Abstract
Significance: Growth factors are very promising molecules for the treatment of skin wounds. However, their translation to clinical use has been seriously limited, facing issues related to safety and cost-effectiveness. These problems may derive from the fact that growth factors are used at vastly supra-physiological levels without optimized delivery systems.
Recent Advances: The extracellular matrix (ECM) plays a fundamental role in coordinating growth factor signaling. Therefore, understanding the mechanisms by which the ECM modulates growth factor activity is key for designing efficient growth factor-based therapies. Recently, several growth factor-binding domains have been discovered within various ECM proteins, and growth factor delivery systems integrating these ECM growth factor-binding domains showed promising results in animal models of skin wound healing. Moreover, a novel strategy consisting of engineering growth factors to target endogenous ECM could substantially enhance their efficacy, even when used at low doses.
Critical Issues: Optimal delivery of growth factors often requires complex engineered biomaterial matrices, which can face regulatory issues for clinical translation. To simplify delivery systems and render strategies more applicable, growth factors can be engineered to optimally function with clinically approved biomaterials or with endogenous ECM present at the delivery site.
Future Directions: Further development and clinical trials will reveal whether growth factor-based therapies can be used as main therapeutic approaches for skin wound healing. The future impact of these therapies will depend on our capacity to deliver growth factors more precisely, to improve efficacy, safety, and cost-effectiveness.

Mikaël M. Martino, PhD

Jeffrey A. Hubbell, PhD
Scope and Significance
Non-healing wounds have an increasing impact in our society and remain a very important clinical challenge. Aging, obesity, diabetes, cardiovascular disorders, sensory neuropathies, and autoimmune diseases are multiple causes that delay wound healing and dramatically increase the global burden of chronic wounds. In fact, chronic wounds affected 6.5 million people and engendered an annual cost of about $20 billion dollars in the United Stated of America in 2009.1,2 Based on the worldwide diabetes prevalence in 2012, we can currently estimate that 22 million people will suffer from infected diabetic foot ulcers—a particular type of chronic wound—in the following years. Consequently, according to Med-Market Diligence,3 the wound care products world market has been projected to exceed $18.5 billion dollars by 2020. During the last decades, the number of wound dressings, biopharmaceutical formulations, and skin substitutes available in the market exploded. However, no generally satisfactory clinical solution for chronic wounds is available today.4,5 A particular interest has been given to growth factors, which are key signaling molecules regulating tissue repair and regeneration.6,7 However, although a number of growth factors involved in skin repair have been identified, their translation to the clinic has been very limited,8,9 additionally facing issues related to safety and cost-effectiveness.10 These problems derive most likely from the fact that the growth factors are used at vastly supra-physiological levels without appropriate delivery systems.8,11 Therefore, design of controlled release strategies for dose reduction in addition to temporal and spatial dose localization is an ongoing challenge. Lately, the extracellular matrix (ECM) has become a source of inspiration to scientists designing growth factor delivery systems. In fact, the ECM plays a fundamental role in coordinating growth factor signaling in vivo, by displaying and releasing them in a highly spatio-temporal controlled manner and also by modulating their intracellular signaling. Thus, understanding how the ECM regulates growth factors and engineering delivery systems integrating these regulatory features could be key to making growth factor-based therapies a reality. In this review, we highlight various ECM-inspired technologies that have been developed to deliver growth factors for skin wound healing.
Translational Relevance
Chronic wounds are stubborn to close, even with extensive medical care. Therefore, since many growth factors have been recognized as key signaling molecules inducing wound healing,12 they have been explored in the clinic to treat chronic wounds.13,14 For example, platelet-derived growth factor-BB (PDGF-BB) is crucial for the formation of granulation tissue and for stem cell recruitment, vascular endothelial growth factor-A (VEGF-A) is necessary to induce the growth of blood vessels that sustain the granulation tissue, and fibroblast growth factors (FGFs), especially FGF-2, are important for both wound reepithelization and angiogenesis.15,16
Clinical Relevance
Even if growth factors are very promising for wound healing, their translation into the clinic has been seriously limited.17 For instance, PDGF-BB (Becaplermin in Regranex®) is commercially available for chronic wound treatment, but the product received a boxed warning from the U.S. Food and Drug Administration and has been withdrawn in Europe due to safety issues. Indeed, PDGF-BB was used as supra-physiological doses and treatment with the growth factor correlated with a five-times increased risk of cancer.17 The problems encountered with PDGF-BB illustrate well the importance of controlling the spatio-temporal release of growth factors at the wound site and overcoming this challenge is probably the key for successful growth factor-based therapies.
