efficient than receptor-mediated entry (40). Thus, increased cell-to-cell transmission could correlate with improved viral fitness.
The HCV envelope glycoproteins are natural targets of neutralizing antibodies (42). Several studies have shown that alterations to E2 especially can cause changes in neutralization sensitivity. This includes deletion of the hypervariable region 1 (HVR1) (43, 44) and specific amino acid changes (33, 34, 45–52). Furthermore, changes to the envelope glycoproteins can also cause changes in the biophysical properties of HCV (43, 44, 53). Thus, changes to the HCV envelope are important to investigate, since they can affect both biophysical and biological properties of the HCV particle.
In this study, we used high-throughput serial passage to generate high-titer virus stocks based on our fittest Core-NS2 JFH1-based HCV recombinant, the genotype 5a recombinant SA13/JFH1C3405G,A3696G (referred to as SA13/JFH1orig) (24). Functional studies revealed that 13 amino acid changes, identified following serial passage, enhanced HCV assembly, specific infectivity, and cell-to-cell transmission, conferring increased HCV genesis and fitness. Furthermore, we found that one of these substitutions, at position 385 in HVR1 of E2, revealed novel changes to the accessibility of neutralizing epitopes and the CD81 binding site of E2. We believe that generation and characterization of highly adapted HCVcc are an important step toward developing more-robust cell culture systems that will facilitate production of HCVcc virions for vaccine studies and morphological studies of the HCV particle.
MATERIALS AND METHODS
Huh7.5 cell cultures.
Human hepatoma Huh7.5 cells were cultured in filter-cap culture flasks (Nunc) in Dulbecco's modified Eagle medium (DMEM) (Gibco/Invitrogen) supplemented with 10% fetal bovine serum (FBS; Sigma), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Gibco/Invitrogen), here referred to as complete DMEM. Cells were split every 2 to 3 days and maintained at 37°C and 5% CO2.
Generation of SA13/JFH1orig-based mutant constructs.
The previously described plasmid pSA13/JFH1orig (24) was used as a backbone for generating mutant constructs. Single point mutations (nucleotide positions refer to the SA13/JFH1 sequence, GenBank accession number FJ393024 [24], unless stated otherwise) A3042G and T2687G were inserted using fusion PCR. All PCRs were performed using Pfu polymerase (Stratagene). Single point mutations C1043G, G1043C, C1493A, A1493C, and A6443G were inserted using the QuikChange II XL site-directed mutagenesis kit (Stratagene/Agilent). Two pUC57 plasmids encoding an SA13 Core-E2 sequence containing mutations C680T, T900C, G1043C, and A1493C (plasmid 1) and a JFH1 NS5A-NS5B sequence containing mutations A7044G, T7086C, T7128C, T7350C, G7895T, A7897G, and T8919C (plasmid 2) were synthesized (GenScript). Target sequence of plasmid 1 was introduced by digestion with KpnI and BlpI FastDigest enzymes (Fermentas). Target sequence of plasmid 2 was introduced by digestion with SanDI and BstZ17I FastDigest enzymes (Fermentas). The HCV sequences of plasmids were verified by sequencing (Macrogen Europe) of the final DNA preparation (HiSpeed Plasmid Maxi kit; Qiagen).
Transfection and infection of Huh7.5 cells.
For transfection of Huh7.5 cells, 350,000 cells/well were seeded in 6-well plates (Nunc) and incubated for 24 h. Plasmids (10 μg) were linearized by overnight digestion with XbaI (New England BioLabs). Linearized plasmids were purified using the Wizard SV gel and PCR cleanup system (Promega). In vitro transcription was performed using T7 RNA polymerase (Promega) for 2 h at 37°C. Transfection was performed by incubating 5 μl Lipofectamine 2000 (Invitrogen) and the RNA transcripts (∼15 μg) in 500 μl serum-free Opti-MEM (Gibco/Invitrogen) for 20 min at room temperature. RNA-Lipofectamine complexes were added to the preplated cells and incubated for 16 to 24 h. Transfection efficiencies and culture spread were determined by HCV NS5A immunostaining (see below). Supernatants were harvested as indicated in the figures, sterile filtered, and stored at −80°C.
For infection of Huh7.5 cells in culture flasks, 3 × 106 cells were seeded in T80 culture flasks (Nunc) and incubated for ∼24 h. Following incubation, cells were infected at a multiplicity of infection (MOI) of either 0.003 or 0.0003 for 3 h (see figure legends for details). Infection spread was monitored by HCV NS5A immunostaining (see below). Supernatants were harvested as indicated in the figures, sterile filtered, and stored at −80°C.
Evaluation of HCV-transfected and -infected cell cultures.
Overall, HCV spread was monitored by HCV NS5A immunostaining as previously described (21, 54). Briefly, cells plated onto chamber slides (Nunc) on the previous day were fixed in ice-cold acetone (Sigma) for 10 min. Fixed cells were stained for 2 h at room temperature, using primary anti-NS5A antibody 9E10 (11). Cells were stained for 10 min using a mix of secondary antibody Alexa Fluor 594-conjugated goat anti-mouse IgG (heavy plus light [H+L]) (Invitrogen) and Hoechst 33342 (Invitrogen). Before microscopy, cells were covered by Fluoromount-G (SouthernBiotech) and a coverslip.
Culture supernatant infectivity titers were determined as focus-forming units (FFU) per milliliter, as previously described (18, 54). Briefly, Huh7.5 cells (6,000 cells/well), seeded the previous day onto poly-d-lysine-coated 96-well plates, were infected with serially diluted supernatants (lowest dilution, 1:2). Forty-eight hours after infection, cells were fixed in ice-cold methanol (Sigma) for 10 min. Fixed cells were incubated for 5 min with 3% H2O2 at room temperature before being stained for 24 h at 4°C using primary anti-NS5A antibody 9E10. Following incubation, cells were stained for 30 min at room temperature using secondary antibody enhanced chemiluminescence (ECL) anti-mouse IgG horseradish peroxidase (HRP)-linked whole antibody (GE Healthcare Amersham). NS5A-positive cells were visualized using a diaminobenzidine (DAB) substrate kit (Dako). FFU were counted automatically, using an Immunospot series 5 UV analyzer (CTL Europe GmbH) with customized software as previously described (55). The lower limit of detection was calculated for each 96-well plate as previously described (19).
Serial passage of SA13/JFH1orig in cell culture flasks and 96-well microtiter plates.
For serial passage in cell culture flasks, 3 × 106 cells were seeded into T80 flasks and infected as described above with a previously described second-passage SA13/JFH1orig stock (18). When infection had peaked as determined by immunostaining, 5 ml supernatant, collected one time point prior to the peak of infection, was used to infect naive cells plated in a new T80 flask for 3 h. This procedure was repeated for serial passage of SA13/JFH1orig in T80 flasks.
For serial passage in 96-well plates, 5,000 cells/well were seeded in a poly-d-lysine-coated 96-well plate (Nunc) and incubated for ∼24 h. Initially, each well was infected for 72 h with 200 μl SA13/JFH1p17 corresponding to an MOI of 0.01. After incubation, supernatant was collected from all wells and stored at −80°C. The infected cells were fixed and stained for HCV NS5A (see above). In subsequent serial passages, 5 to 100 μl of the saved supernatant (the same volume for all wells) was transferred from each well to the corresponding wells of a poly-d-lysine-coated 96-well plate with naive Huh7.5 cells, seeded the previous day. Complete DMEM was added to each well for a total volume of 200 μl, and cells were incubated for 3 h. After incubation, virus was removed and cells were washed in phosphate-buffered saline (PBS) before 200 μl complete DMEM was added, and cells were incubated for 72 h. Following incubation, supernatant was saved from all wells and cells were fixed in ice-cold methanol and stained for NS5A as described above. Single HCV NS5A-positive cells were counted automatically using an Immunospot series 5 UV analyzer with customized software as described previously (19, 55, 56).
Endpoint dilution.
Huh7.5 cells (6,000 cells/well), plated the previous day onto poly-d-lysine-coated 96-well plates, were infected with 12 replicate serial dilutions of SA13/JFH1p31/C5 from 10−1 to 10−8. Forty-eight hours postinfection, supernatant was collected and stored at −80°C. Cells were fixed in ice-cold methanol and HRP stained for HCV NS5A as described above. FFU were counted automatically, as described previously (55). Supernatants from wells with 1 to 2 FFU recorded were selected for testing in culture.
Direct sequencing of SA13/JFH1 HCVcc ORF.
HCV RNA was purified from 200 μl culture supernatant using the High Pure viral nucleic acid kit (Roche Applied Science). Overall, reverse transcription, first-round PCR, and second-round nested PCR were carried out as described previously (21). Primers used to generate cDNA and PCR amplicons spanning the entire ORF have previously been described (21, 24). Direct amplicon sequencing was carried out by Macrogen Europe.
Assays for determination of cell-to-cell transmission.
Following immunostaining of infected cells as described above, the size (square millimeters) of each individual focus was determined automatically, using an Immunospot series 5 UV analyzer (55). For determination of the size of individual foci, we analyzed triplicate wells from 96-well plates that had been used for infectivity titration of the indicated viruses. The numbers of foci/well in wells selected for analyses were between the lower cutoff, determined for the individual plate (see above), and the upper cutoff of 200 foci/well, as this was within the linear range of the assay (19).
Determination of cell-to-cell transmission in infectious coculture assays was performed as previously described (41). In brief, Huh7.5 cells were electroporated with the indicated RNA transcripts 72 h before their use in coculture assays. Huh7.5 cells were seeded in collagen-coated tissue culture plates at 1.2 × 105 cells/cm2 and allowed to adhere for 1 h in the presence of 100 μg/ml pooled immunoglobulin from chronic HCV-infected subjects (anti-HCV Ig) or control uninfected subjects. HCV-infected producer cells were labeled with 5-chloromethylfluorescein diacetate (CMFDA) (Invitrogen) as previously described (41) and cocultured with naive target cells at a ratio of 1:1. After 48 h, the cocultured cells were trypsinized, fixed, and stained for NS5A. De novo transmission events identified as NS5A+/CMFDA− were quantified by flow cytometry as previously described (41). To confirm the neutralizing capacity of the anti-HCV Ig, the extracellular medium was also collected and assessed for levels of infectious virus.
Single-cycle virus production assay in S29 cells.
Transfection of CD81-deficient S29 cells (32) for determination of intra- and extracellular HCV Core titers and infectivity titers, respectively, was carried out as previously described (54, 57, 58). In brief, 400,000 S29 cells were plated in 6-well plates 24 h before transfection. In vitro transcription of plasmids was carried out as described above. RNA transcripts (∼7.5 μg) were transfected into S29 cells using Lipofectamine 2000 as described above, but with the following changes to the protocol: S29 cells were incubated with transfection complexes for 4 h during which complete DMEM had been replaced by Opti-MEM. After 4 h, Opti-MEM was replaced by complete DMEM. Cells were collected at 4, 12, 24, 36, and 48 h posttransfection and prepared for determination of intracellular HCV Core as previously described (54, 57, 58). Intracellular infectivity titers were determined from cell lysates collected at 12, 24, 36, and 48 h posttransfection as previously described (54, 57, 58). Transfection supernatants were collected at 12, 24, 36, and 48 h for determination of extracellular Core and extracellular infectivity titers as previously described (54, 57, 58). Intra- and extracellular Core proteins determined at 12, 24, 36, and 48 h were transformed as percent relative to intracellular Core titers determined 4 h posttransfection. Core titers were determined in duplicate by the Architect HCV antigen (Ag) assay (Abbott). Importantly, cell-to-cell transmission should not affect assays performed in S29 cells (32).
Neutralization assays.
Huh7.5 cells (7,000 cells/well) were seeded onto poly-d-lysine-coated 96-well plates.
(i) Antibody assays.
The following day, IgG purified from serum from genotype 1a-infected patient H, taken 29 years after acute infection (H06 [59]), or monoclonal antibodies AR3A and AR4A (60, 61) were diluted in complete DMEM as specified and mixed 1:1 with 400 FFU of the relevant viruses. IgG-virus mixtures were incubated at 37°C for 1 h before being added onto the preplated cells. Cells were incubated with IgG-virus mixtures for 3 h. After incubation, cells were washed in PBS and complete DMEM was added to all wells.
(ii) sCD81-LEL assay.
The following day, soluble CD81 large extracellular loop (sCD81-LEL) (46, 62) was diluted in complete DMEM as specified and mixed 1:1 with 300 FFU of the relevant viruses. The sCD81-LEL-virus mixtures were incubated at 37°C for 1 h before being added onto the preplated cells. Cells were incubated with sCD81-LEL-virus mixtures for 3 h. After incubation, cells were washed in PBS and complete DMEM was added to all wells.
(iii) Antibody and sCD81-LEL assays.
Cells were fixed 48 h postinfection in ice-cold methanol and HRP stained for HCV NS5A as described above. Single HCV NS5A-positive cells were counted automatically as described previously (19, 56). The percent neutralization was calculated by relating counts of experimental wells to the mean count of at least six replicate wells with untreated control virus. Following logarithmic transformation of x values, variable-slope sigmoidal dose-response curves {y = bottom + [top − bottom]/[1 + 10(log10EC50 − X) × HillSlope]} were fitted using GraphPad Prism 6.0 software. A “bottom” constraint of 0 and a “top” constraint of 100 were introduced, and median inhibitory concentrations (IC50s) were calculated using GraphPad Prism 6.0. Recombinants with envelope mutations (see above) were propagated in Huh7.5 cells. For all virus stocks used for neutralization assays, we confirmed that the entire ORF sequence, determined by direct sequencing, was identical to the plasmid sequence.
