Abstract
Significance: Injuries to the skin as a result of illness or injury, particularly chronic nonhealing wounds, present a major healthcare problem. Traditional wound care approaches attempt to control the underlying causes, such as infection and ischemia, while the application of wound dressings aims to modify a poorly healing wound environment into a microenvironment more closely resembling an acute wound allowing the body to heal the wound naturally.
Recent Advances: Regenerative medicine approaches, such as the use of biologic scaffold materials comprising an intact extracellular matrix (ECM) or individual components of the ECM, are providing new therapeutic options that focus upon the provision of biochemical cues that alter the wound microenvironment to facilitate rapid restoration of normal skin architecture.
Critical Issues: The incidence of chronic nonhealing wounds continues to increase. For example, between 15% and 20% of diabetics are likely to develop chronic, nonhealing foot wounds creating an increasing burden on healthcare systems worldwide.
Future Directions: Developing a thorough understanding of wound microenvironment and the mechanisms by which biologic scaffolds work in vivo has the potential to markedly improve outcomes in the clinical translation for the treatment of chronic wounds.

Stephen F. Badylak, DVM, PhD, MD
Scope and Significance
The use of biologic scaffold materials for a variety of applications has increased dramatically during the past two decades. These scaffolds include those comprising an intact extracellular matrix (ECM) or individual components of the ECM, and those comprising hybrids incorporating a synthetic component with a biologic component.
The mechanisms by which such scaffolds support and promote structural and functional remodeling of injured tissues in vivo are being increasingly understood. The scaffold remodeling process has been described as constructive remodeling, which represents the scenario in which site-appropriate organized tissue with at least some degree of functionality is deposited at the site of scaffold placement. This outcome is in contrast to the default tissue healing response, which would be characterized by scar tissue and general lack of functionality. Each anatomic site and clinical application has distinctive characteristics and the use of biologic scaffolds in these different locations has shown various degrees of success.
Chronic nonhealing wounds are commonly associated with comorbidity characteristics, such as obesity, diabetes, and malnutrition. The success/failure of biologic scaffolds in treating chronic wounds will depend upon a thorough understanding of the mechanisms by which these materials work in vivo, but the potential to markedly improve outcomes in the clinical translation for the treatment of chronic wounds is noteworthy.
Translational Relevance
The outer layer of the skin (epidermis) can regenerate, but injuries that breach the subjacent dermis relegate healing to granulation and scar tissue formation. Biologic scaffolds can transform the default scar tissue response toward one that includes components of true regeneration. This modulation of default healing has been termed as constructive remodeling. A biologic scaffold approach can have direct translational relevance to full thickness, and difficult to heal skin wounds.
Clinical Relevance
In contrast to most wound dressings designed to protect the wound surface and maintain various states of hydration, biologic scaffold materials function as an inductive microenvironment. Mechanisms by which these ECM-based scaffold materials function in soft tissue locations, such as skeletal muscle, tendons, and the gastrointestinal tract, include relatively rapid degradation, which releases embedded cytokines and chemokines, generation of bioactive cryptic peptides with a variety of constructive wound healing properties, modulation of the innate immune response, and recruitment of endogenous stem/progenitor cells to the site of scaffold remodeling. The extent to which these processes occur in the setting of chronic skin wounds with concomitant systemic pathology, such as diabetes, peripheral vascular disease, and malnutrition, has been largely unexplored. The clinical relevance of these biologic scaffolds is predicated upon these scaffold-mediated inductive processes. The potential to change the default healing response may shorten the time to complete healing, prevent advancement of these wounds to deeper structures, and result in improved cosmetic outcomes.
Overview
Injuries to the skin as a result of illness or injury present a major healthcare problem. The incidence of chronic nonhealing wounds, such as venous or diabetic ulcers, continues to increase. Wound management has traditionally involved controlling the underlying causes, such as infection, ischemia, or diabetes, and allowing the body to heal the wound naturally. Regenerative medicine approaches, such as the use of biologic scaffold materials comprising an intact ECM or individual components of the ECM, provide additional therapeutic options. These regenerative medicine approaches focus upon the provision of biochemical cues that alter the wound microenvironment to facilitate rapid restoration of normal skin architecture. The present article describes the various types of scaffold materials available, the mechanisms by which constructive remodeling occurs, and potential future directions.
Discussion
Nonhealing wound microenvironment
Injuries to the skin as a result of illness or injury present a major healthcare problem. Approximately, 12 million wounds will be treated in emergency departments in the United States annually,1 and more than 2 million people will suffer from pressure ulcers.2 Furthermore, the incidence of chronic nonhealing wounds, such as venous or diabetic ulcers, continues to increase. In 2007, in the United States alone, 23.6 million people were diagnosed with diabetes,3 of which between 15% and 20% are likely to develop chronic, nonhealing foot wounds.4 Wound management has traditionally involved controlling the underlying cause, such as infection, ischemia, or diabetes, and allowing the body to heal the wound naturally. Regenerative medicine provides additional therapeutic options, which focus upon the provision of biochemical cues that alter the wound microenvironment to facilitate rapid restoration of normal skin architecture.
The wound repair process is highly complex, requiring the activation and synchronization of multiple biological pathways if tissue integrity and hemostasis are to be restored. Activation of these biological pathways gives rise to a dynamic wound microenvironment that consists of cells and the ECM and a complex milieu of enzymes, growth factors, and cytokines. In healthy individuals, the response to injury occurs in a series of overlapping, but distinct stages of hemostasis, inflammation, new tissue formation, and tissue remodeling.5 Hemostasis and inflammation occur immediately following tissue injury. Platelets initiate the wound healing process through the activation of the coagulation cascade and the release of proinflammatory mediators, including platelet-derived growth factor (PDGF), transforming growth factor β (TGFβ), fibroblast growth factor (FGF), and insulin-like growth factor-1 (IGF-1), among others.2,6 Polymorphonuclear leukocytes migrate to the wound site in response to these secreted factors, followed by macrophages that engulf and attempt to destroy any microbes and necrotic debris. Macrophages play a critical role in the wound healing process by releasing proteases, which degrade damaged ECM as well as a host of proinflammatory and tissue remodeling cytokines.7
Concurrent with the inflammatory response is the commencement of new tissue formation, which is characterized by the migration and proliferation of keratinocytes over the injured dermis and a robust angiogenic response. Fibroblasts begin to replace the fibrin matrix and necrotic tissue of the wound with new ECM initiating the formation of granulation tissue. Some fibroblasts will differentiate into contractile myofibroblasts, which contribute to wound contraction. Key regulators of these processes include hepatocyte growth factor (HGF), FGF-2, -7, and -10, TGFα, and vascular endothelial growth factor A (VEGF-A).
