Abstract
The Caribbean region has the world second highest incidence rate of acquired immunodeficiency syndrome. The island of Hispaniola is composed of two sovereign nations: the Dominican Republic and Haiti. Together, they account for more than 85% of HIV/AIDS cases in the Caribbean; and the Dominican Republic alone has approximately 46,000 (33,000–59,000) HIV-1-infected adults and children. Despite this, the magnitude of the genetic variability and evolution of the HIV-1 virus in the Dominican Republic is unclear. In the current study, we analyzed 195 reverse transcriptase (RT) sequences obtained from the Los Alamos HIV database. The data were used to assess the course of the viral epidemic over time in the Dominican Republic, using a coalescent approach. Based on the data, we estimated that the timing of the most recent common ancestor (tMRCA) of local HIV-1 subtype B emerged in 1963, approximately. In addition, the Bayesian analysis provided new information that suggests that the epidemic in the Dominican Republic experienced a significant decrease in relative genetic diversity in the past 2 decades. The results suggest that adherence to antiretroviral therapy, adequate prevention campaigns, and better access to health care may be altering the virus's evolution in the Dominican Republic.
The Caribbean region has the world's second highest incidence rate of acquired immunodeficiency syndrome. The 2013 UNAIDS report shows that approximately 200,000 to 250,000 people in the region have been infected by the human immunodeficiency virus type 1 (HIV-1).1 The island of Hispaniola, which includes the Dominican Republic (DR) and Haiti (HT), accounts for over 85% of the total HIV-1 cases in the region; and the Dominican Republic alone has approximately 46,000 (33,000–59,000) HIV-1-infected adults and children.1,2 The epidemic in the DR is driven predominantly by HIV-1 subtype B (HIV-1B). The prevalence of HIV infection has been determined to be between 0.71% to 1.0% among the general population, with higher rates of more than 1% occurring in those populations that are most at risk (sex workers, intravenous drug users, men who have sex with men, and transgender individuals).3 In addition, in some regions of DR, 1 in 12 adults (15–49 years old) is living with HIV.4
The earliest reports of HIV-1 infection in the DR date back to the early 1980s; these infections are characterized as having been transmitted by heterosexual contact on bateyes (sugar cane plantations), which are populated by migrant farm workers, primarily from HT.5 A previous molecular clock study estimated that the US HIV-1B founder virus originated in 1967 (CI: 1967–1971), and the introduction of the viral infection in HT is considered to antedate that in the United States.6 Previous phylogenetic studies have suggested that the AIDS epidemic in the United States most probably originated in HT.7
Economic and social-political changes can influence the epidemiology of newly introduced pathogens.8 In addition, since the 1970s, the DR has become the most popular tourist destination in the Caribbean,9 and there is both a large Dominican population in the United States and active migration from the DR to the United States. Although little is known about the history of HIV-1B in the DR, the number of new cases appears to have dropped in the past 2 decades as a result of educational strategies that promoted changes in behavior and improvements in the coordination of care and support services for people living with HIV/AIDS.3 Thus, we hypothesize a decline in the genetic variability of HIV-1 in the DR as a result of adequate prevention campaigns and better access to health care and treatment adherence.
In the present study, we analyzed the 195 reverse transcriptase (RT) sequences, available at the time of study from the Los Alamos HIV database (sampled from 2001 through 2010), using a Bayesian model in order to estimate the HIV-1B coalescent history in the DR (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). This sample size has a confidence level of 85% with a 5% margin of error. The sequences were derived from polymerase chain reaction (PCR) products. The majority of the sequences (166) were from the Santo Domingo area. Phylogeographic analysis was performed to identify DR-specific subtype B clades.
A reference set of 66 sequences was downloaded from the Los Alamos HIV database (www.hiv.lanl.gov). The reference set includes sequences from Haiti (n=30), the United States (n=10), Martinique (n=5), Trinidad-Tobago (n=5), Puerto Rico (n=5), and Jamaica (n=5) (Supplementary Fig. S1). The DR sequences included in this study were from newly diagnosed HIV+ patients who had been infected from an unknown period of time (EU439709, EU439711, EU439715, EU439716, EU439760–EU439772, and EU839596–EU839598) or from patients who had been diagnosed within 3 years from the date of sample collection for sequencing. Although the RT sequences were derived from patients who were either therapy naive (EU439709, EU439711, EU439715, EU439716, EU439760–EU439772, EU839596–EU839598, and JN713567–JN713709), experienced (DQ023719–DQ023738, DQ023740–DQ023756), or not known due to unavailability of the information (DQ023719–DQ023738 and DQ023740–DQ023756), the positions known to be associated with drug resistance mutations were excluded in order to avoid any bias.10,11 The sequences were 532 base pairs long (nucleotides 2673–3205) and covered part of the RT gene, which corresponds to the HXB2 clone.