Equilibrium density gradient ultracentrifugation.
Semicontinuous 10 to 40% iodixanol gradients were prepared as previously described (44, 54). HCVcc-containing supernatants of the indicated viruses were concentrated using Amicon 100-kDa centrifugation filters (Millipore). A final volume of ∼200 μl concentrated supernatant was loaded on top of each gradient. The samples were ultracentrifuged at 151,000 × relative centrifugal force (RCF) for 18 h at 4°C using a Beckman SW-41 rotor mounted in a Beckman XL-70 ultracentrifuge. Following ultracentrifugation, fractions of ∼550 μl were collected from the bottom of the tube. For determination of density, portions of 400 μl of each fraction were weighed (model SI-114; Denver Instruments). Fraction infectivity titers were determined as described above. Iodixanol-containing fractions were diluted to contain ≤10% iodixanol before titration. Core titers in ultracentrifugation fractions were determined by the Architect HCV Ag assay (Abbott). For determination of RNA titers in ultracentrifugation fractions, RNA was extracted using the total nucleic acid isolation kit (Roche Applied Science); RNA titers were determined by TaqMan real-time PCR as previously described (21). For all virus stocks used for ultracentrifugation, we confirmed that the Core-E2 sequence, determined by direct sequencing, was identical to the plasmid sequence.
Receptor blocking assays.
Huh7.5 cells (7,000 cells/well) were seeded onto poly-d-lysine-coated 96-well plates. The following day, purified mouse anti-human CD81 primary antibody (JS-81 [BD Biosciences]) or anti-SR-BI primary antibody (54, 58, 63) was diluted in complete DMEM as specified in the figure and added to the cells for 1 h. Following incubation, 300 FFU of the indicated viruses was added for 3 h. Following incubation, cells were washed in PBS and complete DMEM was added to all wells. Cells were incubated and fixed 48 h postinfection in ice-cold methanol and HRP stained for HCV NS5A. For SR-BI blocking, HRP staining was performed as described above. For CD81 blocking, staining was performed with the following modifications. Following fixation, cells were washed twice in PBS supplemented with 0.1% Tween 20 (Sigma-Aldrich) (termed PBS-Tween), and 3% H2O2 was added for 5 min at room temperature. Cells were washed twice in PBS-Tween, and 300 μl of PBS supplemented with 1% bovine serum albumin (BSA) and 0.2% skim milk (blocking buffer) was added for 1 h at room temperature. Following incubation, the blocking buffer was removed and 10 μg of AffiniPure fragment antigen binding (Fab fragment) goat anti-mouse IgG (H+L) (Jackson ImmunoResearch) was added to the cells at 100 μg/ml and incubated for 1 h at room temperature. Following incubation, cells were washed twice in PBS-Tween and stained for HCV NS5A as described above. Single HCV NS5A-positive cells were counted automatically as described previously (19, 56). The percent blocking was calculated by relating counts of experimental wells to the mean count of eight replicate wells with untreated control virus. Following logarithmic transformation of x values, variable-slope sigmoidal dose-response curves {y = bottom + [top − bottom]/[1 + 10(log10EC50 − X) × HillSlope]} were fitted using GraphPad Prism 6.0 software. A “bottom” constraint of 0 was introduced. For CD81 blocking, a “top” constraint of 100 was introduced. For all virus stocks used for blocking assays, we confirmed that the entire ORF sequence, determined by direct sequencing, was identical to the plasmid sequence.
Statistics.
The nonparametric Mann-Whitney test was used to determine statistical differences between FFU sizes. To accommodate between-plate variation, all spot sizes were standardized to a J6/JFH1 reference virus present on every plate. P values were calculated based on these standardized values. The Mann-Whitney test was performed using GraphPad Prism 6.0 software. An unpaired t test was performed to determine P values for infectious coculture assays. For neutralization assays, statistical analyses were performed as previously described (56). Briefly, log10 IC50s and standard errors of the means (SEM) of log10 IC50s from replicate experiments were used to calculate inverse variance weighted means of log10 IC50s with SEM and 95% confidence intervals (CIs). Mean differences between log10 IC50s of different viruses with SEM and 95% CIs were calculated from the inverse variance weighted mean log10 IC50s. Inverse logarithmic transformation rendered median IC50s with 95% CIs and median fold difference with 95% CIs. P values were determined by the Z test.
RESULTS
Serial passages of HCV SA13/JFH1orig in Huh7.5 cells increase infectivity titers.
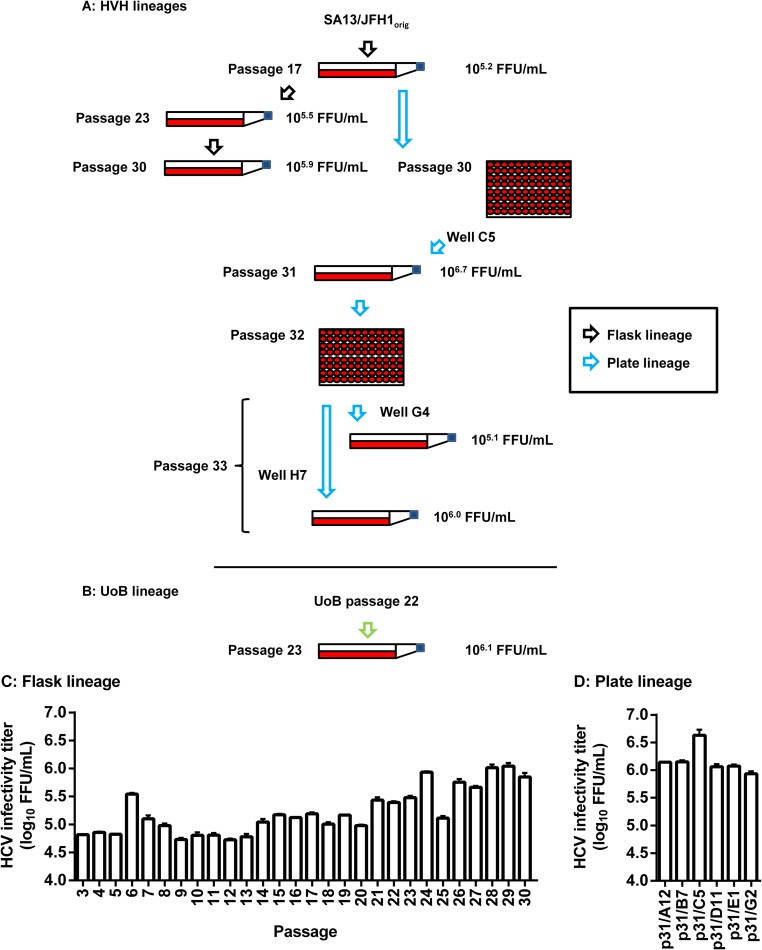
We have previously described the generation of a genotype 5a Core-NS2 JFH1-based HCVcc recombinant (SA13/JFH1orig) that routinely grows to infectivity titers of ∼5.0 log10 FFU/ml, thus representing our fittest JFH1-based recombinant (24). We attempted further adaptation of SA13/JFH1orig to cell culture, by performing serial passage of a previously described second-passage stock (18) in Huh7.5 cells. At Hvidovre Hospital (HVH; Denmark), we employed two different approaches for serial passage (Fig. 1A). For the “flask lineage” (Fig. 1A, black arrows), 30 viral passages in Huh7.5 cells were performed in cell culture flasks. Peak infectivity titers were determined for all passages (Fig. 1C). The highest infectivity titer, 6.0 log10 FFU/ml, was recorded following 29 passages (Fig. 1C). We also employed a high-throughput 96-well plate passage technique, termed the “plate lineage” (Fig. 1A, blue arrows). Passage 17 supernatant (Fig. 1C and Table 1) was used to infect 96 individual Huh7.5 cell cultures in a 96-well plate. Following 13 passages in 96-well plates (yielding a total passage number of 30), we selected six highly infected wells and transferred supernatant from these onto naive Huh7.5 cells in cell culture flasks. We determined peak infectivity titers in these six cultures (Fig. 1D). The highest peak infectivity titer, 6.7 log10 FFU/ml, was recorded in a culture termed SA13/JFH1p31/C5 (Fig. 1D and Table 1). This was ∼1.5 log10 FFU/ml higher than what has typically been observed for SA13/JFH1orig.
FIG 1.
Flow chart and HCV infectivity titers of serial passage lineages of a genotype 5a Core-NS2 recombinant. (A) A previously described second-passage SA13/JFH1orig virus (18) was serially passaged in cell culture flasks for a total of 30 passages as described in Materials and Methods (black arrows). From the 17th passage, the plate lineage was generated (blue arrows). In the plate lineage, virus was passaged in 96-well plates as described in Materials and Methods until passage 30. From the passage 30 plate, six individual wells were used to infect Huh7.5 cultures to generate passage 31 stocks. SA13/JFH1p31/C5 was subjected to endpoint titration in a 96-well plate (passage 32) in order to obtain individual quasispecies. Six individual wells were selected at the highest possible dilution (endpoint) and used to infect Huh7.5 cultures to generate passage 33 stocks. Representative stocks are shown. (B) Simultaneously, an independently serially passaged passage 22 SA13/JFH1orig stock was received from the University of Birmingham (United Kingdom) (UoB lineage, green arrow). This virus stock was used to generate a passage 23 virus stock (SA13/JFH1p23/UoB). (A and B) Selected serially passaged virus stocks from all lineages are shown. The ORFs of RNA genomes recovered from these stocks were all sequenced (Table 1). Furthermore, the infectivities of the viruses were titrated (Table 1 and data not shown); titers are given as focus-forming units per milliliter. (C) Flask lineage peak infectivity titers of passages 3 to 30. (D) Plate lineage peak infectivity titers. Cultures were generated by infection with supernatant from selected wells from the passage 30 plate. Passage cultures are named according to passage number (p31) and the number of the well from which supernatant for infection was recovered (A12, B7, C5, D11, E1, and G2). (C and D) Supernatant infectivity titers are shown as means (focus-forming units per milliliter) from three replicates with SEM.
TABLE 1.
Coding mutations of serially passaged Core-NS2 SA13 (5a) JFH1-based recombinantsg
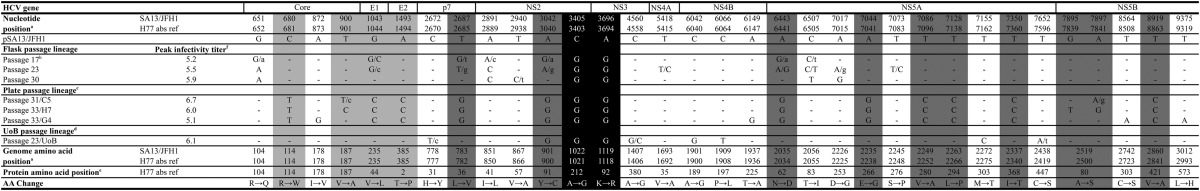
Positions are numbered according to the sequence of pSA13/JFH1 (24) (GenBank accession no. FJ393024). Corresponding H77 absolute reference numbers are given. Only positions with coding mutations present as a 50/50 quasispecies or dominant in at least one isolate are shown. Dashes indicate identity with original plasmid sequence. Positions with quasispecies are written with the dominant sequence in uppercase and the minor sequence in lowercase. The 50/50 quasispecies are shown with two uppercase letters. Shaded positions were introduced back into pSA13/JFH1orig in different combinations. Positions shaded in black represent SA13/JFH1orig cell culture-adaptive mutations described previously (24).
Common 17th passage used for subsequent flask and plate lineage.
Virus stocks are designated according to their passage and the well number used to infect culture. Accordingly, a 31st-passage virus stock derived from well C5 of a 96-well plate is designated p31/C5.
Adapted virus stock received from the University of Birmingham (United Kingdom).
Positions of individual amino acid changes within the given HCV protein are shown as H77 absolute reference numbers.
HCV infectivity titers were determined as described in Materials and Methods. Peak infectivity titers are displayed as log10 focus-forming units per milliliter.
In addition to the mutations shown in the table, the following noncoding nucleotide changes (positions are given as H77 absolute reference numbers, GenBank accession no. AF009606) were observed in the ORFs of the various sequenced virus stocks: G350T, T791C, T2337T/C, T2405C/t, C2468T/c, T2636C, G2807C/g, C3881T/C, A3914G, A4367G, T5231C, T5648C/T, C5750T, T7202C, A7485G/A, T7540C, C7540A/c, T7932C, and A8573G.
We attempted to select individual high-titer quasispecies by performing endpoint dilution of SA13/JFH1p31/C5 as described in Materials and Methods. Supernatant from selected wells was transferred to naive Huh7.5 cells, generating passage 33. For these cultures, the highest infectivity titer recorded was 6.0 log10 FFU/ml (Fig. 1 and Table 1).
We also analyzed a putatively adapted SA13/JFH1orig stock generated independently by 22 serial passages at the University of Birmingham (UoB; United Kingdom). A 23rd passage of this virus was done at HVH (Fig. 1B, green arrow). This was termed the “UoB lineage.” For this virus, a peak infectivity titer of 6.1 log10 FFU/ml was recorded (Fig. 1 and Table 1).
Sequence analysis of serially passaged HCV SA13/JFH1orig.