Background: The ECM as a Regulator of Growth Factor Signaling
Key ECM molecules involved in wound healing
Although skin matrix composition and properties evolve with aging, the environment, and disease, the principal ECM molecules remain qualitatively the same and can be schematically divided in two categories. The first one includes fibrous structural proteins and adhesive glycoproteins, which provide the core structure and tensile strength of the tissue, connect the matrix components, and display adhesion sites for cells. The second category consists of proteoglycans and glycosaminoglycans (GAGs), which are made of highly hydrophilic polysaccharides chains and provide the gel-like compressive properties of the tissue. Collagens are the main class of fibrous proteins composing the healthy dermal ECM, more particularly type I collagen (about 80%) and type III collagen (about 10%) that are organized into a partially cross-linked network. This network is intermingled with bundles of elastic fibers made of a highly cross-linked elastin core surrounded by fibrillin molecules at the periphery. These fibers are essential to provide the skin its stretching and compliance properties. In this collagen-elastin scaffold, glycoproteins such as fibronectin and other matricellular proteins (e.g., osteopontin, vitronectin, and tenascin) are bound. Importantly, most glycoproteins have the ability to interact with soluble signaling molecules such as growth factors and to present cell adhesion sites.18–20 Concerning GAGs, hyaluronan is the predominant one in the dermal interstitial matrix, the others being dermatan-, chondroitin-, heparan-, and keratan-sulfates. The ECM compositions of basement membranes, located beneath the epidermis and surrounding blood and lymphatic vessels, differ from the one described above and are mainly constituted of non-fibrillar collagen IV, laminin, and entactin, and enriched in perlecan and heparan sulfates21–23 (Fig. 1).
Figure 1.
Different ECM compositions in healthy skin and during wound healing. (Top) Locations of the different ECM present in the skin tissue. (Bottom) Schematic representations of the main ECM molecules composing the interstitial matrix (A) and the basal lamina (B) of healthy skin, and the fibrin clot (C) and the granulation tissue (D) during skin wound healing. Stars indicate ECM molecules that have been shown to have a strong affinity for several growth factors. ECM, extracellular matrix.
Following skin injury, the original ECM is damaged and a provisional fibrin matrix is formed within the wound by the coagulation cascade. This matrix is mainly constituted of fibrinogen and contains plasma fibronectin to some extent. During the process of repair, the fibrin matrix is gradually degraded by immune cells migrating into the clot and replaced by a characteristic collagen-based matrix having an excessive proportion of type III collagen (about 20–25%) compared to healthy skin, and the levels of other important ECM proteins, notably fibronectin, tenascin C, and hyaluronan, are also increased. During the subsequent long-term remodeling phase, the network of elastic fibers reestablishes 24 and the matrix reorganizes to reach a composition closer to the initial skin ECM (Fig. 1).
Growth factor regulatory functions of the ECM during wound healing
More than a fiber network providing mechanical cues, the ECM is a highly dynamic microenvironment, which controls a multitude of cellular processes during wound healing. One of the primary functions of the ECM is to provide a scaffold for migrating cells, since collagen and ECM glycoproteins display a number of cell-binding sites such as for integrin receptors. Integrins, which recognize short sequences present in many ECM proteins, such as collagen, fibronectin, and vitronectin,25 are one of the major classes of transmembrane cell surface receptors that allow cell–ECM interactions. Importantly, integrins not only serve for cell adhesion and migration, but they also regulate or induce a number of cellular processes including proliferation and differentiation.
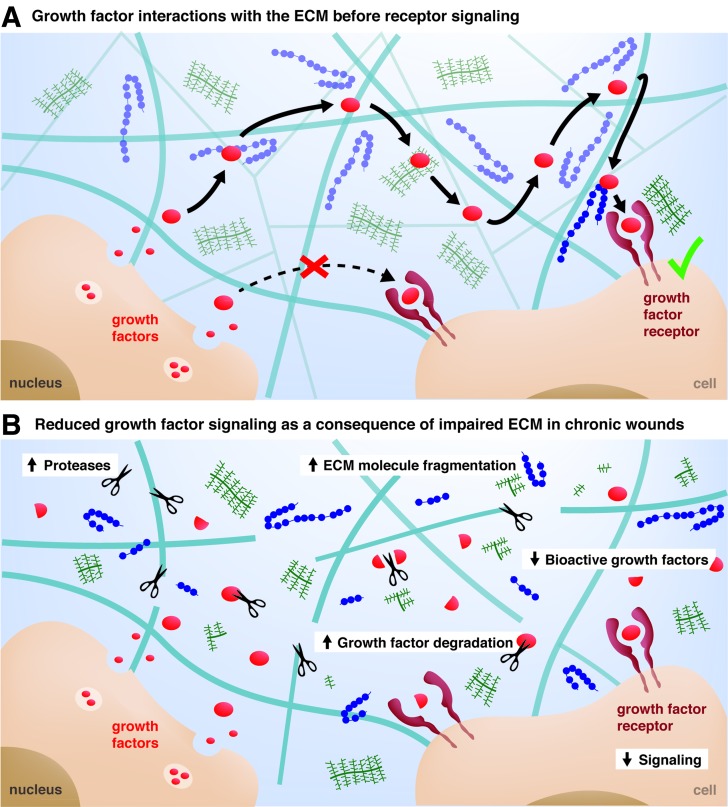
Besides providing cell-adhesion sites, one of the most important functions of the ECM is to act as a reservoir for growth factors. Throughout the different phases of the healing process, immune and tissue-resident cells secrete a multitude of cytokines and growth factors, which strongly modulate cell behavior. Many growth factors have the ability to bind specific sites within the ECM26,27 and will thus first interact with the ECM before finding their cognate cell-surface receptor (Fig. 2A). Several growth factors possess specific interactions with heparan sulfate proteoglycans of the ECM and they are often described as heparin-binding growth factors.28,29 On the other hand, several growth factor-binding sites have been recently discovered within ECM proteins such as fibronectin,18 fibrinogen,30 tenascin C,19 and vitronectin,20 which are present in the provisional matrix and under the basement membrane of the reepithelized wound. Once bound to the ECM, signaling molecules are released depending on their binding-affinity and the action of proteases.10,30 As such, the ECM, with respect to its components, releases signaling molecules at different kinetics and from different locations, which allows an extremely tight spatio-temporal regulation of cell fate within the wound microenvironment.26,31 Moreover, as described below, the formation of molecular complexes between growth factors and components of the ECM can modulate the signaling of growth factor receptors.32,33
Figure 2.