Phylogenetic trees were inferred using the maximum likelihood method implemented by PhyML,12 under a General Time-Reversible (GTR) nucleotide substitution model (suggested by Modeltest) with a gamma-distributed rate variation for each of these tree alignments. A resampling process (100 bootstraps) that started with a BIONJ-based tree was performed to assess the robustness of each node.13 The obtained tree was visualized using FigTree (available at http://tree.bio.ed.ac.uk/software).
The evolution rates (nucleotide substitutions, site, year) and timing of the most recent common ancestor (tMRCA) were estimated using the Bayesian Markov chain Monte Carlo (MCMC) approach implemented in BEAST (v1.4.8), a coalescent inference program.14 The process of coalescent inference can provide the demographic history of a population suggested by the genealogical relationships of samples.15 Data were first converted to the nexus format by Readseq, a biosequence conversion tool, which was provided by the European Bioinformatics Institute (EBI) (www.ebi.ac.uk/cgi-bin/readseq.cgi).
Four different coalescent priors were investigated: constant size, exponential growth, logistic growth, and Bayesian Skyline Plot (BSP).16,17 To measure the evolutionary change over time in our samples, we used either a strict or a relaxed molecular clock, both of which included a gamma-distributed rate.18 The nucleotide substitution model for the alignments was the GTR model suggested by MODELTEST19 (available at www.hiv.lanl.gov/content/sequence/findmodel/findmoedl.html). We obtained serial estimates of effective population size from the time intervals. Sequences were dated according to the sampling date, which as stated above, may not represent the year in which the subject was infected. Markov chains were generated after 200,000,000 generations with a sampling done each 1,000 generations in order to achieve an effective sampling size (ESS). The ESS parameter indicated that the parameter spaces were sufficiently explored.
To summarize the posterior distribution, we created (using TreeAnnotator v5.5.4) a consensus tree, discarding the first 50% as burn-in, and visualized this tree using FigTree (available at http://tree.bio.ed.ac.uk/software). To produce the demographic reconstruction and to calculate the ESS, we used Tracer v1.5.0 (available at http://beast.bio.ed.ac.uk/tracer),20 allowing 10 steps in Nτ over time. All the parameter estimates reached convergence (ESS>200). The ratio of the marginal likelihood with respect to the prior or Bayesian factor (BF) was used to determine the best demographic model using the Newton and Raftery method with the modification proposed by Suchard et al.21,22 Recent studies have demonstrated that the Bayesian coalescent method is an important tool that permits researchers to make inferences as to how a given past epidemic has grown as well as regarding the relationship between coalescent time and population size.23,24
The divergence time analysis was performed with the BEAST software, using the years of isolation as calibration points. The Bayesian method was implemented to estimate phylogeny under both strict and uncorrelated log-normal molecular-clock models. Exponential growth, logistic growth, constant population size, and the nonparametric Bayesian Skyline Plot were used for inferences. The ESS from the exponential relaxed, logistic strict, and logistic relaxed models have not yet been run long enough to obtain a valid estimate of the parameter (x<200). In contrast, the ESS to BSP (strict/relaxed), constant (strict/relaxed), and exponential strict indicated that the parameter spaces were sufficiently explored (ESS>200).