To determine whether serially passaged SA13/JFH1orig had acquired mutations, we sequenced reverse transcription-PCR (RT-PCR) amplicons from selected cultures (Table 1). Compared to the pSA13/JFH1 reference sequence (24), we observed a total of 50 nucleotide changes. Of these 50 nucleotide changes, 19 did not induce amino acid changes whereas the remaining 31 induced amino acid changes at 30 positions, including changes in the structural proteins (Table 1). In all serially passaged viruses, the originally introduced adaptive amino acid changes A1021G and K1118R (24) (these and all subsequent amino acid positions will be given as H77 absolute reference number, GenBank accession no. AF009606) remained stable (Table 1). The UoB lineage displayed a different panel of mutations, except for a single NS2 mutation (Y900C) that was also present as the dominant quasispecies in passage 17 and had become dominant by passage 31 in the plate lineage (Table 1). Interestingly, SA13/JFH1p23/UoB had not acquired any amino acid changes in the structural proteins (Table 1).
Introducing putative adaptive mutations into the HCV SA13/JFH1orig genome increased extracellular infectivity.
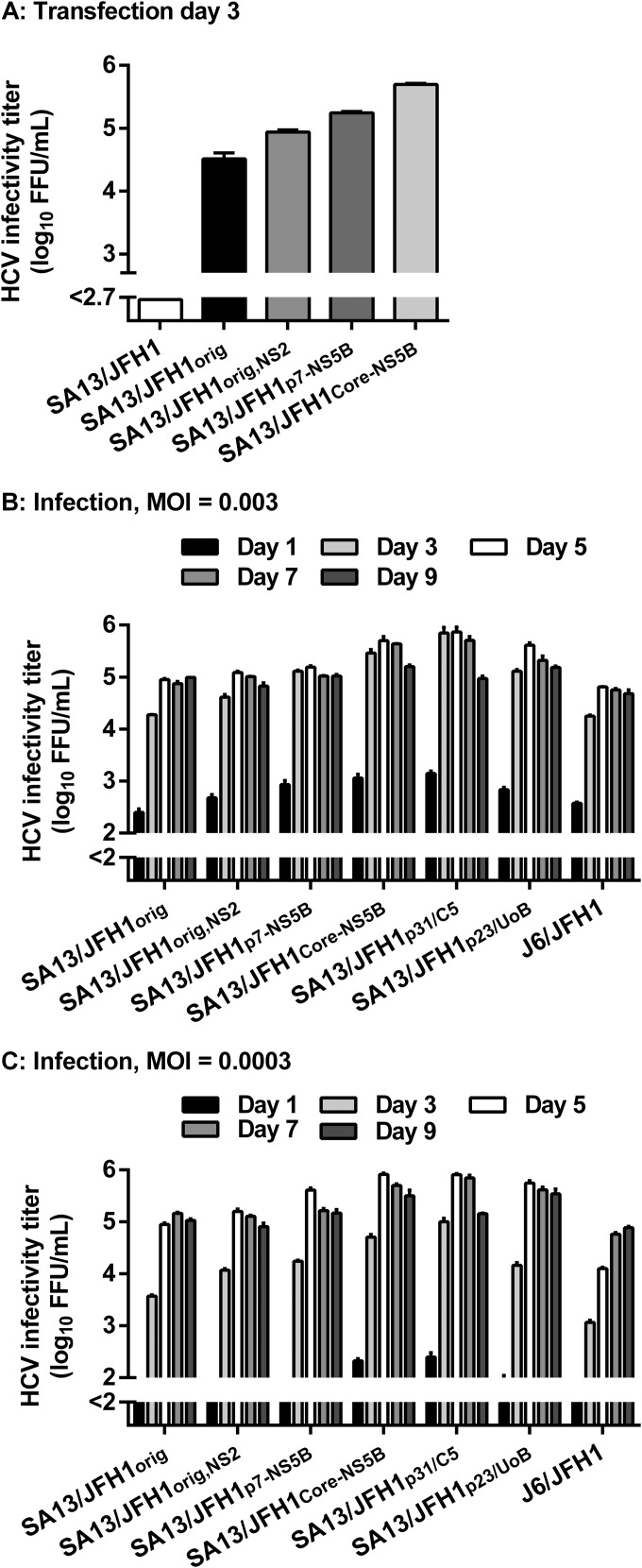
We studied the effect of a panel of 13 amino acid changes (Table 1, shading), identified by sequence analysis of the high-titer virus stock SA13/JFH1p31/C5 and derived stock SA13/JFH1p33/H7 (Table 1). Initially, three different plasmid constructs were made: “pSA13/JFH1orig,NS2,” containing the original adaptive amino acid changes and the single NS2 amino acid change Y900C, present in both the plate lineage and the UoB lineage; “pSA13/JFH1p7-NS5B,” containing the original adaptive mutations and 9 amino acid changes in p7, NS2, NS5A, and NS5B (Table 1, dark gray shading); and “pSA13/JFH1Core-NS5B,” containing the original adaptive mutations and the full panel of 13 amino acid changes in Core, E1, E2, p7, NS2, NS5A, and NS5B (Table 1, shading). RNA transcripts were transfected into Huh7.5 cells together with SA13/JFH1 (SA13/JFH1 plasmid without adaptive mutations) and SA13/JFH1orig (24). On day 3 posttransfection, all cultures were determined to be ∼80 to 90% infected, except for SA13/JFH1, in which the virus had not spread (data not shown). This was in accordance with previously published results for nonadapted SA13/JFH1 (24). The highest infectivity titer, 5.7 log10 FFU/ml, was recorded for SA13/JFH1Core-NS5B (Fig. 2A).
FIG 2.
Adapted SA13/JFH1orig recombinants show accelerated spread and higher infectivity titers after transfection and infection at different MOIs. (A) Different SA13/JFH1orig mutants were generated; HCV RNA transcripts were transfected into Huh7.5 cell cultures as described in Materials and Methods. From transfection cultures, supernatants were collected at day 3 posttransfection, and infectivity titers were determined. (B and C) Second-passage virus stocks generated from transfection supernatants, polyclonal serially passaged virus stocks described in Fig. 1 and Table 1, and control virus stock J6/JFH1 were used to infect Huh7.5 cell cultures at an MOI of 0.003 (B) or 0.0003 (C). From these, supernatants were collected and infectivity titers were determined. (A, B, and C) Supernatant infectivity titers are shown as means (focus-forming units per milliliter) from three replicates with SEM. The lower limits of detection were determined for each individual experiment and are indicated by a y axis break.
We generated and sequenced second-passage stocks of SA13/JFH1orig,NS2, SA13/JFH1p7-NS5B, and SA13/JFH1Core-NS5B (data not shown). Sequence analysis confirmed the presence of all inserted mutations and demonstrated that the three adapted SA13/JFH1orig viruses did not acquire additional mutations during two viral passages in naive Huh7.5 cells.
Comparison of viral spread kinetics and infectivity titers between HCV SA13/JFH1p31/C5 and SA13/JFH1Core-NS5B.
To compare HCV spread kinetics and infectivity titers among the different mutant virus stocks and polyclonal passage 31 (HVH) and 23 (UoB) stocks, we infected Huh7.5 cells at an MOI of 0.003 (Fig. 2B) or 0.0003 (Fig. 2C). We used the sequence-confirmed second-passage SA13/JFH1orig, SA13/JFH1orig,NS2, SA13/JFH1p7-NS5B, and SA13/JFH1Core-NS5B supernatants derived from the transfection experiment shown in Fig. 2A. These were compared to polyclonal SA13/JFH1p31/C5 and SA13/JFH1p23/UoB supernatants (Fig. 1 and Table 1), as well as J6/JFH1 control virus. Infectivity titers were measured for supernatants collected at the indicated time points (Fig. 2B and C). In both experiments, the J6/JFH1 control virus generated comparable peak infectivity titers of 4.8 and 4.9 log10 FFU/ml (Fig. 2B and C). In both experiments, SA13/JFH1Core-NS5B and SA13/JFH1p31/C5 showed the fastest spread kinetics and highest infectivity titers, peaking at ∼5.9 log10 FFU/ml, ∼1.0 log10 higher than SA13/JFH1orig (Fig. 2B and C). Accelerated spread kinetics was evident in the 0.0003-MOI experiment, where only SA13/JFH1Core-NS5B and SA13/JFH1p31/C5 displayed infectivity titers above the cutoff at day 1 postinfection (Fig. 2C). In both experiments, SA13/JFH1orig,NS2 displayed slightly accelerated spread kinetics; however, peak infectivity titers were comparable to those for SA13/JFH1orig (Fig. 2B and C). In both experiments, SA13/JFH1p7-NS5B and SA13/JFH1p23/UoB displayed accelerated spread kinetics and increased infectivity titers, peaking at ∼5.6 log10 FFU/ml. Collectively, these data suggest that the panel of 13 amino acid changes conferred faster spread and increased peak infectivity titers comparable to those of SA13/JFH1p31/C5; however, increased fitness was evident also for SA13/JFH1p7-NS5B and SA13/JFH1p23/UoB, both without envelope amino acid changes.
Adaptive mutations enhance HCV assembly and specific infectivity.
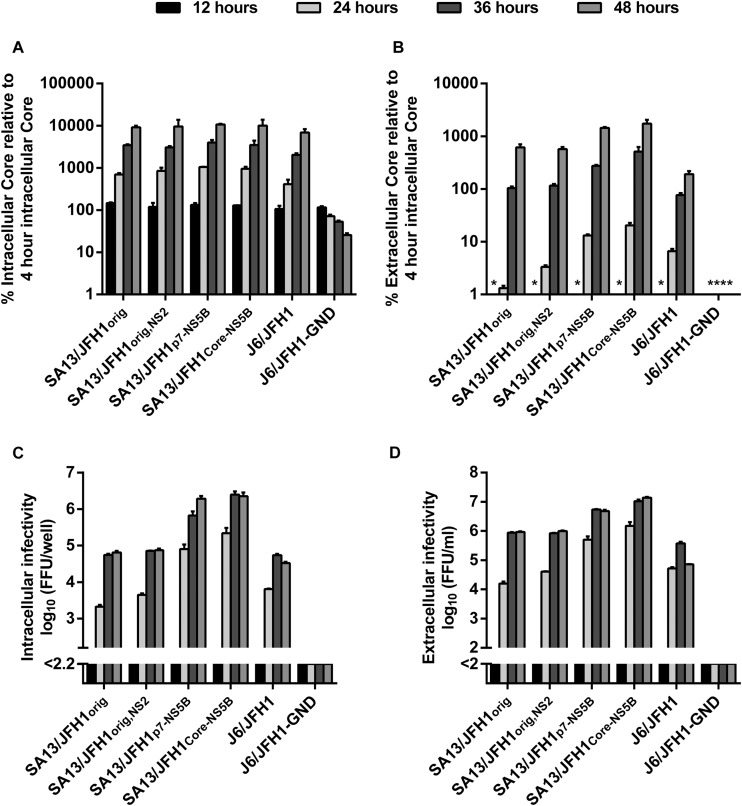
Since the introduced amino acid changes enhanced viral fitness, we investigated which step of the viral life cycle was affected. To this end, we performed single-cycle virus production assays in Huh7-derived CD81-deficient S29 cells (32). S29 cells were transfected with SA13/JFH1orig, SA13/JFH1orig,NS2, SA13/JFH1p7-NS5B, and SA13/JFH1Core-NS5B RNA; J6/JFH1 and J6/JFH1-GND were included as controls (11). To be able to determine differences in, e.g., viral translation/replication occurring at early stages of the viral life cycle, we determined intracellular HCV Core titers at 4 h posttransfection and intra- and extracellular HCV Core titers and infectivity titers at 12, 24, 36, and 48 h posttransfection. While we observed a general increase in intracellular Core titers over time, we observed no differences in intracellular Core titers between the different viruses at any time point, suggesting that HCV replication/translation was not affected by the putative adaptive mutations, even at the early stages of the viral life cycle (Fig. 3A). At 12 h posttransfection, extracellular Core titers were below cutoff for all viruses (Fig. 3B). At 24, 36, and 48 h, we observed a trend toward higher extracellular Core titers for SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B. While this trend could be observed at all time points, it was most pronounced at 24 h posttransfection, where an ∼10-fold increase in extracellular Core could be observed for SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B compared to SA13/JFH1orig (Fig. 3B and Table 2). This suggested that assembly and/or release might be enhanced by the putative adaptive mutations in these two viruses and that this might occur relatively early during the viral life cycle.
FIG 3.
Adaptive mutations result in increased HCV assembly. CD81-deficient S29 cells (32) were transfected with the HCV RNA transcripts indicated, as described in Materials and Methods. (A and B) Intracellular Core titers were determined at 4, 12, 24, 36, and 48 h posttransfection (A), and extracellular Core titers were determined at 12, 24, 36 and 48 h posttransfection (B) as described in Materials and Methods. Intra- and extracellular Core titers are shown as percent Core at the indicated time point, relative to intracellular Core measured 4 h posttransfection. Measurements were performed in duplicate and are shown with SEM. (C and D) Intracellular (C), and extracellular (D) HCV infectivity titers were determined at 12, 24, 36, and 48 h posttransfection. Intracellular infectivity titers are shown as the means (focus-forming units per well) from three replicates with SEM. Extracellular infectivity titers are shown as means (focus-forming units per milliliter) from three replicates with SEM. For infectivity titers, the lower limits of detection were determined for each individual experiment and are indicated by a y axis break. For Core titers, the asterisks indicate values below cutoff.
TABLE 2.