The remodeling phase of wound repair involves maturation of the ECM and restoration of the wound microenvironment toward that of normal skin; a process that may take up to 12 months to complete. However, even in healthy individuals, sebaceous glands, hair follicles, sweat glands, and sensory nerves typically fail to form and wounds that include the full thickness of the dermis never regain the properties of uninjured skin, remaining as dense, fibrous scar tissue.
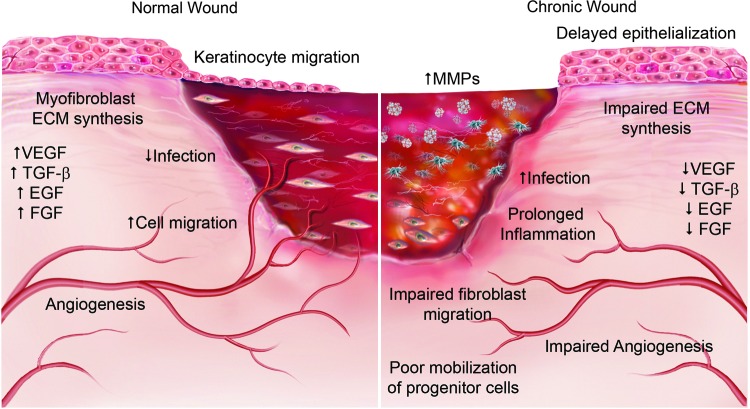
Chronic wounds are defined as wounds that fail to proceed through the normal healing response or fail to produce functional integrity within 3 months. As such, all wounds have the potential to become chronic, although the most common forms of chronic wounds are venous, arterial or diabetic ulcers, and pressure sores.8,9 In these chronic nonhealing wounds, the wound microenvironment is fundamentally altered. The complex interplay of growth factors and cytokines becomes disrupted and the normal phases of wound healing cannot be completed. Figure 1 demonstrates the key differences between the normal wound healing environment and that of a chronic nonhealing wound. Of particular significance in nonhealing wounds are the reduction in angiogenesis, decrease in growth factors, increased incidence of infection, and the increase in the severity and duration of inflammation characterized by a self-sustaining proinflammatory (M1) response. The presence of increased amounts of proteolytic enzymes within chronic wound sites also contributes to delayed healing. These proteolytic enzymes, called matrix metalloproteinases (MMPs), are capable of degrading most of the components of the ECM. In chronic wounds, the increased levels of MMPs degrade and inhibit the deposition of new ECM, which in turn inhibits cell migration. MMPs are also capable of degrading the growth factors and cytokines that regulate the wound microenvironment, further limiting the progression of wound healing.10
Figure 1.
The key differences between the normal wound healing environment and that of a chronic nonhealing wound. Chronic wounds are characterized by prolonged inflammation and infection with delayed epithelialization and impaired fibroblast migration and extracellular matrix (ECM) synthesis. There is increased matrix metalloproteinase (MMP) activity and a decrease in growth factor expression leading to impaired angiogenesis and poor mobilization of circulating progenitor cells. Arrows indicate increase (↑) or decrease (↓). EGF, epidermal growth factor; FGF, fibroblast growth factor; TGFb, transforming growth factor; VEGF, vascular endothelial growth factor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Growth factors and cytokines help regulate the wound healing process through a series of complex feedback mechanisms. In chronic wounds, this growth factor and cytokine milieu is polarized toward inflammation, including increased amounts of interleukin 1α (IL-1α), IL-1β, and tumor necrosis factor α.2,10,11 Many of these cytokines affect the deposition of new ECM either by decreasing the synthesis of ECM molecules or stimulating the synthesis of MMPs. Similarly, decreases in the expression of TGFβ1, IGF-1, VEGF, and PDGF-BB have been shown to correlate with decreased angiogenesis and delayed wound healing in diabetic and venous ulcers.12 Management of these chronic wounds has traditionally required a lengthy complex approach that addresses both the pathophysiological causes, such as debridement of devitalized tissue and infection control, as well as systemic factors such as compromised nutrition and venous insufficiency.
Current treatment of nonhealing wounds
Current best practice for the treatment of dermal wounds depends upon the type of injury. If the wound is larger than 4 cm in diameter, skin grafting may be performed. Tissue flaps can also be used, but must obviously be taken from sites adjacent to or near the wound to minimize differences in skin pigment and composition between the donor and recipient sites. While skin grafting and tissue flaps can be used to treat large, chronic nonhealing wounds, these wounds are typically treated with a more conservative approach with the objective of protecting the wound and preventing desiccation and infection at the wound site, thus allowing the natural healing process to proceed.