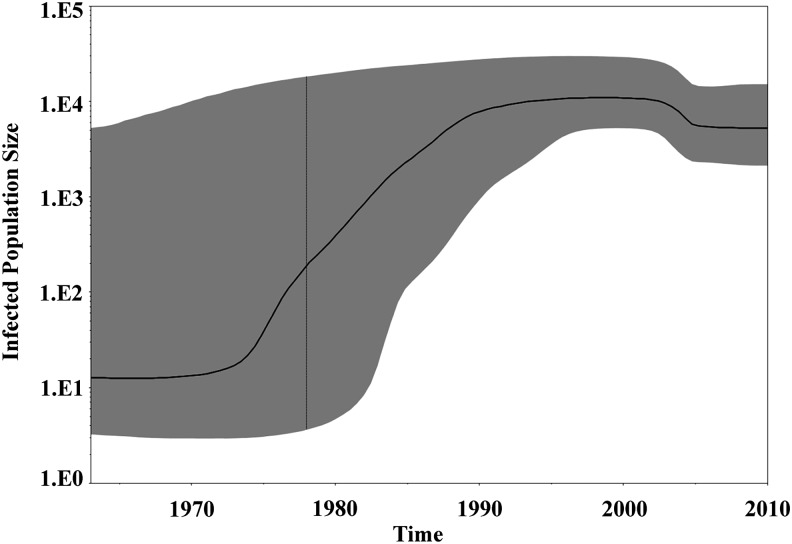
The best model that fits the demographic information was assessed by approximate marginal log-likelihood or by Bayes factor. The BF, which is the difference of the marginal likelihood of null (H0) and the alternative (H1) model,10 showed that the BSP-strict and BSP-relaxed models were strongly supported. Evidence against H0 was assessed in the following way: 0–6 indicates positive evidence for H1, and X>6 indicates strong evidence (Table 1). The demographic reconstruction shows three distinct growth phases. A lag phase was observed between 1963 and 1975; after that the epidemic experienced an increase in the effective population size, with fast exponential growth taking place from 1970 and 1990, followed by an asymptotic phase toward the present (Figs. 1 and 2). This estimation fits with the epidemiological data obtained by surveillance programs in the DR.1,25
Table 1.
Bayes Factor Between Different Molecular Clock Models
| H1/H0a | Constant strict | Constant relaxed | Exponential strict | BSP strict | BSP relaxed |
|---|---|---|---|---|---|
| Constant strict | — | −7.706 | −33.314 | −52.84 | −52.431 |
| Constant relaxed | 7.706 | — | −25.609 | −45.135 | −44.725 |
| Exponential strict | 33.314 | 25.609 | — | −19.526 | −19.116 |
| BSP strict | 52.840 | 45.135 | 19.526 | — | 0.410 |
| BSP relaxed | 52.431 | 44.725 | 19.116 | −0.410 | — |
Bayes factors were estimated by comparing marginal likelihoods of the different evolutionary models (Tracer v1.5.0). The H0 (null) models are in the row while the H1 (alternative) models are in the columns. Evidence against H0 was assessed in the following way: 0–6 indicates positive evidence for H1, and X>6 indicates strong evidence. The relaxed exponential, relaxed logistic, and strict logistic were not included, because the ESS was X<150. The BSP model fitted the data set better than constant, exponential, and logistic.
BSP, Bayesian Skyline Plot.
FIG. 1.
Phylodynamics of HIV-1 subtype B in the Dominican Republic (DR). Time-scaled Bayesian Skyline Plots for the HIV-1B sequences in the DR. The plots represent the estimate of effective number of infections (Y=log10) through time (X=time). The solid line represents the median of effective population size with the 95% upper and lower estimates shaded in gray.