Comparison of Core-NS2 SA13 (5a) JFH1-based recombinants in single-cycle virus production assayse
| Posttransfection time and virus | Intracellular infectivity titera (log10 FFU/well) | Extracellular infectivity titerb (log10 FFU/ml) | Ratio of extracellular/intracellular infectivity | Extracellular corec (log10 amol Core/ml) | Specific infectivity, extracellular (FFU/amol Core) |
|---|---|---|---|---|---|
| 24 h | |||||
| SA13/JFH1orig | 3.3 | 4.2 | 8 | 2.6 | 1/0.03 |
| SA13/JFH1orig,NS2 | 3.6 | 4.6 | 10 | 3.0 | 1/0.03 |
| SA13/JFH1p7-NS5B | 4.9 | 5.7 | 6 | 3.7 | 1/0.01 |
| SA13/JFH1Core-NS5B | 5.3 | 6.2 | 8 | 3.3 | 1/0.001 |
| J6/JFH1 | 3.8 | 4.7 | 8 | 3.6 | 1/0.08 |
| J6/JFH1-GNDd | NA | NA | NA | 1.8 | NA |
| 36 h | |||||
| SA13/JFH1orig | 4.7 | 5.9 | 16 | 4.5 | 1/0.04 |
| SA13/JFH1orig,NS2 | 4.9 | 5.9 | 10 | 4.6 | 1/0.05 |
| SA13/JFH1p7-NS5B | 5.8 | 6.7 | 8 | 5.0 | 1/0.02 |
| SA13/JFH1Core-NS5B | 6.4 | 7.0 | 4 | 4.7 | 1/0.005 |
| J6/JFH1 | 4.7 | 5.6 | 8 | 4.6 | 1/0.1 |
| J6/JFH1-GNDd | NA | NA | NA | 1.8 | NA |
| 48 h | |||||
| SA13/JFH1orig | 4.8 | 6.0 | 16 | 5.3 | 1/0.2 |
| SA13/JFH1orig,NS2 | 4.9 | 6.0 | 13 | 5.3 | 1/0.2 |
| SA13/JFH1p7-NS5B | 6.3 | 6.7 | 3 | 5.7 | 1/0.1 |
| SA13/JFH1Core-NS5B | 6.4 | 7.1 | 5 | 5.2 | 1/0.01 |
| J6/JFH1 | 4.7 | 4.9 | 2 | 5.0 | 1/1.3 |
| J6/JFH1-GNDd | NA | NA | NA | 1.8 | NA |
Intracellular infectivity titers were determined as described in Materials and Methods.
Extracellular infectivity titers were determined as described in Materials and Methods.
HCV Core titers were determined using the Architect HCV Ag assay (Abbott) and displayed as attomoles (amol) per milliliter.
For the J6/JFH1-GND negative control virus, infectivity titers were below cutoff (NA).
S29 cells (32) were transfected with RNA transcripts as described in the Fig. 3 legend and Materials and Methods. Supernatants and cell lysates were collected at 12, 24, 36, and 48 h posttransfection, and infectivity titers and HCV Core titers were determined. Only data from samples collected at 24, 36, and 48 h posttransfection are shown in the table since most data collected at 12 h posttransfection were below the cutoff.
At 12 h posttransfection, intra- and extracellular infectivity titers were below cutoff for all viruses. At 24, 36, and 48 h posttransfection, S29 intracellular infectivity titers were consistently higher for SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B than for SA13/JFH1orig and SA13/JFH1orig,NS2 (Fig. 3C and Table 2), supporting the idea that assembly of intracellular infectious viral particles was enhanced for SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B. The difference was most pronounced at 24 h posttransfection, where intracellular infectivity titers were up to ∼2.0 log10 FFU/well higher for SA13/JFH1Core-NS5B than for SA13/JFH1orig (Fig. 3C and Table 2). Similarly, S29 extracellular infectivity titers were consistently higher for SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B than for SA13/JFH1orig and SA13/JFH1orig,NS2 at all time points (Fig. 3D and Table 2). Again, the difference was most pronounced at 24 h posttransfection, where S29 extracellular infectivity titers were up to ∼2.0 log10 FFU/ml higher for SA13/JFH1Core-NS5B than for SA13/JFH1orig (Fig. 3D and Table 1). The increase in extracellular infectivity titers suggested that viral release was also increased; however, since the ratios between extra- and intracellular infectivity titers did not increase for SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B compared to SA13/JFH1orig and SA13/JFH1orig,NS2 at any time point (Table 2), the effect on release was most likely mediated by the increased assembly and not an independent effect on release itself. In fact, the observation that the ratio seems to decrease for SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B compared to SA13/JFH1orig and SA13/JFH1orig,NS2 would suggest accumulation of intracellular virus due to increased assembly. Finally, the ratio between extracellular infectivity and extracellular Core titers suggested an increase in specific infectivity for extracellular SA13/JFH1Core-NS5B. Specific infectivity was highest for all viruses at 24 h posttransfection and decreased at 36 and 48 h posttransfection; however, the specific infectivity of SA13/JFH1Core-NS5B was consistently ∼10-fold higher than those of SA13/JFH1orig, SA13/JFH1orig,NS2, and SA13/JFH1p7-NS5B at the different time points (Table 2).
Adaptive mutations enhance HCV cell-to-cell transmission.
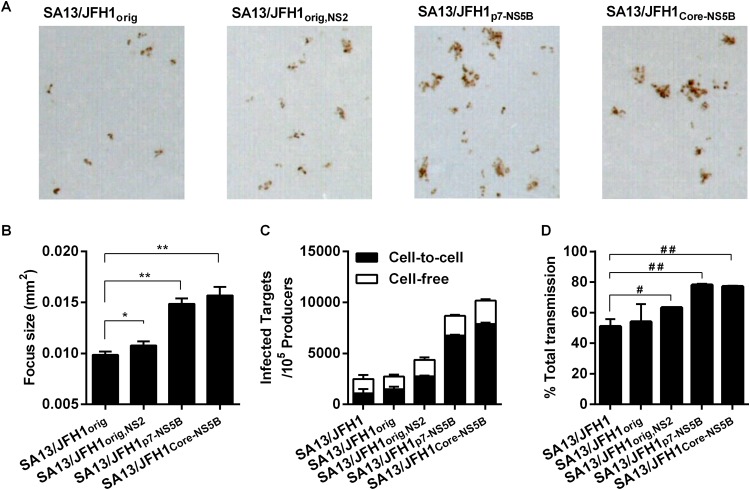
We noted that during infectivity titration of supernatants, individual SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B foci were larger than individual SA13/JFH1orig foci (representative images are shown in Fig. 4A). By measuring the areas of all individual foci automatically in triplicate wells from plates used for infectivity titration of supernatants collected from a first passage of the transfection experiment shown in Fig. 2A, we found that SA13/JFH1orig,NS2 (P = 0.0001; n = 199), SA13/JFH1p7-NS5B (P < 0.0001; n = 486), and SA13/JFH1Core-NS5B (P < 0.0001; n = 145) foci were significantly larger than SA13/JFH1orig foci (n = 158) (Fig. 4B). This indicated that an increase in cell-to-cell transmission could have contributed to the increased viral fitness of the adapted viruses.
FIG 4.
Adaptive mutations enhance cell-to-cell transmission. (A) Representative images of individual foci generated by the indicated viruses. Images were generated using BioSpot 5.0 software for the Immunospot series 5 UV analyzer (CTL Europe GmbH). (B) Individual focus sizes (square millimeters) generated by infection of Huh7.5 cells with the indicated viruses were determined automatically at the peak of infection as described in Materials and Methods. Focus size is shown as the mean of the sizes of all foci in three replicate wells with SEM. The Mann-Whitney test was performed to determine P values as described in Materials and Methods. *, P = 0.0001; **, P < 0.0001. (C and D) Huh7.5 cells were transfected with HCV RNA transcripts, labeled with CMFDA, and cocultured with naive Huh7.5 target cells in the presence or absence of anti-HCV Ig as described in Materials and Methods. (C) The number of newly infected target cells per 105 infected producer cells is presented, where the frequencies of cell-free transmission (white) and cell-to-cell (black) transmission events are shown. (D) Percentage of total transmission events occurring by cell-to-cell transmission. Bars represent the means from two replicate experiments with standard deviations. The unpaired t test was performed to determine P values. #, P < 0.05; ##, P < 0.0001. For panels C and D, data for SA13/JFH1orig were generated in a separate experiment.
To further support this observation, we performed infectious coculture assays in Huh7.5 cells (41), using SA13/JFH1, SA13/JFH1orig, SA13/JFH1orig,NS2, SA13/JFH1p7-NS5B, and SA13/JFH1Core-NS5B. The highly adapted SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B viruses showed the highest increase in infected target cells compared to the control virus, reflecting the accelerated spread kinetics (Fig. 4C). Interestingly, when blocking extracellular virus-mediated transmission by addition of anti-HCV Ig, we observed a significant increase in cell-to-cell transmission events for SA13/JFH1orig,NS2 (P < 0.05), SA13/JFH1p7-NS5B (P < 0.0001), and SA13/JFH1Core-NS5B (P < 0.0001) compared to SA13/JFH1 (Fig. 4C, black bars). We observed no differences between SA13/JFH1 and SA13/JFH1orig. Specifically, the percentages of cell-free and cell-to-cell transmission events were evenly distributed for SA13/JFH1 and SA13/JFH1orig, whereas ∼60% of all transmission events occurred through cell-to-cell transmission for SA13/JFH1orig,NS2 and ∼80% of all transmission events occurred through cell-to-cell transmission for SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B (Fig. 4D). Collectively, these data show that the SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B viruses are capable of increased cell-to-cell transmission.
Increased sensitivity to neutralization with purified patient IgG, human monoclonal IgG, and sCD81-LEL of the highly adapted SA13/JFH1Core-NS5B is caused by a putative adaptive amino acid change in E2.
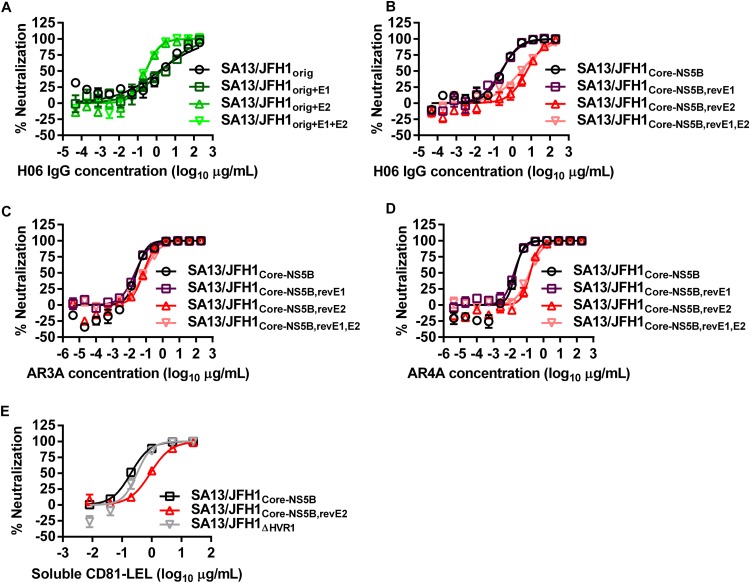
The influence of envelope mutations on the sensitivity of extracellular HCV to neutralizing antibodies has previously been described (33, 34, 45–52). Thus, we investigated if the adaptive amino acid changes present in SA13/JFH1Core-NS5B influenced virus sensitivity to neutralization compared to SA13/JFH1orig. As a neutralizing reagent, we used a well-defined polyclonal IgG purified from patient H serum taken 29 years after the acute infection (59). We observed a significant increase (P = 0.0003) in the neutralization sensitivity of SA13/JFH1Core-NS5B, with a median inhibitory concentration (IC50) of 0.27 μg/ml, compared to SA13/JFH1orig, with an IC50 of 1.4 μg/ml (Fig. 5A and B; Table 3). Thus, mutations in SA13/JFH1Core-NS5B apparently caused increased neutralization sensitivity. To identify the mutation responsible for the increased neutralization sensitivity, we mutated residues at amino acid positions 235 in E1 and 385 in E2 in SA13/JFH1orig. Peak infectivity titers of these recombinants were 5.4 to 5.6 log10 FFU/ml. Interestingly, we observed significantly increased neutralization sensitivity (P = 0.0004) when inserting the T385P in E2 of SA13/JFH1orig (SA13/JFH1orig+E2 [Fig. 5A; Table 3]). The IC50 was determined to be 0.28 μg/ml, similar to that of SA13/JFH1Core-NS5B. Insertion of the E1 mutation V235L (SA13/JFH1orig+E1) did not affect neutralization sensitivity (P = 0.65), whereas insertion of both mutations (SA13/JFH1orig+E1+E2) resulted in significantly increased neutralization sensitivity (P = 0.0002) (Fig. 5A; Table 3), suggesting that the E2 mutation was responsible for the neutralization-sensitive phenotype. We confirmed these findings by reverting the E1 and E2 mutations in SA13/JFH1Core-NS5B. Peak infectivity titers of these recombinants were 5.9 to 6.1 log10 FFU/ml. When reverting P385T in E2 of SA13/JFH1Core-NS5B (SA13/JFH1Core-NS5B,revE2), we observed significantly decreased neutralization sensitivity (P < 0.0001) compared to that of SA13/JFH1Core-NS5B (Fig. 5B; Table 3). Reversion of the E1 mutation L235V (SA13/JFH1Core-NS5B,revE1) did not affect neutralization sensitivity (P = 0.68), whereas reversion of both mutations (SA13/JFH1Core-NS5B,revE1,E2) resulted in significantly decreased neutralization sensitivity (P < 0.0001) (Fig. 5B; Table 3). Thus, the E2 HVR1 amino acid change T385P caused increased sensitivity to neutralizing patient IgG.
FIG 5.