Growth factor journey in healthy and impaired microenvironment. (A) After their secretion by cells, growth factors are sequestered in the ECM and interact with various ECM molecules before reaching their cell-surface receptors, which creates a tight spatio-temporal control of the growth factor delivery by the ECM. (B) Damage of the microenvironment in chronic wounds is a consequence of an increased level of proteases degrading both the ECM and the growth factors, which results in lower growth factor signaling and impaired healing.
Reduced growth factor signaling as a consequence of impaired ECM in chronic wounds
Chronic wounds are defined as wounds that remain unclosed for more than 3 months. Due to a prolonged pathogen invasion or as a consequence of other disease, the wound can sometimes become trapped in a prolonged inflammatory phase.34,35 Although the etiology of these wounds remains only partially elucidated, progress has been made in understanding wound pathology. In 2006, a “unified hypothesis” was presented that observed that four main factors are responsible for most of chronic wounds, namely aging-related cellular and systemic changes, local hypoxia, tissue ischemic-reperfusion repetitions, and bacterial colonization.36
One serious consequence of the prolonged inflammatory phase in chronic wound is the deterioration of the extracellular microenvironment, due an abnormally high level of proinflammatory cytokines and proteases secreted by immune cells. As mentioned above, the ECM plays a dynamic role in delivering growth factors during the repair process, and it has been hypothesized that the degradation of the ECM in chronic wounds is responsible for delayed healing.37 Indeed, the unusually high level of proteolytic enzymes and the imbalance with their inhibitors results in an abnormal degradation of both the matrix and the ECM-bound signaling molecules. For example, reduced levels of growth factors and high fragmentation of ECM molecules have been reported in chronic ulcers.37 Histological analyses of chronic venous ulcers usually show a decreased presence of fibronectin and collagen I, and molecular analyses of wound fluid reveal substantial degradation of fibronectin and vitronectin in diabetic foot ulcers.21 In addition, other ECM alterations such as abnormal matrix glycation and glycosylation, which are found in diabetic patients, increase the matrix instability and adversely influence cell responses by inducing cell senescence and apoptosis.38 In summary, the altered ECM in chronic wounds fails to fulfill its roles in controlling cell and growth factor functions, which may prevent the healing progression beyond the inflammatory phase (Fig. 2B).
ECM-inspired growth factor delivery systems for skin repair
Various techniques have been explored both in research and clinical phases to deliver growth factor proteins, genes encoding them,39,40 or cells engineered to overexpress them.41 Through this, it has become evident that the ECM plays a fundamental role in coordinating growth factor signaling and in guiding injured skin tissue toward healing. Therefore, understanding and mimicking the mechanisms by which the ECM controls growth factors is becoming critical for designing successful growth factor-based therapies.9,42 Consequently, both biomaterial carriers and growth factors have been engineered, taking inspiration from the natural interactions between ECM and growth factors, to achieve both reasonable therapeutic concentrations and spatio-temporal localization. Thus, in the next sections, we will focus on delivery systems that recapitulate those interactions and we will discuss the advantages and limitations of those systems from a clinical perspective.