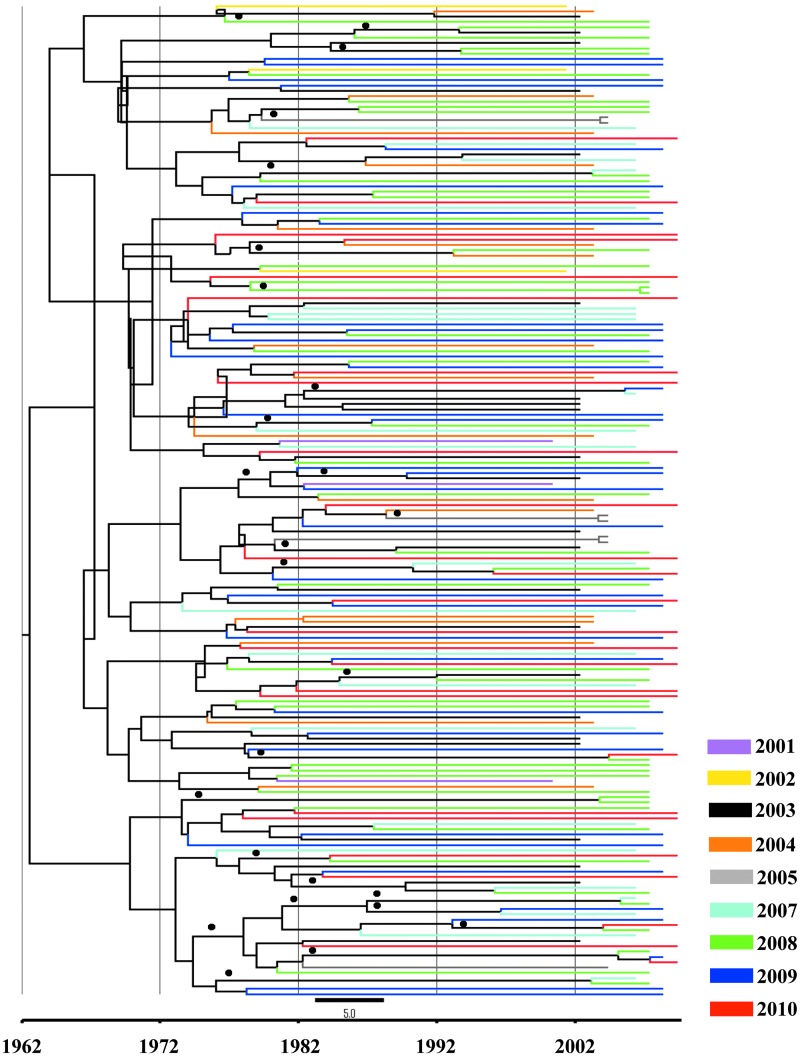
FIG. 2.
Bayesian consensus tree over time of HIV-1B polymerase gene in the DR. A Bayesian consensus tree was selected by TreeAnnotator software employing a Bayesian Skyline Plot (BSP) model. The data set included 195 HIV-1 subtype B sequences from the DR. The Markov chain Monte Carlo (MCMC) chains run 200×106, with a sample every 1,000 generations. The colors indicate the year of the sample, and bullets (●) indicate the branches with a posterior probability value above 90%.
The observed exponential growth coincides with the typical expansion of a new pathogen. Nevertheless, the number of new cases appears to have dropped in the past 2 decades as a result of educational strategies that promoted changes in behavior and improvements in the coordination of care and support services for people living with HIV/AIDS.3 In 1987 and 1989, the government created the National Program to Control AIDS and Sexually Transmitted Diseases (PROCETS) and established a number of STD clinics in state hospitals. In addition, the implementation of universal precautionary and safe blood-handling techniques (1991), the implementation of a condom policy to increase access to condoms, a coordinated effort to provide services to the vulnerable population at the HT/DR border, and strategies aimed at preventing mother-to-child transmission (MTCT) are almost certainly additional factors responsible for slowing the HIV epidemic.3
During that time, local health departments and nongovernmental organizations (national and international) became highly involved in establishing prevention programs to provide care and support after the first cases of AIDS on the island were reported in 1983.26 The above-mentioned initiatives encourage individuals to seek an early diagnosis of HIV, which often results in the early treatment of the infection. In 1999, ART treatment was introduced in the DR to prevent MTCT and has been free of charge to all patients who qualify since 2003.27,28 The scaled-up antiretroviral therapy, improved adherence to ART, and better health care provision post-1990 might have slowed the rate of HIV evolution.
Recently, the government established the new “SIDA” (the initials for AIDS in Spanish) law (2011) in order to support and strengthen the demands of people living with HIV/AIDS. In addition, the 42-01 law (Ley General de Salud) and the 87-01 law (Sistema Dominicano de la Seguridad Social) facilitate prevention, care, and support services for patients.29 Interestingly, during the same period, the HIV prevalence among pregnant women (15–24 years old), female sex workers (FSWs), and the general population presented a similar decreasing pattern.30 Similarly, the growth rates of syphilis and gonorrhea slowed in the late 1990s,25 which should be evidence of the effectiveness of the viral control effort.