Sensitivity to neutralization is affected by a single putative adaptive amino acid change in E2. H06 IgG (A and B), AR3A (C), AR4A (D), and soluble CD81-LEL (E) were mixed and incubated for 1 h with the indicated viruses as described in Materials and Methods. Neutralization reaction mixtures were added to Huh7.5 cells and incubated for 3 h. Neutralization reaction mixtures were removed, and complete DMEM was added to all wells. Cells were fixed 48 h postinfection and stained, and the number of single HCV NS5A-positive cells per well was determined by automated counting as described in Materials and Methods. The percent neutralization was calculated by relating counts of experimental wells to the mean count from six replicate wells with untreated control virus. Data points are means from three replicates with SEM. Following logarithmic transformation of x values, variable-slope sigmoidal concentration-response curves were fitted {y = bottom + [top − bottom]/[1 + 10(log10EC50 − X) × HillSlope]}. “Bottom” was constrained to 0, and “top” was constrained to 100. (A and B) All neutralizations were performed in the same assay but are split into separate panels for visualization purposes. (A to D) IC50s of individual recombinants are shown in Table 3.
TABLE 3.
Neutralization of SA13 Core-NS2 recombinants using chronic-phase patient IgG and human monoclonal antibodiese
| Recombinant virus | H06 purified IgG |
AR3A |
AR4A |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg/ml)a |
Fold differencea |
P valueb | IC50 (μg/ml)a |
Fold differencea |
P valueb | IC50 (μg/ml)a |
Fold differencea |
P valueb | |||||||
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | ||||
| SA13/JFH1orig | 1.4 | 0.61–3.3 | NA | NA | NA | — | — | — | — | — | — | — | — | — | — |
| SA13/JFH1Core-NS5Bc | 0.27 | 0.2–0.37 | 0.2 | 0.08–0.47 | 0.0003 | 0.027 | 0.016–0.044 | NA | NA | NA | 0.02 | 0.013–0.031 | NA | NA | NA |
| SA13/JFH1orig,E1c | 1.8 | 0.92–3.6 | 1.3 | 0.43–3.8 | 0.65 | — | — | — | — | — | — | — | — | — | — |
| SA13/JFH1orig,E2c | 0.28 | 0.2–0.38 | 0.2 | 0.08–0.48 | 0.0004 | — | — | — | — | — | — | — | — | — | — |
| SA13/JFH1orig,E1,E2c | 0.26 | 0.19–0.36 | 0.2 | 0.08–0.46 | 0.0002 | — | — | — | — | — | — | — | — | — | — |
| SA13/JFH1Core-NS5B,revE1d | 0.24 | 0.16–0.37 | 0.9 | 0.53–1.5 | 0.68 | 0.022 | 0.018–0.027 | 0.8 | 0.47–1.4 | 0.46 | 0.017 | 0.013–0.021 | 0.8 | 0.52–1.3 | 0.46 |
| SA13/JFH1Core-NS5B,revE2d | 5.8 | 3.6–9.3 | 21.2 | 12–37.5 | <0.0001 | 0.07 | 0.05–0.097 | 2.6 | 1.4–4.7 | 0.0019 | 0.16 | 0.11–0.23 | 8.0 | 4.6–13.8 | <0.0001 |
| SA13/JFH1Core-NS5B,revE1,E2d | 2.9 | 1.7–4.7 | 10.5 | 5.8–18.9 | <0.0001 | 0.075 | 0.058–0.097 | 2.8 | 1.6–4.9 | 0.0004 | 0.15 | 0.11–0.19 | 7.3 | 4.4–11.9 | <0.0001 |
Median IC50 and median fold differences with 95% confidence interval were calculated as described in Materials and Methods. For calculation of fold differences, the IC50 obtained for the respective recombinant was related to the IC50 obtained for either SA13/JFH1orig or SA13/JFH1Core-NS5B as specified below.
P values were determined using the Z test as described in Materials and Methods. P values of <0.005 were considered significant and are shown in boldface.
For statistical analyses, these recombinants were related to the SA13/JFH1orig recombinant.
For statistical analyses, these recombinants were related to the SA13/JFH1Core-NS5B recombinant.
To investigate whether the substitution at position 385 would affect sensitivity to well-defined human monoclonal IgG, we performed neutralization assays using previously described antibodies AR3A (Fig. 5C) and AR4A (Fig. 5D) (60, 61). Both AR3A and AR4A target conformational epitopes; whereas AR3A binds an epitope on E2 and blocks E2 binding to CD81, AR4A binds only the full E1/E2 heterodimer and does not block E2 binding to CD81 (61). As described above, SA13/JFH1Core-NS5B,revE2 was significantly less sensitive to neutralization with both AR3A and AR4A (P = 0.0019 and P < 0.0001, respectively), with IC50s of 0.07 μg/ml for AR3A and 0.16 μg/ml for AR4A, compared to 0.027 μg/ml (AR3A) and 0.02 μg/ml (AR4A) for SA13/JFH1Core-NS5B (Fig. 5C and D; Table 3). For SA13/JFH1Core-NS5B,revE1, we determined IC50s of 0.022 μg/ml for AR3A and 0.017 μg/ml for AR4A, similar to those of SA13/JFH1Core-NS5B (P = 0.46 for both), whereas SA13/JFH1Core-NS5B,revE1,E2, with IC50s of 0.075 μg/ml for AR3A and 0.15 μg/ml for AR4A, was significantly less sensitive to neutralization (P = 0.0004 and P < 0.0001, respectively) than was SA13/JFH1Core-NS5B (Fig. 5C and D; Table 3).
Finally, we investigated whether the CD81 binding region of E2 was affected by the substitution at position 385 by neutralizing SA13/JFH1Core-NS5B and SA13/JFH1Core-NS5B,revE2 with sCD81-LEL (46, 62). Hypervariable region 1 (HVR1)-deleted SA13/JFH1ΔHVR1 was included as a control, since it was reported that the HVR1 deletion exposed the CD81 binding site of E2 (43). Interestingly, SA13/JFH1Core-NS5B,revE2, with an IC50 of 0.9 μg/ml, was significantly less sensitive to neutralization with sCD81-LEL than were SA13/JFH1Core-NS5B, with an IC50 of 0.2 μg/ml (P < 0.0001), and SA13/JFH1ΔHVR1, with an IC50 of 0.32 μg/ml (P < 0.0001) (Fig. 5E).
It should be noted that neutralizing antibodies and sCD81-LEL were preincubated with the virus for 1 h before the mixture was added onto Huh7.5 cells for a 3-h attachment phase and subsequently removed. Thus, any resistance to neutralizing antibodies caused by differences in cell-to-cell transmission between viruses (41) is not expected to influence the results of these assays. Furthermore, cell-to-cell transmission occurring at later stages of the assay would not affect the outcome of the assay, since all values are related to a virus-specific virus-only control.
Adaptive mutations do not affect the buoyant density of infectious HCVcc particles.
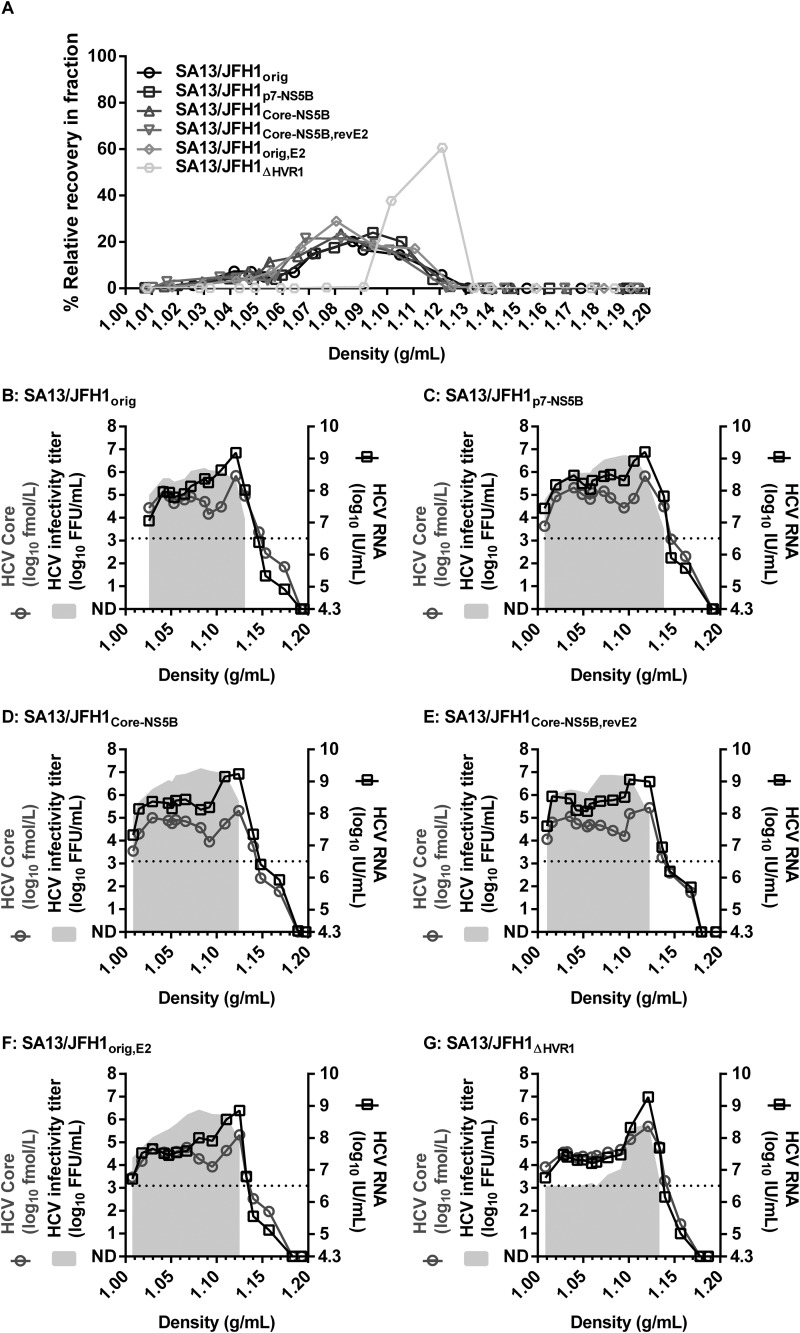
Envelope mutations and deletion of HVR1 from HCVcc have previously been reported to change HCVcc particle density (43, 44, 53). To determine whether the adaptive mutations identified in this study conferred any such changes, we subjected SA13/JFH1orig, SA13/JFH1p7-NS5B, SA13/JFH1Core-NS5B, SA13/JFH1orig,E2, and SA13/JFH1Core-NS5B,revE2 to equilibrium density ultracentrifugation using iodixanol gradients as described in Materials and Methods and measured the level of infectious virus, HCV RNA, and HCV Core antigen in all fractions. We included previously described HVR1-deleted virus (SA13/JFH1ΔHVR1) as a control, since this virus was previously demonstrated to display an altered density profile (44). Overall, mutant viruses displayed similar density profiles with infectious virus present at densities of 1.01 to 1.13 g/ml (Fig. 6A to F). The SA13/JFH1ΔHVR1 virus displayed a characteristic density profile with a single peak at ∼1.10 g/ml (Fig. 6A and G), in accordance with previous observations (44). For mutant viruses, HCV RNA and Core were detected in all fractions containing infectious virus, and both typically peaked at densities of ∼1.1 g/ml (Fig. 6B to F). We did not observe any major differences in distributions of HCV RNA or Core between the mutant viruses, supporting our observation with infectious virus distribution. For the SA13/JFH1ΔHVR1 virus, we observed a peak in HCV RNA and Core at densities corresponding to the peak of infectious virus (Fig. 6G). Thus, the amino acid changes in the structural proteins described in this study did not seem to affect the buoyant density of infectious HCVcc particles.
FIG 6.
Adaptive mutations do not affect buoyant density of infectious HCVcc particles. First-passage virus stocks of the indicated viruses were loaded on top of preformed 10 to 40% iodixanol gradients and subjected to ultracentrifugation as described in Materials and Methods. Fractions were collected from the bottom of the gradients and analyzed by density determination, infectivity titration, RNA titration, and HCV Core titration as described in Materials and Methods. (A) Relative recovery of infectious virus per fraction (percent) was calculated by relating the amount of infectious virus detected in each fraction to the total amount of infectious virus collected and is plotted against the density determined for each fraction. Only fractions with densities of <1.20 g/ml are shown. (B to G) Infectivity titers (gray shading), RNA titers (black squares), and HCV Core titers (gray circles) of individual fractions of the indicated viruses were plotted against the density of the given fraction. Only fractions with densities of <1.20 g/ml are shown. The lower limits of detection were as follows: infectious virus, 3.1 log10 FFU/ml, indicated by dotted line; HCV RNA, 4.3 log10 IU/ml, indicated by beginning of the right y axis. ND, not determinable.