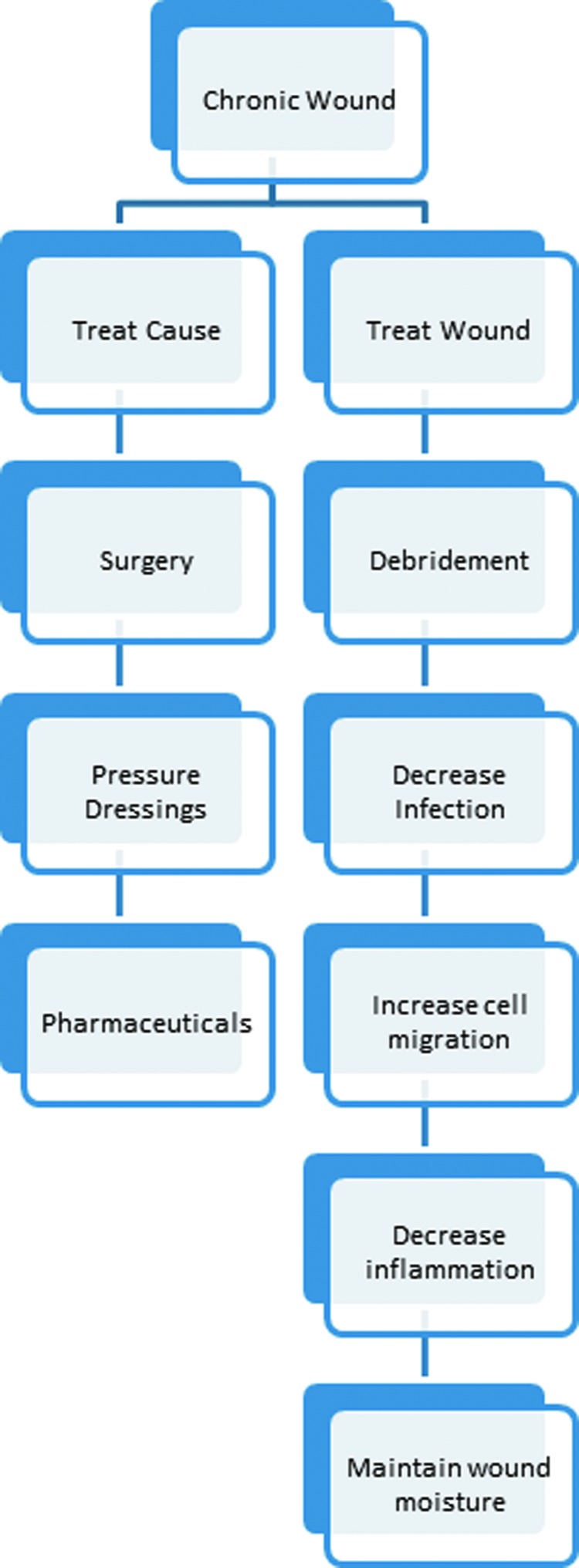
Current standard of care for nonhealing wounds attempts to address key elements of impaired wound healing (Fig. 2). The Wound Healing Society has promoted the use of the acronym T.I.M.E. to refer to these wound healing steps. T stands for tissue and the assessment of the wound site and its defects such as devitalized or necrotic tissue. I refers to the presence of inflammation, infection, or both. M refers to the moisture balance in the wound. E describes the wound edge and the extent of reepithelialization.13 Thus, the first stage in the treatment of any chronic wound involves debridement of the wound site to remove devascularized material, necrotic tissue, and infectious debris. Successful debridement preserves vital tissue and can convert a poorly healing wound environment into a microenvironment more closely resembling an acute wound. In addition, there is increasing evidence to support the use of negative pressure therapy following debridement. This use of negative pressure (i.e., wound VAC) reduces fluid around the wound while promoting granulation tissue formation.14
Figure 2.
Diagram showing the current approaches to wound care. Current treatment options include treating the cause such as improving circulation or treating the wound directly by controlling infection and inflammation to promote healing. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Control of inflammation and infection are equally important to successful wound healing. The presence of β-hemolytic streptococci or a concentration of bacteria, such as staphylococcus aureus, above 105 colony-forming units per gram of tissue is known to impair healing.15 Ultimately, current successful treatment for chronic wounds depends upon the application of an appropriate wound dressing. Choice of wound dressing depends on the stage of wound healing and aims to maintain a moist wound environment while minimizing friction to protect the periulcer skin. These wound dressings range from the relatively simple, such as paraffin gauze, to complex hydrogels and colloids that may or may not contain bioactive factors to promote angiogenesis or reepithelialization. For venous and diabetic ulcers, graduated compression bandages are also used to reduce edema and improve venous return. While hydrogels, foams, and hydrocolloids have been developed in recent years, data suggest that traditional therapies such as saline or paraffin gauze dressings and graduated compression bandages are just as effective. As a result, chronic wounds are still associated with a long recovery period and significant patient morbidity.16 During the last 30 years, advances in cell culture techniques together with a greater understanding of the wound healing process and the factors that constitute the wound microenvironment have led to the development of a number of biologic dressings for wound repair. These biologic materials attempt to restore the wound microenvironment by providing either bioactive factors and ECM components or both to stimulate granulation tissue formation, angiogenesis, and reepithelialization.
Overview of biologic scaffolds
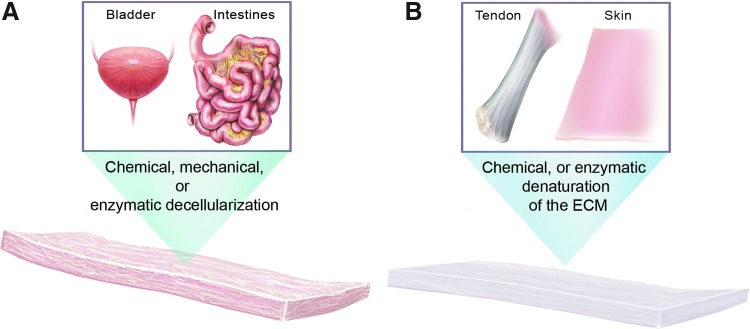
Biologic scaffold materials comprise the ECM or individual components of the ECM, such as collagen, laminin, or hyaluronan (Fig. 3). The ECM represents the secreted products of resident cells within each tissue and organ. These molecular components are arranged in tissue-specific patterns, which are responsive to and optimized for the physiologic and biomechanical requirements of each tissue.17 The three-dimensional ultrastructure of the matrix, including the ligand landscape, available to cells that encounter the ECM is extraordinarily complex. Attempts to synthetically manufacture mimics of the ECM would logically require a comprehensive understanding of both the organization and composition of the matrix at the ultrastructural level. Since such an understanding does not exist, by definition it is not possible to create faithful mimics of the ECM.
Figure 3.
Preparation of biologic scaffolds. Biologic scaffolds can be prepared either by the decellularization of tissues and organs (A) or by degrading the ECM and isolating and purifying individual ECM components (B). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The biologic properties of the matrix, which affect the interaction with resident cells and infiltrating cells, are partially understood. The matrix acts as an orchestrator of cell behavior, including modulation of macrophage phenotype, proliferation, mobilization, differentiation of stem and progenitor cells, and regulation of angiogenesis, among others.18–21 These biologic properties can be retained in biologic scaffolds that are prepared by methods that preserve the composition and structure of the matrix. Stated differently, the methods of manufacturing a biologic scaffold material for therapeutic use play a critical, if not defining role, in the clinical outcomes.