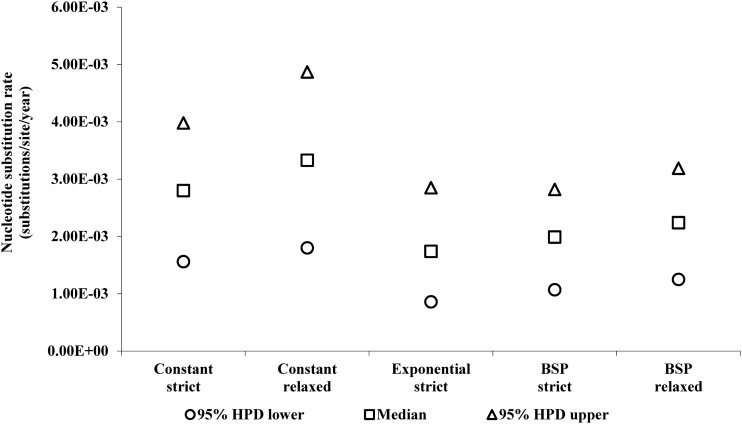
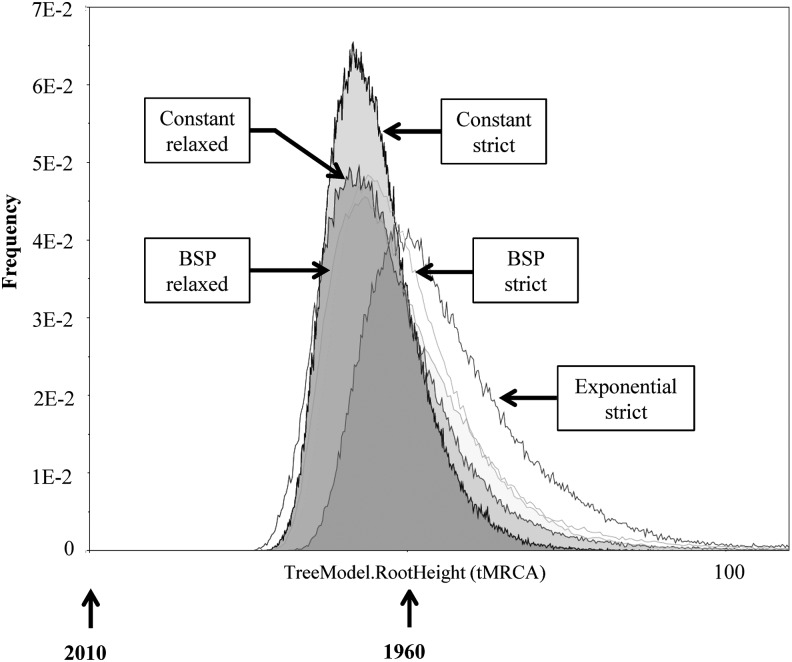
The mean mutation rates of BSP strict and relaxed were estimated to be 2.24×10−3 (95% HPD: 1.07×10−3, 2.82×10−3) and 1.99×10−3 (95% HPD: 1.25×10−3, 3.19×10−3) nucleotide substitutions per site per year, respectively (Fig. 3). The substitution rates obtained (RT region) are consistent with rates previously described.31 Nevertheless, to improve the accuracy of our results, it is necessary to investigate additional sequences from that time period, which sequences are not, unfortunately, available. Additional data should help more precisely define the evolutionary history of the virus. According to the Bayesian analysis, the tMRCA to BSP (strict/relaxed) were estimates in the decade of the 1960s (1963). The marginal distribution analysis for tMRCA shows an overlapping in the marginal densities (Fig. 4), which suggests that the effect of the evolutionary models was limited.32 Our analysis of phylogeographic data demonstrates that the introduction of HIV-1B into the DR dates back to the early 1960s (Table 2)—only a few years earlier than the mean estimated onset date of the spread of HIV-1B in the United States (1968; CI: 1967–1971)7. This fact indicates that HIV-1B apparently was circulating in the DR long before it was officially recognized (1983).
FIG. 3.
The evolution rate of HIV-1 subtype B in the DR. Nucleotide substitution rates between the evolutionary models. The mean mutation rates to BSP strict, BSP relaxed, constant strict, constant relaxed, and exponential strict were estimated as 2.24×10−3 (95% HPD: 1.07×10−3, 2.82×10−3), 1.99×10−3 (95% HPD: 1.25×10−3, 3.19×10−3), 2.80×10−3 (95% HPD: 1.56×10−3, 3.98×10−3), 3.3×10−3 (95% HPD: 1.80×10−3, 4.87×10−3), and 1.7×10−3 (95% HPD: 8.59×10−4, 2.85 x×10−3) nucleotide substitutions per site per year, respectively.