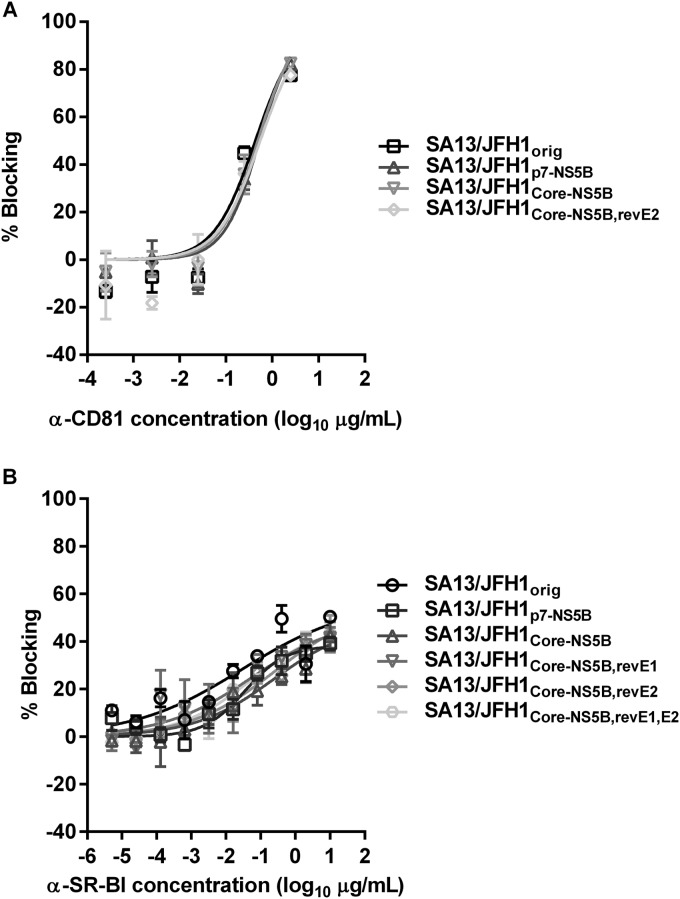
Adaptive mutations did not affect usage of HCV entry receptors CD81 and SR-BI.
HCV E2 is pivotal during entry as it interacts with several HCV coreceptors, including CD81 and SR-BI (64–66). Thus, E2 mutations might influence the usage of these coreceptors. We tested the ability of antibodies to CD81 and SR-BI to block entry of SA13/JFH1orig, SA13/JFH1p7-NS5B, SA13/JFH1Core-NS5B, and SA13/JFH1Core-NS5B,revE2; for SR-BI blocking, we also included viruses SA13/JFH1Core-NS5B,revE1 and SA13/JFH1Core-NS5B,revE1,E2. We observed no major differences between the different viruses when blocking CD81 or SR-BI (Fig. 7). Thus, it seemed that the E2 mutation at position 385 did not affect interactions between E2 and CD81 or SR-BI.
FIG 7.
Adaptive mutations do not affect sensitivity to CD81 and SR-BI blocking. Anti-CD81 (A) or anti-SR-BI (B) was added to Huh7.5 cells at the indicated concentrations and incubated for 1 h. Following incubation, the indicated viruses were added to the Huh7.5 cells, as described in Materials and Methods, and incubated for 3 h. Antibody and virus were removed, and complete DMEM was added to all wells. Cells were fixed 48 h postinfection and stained, and the number of single HCV NS5A-positive cells per well was determined by automated counting as described in Materials and Methods. The percent blocking was calculated by relating counts of experimental wells to the mean count from eight replicate wells with untreated control virus. Data points are means from three replicates with SEM. Following logarithmic transformation of x values, variable-slope sigmoidal concentration-response curves were fitted {y = bottom + [top − bottom]/[1 + 10(log10EC50 − X) × HillSlope]}. “Bottom” was constrained to 0. For CD81 blocking, a “top” constraint of 100 was introduced.
DISCUSSION
In this study, we generated a highly adapted mutant of our fittest JFH1-based Core-NS2 recombinant, the SA13/JFH1orig virus (24). We describe a panel of 13 putative adaptive amino acid changes that in combination enhance viral fitness by increasing HCV assembly, specific infectivity, and cell-to-cell transmission. Furthermore, we propose novel functions of a single amino acid at position 385 in HVR1 of E2 in shielding neutralizing epitopes and the CD81 binding site of E2.
We previously developed a panel of JFH1-based Core-NS2 recombinants containing reference virus stocks of genotypes 1 to 7 (18, 21, 23–25, 28, 67). While this panel has been used in several important studies, one major limitation is the relatively low concentrations of infectious virus particles produced in Huh7.5 cells. Typically, our fittest recombinant, SA13/JFH1orig, yields infectivity titers of approximately 5.0 log10 FFU/ml (Fig. 2); however, other Core-NS2 recombinants typically produce lower infectivity titers in the range of 3.5 to 4.5 log10 FFU/ml or 50% tissue culture infective doses (TCID50)/ml (18, 21, 23, 25, 45, 67–69). While it is important to note that direct comparisons of infectivity titers between laboratories are difficult due to differences in methodologies used for titer determination, as well as variation between Huh7.5 cell cultures in different laboratories, titers of >6.0 log10 TCID50/ml or FFU/ml have been reported in only a few studies for genotype 2a HCVcc JFH1 (30, 37) and J6/JFH1 (35, 38). We studied the SA13/JFH1orig recombinant (24), in which the structural proteins (Core, E1 and E2), p7, and NS2 belong to the poorly characterized genotype 5a. Following 31 viral passages, we were able to generate SA13/JFH1p31/C5 with an infectivity titer of 6.7 log10 FFU/ml, thus improving infectivity titers of our fittest virus by ∼1.5 log10 FFU/ml (Fig. 1D). To our knowledge, this represents one of the most effective HCV cell culture systems described in the literature and the most effective cell culture system representing genotype 5a. While production of HCVcc with titers in the range of 6.5 to 7.0 log10 FFU/ml represents a useful improvement, it might be necessary to further increase production of infectious virus for some research applications. Thus, further adaptation through serial passage could result in generation of even fitter virus clones. Otherwise, it might be necessary to (i) employ alternative methods of adaptation, such as viral codon optimization; (ii) change cell type; or (iii) change cell culture conditions, in order to achieve increased HCV titers in cell culture (14, 54).
We generated SA13/JFH1p7-NS5B by introducing a panel of 9 amino acid changes in the nonstructural proteins (Table 1) and generated SA13/JFH1Core-NS5B by introducing a total of 13 amino acid changes in structural and nonstructural proteins (Table 1). To our knowledge, only amino acid positions 385 and 900 described in this study have been previously investigated by others. While position 385 was studied only in relation to antibody neutralization (see below), the effect of position 900 on assembly was investigated using alanine substitution. Using a single-cycle assay, the authors demonstrated that alanine substitution at this position resulted in a slight reduction (≤1 log10 TCID50/ml) in infectious virus production (70). Thus, this position does not seem to influence assembly significantly, consistent with our findings (Fig. 2 and 3). To discriminate between differences mediated by amino acid changes in the nonstructural proteins alone or in the structural and nonstructural proteins combined, SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B were investigated in parallel. Interestingly, both constructs showed increased viral fitness following transfection, although the complete panel of 13 amino acid changes appeared to generate the fittest construct (Fig. 2A), which was also evident following infection at different MOIs (Fig. 2B and C). Indeed, the SA13/JFH1Core-NS5B recombinant displayed similar accelerated spread kinetics and grew to similar peak titers as the SA13/JFH1p31/C5 virus stock. Thus, the panel of 13 amino acid changes conferred a similar increase in infectivity titers as observed for the fittest polyclonal virus, SA13/JFH1p31/C5 (Fig. 2B and C).
In general, adaptive mutations could enhance interactions between HCV-encoded proteins, increasing compatibility between the genotype-specific proteins Core-NS2 and the JFH1 5′ UTR, 3′ UTR, and NS3-NS5B. Alternatively, the mutations could boost an interaction between HCV and the infected cell. A cross-genotypic comparison of the SA13/JFH1Core-NS5B adaptive mutations to the sequences deposited in the Los Alamos HCV sequence database (71) revealed that at amino acid positions 2238 and 2841 the original amino acids were observed at low frequencies whereas the mutant amino acids were observed at much higher frequencies (Table 4). These changes toward a more conserved amino acid could suggest that these positions are important for interactions between HCV-encoded proteins. We observed several changes toward rare residues (<1% frequency) at amino acid positions 114, 187, 385, 900, 2252, 2266, 2340, and 2500 (Table 4). Such changes might suggest that these positions are important for HCV-host cell interactions. Functionally, such changes in virus-host interactions could have implications for several steps of the viral life cycle. Using single-cycle virus production assays in CD81-deficient S29 cells (32), we observed that HCV assembly might be affected primarily by the putative adaptive amino acid changes present in p7, NS2, NS5A, and NS5B of both SA13/JFH1p7-NS5B and SA13/JFH1Core-NS5B (Fig. 3; Table 1, dark gray shading); all of these proteins have been implicated in HCV assembly (70, 72–78). Furthermore, mutations in Core, E1, and E2 of SA13/JFH1Core-NS5B (Table 1, light gray shading) seemed to mediate a further increase in assembly compared to SA13/JFH1p7-NS5B (Fig. 3). Interestingly, this effect on assembly seemed to be most pronounced at 24 h posttransfection. We also observed an ∼10-fold increase in specific infectivity of SA13/JFH1Core-NS5B compared to all other viruses tested in S29 cells (Table 2). This might suggest that the stability of the virus particle is increased by the amino acid changes in the structural proteins, which have been described previously following cell culture adaptation (35). An important finding was that intracellular Core levels did not change between the 4-h and 12-h samples (Fig. 3A). This suggests that following transfection of the viral RNA, even a highly adapted virus displaying accelerated spread kinetics needs a period of at least 12 h before an increase in HCV protein can be observed, an observation recently described by others as well (79).
TABLE 4.
Frequencies of original and mutated amino acids observed in SA13/JFH1Core-NS5Bb
| HCV protein | Amino acid positiona | Original |
Mutant |
||
|---|---|---|---|---|---|
| Amino acid | Frequency (%) | Amino acid | Frequency (%) | ||
| Core | 114 | R | 91 | W | <1 |
| Core | 187 | V | 48 | A | <1 |
| E1 | 235 | V | 8 | L | 2 |
| E2 | 385 | T | 96 | P | <1 |
| p7 | 782 | L | 43 | V | 4 |
| NS2 | 900 | Y | 16 | C | <1 |
| NS5A | 2034 | N | 5 | D | 3 |
| NS5A | 2238 | E | <1 | G | 99 |
| NS5A | 2252 | V | 24 | A | <1 |
| NS5A | 2266 | L | 1 | P | <1 |
| NS5A | 2340 | I | 3 | T | <1 |
| NS5B | 2500 | A | 99 | S | <1 |
| NS5B | 2841 | V | 19 | A | 81 |
Positions are numbered according to the H77 reference sequence (GenBank accession no. AF009606).
HCV sequence alignments (2008 Web Alignment) of individual HCV proteins were downloaded from the Los Alamos HCV sequence database (71). Amino acid frequency across genotypes at the given positions was determined using BioEdit Sequence Alignment Editor 7.2.5.
While enhanced assembly and increased specific infectivity could account for the increased viral fitness observed, other mechanisms might contribute as well. Indeed, SA13/JFH1orig,NS2, SA13/JFH1p7-NS5B, and SA13/JFH1Core-NS5B were capable of increased cell-to-cell transmission compared to SA13/JFH1 and SA13/JFH1orig (Fig. 4). Cell-to-cell transmission is considered to be more rapid and efficient than cell-free transmission (40). Thus, it is possible that enhanced ability to use cell-to-cell transmission contributes to the increased viral fitness observed for SA13/JFH1p7-NS5B and especially SA13/JFH1Core-NS5B (Fig. 2). In summary, the mutations described in this study might promote efficient interactions between HCV proteins and also their interactions with the host cell, thereby enhancing HCV assembly, specific infectivity, and cell-to-cell transmission, although it remains to be elucidated if several of the mutations identified in this study are needed in combination. It is important to note that since adaptation was performed in Huh7.5 cells, it is possible that the adaptive mutations described in this study increase the fitness of only HCVcc grown in Huh7-derived cells or, even more specifically, Huh7.5 cells. Thus, the effect of these adaptive mutations in other models, such as primary hepatocytes, human liver chimeric SCID-uPA mice, or chimpanzees, could be of interest for future studies.
Due to the lack of immunological pressure in Huh7.5 cells, fitness-enhancing mutations may occur at positions that affect sensitivity to neutralization. For example, amino acid changes in the viral envelope glycoproteins, especially E2, have previously been associated with altered sensitivity to neutralizing antibodies (33, 34, 45–52). The role of changes to the envelope glycoproteins in respect to HCV immunogenicity remains to be fully elucidated. However, immunogenicity studies of other viruses, such as human immunodeficiency virus (80) and respiratory syncytial virus (81), suggest that such changes can be of importance in order to expose otherwise nonaccessible neutralizing epitopes. Indeed, we observed that the E2 HVR1 amino acid change T385P conferred significantly increased neutralization sensitivity toward IgG purified from chronic-phase patient serum (Fig. 5 and Table 3). Alanine substitution at this position was previously shown to confer increased neutralization sensitivity of HCV pseudoparticles toward genotype 1 to 5 patient sera (52). Thus, our results using the HCVcc system support these previous findings in the HCV pseudoparticle system. It should be noted that we observed an increased resistance to H06 IgG for SA13/JFH1Core-NS5B,revE2 when comparing IC50s to SA13/JFH1orig (Table 3). In a repeat experiment, we did not observe this difference between IC50s of the two viruses. In the same experiment, we observed a similar difference in IC50s between SA13/JFH1orig and SA13/JFH1Core-NS5B (data not shown). Thus, this apparent resistance was most likely due to variation in the assay shown in Fig. 5 and Table 3. We further demonstrated that the E2 HVR1 mutation T385P confers a significant increase in sensitivity to neutralization by well-defined human monoclonal antibodies AR3A and AR4A (Fig. 5 and Table 3). Both antibodies target conformational epitopes in E2 (AR3A) or E1E2 (AR4A), and AR3A blocks the CD81 binding site on E2; neither epitope overlaps with position 385 (42, 61). Furthermore, the CD81 binding site of E2 seemed to be more accessible in SA13/JFH1Core-NS5B, as this virus was significantly more sensitive to neutralization with sCD81-LEL than was SA13/JFH1Core-NS5B,revE2 (Fig. 5E). Thus, our findings suggest that the E2 T385P mutation might cause a conformational change in the E1E2 heterodimer or that position 385 is involved in shielding of both the AR3A and AR4A epitopes and the CD81 binding site of E2. Supporting the latter hypothesis is the observation made by others that T385 is a putative site for O-linked glycosylation (52, 82). Thus, even though O-linked glycans have to our knowledge not yet been implicated in shielding epitopes, it is possible that T385P disrupts glycan-mediated shielding of immunogenic epitopes (50, 52). Collectively, these data suggest a novel role for O-linked glycosylation at T385 in the shielding of defined neutralizing conformational epitopes, including epitopes involving the CD81 binding site of E2, an important target of several monoclonal antibodies (42).