Source of growth factors
The source growth factors can be from human or animal tissue, either from blood plasma or extracted within the dermal matrix, or recombinant. Getting growth factors from human blood consists of sampling and treating it to extract the platelet-rich plasma (PRP), which contains a mixture of growth factors. The PRP containing the cocktail of growth factors is then typically administered as a bolus without an additional delivery system. Although PRP-based therapies are used in the clinic on nonhealing ulcers, detailed effects and further characterization are still under investigation.43 On the other hand, decellularized matrices that contain low doses of native growth factors are clinically used as a skin graft substitute for chronic wounds.44 In contrast to these growth factor-based technologies, recombinant growth factors offer more precise characterization and better control on the specific type and doses of factors delivered. Moreover, recombinant growth factors can be engineered with specific features and the use of a synthetic source avoids risk of disease transmission.
Engineering biomaterial matrices to optimize growth factor delivery
When designing a growth factor delivery system, the goal is to deliver sustained low doses of bioactive growth factors at a precise location. In other words, the system aims to deliver optimal concentrations of growth factors within the wound and limit their systemic diffusion, closely resembling what the ECM does under physiological conditions. Therefore, strategies based on biomaterial matrices that can interact with growth factors are appealing. The next sections will focus on biomaterial matrix systems engineered to specifically interact with growth factors.
Increasing biomaterial matrices affinity for growth factors
The release of growth factors from a biomaterial matrix can be controlled by changing the matrix biophysical properties such as its density, porosity, charge, and hydrophobicity8 (Fig. 3A). However, such modifications are often not optimal for cells that should colonize the biomaterial matrix and remodel it. As another approach aiming to slow the release of growth factors, a cell-friendly biomaterial matrix can be functionalized with specific growth factor-binding sites.
Figure 3.
ECM-inspired growth factor delivery systems. (A) The choice of the appropriate biomaterial is central for designing a growth factor delivery system, depending on its ability to retain growth factors while being cell friendly. (B) Further engineering strategies can be implemented to specifically increase the biomaterial affinity for wild-type growth factors. (C) Other strategies are based on the engineering of the growth factors itself, to reduce the complexity of the delivery system.
Since the ECM naturally binds growth factors, useful growth factor-binding domains can be isolated from various ECM molecules. For example, several growth factors possess specific interactions with the heparan sulfate proteoglycans of the ECM.26,28,29 As such, a number of biomaterial matrices have been modified with heparin or heparan sulfate-mimetic molecules to sequester heparin-binding growth factors and control their release. For example, synthetic hydrogel films cross-linked with heparin and derivatives of chondroitin sulfate have been used to successfully control the delivery of FGF-2 in a full-thickness excisional wound model in db/db diabetic mice and showed acceleration of dermis formation and vascularization.45
Recently, several growth factor-binding sites have been discovered within ECM proteins such as fibronectin,18 fibrinogen,30 tenascin C,19 and vitronectin.20 Interestingly, the growth factor-binding sites are often promiscuous in their affinity for multiple growth factors and thus offer the possibility of using them for a multitude of growth factors. For example, fibrin(ogen) has a natural affinity for a number of growth factors and fibrin matrix has been shown to be efficient in delivering low doses of FGF-2 and placenta growth factor-2 (PlGF-2) for wound healing in diabetic mice (db/db).30 Moreover, the growth factor-binding domain of fibrin(ogen) has been isolated and incorporated in a synthetic matrix based on polyethylene glycol (PEG). PEG matrices functionalized with the growth-factor binding domain of fibrin(ogen) were able to sequester growth factors similarly to fibrin. Strikingly, treatment of wounds in diabetic mice by delivering FGF-2 and PlGF-2 through the synthetic matrix performed as well as delivering the growth factors with fibrin. Thus, this approach offers the possibility of replacing fibrin by a completely synthetic matrix that is highly customizable. Moreover, unlike fibrin, which is purified from human plasma, a synthetic fibrin-mimetic matrix could benefit from a more straightforward regulatory path associated with chemical synthesis rather than human sourcing.
Another interesting growth factor-binding ECM protein with a potential for wound healing is vitronectin.10 For example, a complex comprising vitronectin, insulin-like growth factor (IGF), and IGF-binding protein (IGF-BP) and epidermal growth factor (EGF) were assessed as a topical agent for the treatment of deep dermal partial thickness burns in a porcine model.20 Delivery of the complex with low dose of IGF and EGF was observed to significantly accelerate reepithelization of nonhealing ulcers.46 Discovering and integrating ECM growth factor-binding domains into biomaterial matrices or using these domains topically is thus an interesting approach to efficiently deliver low doses of growth factors (Fig. 3B). Moreover, as discussed below, growth factor-binding ECM fragments can be further engineered to enhance growth factor signaling.
