Abstract
Cys-loop receptors are the site of action of many therapeutic drugs. One of these is the smoking cessation agent varenicline, which has its major therapeutic effects at nicotinic acetylcholine (nACh) receptors but also acts at 5-HT3 receptors. Here, we report the X-ray crystal structure of the 5-HT binding protein (5-HTBP) in complex with varenicline, and test the predicted interactions by probing the potency of varenicline in a range of mutant 5-HT3 receptors expressed in HEK293 cells and Xenopus oocytes. The structure reveals a range of interactions between varenicline and 5-HTBP. We identified residues within 5 Å of varenicline and substituted the equivalent residues in the 5-HT3 receptor with Ala or a residue with similar chemical properties. Functional characterization of these mutant 5-HT3 receptors, using a fluorescent membrane potential dye in HEK cells and voltage clamp in oocytes, supports interactions between varenicline and the receptor that are similar to those in 5-HTBP. The structure also revealed C-loop closure that was less than in the 5-HT-bound 5-HTBP, and hydrogen bonding between varenicline and the complementary face of the binding pocket via a water molecule, which are characteristics consistent with partial agonist behavior of varenicline in the 5-HT3 receptor. Together, these data reveal detailed insights into the molecular interaction of varenicline in the 5-HT3 receptor.
Keywords: Ligand-gated ion channel, Cys-loop receptor, serotonin receptor
The 5-HT3 receptors belong to the Cys-loop family of pentameric ligand-gated ion channels, a family that also includes nicotinic acetylcholine (nACh), GABA, and glycine receptors.1 They play important roles in fast neurotransmission in the central and peripheral nervous systems. There are five 5-HT3 receptor subtypes (5-HT3A–5-HT3E). Subunits of 5-HT3A can assemble to form homopentamers, but the other subtypes must coassemble with 5-HT3A subunits to form functional receptors. However, it is as yet unclear what effect subunits 5-HT3C-5-HT3E have on the receptor function.2,3
Varenicline is a high affinity partial agonist of the α4β2 nACh receptor in clinical use for smoking cessation.4 It is also a full agonist at the α7 nACh receptor.5 There is considerable overlap between compounds that act at α7 nACh and 5-HT3 receptors,6 and recently, we have shown that varenicline is also a high affinity agonist at the human 5-HT3 receptor.7 5-HT3 receptors are located in the chemoreceptor trigger zone in the area postrema and in the gastrointestinal tract, where they have roles in regulating gut motility and the emesis reflex.8 Antagonists of 5-HT3 receptors, such as ondansetron and palonosetron, are in clinical use as antiemetics to treat postoperative nausea and vomiting and chemotherapy-induced nausea and vomiting.9 Nausea is a common adverse effect reported from patients receiving varenicline,4 and it is likely that this is due to its actions at the 5-HT3 receptor. The design of improved smoking cessation drugs that do not have such cross-reactivity at the 5-HT3 receptor would clearly be of benefit.
For this aim to be achieved, further information is needed about the structure–activity relationships of varenicline binding to different receptors. Here, we present the structure of varenicline bound to 5-HTBP, an acetylcholine binding protein (AChBP) from Aplysia californica (Ac) engineered to bind 5-HT with high affinity.10 This protein carries the mutations S92E, V140L, K141T, and Q55R (A1B2D1R) and displays affinities for the 5-HT3 receptor antagonist granisetron and for 5-HT that are 21- and 4.3-fold higher than for native AChBP, respectively. We have also performed a thorough mutagenesis study on the human 5-HT3 receptor of residues predicted to be near the ligand-binding site, characterizing these mutants both in HEK293 cells using a fluorescent membrane potential dye and in oocytes using two-electrode voltage clamp electrophysiology. The data suggest that 5-HTBP is a good structural model for examining ligand binding interactions, and functional experiments on the 5-HT3 receptors yield additional information on binding modes that cannot readily be ascertained from structural information.
Results and Discussion
X-ray Crystal Structure of Varenicline-Bound 5-HTBP
Several crystals were obtained from crystallization screens. One of these crystals diffracted to a resolution of 2.3 Å, and a complete diffraction data set was collected, which allowed for the determination of the three-dimensional structure of 5-HTBP in complex with varenicline. The crystal belongs to the space group P41212, and the crystallographic unit cell has the dimensions 72.8 Å (a), 72.8 Å (b), 479.22 Å (c), 90° (α), 90° (β), 90° (γ) and contains one pentamer per asymmetric unit. The electron density map was of excellent quality and difference density could be clearly observed for varenicline in all five orthosteric ligand binding sites (Figure S1, Supporting Information). The orientation of varenicline in this structure is similar to that in the two structures of varenicline bound to AChBP11,12 (Figure S2, Supporting Information). In these structures, however, varenicline is slightly further away from the principal binding face.
Varenicline Interactions at the Principal Binding Face
Varenicline is located at the interface between two subunits and has interactions on the principal face with aromatic residues, which are conserved within the Cys-loop family. The 5-HTBP–varenicline structure shows hydrogen bonds between the benzazepine nitrogen of varenicline and the hydroxyl of Y91 (loop A) and the backbone carbonyl of W145 (loop B), and one a pyrazine nitrogen hydrogen bonds to Y193 (loop C) (Figure 1). The equivalent of these and other residues within 5 Å of bound varenicline were mutated in the 5-HT3 receptor to verify the orientation of varenicline; substitutions were made to residues N128 in loop A, W183 and L184 in loop B, and F226, M228, E229, and Y234 in loop C. The parameters obtained from concentration response curves determined from these mutant receptors expressed in HEK cells are in Tables 1 and 2, and typical responses and curves are shown in Figure 2.