While T385P had a significant impact on neutralization sensitivity, it did not influence the usage of CD81 as all tested viruses were equally sensitive to antibody-mediated blocking (Fig. 7A). Thus, even though the CD81 binding site of E2 was more accessible for viruses carrying the T385P mutation, this did not influence the binding properties. Furthermore, unlike deletion of HVR1, which seems to reduce the usage of SR-BI for some genotypes (58), T385P, localizing to the second amino acid in HVR1 of E2, did not have any major effects on SR-BI usage (Fig. 7B).
Amino acid changes in E2, as well as deletion of HVR1, have previously been associated with changes in HCVcc biophysical properties, suggested to be due to altered lipoprotein association (43, 44, 53). Since one of the main applications of high-titer HCVcc recombinants would be to facilitate morphological studies of the virus particle, it is of great importance to determine whether the biophysical properties of the virus change with the introduction of putative adaptive amino acid changes in the structural proteins. We did not observe any differences in HCVcc particle density distribution between SA13/JFH1orig, SA13/JFH1p7-NS5B, SA13/JFH1Core-NS5B, SA13/JFH1orig,E2, and SA13/JFH1Core-NS5B,revE2 (Fig. 6), suggesting that the E2 mutation at position 385 or other adaptive mutations described in this study did not affect the morphology of the virus particles. It is possible that minor differences in the compositions of the virus particles would become apparent using more sensitive methods, such as mass spectrometry (83); however, such analyses were considered outside the scope of this study.
In summary, we have demonstrated high-throughput adaptation of a JFH1-based HCVcc recombinant containing Core-NS2 of poorly characterized genotype 5a. We have studied a panel of 13 amino acid changes which were sufficient to confer an increase in viral fitness, probably by enhancing HCV assembly and cell-to-cell transmission. The panel of amino acid changes did not affect HCVcc particle density or usage of coreceptors CD81 and SR-BI. We propose novel functions of a putative O-linked glycosylation site at T385 in E2 in shielding of at least two well-defined neutralizing conformational epitopes as well as the CD81 binding site of E2. We believe that production and characterization of high-titer virus stocks are of great importance for research aimed at vaccine development and morphological characterization that currently involves production of large volumes of HCVcc followed by cumbersome concentration procedures. Thus, we believe that cell culture adaptation of JFH1-based HCVcc recombinants might facilitate improvement of the HCVcc system in order to produce sufficient amounts of virus particles of different genotypes for such studies.
ACKNOWLEDGMENTS
We are grateful to Lubna Ghanem and Lotte Mikkelsen for skilled technical assistance, to Anna-Louise Sørensen for general laboratory support, to Steen Ladelund for assistance in statistics, and to Jens Ole Nielsen, Bjarne Ørskov Lindhardt, and Ove Andersen (all from Copenhagen University Hospital, Hvidovre, Denmark) and Carsten Geisler (University of Copenhagen, Denmark) for their support of the project. We are grateful to Harvey Alter, Suzanne U. Emerson, and Robert H. Purcell (all of the National Institutes of Health, USA), Alfredo Nicosia (CEINGE, University of Naples, Italy), Mansun Law (The Scripps Institute, USA), Steven K. H. Foung (Stanford University, USA), and Charles Rice (Rockefeller University, USA) for providing reagents.
This work was supported by the A. P. Møller and Chastine McKinney-Møllers Foundation for Medical Sciences (J.M.G. and J.B.), Hvidovre Hospital, Research Foundation (C.K.M.), Region Hovedstaden Proof-of-Concept Grants (J.M.G. and J.B.), The Danish Cancer Society (J.M.G. and J.B.), The Danish Council for Independent Research, Medical Sciences (J.B.), The Lundbeck Foundation (J.M.G. and J.B.), The Novo Nordisk Foundation (J.M.G. and J.B.), Novo Nordisk Pre-seed Grant (J.M.G. and J.B.), Ph.D. stipends from the Faculty of Health and Medical Sciences, University of Copenhagen (C.K.M. and T.B.J.), and a postdoctoral stipend from The Danish Council of Independent Research (J.P.). J.B. is the 2014 recipient of an advanced-top researcher grant from the Danish Council for Independent Research and the 2015 recipient of the Novo Nordisk Prize. Research in the McKeating laboratory is funded by the Medical Research Council (G1100247), MRC grant G1100247, EU FP7-funded PathCO HEALTH F3-2012-305578, and Birmingham NIHR BRU.
There is no conflict of interest to be reported.
REFERENCES
- 1.Alter HJ, Seeff LB. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 2.Brown K. 2005. Liver transplantation. Curr Opin Gastroenterol 21:331–336. doi: 10.1097/01.mog.0000159830.36793.2b. [DOI] [PubMed] [Google Scholar]
- 3.Gottwein JM, Bukh J. 2008. Cutting the Gordian knot—development and biological relevance of hepatitis C virus cell culture systems. Adv Virus Res 71:51–133. doi: 10.1016/S0065-3527(08)00002-X. [DOI] [PubMed] [Google Scholar]
- 4.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. 2014. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology 59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajarizadeh B, Grebely J, Dore GJ. 2013. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 6.Antaki N, Craxi A, Kamal S, Moucari R, Van der Merwe S, Haffar S, Gadano A, Zein N, Lai CL, Pawlotsky J-M, Heathcote EJ, Dusheiko G, Marcellin P. 2010. The neglected hepatitis C virus genotypes 4, 5 and 6: an international consensus report. Liver Int 30:342–355. doi: 10.1111/j.1478-3231.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 7.Bukh J, Apgar CL, Engle R, Govindarajan S, Hegerich PA, Tellier R, Wong DC, Elkins R, Kew MC. 1998. Experimental infection of chimpanzees with hepatitis C virus of genotype 5a: genetic analysis of the virus and generation of a standardized challenge pool. J Infect Dis 178:1193–1197. doi: 10.1086/515683. [DOI] [PubMed] [Google Scholar]
- 8.Bukh J, Meuleman P, Tellier R, Engle RE, Feinstone SM, Eder G, Satterfield WC, Govindarajan S, Krawczynski K, Miller RH, Leroux-Roels G, Purcell RH. 2010. Challenge pools of hepatitis C virus genotypes 1–6 prototype strains: replication fitness and pathogenicity in chimpanzees and human liver-chimeric mouse models. J Infect Dis 201:1381–1389. doi: 10.1086/651579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinge CNW, Espiritu C, Prabdial-Sing N, Sithebe NP, Saeed M, Rice CM. 2014. Hepatitis C virus genotype 5a subgenomic replicons for evaluation of direct-acting antiviral agents. Antimicrob Agents Chemother 58:5386–5394. doi: 10.1128/AAC.03534-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 12.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. 2005. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A 102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottwein JM, Jensen SB, Li Y-P, Ghanem L, Scheel TKH, Serre SBN, Mikkelsen L, Bukh J. 2013. Combination treatment with hepatitis C virus protease and NS5A inhibitors is effective against recombinant genotype 1a, 2a, and 3a viruses. Antimicrob Agents Chemother 57:1291–1303. doi: 10.1128/AAC.02164-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmann V, Bartenschlager R. 2014. On the history of hepatitis C virus cell culture systems. J Med Chem 57:1627–1642. doi: 10.1021/jm401401n. [DOI] [PubMed] [Google Scholar]
- 15.Li Y-P, Ramirez S, Humes D, Jensen SB, Gottwein JM, Bukh J. 2014. Differential sensitivity of 5′UTR-NS5A recombinants of hepatitis C virus genotypes 1–6 to protease and NS5A inhibitors. Gastroenterology 146:812–821. doi: 10.1053/j.gastro.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez S, Li Y-P, Jensen SB, Pedersen J, Gottwein JM, Bukh J. 2014. Highly efficient infectious cell culture of three hepatitis C virus genotype 2b strains and sensitivity to lead protease, nonstructural protein 5A, and polymerase inhibitors. Hepatology 59:395–407. doi: 10.1002/hep.26660. [DOI] [PubMed] [Google Scholar]
- 17.Li Y-P, Ramirez S, Mikkelsen L, Bukh J. 2015. Efficient infectious cell culture systems of the hepatitis C virus (HCV) prototype strains HCV-1 and H77. J Virol 89:811–823. doi: 10.1128/JVI.02877-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottwein JM, Scheel TK, Jensen TB, Lademann JB, Prentoe JC, Knudsen ML, Hoegh AM, Bukh J. 2009. Development and characterization of hepatitis C virus genotype 1–7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology 49:364–377. doi: 10.1002/hep.22673. [DOI] [PubMed] [Google Scholar]
- 19.Scheel TKH, Gottwein JM, Mikkelsen LS, Jensen TB, Bukh J. 2011. Recombinant HCV variants with NS5A from genotypes 1–7 have different sensitivities to an NS5A inhibitor but not interferon-α. Gastroenterology 140:1032–1042. doi: 10.1053/j.gastro.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Galli A, Scheel TKH, Prentoe JC, Mikkelsen LS, Gottwein JM, Bukh J. 2013. Analysis of hepatitis C virus core/NS5A protein co-localization using novel cell culture systems expressing core-NS2 and NS5A of genotypes 1–7. J Gen Virol 94:2221–2235. doi: 10.1099/vir.0.053868-0. [DOI] [PubMed] [Google Scholar]
- 21.Gottwein JM, Scheel TK, Hoegh AM, Lademann JB, Eugen-Olsen J, Lisby G, Bukh J. 2007. Robust hepatitis C genotype 3a cell culture releasing adapted intergenotypic 3a/2a (S52/JFH1) viruses. Gastroenterology 133:1614–1626. doi: 10.1053/j.gastro.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Yi M, Ma Y, Yates J, Lemon SM. 2007. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J Virol 81:629–638. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheel TK, Gottwein JM, Jensen TB, Prentoe JC, Hoegh AM, Alter HJ, Eugen-Olsen J, Bukh J. 2008. Development of JFH1-based cell culture systems for hepatitis C virus genotype 4a and evidence for cross-genotype neutralization. Proc Natl Acad Sci U S A 105:997–1002. doi: 10.1073/pnas.0711044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen TB, Gottwein JM, Scheel TKH, Hoegh AM, Eugen-Olsen J, Bukh J. 2008. Highly efficient JFH1-based cell-culture system for hepatitis C virus genotype 5a: failure of homologous neutralizing-antibody treatment to control infection. J Infect Dis 198:1756–1765. doi: 10.1086/593021. [DOI] [PubMed] [Google Scholar]
- 25.Scheel TKH, Gottwein JM, Carlsen THR, Li Y-P, Jensen TB, Spengler U, Weis N, Bukh J. 2011. Efficient culture adaptation of hepatitis C virus recombinants with genotype-specific core-NS2 by using previously identified mutations. J Virol 85:2891–2906. doi: 10.1128/JVI.01605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan K, Cheng G, Beran RKF, Yang H, Appleby TC, Pokrovskii MV, Mo H, Zhong W, Delaney WE IV. 2012. An adaptive mutation in NS2 is essential for efficient production of infectious 1b/2a chimeric hepatitis C virus in cell culture. Virology 422:224–234. doi: 10.1016/j.virol.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Li Y-P, Gottwein JM, Scheel TK, Jensen TB, Bukh J. 2011. MicroRNA-122 antagonism against hepatitis C virus genotypes 1–6 and reduced efficacy by host RNA insertion or mutations in the HCV 5′ UTR. Proc Natl Acad Sci U S A 108:4991–4996. doi: 10.1073/pnas.1016606108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen J, Carlsen THR, Prentoe J, Ramirez S, Jensen TB, Forns X, Alter H, Foung SKH, Law M, Gottwein J, Weis N, Bukh J. 2013. Neutralization resistance of hepatitis C virus can be overcome by recombinant human monoclonal antibodies. Hepatology 58:1587–1597. doi: 10.1002/hep.26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong J, Gastaminza P, Chung J, Stamataki Z, Isogawa M, Cheng G, McKeating JA, Chisari FV. 2006. Persistent hepatitis C virus infection in vitro: coevolution of virus and host. J Virol 80:11082–11093. doi: 10.1128/JVI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaul A, Woerz I, Meuleman P, Leroux-Roels G, Bartenschlager R. 2007. Cell culture adaptation of hepatitis C virus and in vivo viability of an adapted variant. J Virol 81:13168–13179. doi: 10.1128/JVI.01362-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgrange D, Pillez A, Castelain S, Cocquerel L, Rouillé Y, Dubuisson J, Wakita T, Duverlie G, Wychowski C. 2007. Robust production of infectious viral particles in Huh-7 cells by introducing mutations in hepatitis C virus structural proteins. J Gen Virol 88:2495–2503. doi: 10.1099/vir.0.82872-0. [DOI] [PubMed] [Google Scholar]