FIG. 4.
Marginal density of timing of the most recent common ancestor (tMRCA) obtained from the DR sequences. The diagram was generated using Tracer v1.5.0 and shows the marginal distribution of the most common ancestor plotted against frequency of trees. The tMRCAs of BSP (both strict and relaxed), constant strict, constant relaxed, and exponential strict were estimated in 1963, 1967, 1966, and 1957, respectively.
Table 2.
Bayesian Coalescent Results (Timing of the Most Recent Common Ancestor) of Evolutionary Parameters for HIV-1B in the Dominican Republic
| Summary statistica | Constant strict | Constant relaxed | Exponential strict | BSP strict | BSP relaxed |
|---|---|---|---|---|---|
| Median | 43.71 (1967) | 44.66 (1966) | 53.61 (1957) | 47.63 (1963) | 47.02 (1963) |
| 95% HPD lower | 32.496 | 29.594 | 36.562 | 33.317 | 32.016 |
| 95% HPD upper | 58.957 | 67.108 | 83.458 | 70.309 | 74.220 |
| Effective sample size (ESS) | 437.382 | 629.790 | 303.579 | 855.570 | 416.086 |
The ESS parameter indicated that the parameter (Threelength) for each model was sufficiently explored (X>200). To produce the demographic reconstruction and to calculate the ESS, we used Tracer v1.5.0, allowing 10 steps in Nτ over time. According to the Bayesian analysis, the tMRCA to BSP (strict/relaxed) were estimates in the decade of the 1960s (1963).
The relationship between immigration and health in the DR has been a delicate and controversial subject over the years.5 Previous phylogenetic studies have suggested that the AIDS epidemic in the United States was most probably introduced from HT, the country that coexists with the DR on the island of Hispaniola.6,7 Nevertheless, it is reasonable that the booming tourist industry and the high levels of population movement between HT and the DR in the 1950s might have created conditions for the entry of pathogens earlier than has been estimated in the previous literature.33 In addition, the 1960s was a turbulent decade, beginning, as it did, with the fall of a 3-decades-long dictatorship, a U.S. military intervention, and a new constitution that marked the beginning of civil liberties and included greater tolerance for sexual preferences and expression, among others.34,35 This climate of experimentation and new liberties may have been ideal for the transmission en masse of a preexisting pathogen.
Nevertheless, in the highly mobile world of today, it is important to keep the rate of HIV evolution to a minimum so that the emergence of any highly unusual strain can be avoided. Recent reports suggest that there is an ecological association between tourist areas and high rates of high-risk sexual behaviors in the DR.9,36 Since the 1970s, the DR has become the most popular tourist destination in the Caribbean.37 According to the International Organization for Migration (IOM) and Minority Rights Group International (MRG), the current migration countries of origin are HT, China, Cuba, Europe, Puerto Rico, and the United States.4,38 Because of the high levels of migration and immigration, it is important to understand how the virus evolves. In addition, because of poverty and the poor quality of life in HT, many Haitians cross the border into the DR every year.4,25
In the absence of close virological monitoring, drug resistance mutations may selectively expand, rekindling viral evolution. Furthermore, although in the DR the rate of new infection fell by 73% (adults 15–49 years) and there are 61% fewer people dying from AIDS-related causes, routine surveillance of the genetic diversity of HIV-1B is necessary in order to initiate public health efforts.1,39 It is important to understand the fact that educating the community about risk factors, condom use, needle-exchange programs, and adherence to HIV treatment regimens (ART) constitutes vital elements that are necessary for the control of the evolution of the HIV-1B virus. Nevertheless, poor access to health care, poor medication compliance, stigmatization, and the lack of access to the migrant population might be important obstacles to achieving these objectives in the DR.40,41 Similarly important is the constant monitoring of viral evolution, especially in high-risk populations. In the absence of virological monitoring, drug resistance mutations and other significant changes can accumulate, the occurrence of which would necessitate implementing regionally adapted strategies.