Biologic scaffolds manufactured for the treatment of nonhealing wounds are typically designed to degrade. The degradation process of these scaffolds is particularly important for the release of embedded signaling molecules and the generation of cryptic peptide molecules by enzymatic cleavage of parent molecules, such as collagen and laminin. These bioactive cryptic peptides play a key role in the observed biological activity of these materials.22,23 The following section provides more detail regarding the mechanisms by which biologic scaffolds support constructive remodeling processes, especially in the context of the integumentary system.
Use of biologic scaffolds in wound repair
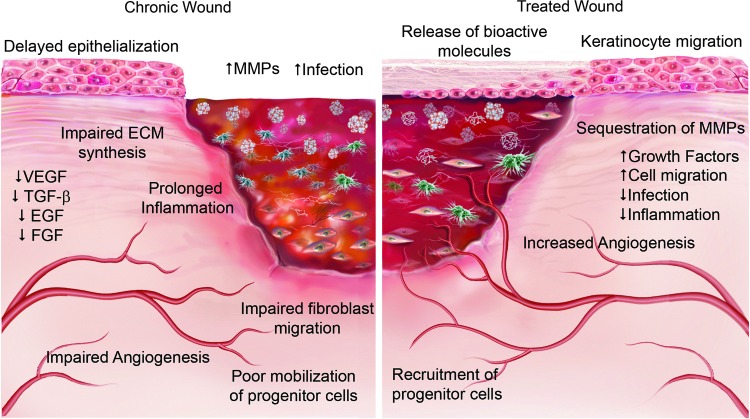
At its simplest, the objective of all wound repair approaches is to create an environment that minimizes infection, promotes the correct moisture balance, and facilitates reepithelialization of the wound. Although a comprehensive understanding of the microenvironmental conditions that exist within mammalian tissue in both healthy and injured tissue is still lacking, the convergence of recent findings in the field of stem cell biology and regenerative medicine has highlighted the importance of the ECM in providing such a friendly microenvironment and regulating cell behavior and promoting tissue repair (Fig. 4). The recognition of the importance of the ECM in wound healing has led to the development of numerous wound healing products derived from mammalian ECM. These products may be biologic and derived from animal or human tissues or they could be composites containing a combination of biologic and synthetic products. For the purpose of this review, the term biologic scaffold will refer to all ECM-based materials and engineered ECM scaffolds will refer to materials produced ex vivo from individual ECM components with or without synthetic components. At least five different ECM components have been identified as important mediators of wound healing: (1) structural proteins such as collagen and elastin, (2) glycoproteins such as fibronectin and laminin, (3) glycosaminoglycans (GAGs), (4) proteoglycans, and (5) matricellular peptides. Engineered ECM scaffolds have been fabricated from each of these individual components.
Figure 4.
Comparison of a chronic wound and one treated with a biologic scaffold highlighting the importance of the ECM in providing a friendly microenvironment and regulating cell behavior. As the biologic scaffold is degraded, it releases bioactive ECM fragments that sequester MMPs, decrease inflammation, and promote increased cell migration and angiogenesis. Arrows indicate increase (↑) or decrease (↓). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Engineered ECM scaffolds, to date, include natural materials, such as collagen, fibronectin, and hyaluronan, with and without synthetic materials, such as polyglycolic acid, polylactic acid, nylon, and silicone.24 Collagen is perhaps the most widely used ECM component in wound dressings and scaffolds.25 Products, such as Puracol Plus® (Medline Industries), Fibracol Plus® (Systagenix) or BIOPAD® (Angelini Pharma), are designed as topical wound dressings. These dressings comprising a microfibrous type I collagen sponge are perhaps the simplest biologic scaffold and primarily function to absorb wound exudate and prevent desiccation of the wound rather than provide any bioactive component. The benefit of these materials over standard wound dressings is that as the scaffold absorbs liquid the collagen forms a hydrogel, which protects the wound. The collagen also sequesters MMPs from the wound environment, preventing further wound breakdown. However, they are typically replaced after only a few days, and so do not function as a typical scaffold to direct tissue repair. Attempts have been made to use collagen in combination with a GAG matrix, which showed the ability to regenerate injured dermis.26 However, it has been suggested that a construct composed entirely of type I collagen may not be optimal to promote regeneration.6,27 Similarly, hydrogel dressings, such as Tegagel™ (3M) and Vigilon® (CR Bard), have become common treatments for wound care. These hydrogels can be manufactured from biologic materials, such as alginate, or synthetic materials such as polyethylene oxide. Few bioactive hydrogels that actively promote wound healing have made it to market. One example is Xelma® (Mölnlycke Health Care), which consists of amelogenin peptides within a polyethylene glycol–alginate gel.28 Amelogenin proteins have been shown to stimulate growth factor release and proliferation of fibroblasts, and Xelma been shown to significantly reduce the size of venous ulcers.29,30
Integra™ (Integra Life Sciences)31 is a composite of cross-linked bovine collagen and chondroitin 6-sulfate isolated from shark skin, with a silicone covering. The silicone covering is semipermeable, controlling water vapor loss from the wound and providing structural strength to the graft. After placement on the wound, the collagen and chondroitin matrix recruits dermal fibroblasts to the wound, which then secrete new ECM to restore the dermal bed. The graft matrix degrades in ∼2–3 weeks leaving only the silicone covering. The silicone sheet can then be removed. A related product is Biobrane® (Smith & Nephew), which consists of a silicone sheet with a partially embedded nylon fabric embedded on the surface.32 This nylon fabric is then coated with porcine dermal collagen. Blood and sera from the wound form a clot within the nylon fabric attaching the dressing to the wound, while the collagen promotes fibroblast growth and maintains a hydrated environment. This product has been targeted toward healing donor skin graft sites and skin slough disorders,33,34 but has been shown to promote granulation tissue in hard-to-heal lower extremity wounds.35
Wound dressings based on natural ECM materials derived from decellularized tissues share a number of similarities in their mode of action in promoting wound healing; however, as described previously, these products differ greatly in their source material and preparation and processing.36 Although well-designed and informative studies have been conducted on a variety of decellularized tissues, perhaps the most comprehensively studied matrices regarding biological activity, composition, mechanical properties, and macro- and ultrastructure are those derived from the porcine small intestinal submucosa (SIS)19,37–45 and from the porcine urinary bladder matrix (UBM).46–53 By dry weight, SIS-ECM comprises more than 90% collagen with the majority of that being type I collagen. Small amounts of type III, IV, V, and VI collagens are also present,54 as are adhesive proteins such as fibronectin and laminin.38,55 Natural ECM scaffolds also contain a variety of GAGs, including heparin, heparan sulfate, chondroitin sulfate, and hyaluronic acid.41 The amount of these GAGs remaining in the tissue following decellularization depends greatly on the methods of decellularization, especially those methods, which use ionic detergents known to remove GAGs from the ECM.36 Various growth factors have been identified within these biologic matrices, even following dehydration and terminal sterilization. These growth factors include TGFβ,56 basic FGF,57 and VEGF.58 In addition, cryptic peptides released by the ECM degradation process have been shown to initiate and sustain the recruitment of bone marrow-derived cells, which actively participate in long-term tissue remodeling.21,59,60