Engineering the signaling microenvironment of growth factors
Besides the fact that the ECM binds growth factors and controls their bioavailability, the ECM can also modulate growth factor receptor signaling.47 Indeed, the signaling of many growth factors is regulated by the dynamic interactions between growth factors, ECM proteins, adhesion receptors, and growth factor receptors.31,48,49 Interestingly, the formation of molecular complexes between growth factors and ECM proteins such as fibronectin50,51 and vitronectin20,46 can considerably enhance growth factor signaling. In particular, ECM protein-growth factor complexes can induce the formation of clusters between growth factor-receptors and integrins. Because the signaling machinery of growth factor receptors and integrins shares several common molecules, the formation of such clusters enhances and prolongs signaling (Fig. 4).32,33,52 Therefore, one can exploit this synergy to have a strong signaling with low doses of growth factors. For example, to promote synergistic signaling between integrins and growth factor receptors, a multifunctional recombinant fragment of fibronectin was engineered to comprise a fibrin-binding sequence, the major integrin-binding domain of fibronectin, and one of the growth factor-binding domains of fibronectin. In a model of chronic wounds in db/db mouse, codelivery of VEGF-A and PDGF-BB with the multifunctional fibronectin fragment was able to induce skin repair at low doses, where the growth factors delivered without the fragment had no significant effect.33
Figure 4.
Engineering of the growth factor signaling microenvironment. Cosignaling of integrins and growth factor receptors has been shown to trigger a synergistic effect that increase and prolong growth factor signaling. The recruitment of common molecules from both signaling cascade induces an enhanced effect of growth factor. Exploiting this synergistic signaling permits to lower the effective dose of growth factors in wound healing therapies.
Engineering growth factors to interact with biomaterial matrices and the ECM
Instead of modifying the biomaterial matrices for enhancing their affinity for growth factors, growth factors can be directly engineered to increase their affinity for biomaterials or endogenous matrices. As a first approach, growth factors can be covalently immobilized into a biomaterial matrix using chemical or enzymatic reactions. The second approach consists of engineering the growth factor to enhance its affinity for a biomaterial matrix or for the endogenous ECM.
Engineering growth factors to bind biomaterial matrices
While a variety of chemical conjugation methods have been developed, a potential limitation of these strategies is that growth factors may lose their biological activity after chemical coupling. To address this limitation, a technique has been developed to covalently cross-link growth factors into fibrin matrices through a specific transglutaminase peptide sequence. The growth factor is recombinantly produced to contain a substrate sequence for factor XIIIa derived from alpha-2-plasmin inhibitor (NQEQVSPL). Thus, the engineered growth factor can be incorporated into fibrin during the natural matrix polymerization and cross-linking process, which is mediated by the transglutaminase factor XIIIa (Fig. 3C). For example, this specific enzymatic cross-linking of growth factors into fibrin has demonstrated to be effective to deliver VEGF-A in wound healing models.53–55
In the case of growth factors covalently bound to a biomaterial matrix, growth factor release will depend on the matrix degradation rate. For example, growth factors covalently bound to fibrin are released by the action of cell-secreted or cell-activated proteases such as matrix metalloproteinases and plasmin, which degrade the matrix. To have a better control of growth factor release and to have release proceed upon cellular demand, growth factors can be engineered to incorporate a protease sensitive site between the growth factor and the fibrin-coupling site (Fig. 3C).53,54
Engineering growth factors to bind endogenous matrices
As described in the previous sections, optimal delivery of growth factors often requires engineering of complex biomaterial matrix systems, which can face regulatory challenges for clinical translation. To simplify development of delivery systems and make them more suitable for clinical applications, growth factors can be engineered to optimally bind to clinically available biomaterial matrices such as fibrin or directly to the endogenous ECM at the delivery site.
Taking inspiration of heparin-binding growth factors that extend their half-life by being protected in the matrix, bioengineers have modified non-heparin-binding growth factors to increase their affinity to endogenous heparan sulfate and GAGs in vivo. To our knowledge, this concept has not been studied in wound healing therapies yet, but it has been applied in cartilage tissue engineering. Indeed, the engineering of a heparin-binding IGF-1 (HB-IGF-1) variant has shown an improved retention in proteoglycan-rich environments and sustained bioactivity.56 In dermal wound healing, IGF-1 is also a key factor that promotes type I collagen synthesis, and fibroblasts and keratinocytes proliferation. Its topical application on nonhealing diabetic skin has been correlated with a faster reepithelization and enhanced scarring in rat model.57 These observations suggest that the delivery of HB-IGF-1 variant in chronic wounds may have an improved interaction with GAGs and a prolonged effect in comparison to the wild-type IGF-1.