Supplementary Material
Acknowledgments
We thank Mr. Bob Ritchie (PHSU Editor) and Dr. Aida Mencia for their assistance in editing and reviewing drafts of the manuscript. The study was made possible by the research infrastructure grant provided under NCRR/RCMI (G12 RR003050) and PRCTRC (8U54MD007587-03).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic, 2013 [Google Scholar]
- 2.Hunter LM, Reid-Hresko J, and Dickinson : Environmental change, risky sexual behavior, and the HIV/AIDS pandemic: Linkages through livelihoods in rural Haiti. Popul Res Policy Rev 2011;30(5):729–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS, COPRESIDA, DIGECITSS: HIV Modes of Transmission Model: Analysis of the distribution of new HIV infections in the Dominican Republic and recommendations for prevention, 2010 [Google Scholar]
- 4.Borland R, Faas L, Marshall D, et al. : HIV/AIDS and Mobile Populations in the Caribbean : A Baseline Assessment 2004. Santo Domingo, Dominican Republic, IOM (International Organization for Migration) [Google Scholar]
- 5.Koenig RE, Pittaluga J, Bogart M, et al. : Prevalence of antibodies to the human immunodeficiency virus in Dominicans and Haitians in the Dominican Republic. JAMA 1987;257(5):631–634 [PubMed] [Google Scholar]
- 6.Gilbert MT, Rambaut A, Wlasiuk G, et al. : The emergence of HIV/AIDS in the Americas and beyond. Proc Natl Acad Sci USA 2007;104(47):18566–18570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins KE, Lemey P, Pybus OG, et al. :. U.S. Human immunodeficiency virus type 1 epidemic: Date of origin, population history, and characterization of early strains. J Virol 2003;77(11):6359–6366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajoge HO, Gordon ML, de Oliveira T, et al. : Genetic characteristics, coreceptor usage potential and evolution of Nigerian HIV-1 subtype G and CRF02_AG isolates. PLoS One 2011;6(3):e17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padilla MB, Guilamo-Ramos V, Bouris A, and Reyes AM: HIV/AIDS and tourism in the Caribbean: An ecological systems perspective. Am J Public Health 2010;100(1):70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bello G, Aulicino PC, Ruchansky D, et al. : Phylodynamics of HIV-1 circulating recombinant forms 12_BF and 38_BF in Argentina and Uruguay. Retrovirology 2010;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalmet K, Staelens D, Blot S, et al. : Epidemiological study of phylogenetic transmission clusters in a local HIV-1 epidemic reveals distinct differences between subtype B and non-B infections. BMC Infect Dis 2010;10:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guindon S. and Gascuel O: A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 2003;52(5):696–704 [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein J: Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985;39(4):783–791 [DOI] [PubMed] [Google Scholar]
- 14.Drummond AJ. and Rambaut A: BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 2007;7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salemi M, Goodenow MM, Montieri S, et al. : The HIV type 1 epidemic in Bulgaria involves multiple subtypes and is sustained by continuous viral inflow from West and East European countries. AIDS Res Hum Retroviruses 2008;24(6):771–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond AJ, Rambaut A, Shapiro B, and Pybus OG: Bayesian coalescent inference of past population dynamics from molecular sequences. Mol Biol Evol 2005;22(5):1185–1192 [DOI] [PubMed] [Google Scholar]
- 17.Purdy MA. and Khudyakov YE: Evolutionary history and population dynamics of hepatitis E virus. PLoS One 2010;5(12):e14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tully DC. and Wood C: Chronology and evolution of the HIV-1 subtype C epidemic in Ethiopia. AIDS 2010;24(10):1577–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posada D. and Crandall KA: MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998;14(9):817–818 [DOI] [PubMed] [Google Scholar]
- 20.Drummond AJ, Suchard MA, Xie D, and Rambaut A: Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 2012;29(8):1969–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suchard MA, Weiss RE, and Sinsheimer JS: Bayesian selection of continuous-time Markov chain evolutionary models. Mol Biol Evol 2001;18(6):1001–1013 [DOI] [PubMed] [Google Scholar]