- 32.Russell RS, Meunier J-C, Takikawa S, Faulk K, Engle RE, Bukh J, Purcell RH, Emerson SU. 2008. Advantages of a single-cycle production assay to study cell culture-adaptive mutations of hepatitis C virus. Proc Natl Acad Sci U S A 105:4370–4375. doi: 10.1073/pnas.0800422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao W, Xu C, Ding Q, Li R, Xiang Y, Chung J, Zhong J. 2009. A single point mutation in E2 enhances hepatitis C virus infectivity and alters lipoprotein association of viral particles. Virology 395:67–76. doi: 10.1016/j.virol.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Dhillon S, Witteveldt J, Gatherer D, Owsianka AM, Zeisel MB, Zahid MN, Rychłowska M, Foung SKH, Baumert TF, Angus AGN, Patel AH. 2010. Mutations within a conserved region of the hepatitis C virus E2 glycoprotein that influence virus-receptor interactions and sensitivity to neutralizing antibodies. J Virol 84:5494–5507. doi: 10.1128/JVI.02153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pokrovskii MV, Bush CO, Beran RKF, Robinson MF, Cheng G, Tirunagari N, Fenaux M, Greenstein AE, Zhong W, Delaney WE, Paulson MS. 2011. Novel mutations in a tissue culture-adapted hepatitis C virus strain improve infectious-virus stability and markedly enhance infection kinetics. J Virol 85:3978–3985. doi: 10.1128/JVI.01760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Xiao L, Nelson C, Hagedorn C. 2012. A cell culture adapted HCV JFH1 variant that increases viral titers and permits the production of high titer infectious chimeric reporter viruses. PLoS One 7:e44965. doi: 10.1371/journal.pone.0044965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, Luo G. 2012. Cell culture-adaptive mutations promote viral protein-protein interactions and morphogenesis of infectious hepatitis C virus. J Virol 86:8987–8997. doi: 10.1128/JVI.00004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horwitz JA, Dorner M, Friling T, Donovan BM, Vogt A, Loureiro J, Oh T, Rice CM, Ploss A. 2013. Expression of heterologous proteins flanked by NS3-4A cleavage sites within the hepatitis C virus polyprotein. Virology 439:23–33. doi: 10.1016/j.virol.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douam F, Lavillette D, Cosset F-L. 2015. The mechanism of HCV entry into host cells. Prog Mol Biol Transl Sci 129:63–107. doi: 10.1016/bs.pmbts.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, Schwarz A, Desombere I, Roels GL, Balfe P, McKeating JA. 2008. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 47:17–24. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- 41.Brimacombe CL, Grove J, Meredith LW, Hu K, Syder AJ, Flores MV, Timpe JM, Krieger SE, Baumert TF, Tellinghuisen TL, Wong-Staal F, Balfe P, McKeating JA. 2011. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol 85:596–605. doi: 10.1128/JVI.01592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahid A, Dubuisson J. 2013. Virus-neutralizing antibodies to hepatitis C virus. J Viral Hepat 20:369–376. doi: 10.1111/jvh.12094. [DOI] [PubMed] [Google Scholar]
- 43.Bankwitz D, Steinmann E, Bitzegeio J, Ciesek S, Friesland M, Herrmann E, Zeisel MB, Baumert TF, Keck Z, Foung SKH, Pécheur E-I, Pietschmann T. 2010. Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol 84:5751–5763. doi: 10.1128/JVI.02200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prentoe J, Jensen TB, Meuleman P, Serre SBN, Scheel TKH, Leroux-Roels G, Gottwein JM, Bukh J. 2011. Hypervariable region 1 differentially impacts viability of hepatitis C virus strains of genotypes 1 to 6 and impairs virus neutralization. J Virol 85:2224–2234. doi: 10.1128/JVI.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen J, Jensen TB, Carlsen THR, Schønning K, Christensen PB, Laursen AL, Krarup H, Bukh J, Weis N. 2013. Neutralizing antibodies in patients with chronic hepatitis C, genotype 1, against a panel of genotype 1 culture viruses: lack of correlation to treatment outcome. PLoS One 8:e62674. doi: 10.1371/journal.pone.0062674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keck Z, Xia J, Wang Y, Wang W, Krey T, Prentoe J, Carlsen T, Li AY-J, Patel AH, Lemon SM, Bukh J, Rey FA, Foung SKH. 2012. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog 8:e1002653. doi: 10.1371/journal.ppat.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong L, Giang E, Robbins JB, Stanfield RL, Burton DR, Wilson IA, Law M. 2012. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proc Natl Acad Sci U S A 109:9499–9504. doi: 10.1073/pnas.1202924109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duan H, Struble E, Zhong L, Mihalik K, Major M, Zhang P, Feinstone S, Feigelstock D. 2010. Hepatitis C virus with a naturally occurring single amino-acid substitution in the E2 envelope protein escapes neutralization by naturally-induced and vaccine-induced antibodies. Vaccine 28:4138–4144. doi: 10.1016/j.vaccine.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 49.Grove J, Nielsen S, Zhong J, Bassendine MF, Drummer HE, Balfe P, McKeating JA. 2008. Identification of a residue in hepatitis C virus E2 glycoprotein that determines scavenger receptor BI and CD81 receptor dependency and sensitivity to neutralizing antibodies. J Virol 82:12020–12029. doi: 10.1128/JVI.01569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helle F, Goffard A, Morel V, Duverlie G, McKeating J, Keck Z-Y, Foung S, Penin F, Dubuisson J, Voisset C. 2007. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol 81:8101–8111. doi: 10.1128/JVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koutsoudakis G, Dragun J, Pérez-del-Pulgar S, Coto-Llerena M, Mensa L, Crespo G, González P, Navasa M, Forns X. 2012. Interplay between basic residues of hepatitis C virus glycoprotein E2 with viral receptors, neutralizing antibodies and lipoproteins. PLoS One 7:e52651. doi: 10.1371/journal.pone.0052651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falkowska E, Kajumo F, Garcia E, Reinus J, Dragic T. 2007. Hepatitis C virus envelope glycoprotein E2 glycans modulate entry, CD81 binding, and neutralization. J Virol 81:8072–8079. doi: 10.1128/JVI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gastaminza P, Dryden KA, Boyd B, Wood MR, Law M, Yeager M, Chisari FV. 2010. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol 84:10999–11009. doi: 10.1128/JVI.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathiesen CK, Jensen TB, Prentoe J, Krarup H, Nicosia A, Law M, Bukh J, Gottwein JM. 2014. Production and characterization of high-titer serum-free cell culture grown hepatitis C virus particles of genotype 1–6. Virology 458-459:190–208. doi: 10.1016/j.virol.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gottwein JM, Scheel TKH, Callendret B, Li Y-P, Eccleston HB, Engle RE, Govindarajan S, Satterfield W, Purcell RH, Walker CM, Bukh J. 2010. Novel infectious cDNA clones of hepatitis C virus genotype 3a (strain S52) and 4a (strain ED43): genetic analyses and in vivo pathogenesis studies. J Virol 84:5277–5293. doi: 10.1128/JVI.02667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gottwein JM, Scheel TKH, Jensen TB, Ghanem L, Bukh J. 2011. Differential efficacy of protease inhibitors against HCV genotypes 2a, 3a, 5a, and 6a NS3/4A protease recombinant viruses. Gastroenterology 141:1067–1079. doi: 10.1053/j.gastro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Serre SBN, Krarup HB, Bukh J, Gottwein JM. 2013. Identification of alpha interferon-induced envelope mutations of hepatitis C virus in vitro associated with increased viral fitness and interferon resistance. J Virol 87:12776–12793. doi: 10.1128/JVI.00901-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prentoe J, Serre SBN, Ramirez S, Nicosia A, Gottwein JM, Bukh J. 2014. Hypervariable region 1 deletion and required adaptive envelope mutations confer decreased dependency on scavenger receptor class B type I and low-density lipoprotein receptor for hepatitis C virus. J Virol 88:1725–1739. doi: 10.1128/JVI.02017-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meuleman P, Bukh J, Verhoye L, Farhoudi A, Vanwolleghem T, Wang RY, Desombere I, Alter H, Purcell RH, Leroux-Roels G. 2011. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology 53:755–762. doi: 10.1002/hep.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, Ball JK, McKeating JA, Kneteman NM, Burton DR. 2008. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med 14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 61.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, Rice CM, Ploss A, Burton DR, Law M. 2012. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A 109:6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlsen THR, Scheel TKH, Ramirez S, Foung SKH, Bukh J. 2013. Characterization of hepatitis C virus recombinants with chimeric E1/E2 envelope proteins and identification of single amino acids in the E2 stem region important for entry. J Virol 87:1385–1399. doi: 10.1128/JVI.00684-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Catanese MT, Graziani R, von Hahn T, Moreau M, Huby T, Paonessa G, Santini C, Luzzago A, Rice CM, Cortese R, Vitelli A, Nicosia A. 2007. High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J Virol 81:8063–8071. doi: 10.1128/JVI.00193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. 1998. Binding of hepatitis C virus to CD81. Science 282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 65.Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J 21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thi VLD, Granier C, Zeisel MB, Guerin M, Mancip J, Granio O, Penin F, Lavillette D, Bartenschlager R, Baumert TF, Cosset F-L, Dreux M. 2012. Characterization of hepatitis C virus particle sub-populations reveals multiple usage of the scavenger receptor BI for entry steps. J Biol Chem 287:31242–31257. doi: 10.1074/jbc.M112.365924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carlsen THR, Pedersen J, Prentoe JC, Giang E, Keck Z-Y, Mikkelsen LS, Law M, Foung SKH, Bukh J. 2014. Breadth of neutralization and synergy of clinically relevant human monoclonal antibodies against HCV genotypes 1a, 1b, 2a, 2b, 2c, and 3a. Hepatology 60:1551–1562. doi: 10.1002/hep.27298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset F-L, Bartenschlager R. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A 103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu J, Tao W, Li R, Xiang Y, Zhang N, Xiang X, Xie Q, Zhong J. 2013. Construction and characterization of infectious hepatitis C virus chimera containing structural proteins directly from genotype 1b clinical isolates. Virology 443:80–88. doi: 10.1016/j.virol.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 70.de la Fuente C, Goodman Z, Rice CM. 2013. Genetic and functional characterization of the N-terminal region of the hepatitis C virus NS2 protein. J Virol 87:4130–4145. doi: 10.1128/JVI.03174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuiken C, Yusim K, Boykin L, Richardson R. 2005. The Los Alamos hepatitis C sequence database. Bioinformatics 21:379–384. doi: 10.1093/bioinformatics/bth485. [DOI] [PubMed] [Google Scholar]
- 72.Appel N, Zayas M, Miller S, Krijnse-Locker J, Schaller T, Friebe P, Kallis S, Engel U, Bartenschlager R. 2008. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog 4:e1000035. doi: 10.1371/journal.ppat.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tellinghuisen TL, Foss KL, Treadaway JC, Rice CM. 2008. Identification of residues required for RNA replication in domains II and III of the hepatitis C virus NS5A protein. J Virol 82:1073–1083. doi: 10.1128/JVI.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masaki T, Suzuki R, Murakami K, Aizaki H, Ishii K, Murayama A, Date T, Matsuura Y, Miyamura T, Wakita T, Suzuki T. 2008. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J Virol 82:7964–7976. doi: 10.1128/JVI.00826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jirasko V, Montserret R, Lee JY, Gouttenoire J, Moradpour D, Penin F, Bartenschlager R. 2010. Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog 6:e1001233. doi: 10.1371/journal.ppat.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim S, Welsch C, Yi M, Lemon SM. 2011. Regulation of the production of infectious genotype 1a hepatitis C virus by NS5A domain III. J Virol 85:6645–6656. doi: 10.1128/JVI.02156-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boson B, Granio O, Bartenschlager R, Cosset F-L. 2011. A concerted action of hepatitis C virus P7 and nonstructural protein 2 regulates core localization at the endoplasmic reticulum and virus assembly. PLoS Pathog 7:e1002144. doi: 10.1371/journal.ppat.1002144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gouklani H, Bull RA, Beyer C, Coulibaly F, Gowans EJ, Drummer HE, Netter HJ, White PA, Haqshenas G. 2012. Hepatitis C virus nonstructural protein 5B is involved in virus morphogenesis. J Virol 86:5080–5088. doi: 10.1128/JVI.07089-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Afzal MS, Alsaleh K, Farhat R, Belouzard S, Danneels A, Descamps V, Duverlie G, Wychowski C, Zaidi NUSS, Dubuisson J, Rouillé Y. 2015. Regulation of core expression during the hepatitis C virus life cycle. J Gen Virol 96:311–321. doi: 10.1099/vir.0.070433-0. [DOI] [PubMed] [Google Scholar]
- 80.Blish CA, Nguyen M-A, Overbaugh J. 2008. Enhancing exposure of HIV-1 neutralization epitopes through mutations in gp41. PLoS Med 5:e9. doi: 10.1371/journal.pmed.0050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang G-Y, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko S-Y, Todd J-P, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bräutigam J, Scheidig AJ, Egge-Jacobsen W. 2013. Mass spectrometric analysis of hepatitis C viral envelope protein E2 reveals extended microheterogeneity of mucin-type O-linked glycosylation. Glycobiology 23:453–474. doi: 10.1093/glycob/cws171. [DOI] [PubMed] [Google Scholar]
- 83.Merz A, Long G, Hiet M-S, Brugger B, Chlanda P, Andre P, Wieland F, Krijnse-Locker J, Bartenschlager R. 2011. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem 286:3018–3032. doi: 10.1074/jbc.M110.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]