Oasis® Wound Matrix (Healthpoint) is an SIS-ECM graft consisting of noncross-linked porcine SIS-ECM. Oasis has been shown to significantly reduce healing times in full-thickness venous ulcers61 and has also been shown to be effective as an alternative to split-thickness skin grafting.62 The added benefit of Oasis is that multiple treatments can be performed without removal of previous Oasis dressings due to the very rapid degradation of the SIS material. In vitro, SIS-ECM has been shown to support epithelial cell differentiation and formation of a new basement membrane.42 Studies utilizing SIS-ECM for the treatment of tissues other than skin have shown that this material also supports angiogenesis, neurogenesis, and the repopulation of the wound site with multiple tissue-specific cell types primarily through the migration of tissue resident and circulating progenitor cells.54,63–67
Matristem® is a natural ECM scaffold consisting of noncross-linked porcine UBM-ECM. The primary advantage of UBM-ECM over other natural ECM products is that UBM-ECM retains the basement membrane component of the urinary bladder on one surface of the biologic scaffold. The basement membrane functions to anchor epithelial tissues to the ECM in organs such as the skin and blood vessels. Basement membrane proteins are also potent promoters of angiogenesis, and so retention of the basement membrane has been suggested to be advantageous in enhancing tissue repair and wound healing. Matristem has been extensively studied as a wound dressing for venous, diabetic, and ischemic ulcers. Lecheminant and Field performed a retrospective study on the use of Matristem and found that application of the product significantly reduced healing time from an average of 25.5–9.8 weeks, although their study also highlighted a number of risk factors that should be considered and addressed to maximize the effectiveness of the Matristem product.52 Other case reports have described similar successful applications for other hard-to-heal wounds, such as radiation wounds,68 open pilonidal wounds,69 and chronic wounds, in patients with a mixed etiology, including diabetes, hypertension, and peripheral vascular disease.70
Primatrix™ (TEI Biosciences) is a noncross-linked ECM derived from decellularized fetal bovine dermis intended for the treatment of nonhealing ulcers, second-degree burns, and surgical wounds. The product is distinctive, in that it utilizes a fetal tissue that is rich in type III collagen, which is the first type of collagen synthesized during both embryonic development and wound healing.71,72 The fetal bovine dermis ECM in Primatrix is ∼30% type III collagen.73 In addition to providing elasticity to the ECM, type III collagen has been shown to promote migration of fibroblasts74 and to be an essential regulator of ECM deposition and organization.75,76 Primatrix becomes incorporated into the wound and rapidly degrades and has shown success for the treatment of acute full-thickness wounds.77
Alloderm® (Lifecell), GraftJacket® (Wright Medical Technology), and DermaMatrix™ (Synthes) are decellularized dermal constructs derived from human dermis. GraftJacket is chemically cross-linked to maintain the collagen architecture, while Alloderm and DermaMatrix are noncross-linked. Unlike xenogeneic ECM scaffolds, constructs derived from human tissue are classified as tissue transplants rather than surgical mesh devices (i.e., medical devices) and therefore are not regulated by the FDA. As a result, there are fewer restrictions on the applications for which these devices can be used. However, since these materials are derived from human tissue, careful screening is required to ensure that the source tissue is free from transmissible pathogens, such as Hepatitis B and C viruses, HIV, and syphilis. Clinically, these products have been indicated for the treatment of nonhealing nonischemic ulcers and other dermal wounds and have shown the ability to significantly reduce healing time, wound volume, and depth.78 GraftJacket increased the probability of successful wound healing of diabetic foot ulcers within 12 weeks by 2.7-fold with a significant increase in the total number of successfully healed wounds.79
The use of biologic therapies for wound repair has shown notable advantages over traditional therapies in terms of the quality of the remodeled tissue and speed of repair. However, ease of handling, cost, and shelf life are of concern. It is clear that over the last 30 years the need for optimal wound care and the concept of moist interactive wound dressings have been recognized. However, the complexities of different wound environments, coupled with the plethora of biologic materials that differ by source, method, or preparation, and method of sterilization, have created a complex range of products, which although similar in nature vary greatly in their ability to promote tissue regeneration. Although clinical studies have been generally positive, results have been mixed, and today, 50% of all wounds still do not receive appropriate care. Biologic wound dressings, when used appropriately, have the potential to change the default wound healing response, but the full ability of these materials to promote constructive remodeling will not be realized until a comprehensive understanding of the biology of the ECM and wound microenvironment is acquired.
Future Directions
The development of bioactive wound dressings has increased in recent years, in part, due to increased research on wound dressings to treat the complex extremity injuries suffered as a consequence of modern warfare. Increasing focus is being placed on the development of a multifunctional device that can promote rapid wound healing and does not need to be replaced. These multifunctional devices may include the ability to control hemostasis, deliver local analgesia or infection control for extended periods, regulate the inflammatory response, and stimulate the reconstruction of functional site-appropriate tissue.