- 22.Kass RE. and Raftery AE: Bayes factor. J Am Stat Assoc 1995;90(430):773–795 [Google Scholar]
- 23.Esbjornsson J, Mild M, Mansson F, et al. : HIV-1 molecular epidemiology in Guinea-Bissau, West Africa: Origin, demography and migrations. PLoS One 2011;6(2):e17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worobey M, Gemmel M, Teuwen DE, et al. : Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature 2008;455(7213):661–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halperin DT, de Moya EA, Perez-Then E, et al. : Understanding the HIV epidemic in the Dominican Republic: A prevention success story in the Caribbean? J Acquir Immune Defic Syndr 2009;51(Suppl 1):S52–59 [DOI] [PubMed] [Google Scholar]
- 26.Brewer TH, Hasbun J, Ryan CA, et al. : Migration, ethnicity and environment: HIV risk factors for women on the sugar cane plantations of the Dominican Republic. AIDS 1998;12(14):1879–1887 [DOI] [PubMed] [Google Scholar]
- 27.Myers JE, Taylor BS, Rojas Fermin RA, et al. : Transmitted drug resistance among antiretroviral-naive patients with established HIV type 1 infection in Santo Domingo, Dominican Republic and review of the Latin American and Caribbean literature. AIDS Res Hum Retroviruses 2012;28(7):667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenzo O, Beck-Sague CM, Bautista-Soriano C, et al. : Progress towards elimination of HIV mother-to-child transmission in the Dominican Republic from 1999 to 2011. Infect Dis Obstet Gynecol 2012;2012:543916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UNGASS: Seguimiento a la Declaracion de Compromiso sobre el VIH/SIDA. Republica Dominicana Informe Nacional sobre los Progresos Realizados en el Pais [www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_DO_Narrative_Report[1].pdf
- 30.SESPAS, DIGECITSS: Resultados de la “XV Encuesta Serologica de Vigilancia Centinela Segunda Generacion. www.portalsida.org/repos/XV_ENCUESTA_SEROL%c3%93GICA_DE_VIGILANCIA_CENTINELA_SEGUNDA_GENERACI%c3%93N[1].pdf
- 31.Abecasis AB, Vandamme AM, and Lemey P: Quantifying differences in the tempo of human immunodeficiency virus type 1 subtype evolution. J Virol 2009;83(24):12917–12924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemey P, Pybus OG, Rambaut A, et al. : The molecular population genetics of HIV-1 group O. Genetics 2004;167(3):1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez F: 1950, Una Decada Marcada. In Cincuenta Años de Vida Sindical, 2007 [Google Scholar]
- 34.Forsythe S, Hasbun J, and Butler de Lister M: Protecting paradise: Tourism and AIDS in the Dominican Republic. Health Policy Plan 1998;13(3):277–286 [DOI] [PubMed] [Google Scholar]
- 35.Washington DCIA: The World Factbook 2013–2014: Dominican Republic, 2013 [Google Scholar]
- 36.Padilla MB, Reyes AM, Connolly M, et al. : Examining the policy climate for HIV prevention in the Caribbean tourism sector: A qualitative study of policy makers in the Dominican Republic. Health Policy Plan 2012;27(3):245–255 [DOI] [PubMed] [Google Scholar]
- 37.World Trade Organization: Trade Policy Review: Dominican Republic, 2002 [Google Scholar]
- 38.Ferguson J: Migration in the Caribbean: Haiti, the Dominican Republic and Beyond 2003. Minority Rights Group International [Google Scholar]
- 39.Kao CF, Chang SY, Hsia KT, et al. : Surveillance of HIV type 1 recent infection and molecular epidemiology among different risk behaviors between 2007 and 2009 after the HIV type 1 CRF07_BC outbreak in Taiwan. AIDS Res Hum Retroviruses 2011;27(7):745–749 [DOI] [PubMed] [Google Scholar]
- 40.Cubano LA, Sepulveda-Torres Ldel C, Sosa G, et al. : Prevalence of drug resistance and associated mutations in HIV-positive Puerto Ricans: Sex variations. Ethn Dis 2008;18(2 Suppl 2):S2-132–136 [PubMed] [Google Scholar]
- 41.Baez-Feliciano DV, Thomas JC, Gomez MA, et al. : Changes in the AIDS epidemiologic situation in Puerto Rico following health care reform and the introduction of HAART. Rev Panam Salud Publica 2005;17(2):92–101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.