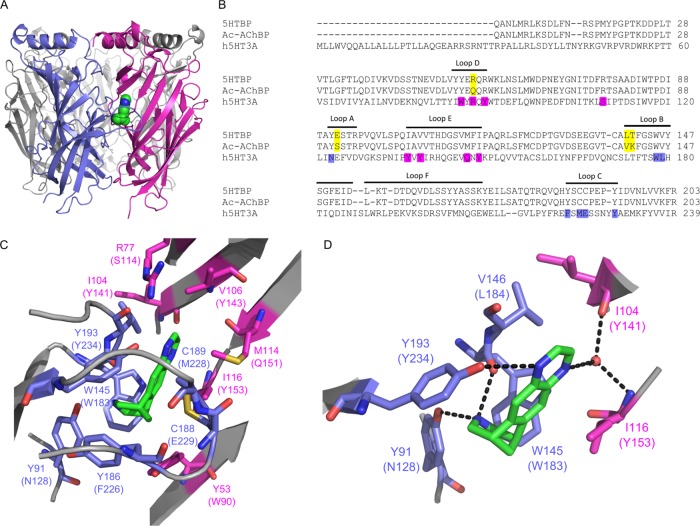
Figure 1.
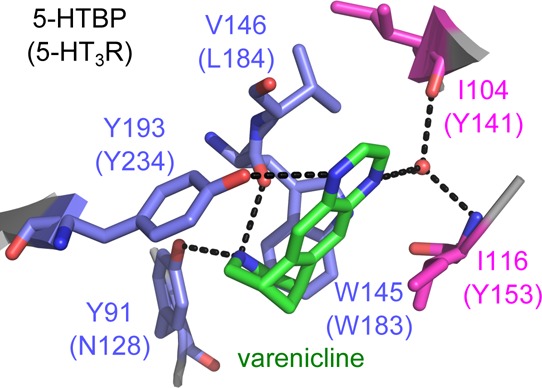
Varenicline bound to 5-HTBP. (A) Location of varenicline (green) at the interface between two subunits in the orthosteric 5-HTBP binding site. (B) Alignment of 5-HTBP, AChBP, and the extracellular domain of the 5-HT3A receptor subunit showing the approximate location of the A–E binding loops. The residues mutated in this study are highlighted in purple, and the residues that differ between 5-HTBP and AChBP in yellow. (C) The 5-HTBP binding pocket showing the orientation of varenicline (green) and nearby residues on the principal (blue) and complementary (magenta) faces. The corresponding 5-HT3 receptor residues are in parentheses. (D) Hydrogen bonds are present between varenicline and residues Y91, Y193, and W145 on the principal face and residues I104 and I116 on the complementary face via a water molecule.
Table 1. Concentration Response Parameters of 5-HT Obtained from HEK Cells.
| loop | mutant | 5-HT pEC50a (M) | 5-HT EC50 (μM) | n | |
|---|---|---|---|---|---|
| WT | 6.51 ± 0.03 | 0.31 | 5 | ||
| A | N128 | N128A | 5.47 ± 0.10b | 3.4 | 4 |
| N128Q | 4.53 ± 0.02b | 29 | 4 | ||
| B | W183 | W183A | 3.88 ± 0.05b | 130 | 4 |
| W183Y | 4.60 ± 0.03b | 25 | 5 | ||
| B | L184 | L184A | NRc | NRc | 6 |
| L184I | 5.56 ± 0.05b | 2.8 | 4 | ||
| C | F226 | F226A | 4.18 ± 0.05b | 170 | 6 |
| F226Y | 5.72 ± 0.06b | 1.9 | 7 | ||
| C | M228 | M228A | 5.03 ± 0.04b | 9.4 | 4 |
| M228C | NRc | NRc | 6 | ||
| C | E229 | E229A | 5.63 ± 0.04b | 2.3 | 6 |
| E229D | 6.58 ± 0.05 | 0.27 | 3 | ||
| C | Y234 | Y234A | NRc | NRc | 6 |
| Y234F | 5.89 ± 0.03b | 1.3 | 4 | ||
| Y234S | NRc | NRc | 6 | ||
| D | W90 | W90A | NRc | NRc | 6 |
| W90Y | 5.66 ± 0.05b | 2.2 | 5 | ||
| D | R92 | R92A | 6.06 ± 0.04b | 0.87 | 4 |
| R92Q | 4.82 ± 0.04b | 15 | 4 | ||
| D | Y94 | Y94A | 7.24 ± 0.03b | 0.058 | 5 |
| Y94F | 6.84 ± 0.04 | 0.14 | 6 | ||
| Y94S | 7.03 ± 0.06b | 0.093 | 6 | ||
| S114 | S114A | 6.31 ± 0.05 | 0.48 | 7 | |
| S114T | 5.87 ± 0.27b | 1.4 | 3 | ||
| E | Y141 | Y141A | NRc | NRc | 6 |
| Y141F | 6.83 ± 0.03 | 0.15 | 3 | ||
| E | Y143 | Y143A | NRc | NRc | 6 |
| Y143F | 4.98 ± 0.09b | 11 | 4 | ||
| Y143S | 4.35 ± 0.03b | 45 | 3 | ||
| E | Q151 | Q151A | 6.13 ± 0.06 | 0.75 | 4 |
| Q151N | 6.34 ± 0.03 | 0.45 | 3 | ||
| E | Y153 | Y153A | 4.73 ± 0.03b | 19 | 3 |
| Y153F | 5.54 ± 0.03b | 2.9 | 3 | ||
| Y153S | 5.32 ± 0.06b | 4.8 | 3 |
Data = mean ± SEM.