Scar-free healing is a primary goal in developing new wound healing strategies. While embryonic skin has the ability to repair without scarring, adult skin does not have this ability due, in part, to the immune response and inflammatory cascade. TGFβ1–3 and PDGF seem to play prominent roles. It has been proposed that scar-free healing can be achieved through the addition of TGFβ3.80–82 However, while TGFβ3-mediated healing has been successfully demonstrated in rodents, pigs, and healthy human volunteers, phase III clinical trials failed to show any significant improvements and primary and secondary end points were not met.83 Other groups have investigated the use of other growth factors, including epithelial growth factor84 and FGF,85 for the treatment of chronic nonhealing wounds. Studies using PDGF have shown significant improvements in healing of chronic ulcers in the clinical trial.86
Control of hemostasis and wound hydration has led to the investigation of chitosan as a wound dressing for nonhealing wounds.87–90 The benefit of these scaffolds is their ability to rapidly control hemostasis while also modulating the wound environment to maintain an optimal hydration level and prevent desiccation. Furthermore, these chitosan materials can be combined with other bioactive molecules to provide combination dressings that promote wound healing while also combatting infection87 or modulating inflammation.88 The potential for other hydrogel materials to promote wound healing while delivering bioactive factors, such as antibiotics,91 or to deliver analgesia to the wound site for prolonged periods92 are also being investigated. However, it is likely that the greater advances in wound healing will come as a result of studies that characterize the wound microenvironment and elucidate the molecular and biochemical pathways involved in the regulation of adult wound healing versus regeneration.11,93,94
Summary
In contrast to most wound dressings designed to protect the wound surface and maintain various states of hydration, biologic scaffold materials function as an inductive microenvironment. The constructive remodeling of biologic scaffolds represents a scenario in which site-appropriate organized tissue forms at the site of scaffold placement and offers new therapeutic options for the treatment of chronic nonhealing wounds. By altering the wound microenvironment to one that promotes functional tissue formation, biologic scaffolds have the potential to shorten the time to complete healing, prevent advancement of these wounds to deeper structures, and result in improved cosmetic outcomes.
TAKE-HOME MESSAGES.
• Tissue Engineering Regenerative Medicine (TERM) approaches for chronic nonhealing skin wounds holds therapeutic promise.
• True regeneration is limited to the epidermis; deeper wounds heal by inflammation and scar tissue formation.
• Biologic scaffolds comprising the ECM have been shown to promote constructive remodeling in nondermal tissues.
• Degradation of ECM scaffolds results in the formation of cryptic peptides with relevant biologic activities.
• Signaling molecules released from biologic scaffolds contribute to recruitment of endogenous stem cells to the site of interest.
• Signaling molecules from biologic scaffolds modulate the innate immune response from an M1 phenotype toward a more constructive M2 phenotype.
• Optimal formulations of ECM scaffolds for the treatment of nonhealing wounds have yet to be identified, but even suboptimal use has resulted in promising outcomes.
Abbreviations and Acronyms
- ECM
extracellular matrix
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- GAG
glycosaminoglycan
- HGF
hepatocyte growth factor
- IGF
insulin-like growth factor
- IL
interleukin
- MMP
matrix metalloproteinase
- PDGF
platelet-derived growth factor
- SIS
small intestinal submucosa
- TERM
tissue engineering and regenerative medicine
- TGF
transforming growth factor
- UBM
urinary bladder matrix
- VEGF
vascular endothelial growth factor
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Stephen F. Badylak, DVM, PhD, MD, is a professor in the Departments of Surgery and Bioengineering at the University of Pittsburgh. He is also the Deputy Director at the McGowan Institute for Regenerative Medicine. Neill J. Turner, PhD, is a research assistant professor in the Department of Surgery at the University of Pittsburgh and affiliated member of the McGowan Institute for Regenerative Medicine.
References
- 1.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Report 1–31, 2008 [PubMed] [Google Scholar]
- 2.Lawrence WT, Diegelmann RF. Growth factors in wound healing. Clin Dermatol 1994;12:157–169 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: Department of Health and Human Services, 2008 [Google Scholar]
- 4.Reiber GE, Boyko EJ, Smith DG. Lower-extremity foot ulcers and amputations in diabetes. In: Group NDD, ed. Diabetes in America, Second Edition. Washington, DC: National Institutes of Health, 1995:409–428 [Google Scholar]
- 5.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 6.Clark RA. Biology of dermal wound repair. Dermatol Clin 1993;11:647–666 [PubMed] [Google Scholar]
- 7.Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology 2011;216:753–762 [DOI] [PubMed] [Google Scholar]
- 8.Montfrans CV, Stok M, Geerkens M. Biology of chronic wounds and new treatment strategies. Phlebology 2014;29:165–167 [DOI] [PubMed] [Google Scholar]
- 9.Lawall H. Treatment of chronic wounds. VASA 2012;41:396–409 [DOI] [PubMed] [Google Scholar]
- 10.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 2012;25:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junker JP, Caterson EJ, Eriksson E. The microenvironment of wound healing. J Craniofac Surg 2013;24:12–16 [DOI] [PubMed] [Google Scholar]
- 12.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 1998;176:26S–38S [DOI] [PubMed] [Google Scholar]
- 13.Dowsett C, Ayello E. TIME principles of chronic wound bed preparation and treatment. Br J Nurs 2004;13:S16–S23 [DOI] [PubMed] [Google Scholar]
- 14.Jerome D. Advances in negative pressure wound therapy: the VAC instill. J Wound Ostomy Continence Nurs 2007;34:191–194 [DOI] [PubMed] [Google Scholar]
- 15.Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997;77:637–650 [DOI] [PubMed] [Google Scholar]
- 16.Queen D, Orsted H, Sanada H, Sussman G. A dressing history. Int Wound J 2004;1:59–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials 2007;28:3587–3593 [DOI] [PubMed] [Google Scholar]