Recently, a proof of concept study demonstrated that the simultaneous targeting of endogenous ECM proteins and GAGs could enhance their efficacy when used at low doses.10 In this study, 25 growth factors were screened for their binding to key ECM proteins, namely fibronectin, vitronectin, tenascin C, osteopontin, fibrinogen, and collagen I. Among all the growth factors, PlGF-2 displayed the strongest binding to all the ECM proteins tested. Indeed, the heparin-binding sequence of PlGF-2 (PlGF-2123-144) was responsible for the binding characteristics of the growth factor to ECM proteins. Based on this finding, and using rational protein engineering, PlGF-2123-144 has been incorporated as a fusion into growth factors that bear clinical translation limitations, namely VEGF-A and PDGF-BB (Fig. 5A). Insertion of the PlGF-2123-144 domain conferred super-affinity for ECM proteins and heparan sulfate (Fig. 5B) and the PlGF-2123-144-fused growth factors were strongly retained in a fibrin matrix. Strikingly, skin wounds in diabetic mice treated with a low dose of PlGF-2123-144-fused PDGF-BB and VEGF-A led to significantly faster wound closure and to more granulation tissue compared to wild-type growth factors, both topically and in fibrin. Furthermore, one of the critical clinical limitations of VEGF-A, that is, its induction of vascular hyperpermeability, was ameliorated through this growth factor engineering concept.10
Figure 5.
Growth factors engineered for super-affinity to the ECM. (A) Fusing an ECM-binding domain on growth factors enhances their binding to endogenous matrices, which improves their retention and tissue healing effect in vivo. (B) Immunostaining of VEGF-A variants having different affinity for the ECM after their incubation on ear skin matrix. Super-affinity engineered VEGF-A shows a drastically increased retention in the matrix compared to wild-type VEGF-A. Scale bar=50 μm. VEGF-A, vascular endothelial growth factor-A.
Targeting of endogenous matrices is thus an interesting alternative to develop carrier-free growth factor delivery systems. Such systems are highly versatile since ECM-binding growth factors may be delivered by direct topical application on wounds (as biomaterial-free systems) or using natural or ECM-mimicking biomaterials such as fibrin hydrogels (as biomaterial-based systems). Although a biomaterial-based delivery system is surely important for biomechanical support and to provide a scaffold for migrating cells, the complexity of the delivery method is substantially reduced when using only engineered super-affinity growth factors to target endogenous ECM. In terms of regulatory constraints, such an approach could greatly simplify growth factor path toward clinical translation.
Future Directions
Tissue repair and regeneration involves the sequential signaling of multiple growth factors and the delivery of a single type of growth factor could be insufficient. Therefore, delivering multiple growth factors simultaneously or sequentially may be required to build an efficient and proper regenerative microenvironment.58 However, the challenge is to understand which optimal concentrations of the right growth factors would be detected by the right cells at the right time. As a relevant process taking part during wound healing, the beginning of angiogenesis requires VEGF, FGF-2, and angiopoietin-2 to disrupt the structure of preexisting blood vessels and to promote the proliferation and migration of endothelial cells to form new immature vessels. Then, angiopoietin-1 and PDGF-BB stabilize these newly formed blood vessels by recruiting smooth muscle cells.59,60 Therefore, systems engineered to reproduce the sequential presentation of the growth factors involved in angiogenesis may promote a more physiological vascularization. For example, in models of myocardial infarction, sequential delivery of VEGF-A and PDGF-BB, or IGF-1 and hepatocyte growth factor from alginate hydrogel systems induced the formation of mature vessels and improved cardiac function more efficiently than each factor separately.61,62 It could be analogously interesting to engineer growth factor spatio-temporal delivery systems for skin wound healing.
Summary
While growth factors are very promising for the treatment of skin wounds, their translation to the clinic has been limited, in part due to the lack of appropriate delivery systems. The ECM is critical in guiding injured skin tissue toward the reparative or regenerative path and it has become evident that one of its main functions is to coordinate and modulate growth factor signaling. Growth factor delivery systems mimicking the ECM's growth factor regulatory functions are proving to be successful, at least in animal models. Further development and clinical trials will thus reveal if growth factor-based therapies can be used as primary therapeutics for wound healing. Indeed, the future impact of these therapies will most likely depend on our capacity to deliver growth factors precisely, to improve efficacy, safety, and cost-effectiveness. The main challenges are still to control the local delivery, reduce the doses, and to achieve spatio-temporal control of delivery of multiple growth factors.
Take-Home Messages.
• While growth factors are promising for the treatment of skin wounds, their translation to the clinic has been limited, in part due to issues related to efficacy, safety, and cost-effectiveness.
• One of the main functions of the ECM is to coordinate and modulate growth factor signaling.
• Understanding the mechanisms by which the ECM controls growth factors is proving to be key for designing efficient growth factor-based therapies.
• Growth factor delivery systems mimicking the natural interaction between ECM and growth factors have shown to be efficient in animal models of wound healing.
• The future impact of growth factor-based therapies will depend on our capacity to deliver low doses of these molecules in a precise spatio-temporal frame, to improve efficacy, safety, and cost-effectiveness.