Significantly different (p < 0.05) than that of WT 5-HT3A receptors.
NR = no response at 100 mM 5-HT.
Table 2. Varenicline Concentration Response Parameters Obtained from HEK Cells.
| loop | mutant | varenicline pEC50a (M) | varenicline EC50 (μM) | n | |
|---|---|---|---|---|---|
| WT | 6.38 ± 0.02 | 0.42 | 5 | ||
| A | N128 | N128A | 5.36 ± 0.05b | 4.4 | 3 |
| N128Q | 4.58 ± 0.03b | 26 | 3 | ||
| B | W183 | W183A | NRc | NRc | 6 |
| W183Y | 4.43 ± 0.04b | 38 | 5 | ||
| B | L184 | L184A | NRc | NRc | 6 |
| L184I | 5.45 ± 0.04b | 3.6 | 5 | ||
| C | F226 | F226A | 4.36 ± 0.12b | 44 | 3 |
| F226Y | 5.99 ± 0.05b | 1.0 | 3 | ||
| C | M228 | M228A | 4.53 ± 0.04b | 30 | 3 |
| M228C | NRc | NRc | 6 | ||
| C | E229 | E229A | 5.38 ± 0.18b | 4.2 | 3 |
| E229D | 5.98 ± 0.05b | 1.1 | 3 | ||
| C | Y234 | Y234A | NRc | NRc | 6 |
| Y234F | 5.96 ± 0.04b | 1.1 | 4 | ||
| Y234S | NRc | NRc | 6 | ||
| D | W90 | W90A | NRc | NRc | 6 |
| W90Y | 5.54 ± 0.03b | 2.9 | 4 | ||
| D | R92 | R92A | 6.49 ± 0.06 | 0.39 | 4 |
| R92Q | 5.19 ± 0.03b | 6.4 | 4 | ||
| D | Y94 | Y94A | 6.89 ± 0.02b | 0.13 | 5 |
| Y94F | 6.43 ± 0.02 | 0.37 | 5 | ||
| Y94S | 6.82 ± 0.04b | 0.15 | 6 | ||
| S114 | S114A | 6.16 ± 0.05 | 0.95 | 3 | |
| S114T | 5.36 ± 0.05b | 4.3 | 4 | ||
| E | Y141 | Y141A | NRc | NRc | 6 |
| Y141F | 6.56 ± 0.07 | 0.28 | 4 | ||
| E | Y143 | Y143A | NRc | NRc | 6 |
| Y143F | SRd | SRd | 6 | ||
| Y143S | SRd | SRd | 6 | ||
| E | Q151 | Q151A | 6.22 ± 0.05 | 0.61 | 3 |
| Q151N | 5.67 ± 0.06b | 2.1 | 4 | ||
| E | Y153 | Y153A | 5.03 ± 0.04b | 9.4 | 3 |
| Y153F | 5.50 ± 0.06b | 3.2 | 3 | ||
| Y153S | 5.38 ± 0.04b | 4.2 | 3 |
Data = mean ± SEM.
Significantly different (p < 0.05) than that of WT 5-HT3A receptors.
NR = no response at 100 mM varenicline
SR = responses too small to obtain parameters.
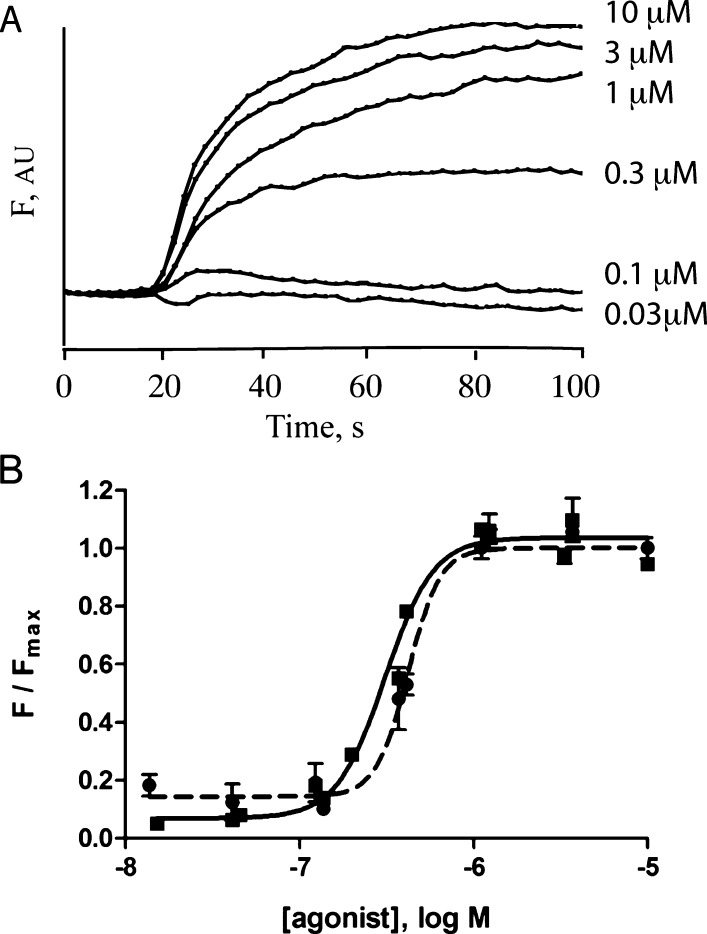
Figure 2.