- 18.Agrawal V, Brown BN, Beattie AJ, Gilbert TW, Badylak SF. Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J Tissue Eng Regen Med 2009;3:590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater 2009;5:1–13 [DOI] [PubMed] [Google Scholar]
- 20.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol 2008;20:109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A 2009;15:1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis GE. Matricryptic sites control tissue injury responses in the cardiovascular system: relationships to pattern recognition receptor regulated events. J Mol Cell Cardiol 2010;48:454–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol 2000;156:1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005;23:47–55 [DOI] [PubMed] [Google Scholar]
- 25.Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol 2005;23:403–412 [DOI] [PubMed] [Google Scholar]
- 26.Butler CE, Orgill DP. Simultaneous in vivo regeneration of neodermis, epidermis, and basement membrane. Adv Biochem Eng Biotechnol 2005;94:23–41 [DOI] [PubMed] [Google Scholar]
- 27.Welch MP, Odland GF, Clark RA. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol 1990;110:133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirastschijski U, Konrad D, Lundberg E, Lyngstadaas SP, Jorgensen LN, Agren MS. Effects of a topical enamel matrix derivative on skin wound healing. Wound Repair Regen 2004;12:100–108 [DOI] [PubMed] [Google Scholar]
- 29.Romanelli M, Kaha E, Stege H, Wnorowski JW, Vowden P, Majamaa H, et al. . Effect of amelogenin extracellular matrix protein and compression on hard-to-heal venous leg ulcers: follow-up data. J Wound Care 2008;17:17–18, 20–23. [DOI] [PubMed] [Google Scholar]
- 30.Vowden P, Romanelli M, Peter R, Bostrom A, Josefsson A, Stege H. The effect of amelogenins (Xelma) on hard-to-heal venous leg ulcers. Wound Repair Regen 2006;14:240–246 [DOI] [PubMed] [Google Scholar]
- 31.Burke JF, Yannas IV, Quinby WC, Jr., Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 1981;194:413–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavis MJ, Thornton JW, Bartlett RH, Roth JC, Woodroof EA. A new composite skin prosthesis. Burns 1980;7:123–130 [Google Scholar]
- 33.Arevalo JM, Lorente JA. Skin coverage with Biobrane biomaterial for the treatment of patients with toxic epidermal necrolysis. J Burn Care Rehabil 1999;20:406–410 [DOI] [PubMed] [Google Scholar]
- 34.McHugh TP, Robson MC, Heggers JP, Phillips LG, Smith DJ, Jr., McCollum MC. Therapeutic efficacy of Biobrane in partial- and full-thickness thermal injury. Surgery 1986;100:661–664 [PubMed] [Google Scholar]
- 35.Kirwan L. Management of difficult wounds with Biobrane. Conn Med 1995;59:523–529 [PubMed] [Google Scholar]
- 36.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 2006;27:3675–3683 [DOI] [PubMed] [Google Scholar]
- 37.Badylak SF, Record R, Lindberg K, Hodde J, Park K. Small intestinal submucosa: a substrate for in vitro cell growth. J Biomater Sci Polym Ed 1998;9:863–878 [DOI] [PubMed] [Google Scholar]
- 38.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng 2006;12:519–526 [DOI] [PubMed] [Google Scholar]
- 39.Freytes DO, Badylak SF, Webster TJ, Geddes LA, Rundell AE. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials 2004;25:2353–2361 [DOI] [PubMed] [Google Scholar]
- 40.Grimes M, Pembroke JT, McGloughlin T. The effect of choice of sterilisation method on the biocompatibility and biodegradability of SIS (small intestinal submucosa). Biomed Mater Eng 2005;15:65–71 [PubMed] [Google Scholar]
- 41.Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng 1996;2:209–217 [DOI] [PubMed] [Google Scholar]
- 42.Lindberg K, Badylak SF. Porcine small intestinal submucosa (SIS): a bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns 2001;27:254–266 [DOI] [PubMed] [Google Scholar]
- 43.Pu LL. Small intestinal submucosa (Surgisis) as a bioactive prosthetic material for repair of abdominal wall fascial defect. Plast Reconstr Surg 2005;115:2127–2131 [DOI] [PubMed] [Google Scholar]
- 44.Yu J, Zeng Y, Zhao J, Liao D, Gregersen H. Quantitative analysis of collagen fiber angle in the submucosa of small intestine. Comput Biol Med 2004;34:539–550 [DOI] [PubMed] [Google Scholar]
- 45.Sacks MS, Gloeckner DC. Quantification of the fiber architecture and biaxial mechanical behavior of porcine intestinal submucosa. J Biomed Mater Res 1999;46:1–10 [DOI] [PubMed] [Google Scholar]
- 46.Obara T, Matsuura S, Narita S, Satoh S, Tsuchiya N, Habuchi T. Bladder acellular matrix grafting regenerates urinary bladder in the spinal cord injury rat. Urology 2006;68:892–897 [DOI] [PubMed] [Google Scholar]
- 47.Freytes DO, Stoner RM, Badylak SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater 2008;84:408–414 [DOI] [PubMed] [Google Scholar]
- 48.Freytes DO, Tullius RS, Valentin JE, Stewart-Akers AM, Badylak SF. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J Biomed Mater Res Part A 2008;87:862–872 [DOI] [PubMed] [Google Scholar]
- 49.Rosario DJ, Reilly GC, Ali Salah E, Glover M, Bullock AJ, Macneil S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen Med 2008;3:145–156 [DOI] [PubMed] [Google Scholar]
- 50.Parekh A, Mantle B, Banks J, Swarts JD, Badylak SF, Dohar JE, et al. . Repair of the tympanic membrane with urinary bladder matrix. Laryngoscope 2009;119:1206–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnes CA, Brison J, Michel R, Brown BN, Castner DG, Badylak SF, et al. . The surface molecular functionality of decellularized extracellular matrices. Biomaterials 2011;32:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lecheminant J, Field C. Porcine urinary bladder matrix: a retrospective study and establishment of protocol. J Wound Care 2012;21:476, 478–480, 482 [DOI] [PubMed] [Google Scholar]
- 53.Marcal H, Ahmed T, Badylak SF, Tottey S, Foster LJ. A comprehensive protein expression profile of extracellular matrix biomaterial derived from porcine urinary bladder. Regen Med 2012;7:159–166 [DOI] [PubMed] [Google Scholar]
- 54.Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, et al. . The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res 1995;29:977–985 [DOI] [PubMed] [Google Scholar]
- 55.Hodde JP, Record RD, Tullius RS, Badylak SF. Retention of endothelial cell adherence to porcine-derived extracellular matrix after disinfection and sterilization. Tissue Eng 2002;8:225–234 [DOI] [PubMed] [Google Scholar]
- 56.McDevitt CA, Wildey GM, Cutrone RM. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res Part A 2003;67:637–640 [DOI] [PubMed] [Google Scholar]
- 57.Hodde JP, Ernst DM, Hiles MC. An investigation of the long-term bioactivity of endogenous growth factor in OASIS Wound Matrix. J Wound Care 2005;14:23–25 [DOI] [PubMed] [Google Scholar]