Abbreviations and Acronyms
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FGF-2
fibroblast growth factor-2
- GAG
glycosaminoglycans
- HB-IGF-1
heparin-binding insulin-like growth factor-1
- IGF-BP
insulin-like growth factor-binding protein
- IGF-1
insulin-like growth factor
- PDGF-AB/-BB
platelet-derived growth factor-AB/-BB
- PEG
polyethylene glycol
- PlGF-2
placenta growth factor-2
- PlGF-2123-144
ECM-binding domain of PLGF-2
- PRP
platelet-rich plasma
- VEGF-A
vascular endothelial growth factor-A
Acknowledgments and Funding Sources
The authors would like to acknowledge Dr. Hans M. Larsson and Dr. Federico Tortelli for careful reading of the article. This work was supported in part by the European Research Council under the Advanced Grant Cytrix and by the Swiss National Science Foundation.
Author Disclosure and Ghostwriting
No competing financial interests exist. The authors listed expressly wrote the content of this article. No ghostwriters were used to write this article.
About the Authors
Priscilla S. Briquez is a graduate student specialized in regenerative medicine and biomedical technologies, currently in the Biotechnology and Bioengineering program at the Ecole Polytechnique Fédérale de Lausanne (EPFL, Switzerland). Her work focuses on growth factor engineering and the development of smart delivery systems for growth factors. Mikaël M. Martino, received his Ph.D. in Biotechnology and Bioengineering from the École Polytechnique Fédérale de Lausanne (EPFL, Switzerland) where he principally focused on growth factor signaling and growth factor use in regenerative medicine. He has designed new approaches to improve the efficacy and safety of growth factor-based therapies for impaired wound healing, bone defects, and angiogenesis. Currently, he is a Swiss National Science Foundation fellow at Osaka University (Japan). His research focuses on the immune regulation of tissue repair and regeneration. Jeffrey A. Hubbell is professor at the Institute for Molecular Engineering, University of Chicago and at the Institute of Bioengineering at the École Polytechnique Fédérale de Lausanne (EPFL, Switzerland), where his research is directed toward biomaterials and protein engineering in regenerative medicine and immunotherapeutics.
References
- 1.Werdin F, Tennenhaus M, Schaller HE. Evidence-based management strategies for treatment of chronic wounds. Eplasty 2009;8:26. [PMC free article] [PubMed] [Google Scholar]
- 2.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MedMarket Diligence LLC. Worldwide wound management, Forecast to 2021, Report #S249. Wound Management 2013:1–6 [Google Scholar]
- 4.Powers JG, Morton LM, Phillips TJ. Dressings for chronic wounds. Dermatol Ther 2013;26:197–206 [DOI] [PubMed] [Google Scholar]
- 5.Moura LIF, Dias AMA, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomater 2013;9:7093–7114 [DOI] [PubMed] [Google Scholar]
- 6.Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care 2004;17:24–35 [DOI] [PubMed] [Google Scholar]
- 7.Carter K. Growth factors: the wound healing therapy of the future? Br J Commun Nurs 2003;8:S15-6, S18-9, S22-3. [DOI] [PubMed] [Google Scholar]
- 8.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 2011;8:153–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater 2013;2:57–71 [DOI] [PubMed] [Google Scholar]
- 10.Martino MM, Briquez PS, Guc E, et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 2014;343:885–888 [DOI] [PubMed] [Google Scholar]
- 11.Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg 2003;90:133–146 [DOI] [PubMed] [Google Scholar]
- 12.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care 2012;25:349–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchberger B, Follmann M, Freyer D, Huppertz H, Ehm A, Wasem J. The importance of growth factors for the treatment of chronic wounds in the case of diabetic foot ulcers. GMS Health Technol Assess 2010;6:Doc12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cugat R, Garcia-Balletbo M. Growth factors—a brief review. Eur Musculoskelet Rev 2010;5:32–35 [Google Scholar]
- 15.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870 [DOI] [PubMed] [Google Scholar]
- 16.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 17.Traversa B, Sussman G. The role of growth factors, cytokines and proteases in wound management. AWMA J 2001;9:161–167 [Google Scholar]
- 18.Martino MM, Hubbell JA. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J 2010;24:4711–4721 [DOI] [PubMed] [Google Scholar]
- 19.De Laporte L, Rice JJ, Tortelli F, Hubbell JA. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS One 2013;8:e62076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Upton Z, Cuttle L, Noble A, et al. Vitronectin: growth factor complexes hold potential as a wound therapy approach. J Invest Dermatol 2008;128:1535–1544 [DOI] [PubMed] [Google Scholar]
- 21.Schultz GS, Ladwig G, Wysocki A. Extracellular matrix: review of its roles in acute and chronic wounds. World Wide Wounds 2005. www.worldwidewounds.com/2005/august/Schultz/Extrace-Matric-Acute-Chronic-Wounds.html
- 22.Agren MS, Werthen M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int J Low Extrem Wounds 2007;6:82–97 [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Professional Edition. Philadelphia, PA: Elsevier Health Sciences; 2009 [Google Scholar]