HEK cell data. (A) Typical traces from HEK cells transfected with WT 5-HT3 receptor cDNA, loaded with membrane potential dye, and stimulated at 20 s with various concentrations of varenicline. F = arbitrary fluorescent units. (B) Concentration–response curves constructed from FlexStation responses to 5-HT (squares, filled line) and varenicline (circles, dashed line). Data = mean ± SEM, n = 4.
The functional data from 5-HT3N128A receptors support the presence of a hydrogen bond here, as observed in 5-HTBP, because the EC50 in this mutant is increased 10-fold compared to that of the WT. However, an even larger change in the varenicline EC50 was seen with N128Q, which was unexpected as this residue also has hydrogen bonding ability. We propose that this larger amino acid is positioned incorrectly and is unable to form an H bond. Examination of the published 5-HT3 receptor structure13 reveals that N128 is probably too far from the center of the binding site to form a hydrogen bond with smaller ligands, but this structure is in an unbound (apo) state and thus movements induced by agonist binding could bring N128 within hydrogen bonding distance. Such movement would be consistent with previous studies that show this residue is important for gating but not binding.14
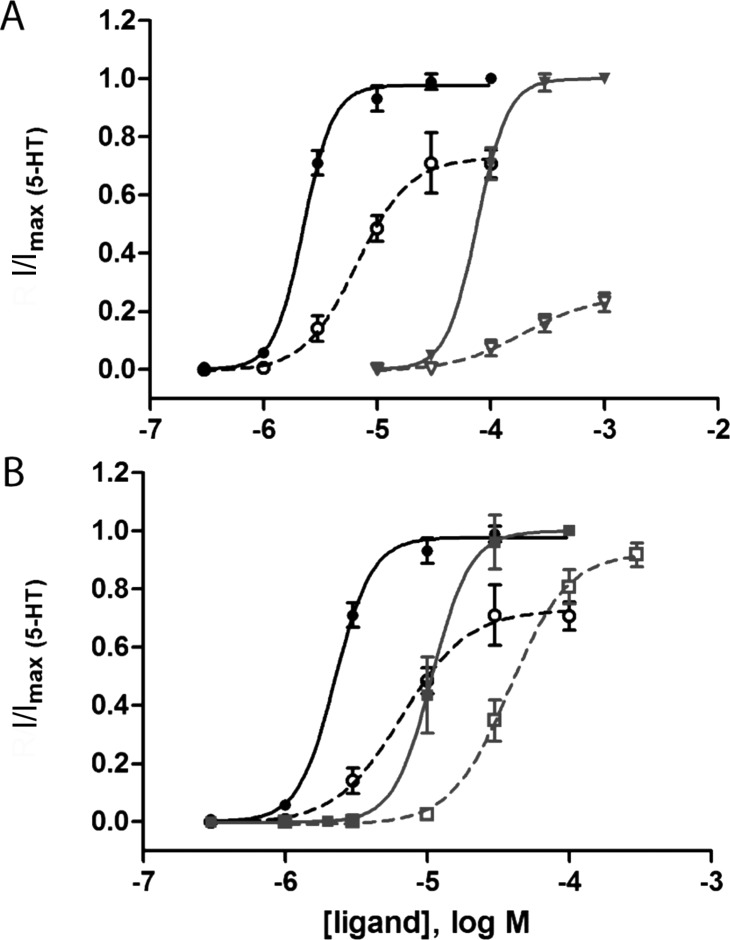
The Trp residue in loop B forms cation-π interactions with various agonists in a number of Cys-loop receptors (including 5-HT and 5-HT315). In 5-HTBP, we observe such an interaction between this residue and the protonated benzazepine nitrogen of varenicline. The same interaction in 5-HT3 receptors is supported by a lack of function in 5-HT3W183A receptors and is also consistent with data from varenicline studies at the α4β2 nACh receptor,16 although varenicline does not participate in a cation-π interaction with the TrpB residue in the α7 nACh receptor.17 Another important loop B residue is L184. Substitution of this residue to Ile resulted in an ∼10 fold increase in EC50 with receptors expressed in HEK cells, but no response was observed when we substituted this residue with Ala. We did, however, observe responses in oocytes (Tables 3 and 4), which revealed a 46-fold increase in EC50 and a decrease in Rmax/Rmax,5-HT from 0.73 to 0.26 compared to those of WT receptors (Figure 3). A previous study has shown that a backbone L184 mutation causes partial loss of function and converts the partial agonist mCPBG into an antagonist.18 There is also increasing evidence that loop B is important for the structure rather than the function of the receptor.19 We therefore suggest that the role of L184 may be to define the correct structure of the binding site.
Table 3. Concentration Response Parameters of 5-HT Obtained from Oocytes.
| mutant | 5-HT pEC50a (M) | 5-HT EC50 (μM) | n | 5-HT fold change (EC50,mut/EC50,WT) |
|---|---|---|---|---|
| WT | 5.65 ± 0.03 | 2.2 | 5 | |
| L184A | 4.12 ± 0.03b | 76 | 5 | 40 |
| W90Y | 4.97 ± 0.04b | 11 | 3 | 5 |
| Y143F | 4.78 ± 0.10b | 17 | 4 | 7.7 |
| Y143S | 4.22 ± 0.09b | 60 | 4 | 27 |
| Y153F | 4.66 ± 0.04b | 22 | 4 | 10 |
Data = mean ± SEM.
Significantly different (p < 0.05) than that of WT 5-HT3A receptors.