- 58.Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium 2001;8:11–24 [DOI] [PubMed] [Google Scholar]
- 59.Brennan EP, Tang XH, Stewart-Akers AM, Gudas LJ, Badylak SF. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med 2008;2:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. . A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313 [DOI] [PubMed] [Google Scholar]
- 61.Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 2005;41:837–843 [DOI] [PubMed] [Google Scholar]
- 62.Barendse-Hofmann MG, van Doorn LP, Oskam J, Steenvoorde P. Extracellular matrix prevents split-skin grafting in selected cases. J Wound Care 2007;16:455–458 [DOI] [PubMed] [Google Scholar]
- 63.Gilbert TW, Nieponice A, Spievack AR, Holcomb J, Gilbert S, Badylak SF. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res 2008;147:61–67 [DOI] [PubMed] [Google Scholar]
- 64.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am 2007;89:621–630 [DOI] [PubMed] [Google Scholar]
- 65.Kropp BP, Rippy MK, Badylak SF, Adams MC, Keating MA, Rink RC, et al. . Regenerative urinary bladder augmentation using small intestinal submucosa: urodynamic and histopathologic assessment in long-term canine bladder augmentations. J Urol 1996;155:2098–2104 [DOI] [PubMed] [Google Scholar]
- 66.Suckow MA, Voytik-Harbin SL, Terril LA, Badylak SF. Enhanced bone regeneration using porcine small intestinal submucosa. J Invest Surg 1999;12:277–287 [DOI] [PubMed] [Google Scholar]
- 67.Wood JD, Simmons-Byrd A, Spievack AR, Badylak SF. Use of a particulate extracellular matrix bioscaffold for treatment of acquired urinary incontinence in dogs. J Am Vet Med Assoc 2005;226:1095–1097 [DOI] [PubMed] [Google Scholar]
- 68.Rommer EA, Peric M, Wong A. Urinary bladder matrix for the treatment of recalcitrant nonhealing radiation wounds. Adv Skin Wound Care 2013;26:450–455 [DOI] [PubMed] [Google Scholar]
- 69.Sasse KC, Brandt J, Lim DC, Ackerman E. Accelerated healing of complex open pilonidal wounds using MatriStem extracellular matrix xenograft: nine cases. J Surg Case Rep 2013; DOI: 10.1093/jscr/rjt025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimmel H, Rahn M, Gilbert TW. The clinical effectiveness in wound healing with extracellular matrix derived from porcine urinary bladder matrix: a case series on severe chronic wounds. J Am Coll Certif Wound Spec 2010;2:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith LT, Holbrook KA, Madri JA. Collagen types I, III, and V in human embryonic and fetal skin. Am J Anat 1986;175:507–521 [DOI] [PubMed] [Google Scholar]
- 72.Sykes B, Puddle B, Francis M, Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun 1976;72:1472–1480 [DOI] [PubMed] [Google Scholar]
- 73.Ramshaw JA. Distribution of type III collagen in bovine skin of various ages. Connect Tissue Res 1986;14:307–314 [DOI] [PubMed] [Google Scholar]
- 74.Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci U S A 1978;75:871–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A 1997;94:1852–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fleischmaher R, MacDonald ED, Perlish JS, Brugeson RE. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J Struct Biol 1990;105:162–169 [DOI] [PubMed] [Google Scholar]
- 77.Wanitphakdeedecha R, Chen TM, Nguyen TH. The use of acellular, fetal bovine dermal matrix for acute, full-thickness wounds. J Drugs Dermatol 2008;7:781–784 [PubMed] [Google Scholar]
- 78.Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int Wound J 2006;3:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, et al. . Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J 2009;6:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Occleston NL, Fairlamb D, Hutchison J, O'Kane S, Ferguson MW. Avotermin for the improvement of scar appearance: a new pharmaceutical in a new therapeutic area. Expert Opin Investig Drugs 2009;18:1231–1239 [DOI] [PubMed] [Google Scholar]
- 81.Shah M, Revis D, Herrick S, Baillie R, Thorgeirson S, Ferguson M, et al. . Role of elevated plasma transforming growth factor-beta1 levels in wound healing. Am J Pathol 1999;154:1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol 1997;29:63–78 [DOI] [PubMed] [Google Scholar]
- 83.Renovo Slashes Jobs after Trial Failure. Chem Eng News 2011;89:23 [Google Scholar]
- 84.Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen 2000;8:347–352 [PubMed] [Google Scholar]
- 85.Robson MC, Phillips LG, Lawrence WT, Bishop JB, Youngerman JS, Hayward PG, et al. . The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Ann Surg 1992;216:401–406; discussion 6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Embil JM, Papp K, Sibbald G, Tousignant J, Smiell JM, Wong B, et al. . Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open-label clinical evaluation of efficacy. Wound Repair Regen 2000;8:162–168 [DOI] [PubMed] [Google Scholar]
- 87.Zhao R, Li X, Sun B, Zhang Y, Zhang D, Tang Z, et al. . Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int J Biol Macromol 2014;68:92–97 [DOI] [PubMed] [Google Scholar]
- 88.Moura LI, Dias AM, Leal EC, Carvalho L, de Sousa HC, Carvalho E. Chitosan-based dressings loaded with neurotensin—an efficient strategy to improve early diabetic wound healing. Acta Biomater 2014;10:843–857 [DOI] [PubMed] [Google Scholar]
- 89.Mayol L, De Stefano D, Campani V, De Falco F, Ferrari E, Cencetti C, et al. . Design and characterization of a chitosan physical gel promoting wound healing in mice. J Mater Sci 2014;25:1483–1493 [DOI] [PubMed] [Google Scholar]
- 90.Kirichenko AK, Bolshakov IN, Ali-Riza AE, Vlasov AA. Morphological study of burn wound healing with the use of collagen-chitosan wound dressing. Bull Exp Biol Med 2013;154:692–696 [DOI] [PubMed] [Google Scholar]
- 91.Mori M, Rossi S, Bonferoni MC, Ferrari F, Sandri G, Riva F, et al. . Calcium alginate particles for the combined delivery of platelet lysate and vancomycin hydrochloride in chronic skin ulcers. Int J Pharm 2014;461:505–513 [DOI] [PubMed] [Google Scholar]
- 92.Heilmann S, Kuchler S, Wischke C, Lendlein A, Stein C, Schafer-Korting M. A thermosensitive morphine-containing hydrogel for the treatment of large-scale skin wounds. Int J Pharm 2013;444:96–102 [DOI] [PubMed] [Google Scholar]
- 93.Martins-Green M, Petreaca M, Wang L. Chemokines and their receptors are key players in the orchestra that regulates wound healing. Adv Wound Care 2013;2:327–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Plantman S. Proregenerative properties of ECM molecules. Biomed Res Int 2013;2013:981695. [DOI] [PMC free article] [PubMed] [Google Scholar]