- 24.Shuttleworth L, Black RA. Deposition of elastic fibres in a murine cutaneous wound healing model. Int J Exp Pathol 2005;86:A68 [Google Scholar]
- 25.Humphries JD. Integrin ligands at a glance. J Cell Sci 2006;119:3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–162 [DOI] [PubMed] [Google Scholar]
- 27.Schönherr E, Hausser H. Extracellular matrix and cytokines: a functional unit. Dev Immunol 2000;7:89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capila I, Linhardt RJ. Heparin–protein interactions. Angew Chem Int Ed 2002;41:390–412 [DOI] [PubMed] [Google Scholar]
- 29.Macri L, Silverstein D, Clark RAF. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev 2007;59:1366–1381 [DOI] [PubMed] [Google Scholar]
- 30.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A 2013;110:4563–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009;326:1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol 2002;4:E75–E76 [DOI] [PubMed] [Google Scholar]
- 33.Martino MM, Tortelli F, Mochizuki M, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 2011;3:100ra89. [DOI] [PubMed] [Google Scholar]
- 34.Broderick N. Understanding chronic wound healing. Nurse Pract 2009;34:16 [Google Scholar]
- 35.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–1743 [DOI] [PubMed] [Google Scholar]
- 36.Mustoe T, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 2006;117(7 Suppl):35S–41S [DOI] [PubMed] [Google Scholar]
- 37.Harding KG, Morris HL, Patel GK. Healing chronic wounds. BMJ 2002;324:160–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 2012;25:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferraro B, Cruz YL, Coppola D, Heller R. Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol Ther 2009;17:651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee P-Y, Li Z, Huang L. Thermosensitive hydrogel as a Tgf-β1 gene delivery vehicle enhances diabetic wound healing. Pharm Res 2003;20:1995–2000 [DOI] [PubMed] [Google Scholar]
- 41.Breitbart AS, Laser J, Parrett B, et al. Accelerated diabetic wound healing using cultured dermal fibroblasts retrovirally transduced with the platelet-derived growth factor B gene. Ann Plast Surg 2003;51:409. [DOI] [PubMed] [Google Scholar]
- 42.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005;23:47–55 [DOI] [PubMed] [Google Scholar]
- 43.Arora NS, Ramanayake T, Ren Y-F, Romanos GE. Platelet-rich plasma: a literature review. Implant Dent 2009;18:303–310 [DOI] [PubMed] [Google Scholar]
- 44.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 2010;7:229–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Cai S, Shu XZ, Shelby J, Prestwich GD. Release of basic fibroblast growth factor from a crosslinked glycosaminoglycan hydrogel promotes wound healing. Wound Repair Regen 2007;15:245–251 [DOI] [PubMed] [Google Scholar]
- 46.Upton Z, Wallace HJ, Shooter GK, et al. Human pilot studies reveal the potential of a vitronectin: growth factor complex as a treatment for chronic wounds. Int Wound J 2011;8:522–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Clark RAF. Fibronectin at select sites binds multiple growth factors and enhances their activity: expansion of the collaborative ECM-GF paradigm. J Invest Dermatol 2014;134:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 2011;209:139–151 [DOI] [PubMed] [Google Scholar]
- 49.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 2003;19:173–206 [DOI] [PubMed] [Google Scholar]
- 50.Lin F, Ren X-D, Pan Z, et al. Fibronectin growth factor-binding domains are required for fibroblast survival. J Invest Dermatol 2011;131:84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wijelath ES, Rahman S, Namekata M, et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res 2006;99:853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol 2003;15:565–571 [DOI] [PubMed] [Google Scholar]
- 53.Ehrbar M, Djonov VG, Schnell C, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res 2004;94:1124–1132 [DOI] [PubMed] [Google Scholar]
- 54.Traub S, Morgner J, Martino MM, et al. The promotion of endothelial cell attachment and spreading using FNIII10 fused to VEGF-A165. Biomaterials 2013;34:5958–5968 [DOI] [PubMed] [Google Scholar]
- 55.Sacchi V, Mittermayr R, Hartinger J, et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc Natl Acad Sci U S A 2014;111:6952–6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tokunou T, Miller R, Patwari P, et al. Engineering insulin-like growth factor-1 for local delivery. FASEB J 2008;22:1886–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Achar RAN, Silva TC, Achar E, Martines RB, Machado JLM. Use of insulin-like growth factor in the healing of open wounds in diabetic and non-diabetic rats. Acta Cir Bras 2014;29:125–131 [DOI] [PubMed] [Google Scholar]
- 58.Chen F-M, Zhang M, Wu Z-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010;31:6279–6308 [DOI] [PubMed] [Google Scholar]
- 59.Carmeliet P. Angiogenesis in health and disease. Nat Med 2003;9:653–660 [DOI] [PubMed] [Google Scholar]
- 60.Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 2011;12:551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao X, Silva EA, Månsson-Broberg A, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res 2007;75:178–185 [DOI] [PubMed] [Google Scholar]
- 62.Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 2011;32:565–578 [DOI] [PubMed] [Google Scholar]