Table 4. Varenicline Concentration Response Parameters Obtained from Oocytes.
| mutant | varenicline pEC50a (M) | varenicline EC50 (μM) | varenicline fold change (EC50,mut/EC50,WT) | varenicline efficacy (Rmax/Rmax,5-HT) |
|---|---|---|---|---|
| WT | 5.18 ± 0.08 | 6.6 | 0.73 ± 0.05 | |
| L184A | 3.71 ± 0.03b | 193 | 46 | 0.26 ± 0.03b |
| W90Y | 4.42 ± 0.05b | 38 | 5.8 | 0.92 ± 0.05b |
| Y143F | 4.01 ± 0.07b | 97 | 15 | 0.13 ± 0.01b |
| Y143S | 3.85 ± 0.10b | 142 | 22 | 0.33 ± 0.04b |
| Y153F | 4.55 ± 0.05b | 28 | 4.2 | 0.76 ± 0.04 |
Data = mean ± SEM.
Significantly different (p < 0.05) than that of WT 5-HT3A receptors.
Figure 3.
Concentration response curves for WT and mutant 5-HT3 receptors expressed in oocytes. Concentration response curves for (A) 5-HT3A L184A and (B) 5-HT3A W90Y mutant receptors (gray lines) compared to WT 5-HT3A receptors (black lines) for 5-HT (solid lines) and varenicline (dashed lines) reveal differences in EC50 and Rmax/Rmax,5-HT. Data = mean ± SEM, n = 3–6.
The data show that the aromatic rings of the loop C residues F226 and Y234 are important. Conservation of aromaticity in the 5-HT3F226Y receptor had little effect on the EC50, whereas a non-aromatic residue ablated agonist-gated currents, suggesting a critical hydrophobic interaction. The important role of Y234 has been shown in many studies, and the data were similar here. Both an aromatic and a hydroxyl group play a role: removal of the former in 5-HT3Y234A receptors ablated function, and removal of the latter in 5-HT3Y234F receptors increased the EC50. In 5-HTBP, the equivalent residue forms a hydrogen bond with varenicline, and we propose a similar role here. In the α7 nACh receptor, however, the residue equivalent to Y234 (TyrC2) is not important for binding varenicline (although it is involved in the binding of ACh and epibatidine).17,20 In the previously reported varenicline-bound AChBP structures (4AFG11 and 4AFT12), varenicline is further away from this residue; a H bond is only predicted in one of the five binding sites for Ac-AChBP (4AFT) and may occur via a bridging water molecule in one of the Capitella telata–AChBP sites (Figure S2, Supporting Information). Thus, it seems that varenicline may have different interactions with different receptors.
Varenicline Interactions at the Complementary Binding Face
The structure revealed hydrogen bonds via a water molecule to the backbone OH of I104 and NH of I116 (loop E), and Y53 forms part of the aromatic box that surrounds varenicline. To probe interactions with these and other residues in the 5-HT3 receptor, we mutated W90, R92, and Y94 in loop D, S114 (not in a binding loop), and Y141, Y143, Q151, and Y153 in loop E, all of which are within 5 Å of varenicline.
Of the loop D residues, W90 (equivalent to 5-HTBP Y53) appears to be the most important for functional responses to 5-HT and varenicline. W90 has been previously investigated in mouse 5-HT3 receptors, and its aromatic group is essential for binding.21 Here, we show that substitution with Tyr, which has only a small effect on EC50, significantly increases the efficacy of varenicline, making it a full agonist (Figure 3, Table 4). In the α4β2 nACh receptor, mutation of the equivalent Trp residue also increased efficacy, in this case from 15 to 125%.11 A different effect was observed in the α7 nACh receptor, where the equivalent mutation causes the EC50 of varenicline to increase much more than it does for ACh.17 These agonist-specific effects at multiple receptors indicate that this residue may be an important determinant of affinity and efficacy across the superfamily, even though it may interact differently with different ligands.
Substitution of the loop E residues Y141, Y143, and Y153 causes large changes in EC50 or even ablates function. Of these residues, Y143 appears to play the biggest role as mutating this residue in the 5-HT3 receptor has large effects on both the EC50 and the Rmax of varenicline, which is only 13% in Y143F mutant receptors. Previously, we suggested that a hydrogen bond formed by this residue was essential for receptor gating.22 With the benefit of the recent high-resolution structure of the 5-HT3 receptor,13 we can see that the Y143 hydroxyl does indeed form hydrogen bonds to D189 and T186 backbone atoms across the binding interface (Figure 4). Because both of these residues have been previously identified as important for receptor function,23,24 it seems likely that a network of hydrogen bonds in this region are critical for receptor gating transitions. This network may also involve Y141, which forms hydrogen bonds with the backbone of V122, also across the interface, although the hydroxyl group of this residue appears to be less critical.22
Figure 4.
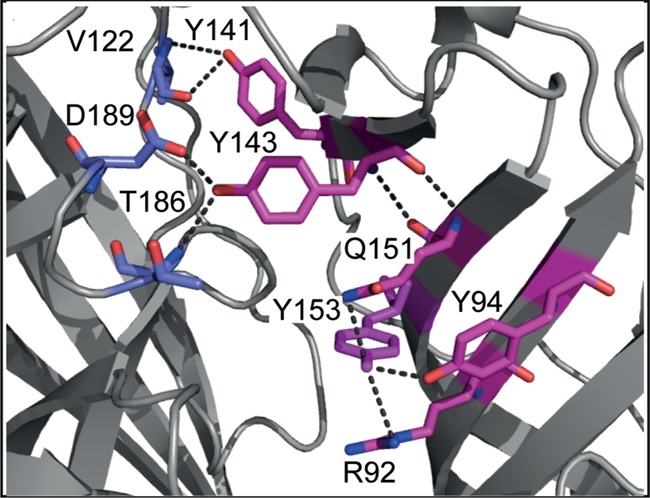
The 5-HT3 receptor loop E region. Structural data from the mouse 5-HT3A receptor structure (PDB: 4PIR) reveal a network of hydrogen bonds involving the loop E aromatic residues Y141, Y143, and Y153. Functional data show the importance of these residues for proper functioning of the receptor.
The hydroxyl group of Y153 is also important; its removal through the Y153F mutation caused 4–10 fold changes in EC50. The structure of the 5-HT3 receptor13 shows this group forming hydrogen bond interactions with other residues (Q151, R92, and Y94; Figure 4) on the complementary face. The residue equivalent to Y153 has been extensively studied in AChBP and in nACh receptors. In AChBP, many structures with bound ligands show direct or water-mediated hydrogen bonds to the backbone NH of this residue, including the varenicline-bound Ct-AChBP structure11 (Figure S2B, Supporting Information), and experimental evidence for such an interaction with the backbone NH of the equivalent residue in α4β2 nACh receptors has been demonstrated using unnatural amino acid mutagenesis.25 In the α7 nACh receptor, the equivalent mutation shows that the EC50 for varenicline increases greater than 10-fold compared to that of ACh.17 Thus, different interactions with this residue in different receptors likely contribute to drug selectivity and the observed differences in efficacy.
Possible Explanations for the Partial Agonist Behavior of Varenicline
There is substantial evidence that agonists and antagonists induce different conformational changes in the region of the C-loop with agonists causing C-loop closure around the bound ligand.26,27 There is also increasing evidence that partial agonists are unable to achieve the same extent of C-loop closure seen with full agonists,28 although there do appear to be some exceptions.12,26 In the varenicline-bound structure presented here, there appears to be a small increase in the C188–W145 distance compared to that of the 5-HT bound structure. The distance between the γ-sulfur of C188 and the carbonyl oxygen of W145 was found to be 7.34 Å (an average from 5 binding sites) compared to an average distance of 6.89 Å for the 10 binding sites in the 5-HT-bound 5-HTBP structure (PDB: 2YMD), indicating that the C-loop in 5-HTBP contracts more fully over 5-HT than varenicline (although we cannot show statistical significance here due to errors associated with the resolution and R-factor). A similarly increased distance has been observed for the varenicline-bound relative to the ACh-bound AChBP structure.11 This intermediate level of closure of the C-loop could provide an explanation for the partial agonism achieved by this drug at the 5-HT3 receptor. It may be, however, that the partial agonism is due to differences in interactions with the complementary face; Rohde et al.29 did not observe large differences in the C188–W145 distances of a range of structures of partial agonists with varying efficacies, but did report a correlation between the strength of interactions with residues on the complementary face and the degree of efficacy. Thus, it may be that the difference between water-mediated and direct hydrogen bonds to complementary face residues with varenicline and 5-HT, respectively, results in the partial agonist activity of the former.
Conclusions
In conclusion, we show here a high resolution structure of 5-HTBP in complex with varenicline. Substitution and functional characterization of 5-HT3 receptor residues that are equivalent to those observed in the 5-HTBP binding site suggest that the orientation of varenicline is similar in 5-HT3 receptors, where it is a reasonably potent albeit a partial agonist. Differences in the extent of C-loop closure and/or differences in interactions with the complementary face residues could explain this partial agonist activity.
Methods
Protein Expression and Crystallization
5-HTBP was expressed and purified as previously described.10 A stock solution of varenicline tartrate (Sigma) was made in water at a concentration of 500 mM and mixed with 6 mg/mL 5-HTBP to yield a final varenicline concentration of 5 mM. Cryoprotection of the crystals was achieved by equilibration of the mother liquor in 5% increments of glycerol to a final concentration of 30% glycerol, and crystals were immersed in liquid nitrogen prior to X-ray diffraction data collection. The structure was determined by molecular replacement using the published structure of 5-HTBP as the template (PDB: 2YMD). After placing the ligand into the electron density map, the model of the complex was manually rebuilt and refined through iterative cycles in Coot and Phenix. The final model has an Rwork value of 18.7% and Rfree value of 24.4%. Model validation was carried out in Molprobity, and the model has a score of 1.75, which is in the 97th percentile for this resolution range. Figures were prepared with Pymol.
Mutagenesis
All mutant receptors were created using QuikChange mutagenesis (Agilent). Residue numbering was altered to be consistent with mouse 5-HT3 receptor numbering.
Cell Culture
Human embryonic kidney (HEK) 293 cells were maintained on 90 mm tissue culture plates at 37 °C and 7% CO2 in a humidified atmosphere. They were cultured in Dulbecco’s Modified Eagle’s Medium/Nutrient Mix F12 (1:1) with GlutaMAX I media (Life Technologies, Paisley, UK) containing 10% HyClone fetal calf serum (GE Healthcare). For FlexStation studies, cells were transfected using polyethylenimine (PEI; Polysciences). PEI (30 μL at 1 mg/mL), cDNA (5 μL at 1 mg/mL; subcloned into pcDNA3.1), and DMEM (1 mL) were incubated for 10 min at room temperature, added dropwise to a 70–90% confluent plate, and incubated for 2 days. Cells were then transferred to poly-l-lysine (Cultrex)-coated 96-well plates and allowed to adhere overnight before use.
FlexStation Analysis
The methods were used essentially as described previously.30 In brief, fluorescent membrane potential dye (Membrane Potential Blue kit, Molecular Devices) was diluted in flex buffer (10 mM HEPES, 115 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM glucose, pH 7.4), and 100 μL was added to each well of transfected cells. The cells were incubated at 37 °C for 45 min, and then fluorescence was measured in a FlexStation 3 microplate reader (Molecular Devices) at 2 s intervals for 200 s. Varenicline tartrate (Tocris) or 5-HT (Sigma) was added to each well after 20 s. The change in fluorescence (ΔF) was defined as Fmax (peak fluorescence) – Fmin (baseline fluorescence). Data were normalized to the maximum ΔF with the highest concentration of 5-HT. Concentration–response data were fitted to the four-parameter logistic equation using Prism (GraphPad Software Inc., San Diego, CA).
Oocyte Maintenance and RNA Preparation
Xenopus laevis oocytes were purchased from Ecocyte Bioscience (Austin, TX) and stored in Barth’s solution (88 mM NaCl, 2.4 mM NaHCO3, 1 mM KCl, 0.33 mM Ca(NO3)·4H2O, 0.41 mM CaCl2·2H2O, 0.82 mM MgSO4·7H2O, and 5 mM Tris/HCl, pH 7.5) containing 2.5 mM sodium pyruvate, 50 mM gentamicin, and 0.7 mM theophylline. RNA was transcribed in vitro from Sph I-linearized plasmid cDNA template using the mMessage mMachine T7 transcription kit (Ambion, Austin, TX). Oocytes were injected with 5–20 ng of cRNA, and currents were recorded 1–2 days post injection.
Electrophysiology
Oocytes were clamped at −60 mV using a Roboocyte (Multi Channel Systems), an automated two-electrode voltage clamp workstation. Concentration–response data for each oocyte were normalized to the maximum current for that oocyte. The mean and SEM for a series of oocytes were plotted against agonist concentration and iteratively fitted to the four-parameter logistic equation using Prism. Values are presented as mean ± SEM.
Acknowledgments
We thank local contacts at beamline ID23-1 of the European Synchrotron Radiation Facility (ESRF, Grenoble) for assistance during data collection. Structure factors and atomic coordinates are deposited with the Protein Data Bank (PDB ID = 5AIN).
Glossary
Abbreviations:
- 5-HT
5-hydroxytryptamine
- nACh receptor
nicotinic acetylcholine
- GABA
gamma-aminobutyric acid
- HEK
human embryonic kidney
- AChBP
acetylcholine binding protein
Supporting Information Available
Details of the crystallographic data, an image of the electron density of varenicline bound in 5-HTBP, and an image showing varenicline bound in AChBP. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
Participated in research design: S.C.R.L., K.L.P., and C.U. Conducted experiments: R.K.L., K.L.P., C.U., and S.C.R.L. Performed data analysis: R.K.L., K.L.P., C.U., S.C.R.L. Wrote or contributed to the writing of the manuscript: S.C.R.L., K.L.P., and C.U.
Supported by grants from the Wellcome Trust (81925) and the MRC to S.C.R.L.
The authors declare no competing financial interest.
Supplementary Material
References
- Lummis S. C. (2012) 5-HT3 receptors. J. Biol. Chem. 287, 40239–40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler B.; Walstab J.; Combrink S.; Möller D.; Kapeller J.; Rietdorf J.; Bönisch H.; Göthert M.; Rappold G.; Brüss M. (2007) Characterization of the novel human serotonin receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. Mol. Pharmacol. 72, 8–17. [DOI] [PubMed] [Google Scholar]
- Holbrook J. D.; Gill C. H.; Zebda N.; Spencer J. P.; Leyland R.; Rance K. H.; Trinh H.; Balmer G.; Kelly F. M.; Yusaf S. P.; Courtenay N.; Luck J.; Rhodes A.; Modha S.; Moore S. E.; Sanger G. J.; Gunthorpe M. J. (2009) Characterisation of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: evolution, distribution and function. J. Neurochem. 108, 384–396. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K.; Hughes J. (2008) Varenicline in the treatment of tobacco dependence. Neuropsychiatr. Dis. Treat. 4, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak K. B.; Carroll F. I.; Luetje C. W. (2006) Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol. Pharmacol. 70, 801–805. [DOI] [PubMed] [Google Scholar]
- Gurley D. A.; Lanthorn T. H. (1998) Nicotinic agonists competitively antagonize serotonin at mouse 5-HT3 receptors expressed in Xenopus oocytes. Neurosci. Lett. 247, 107–110. [DOI] [PubMed] [Google Scholar]
- Lummis S. C.; Thompson A. J.; Bencherif M.; Lester H. A. (2011) Varenicline is a potent agonist of the human 5-hydroxytryptamine3 receptor. J. Pharmacol. Exp. Ther. 339, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. J.; Lummis S. C. (2006) 5-HT3 receptors. Curr. Pharm. Des. 12, 3615–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machu T. K. (2011) Therapeutics of 5-HT3 receptor antagonists: current uses and future directions. Pharmacol Ther 130, 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesters D.; Thompson A. J.; Brams M.; van Elk R.; Spurny R.; Geitmann M.; Villalgordo J. M.; Guskov A.; Danielson U. H.; Lummis S. C.; Smit A. B.; Ulens C. (2013) Structural basis of ligand recognition in 5-HT3 receptors. EMBO Rep. 14, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen B.; Spurny R.; Brams M.; van Elk R.; Valera-Kummer S.; Yakel J. L.; Voets T.; Bertrand D.; Smit A. B.; Ulens C. (2012) Molecular actions of smoking cessation drugs at α4β2 nicotinic receptors defined in crystal structures of a homologous binding protein. Proc. Natl. Acad. Sci. U.S.A. 109, 9173–9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucktooa P.; Haseler C. A.; van Elk R.; Smit A. B.; Gallagher T.; Sixma T. K. (2012) Structural characterization of binding mode of smoking cessation drugs to nicotinic acetylcholine receptors through study of ligand complexes with acetylcholine-binding protein. J. Biol. Chem. 287, 23283–23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassaine G.; Deluz C.; Grasso L.; Wyss R.; Tol M. B.; Hovius R.; Graff A.; Stahlberg H.; Tomizaki T.; Desmyter A.; Moreau C.; Li X. D.; Poitevin F.; Vogel H.; Nury H. (2014) X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 512, 276–281. [DOI] [PubMed] [Google Scholar]
- Price K. L.; Bower K. S.; Thompson A. J.; Lester H. A.; Dougherty D. A.; Lummis S. C. (2008) A hydrogen bond in loop A is critical for the binding and function of the 5-HT3 receptor. Biochemistry 47, 6370–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beene D. L.; Brandt G. S.; Zhong W.; Zacharias N. M.; Lester H. A.; Dougherty D. A. (2002) Cation-π Interactions in Ligand Recognition by Serotonergic (5-HT3A) and Nicotinic Acetylcholine Receptors: The Anomalous Binding Properties of Nicotine. Biochemistry 41, 10262–10269. [DOI] [PubMed] [Google Scholar]
- Tavares X. D. S.; Blum A. P.; Nakamura D. T.; Puskar N. L.; Shanata J. A. P.; Lester H. A.; Dougherty D. A. (2012) Variations in Binding Among Several Agonists at Two Stoichiometries of the Neuronal, α4β2 Nicotinic Receptor. J. Am. Chem. Soc. 134, 11474–11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Arnam E. B.; Blythe E. E.; Lester H. A.; Dougherty D. A. (2013) An unusual pattern of ligand-receptor interactions for the α7 nicotinic acetylcholine receptor, with implications for the binding of varenicline. Mol. Pharmacol. 84, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles T. F.; Bower K. S.; Lester H. A.; Dougherty D. A. (2012) A coupled array of noncovalent interactions impacts the function of the 5-HT3A serotonin receptor in an agonist-specific way. ACS Chem. Neurosci. 3, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. J.; Price K. L.; Reeves D. C.; Chan S. L.; Chau P. L.; Lummis S. C. (2005) Locating an antagonist in the 5-HT3 receptor binding site using modeling and radioligand binding. J. Biol. Chem. 280, 20476–20482. [DOI] [PubMed] [Google Scholar]
- Puskar N. L.; Xiu X.; Lester H. A.; Dougherty D. A. (2011) Two neuronal nicotinic acetylcholine receptors, α4β4 and α7, show differential agonist binding modes. J. Biol. Chem. 286, 14618–14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier A. D.; Lummis S. C. (2000) The role of tryptophan residues in the 5-Hydroxytryptamine3 receptor ligand binding domain. J. Biol. Chem. 275, 5620–5625. [DOI] [PubMed] [Google Scholar]
- Beene D. L.; Price K. L.; Lester H. A.; Dougherty D. A.; Lummis S. C. (2004) Tyrosine residues that control binding and gating in the 5-hydroxytryptamine3 receptor revealed by unnatural amino acid mutagenesis. J. Neurosci. 24, 9097–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. J.; Lochner M.; Lummis S. C. (2008) Loop B is a major structural component of the 5-HT3 receptor. Biophys. J. 95, 5728–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P. R.; Suryanarayanan A.; Hazai E.; Schulte M. K.; Maksay G.; Bikadi Z. (2006) Interactions of granisetron with an agonist-free 5-HT3A receptor model. Biochemistry 45, 1099–1105. [DOI] [PubMed] [Google Scholar]
- Blum A. P.; Lester H. A.; Dougherty D. A. (2010) Nicotinic pharmacophore: the pyridine N of nicotine and carbonyl of acetylcholine hydrogen bond across a subunit interface to a backbone NH. Proc. Natl. Acad. Sci. U.S.A. 107, 13206–13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. B.; Sulzenbacher G.; Huxford T.; Marchot P.; Taylor P.; Bourne Y. (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 24, 3635–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brams M.; Pandya A.; Kuzmin D.; van Elk R.; Krijnen L.; Yakel J. L.; Tsetlin V.; Smit A. B.; Ulens C. (2011) A structural and mutagenic blueprint for molecular recognition of strychnine and d-tubocurarine by different Cys-loop receptors. PLoS Biol. 9, e1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs R. E.; Sulzenbacher G.; Shi J.; Talley T. T.; Conrod S.; Kem W. R.; Taylor P.; Marchot P.; Bourne Y. (2009) Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal α7 nicotinic acetylcholine receptor. EMBO J. 28, 3040–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde L. A.; Ahring P. K.; Jensen M. L.; Nielsen E. O.; Peters D.; Helgstrand C.; Krintel C.; Harpsoe K.; Gajhede M.; Kastrup J. S.; Balle T. (2012) Intersubunit bridge formation governs agonist efficacy at nicotinic acetylcholine α4β2 receptors: unique role of halogen bonding revealed. J. Biol. Chem. 287, 4248–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. L.; Lummis S. C. (2005) FlexStation examination of 5-HT3 receptor function using Ca2+ - and membrane potential-sensitive dyes: advantages and potential problems. J. Neurosci. Methods 149, 172–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.