Abstract
We compared the Framingham risk score (FRS) for 10-year coronary heart disease (CHD) risk in age- and race-matched hepatitis C virus (HCV)-infected and HCV-uninfected persons: 114,073 HCV-infected (111,436 HCV-monoinfected and 2,637 HIV/HCV-coinfected) and 122,996 HCV-uninfected (121,380 HIV and HCV-uninfected and 1,616 HIV-monoinfected) males without cardiovascular disease, diabetes, or hepatitis B. In unadjusted analyses, FRS was similar between the HCV-infected and HCV-uninfected groups [median (interquartile range, IQR) risk points 13 (10–14) vs. 13 (10–14), p=0.192]. Cholesterol levels were lower and current smoking more prevalent in the HCV groups (both HCV and HIV/HCV) compared with the uninfected groups (p<0.001 for both). Prevalence of non-FRS CHD risk factors, such as substance abuse and chronic kidney disease, in the cohort was high, and differed by HCV and HIV status. Adjusting for age, race/ethnicity, body mass index, chronic kidney disease, drug and alcohol use, and HIV status, HCV infection was associated with minimally lower FRS (β=−0.095 risk points, p<0.001), suggesting a small but significant difference in 10-year CHD risk estimation in HCV-infected as compared to HCV-uninfected persons when measuring risk by FRS. Given the complex relationship between HCV, HIV, and CHD risk factors, some of which are not captured by the FRS, the FRS may underestimate CHD risk in HCV-monoinfected and HIV/HCV-coinfected persons. HCV- and HIV/HCV-specific risk scores may be needed to optimize CHD risk stratification.
As persons with HIV infection are living longer due to advances in antiretroviral therapy (ART), cardiovascular disease (CVD) has emerged as a major cause of morbidity and mortality in HIV-infected persons.1,2 In the United States, about 30% of persons with HIV infection are coinfected with hepatitis C virus (HCV)3 and at risk for increased mortality—not only liver-associated mortality, but also non-liver-associated mortality, of which CVD is a leading etiology.4,5 Data from observational studies suggest that chronic HCV infection is independently associated with increased CVD risk6–10 and thus HCV coinfection may exacerbate CVD risk in HIV-infected persons.11,12
The Framingham risk score (FRS) is a widely used global risk score for 10-year coronary heart disease (CHD) risk, validated for use in the general, non-HIV-infected population.13,14 In HIV-infected persons, it has been increasingly recognized that modified risk prediction tools may be needed for optimal CVD risk assessment.15 Similarly, HCV infection may modify CVD risk and new tools may be needed to optimize CVD risk assessment in this population. Two of the FRS components, blood pressure and total cholesterol, are decreased in the setting of HCV infection,7 suggesting a protective effect for CHD if interpreted traditionally, and in contrast to the apparent increased risk noted in an increasing number of observational studies. We hypothesized that FRS is lower in HCV-infected persons compared with HCV-uninfected persons, and thus may underestimate the risk of CHD events.
We conducted a retrospective cohort study utilizing the national Electronically Retrieved Cohort of HCV Infected Veterans (ERCHIVES), 2001–2009. The creation of ERCHIVES has previously been described.7,16–18 Inpatient and outpatient data were derived from the Veteran Affairs (VA) National Patient Care Database, the VA Pharmacy Benefits Management database, and the Decisions Support System database in VA fiscal years 2001–2009. Discharge diagnoses and diagnoses from outpatient records are coded according to the International Classification of Diseases, 9th Revision (ICD-9). The validity of the administrative, pharmacy, and laboratory data has previously been reported,17,19,20 as has the validity of the ICD-9 codes for select comorbid conditions, including myocardial infarction (MI).19
HCV infection was defined by the presence of HCV antibody or a positive result of qualitative or quantitative testing for HCV RNA. Controls are matched by age (in 5-year increments), sex, race, and year of HCV diagnosis in the VA health care system. Subjects with baseline diabetes, cardiovascular disease [defined as peripheral vascular disease, MI, congestive heart failure, and history of coronary artery bypass grafting (CABG), or percutaneous transluminal coronary angioplasty (PTCA), or stroke], and chronic hepatitis B were excluded. Men ages 20–79 years were included; only men were included as 97% of the ERCHIVES participants are male. Primary comparison was of baseline FRS between HCV-infected and uninfected persons, including HIV, with additional comparison between four mutually exclusive groups: uninfected, HCV-monoinfected, HIV-monoinfected, and HIV/HCV-coinfected. The FRS defines 10-year CHD risk from 0 to ≥30%. FRS were categorized into low (<10%), medium (10–20%), and high (>20%) predicted risk.
Ten-year CHD risk was calculated using the male-specific Framingham equation, using age, systolic blood pressure (SBP), antihypertensive treatment status, smoking status, high-density lipoprotein cholesterol (HDL-C), and total cholesterol level.21 Subjects for whom any of the variables required to calculate FRS were missing were excluded. Baseline variables abstracted included race/ethnicity, diabetes, height, weight, alanine aminotransferase (ALT) level, renal function categorized as chronic kidney disease (CKD) stages 0–2 or 3–5 [stages 0–2 defined as glomerular filtration rate (GFR) ≥60 ml/min/1.73 m2, stages 3–5 as GFR <60 or on dialysis], alcohol- and drug-related diagnoses, HCV viral load, HIV viral load, CD4 T cell count, and antiretroviral and lipid-lowering therapy. Smoking status was defined as current, former, or never, documented within a 5-year window of study entry.
Descriptive statistics (mean, standard deviation, median, interquartile range, and frequency distribution) were generated for baseline demographic and clinical information among the four groups to characterize the study population. Continuous variables were compared by Wilcoxon rank-sum and Kruskal–Wallis tests and categorical variables were compared by the Chi-squared test. Regression analyses were conducted to examine differences in FRS adjusting for age, race/ethnicity, body mass index (BMI) (dichotomized as <30 or ≥30), CKD stage, drug and alcohol abuse, and HIV and HCV status.
The study was determined by the University of California Los Angeles Institutional Review Board (IRB) to be exempt from IRB review. ERCHIVES is approved by the IRB at VA Pittsburgh Healthcare System.
Of 316,514 eligible subjects, 79,445 (25.1%) were excluded as FRS could not be calculated, leaving a cohort of 237,069 persons: 121,380 HIV/HCV uninfected, 111,436 HCV monoinfected, 1,616 HIV monoinfected, and 2,637 HIV/HCV coinfected. The primary reason for missing FRS calculation was missing cholesterol levels and similar between groups: missing total cholesterol in 14,475 (9.2%) uninfected, 20,549 (13.4%) HCV, 224 (10.7%) HIV, and 428 (12%) HIV/HCV; missing HDL-C in 26,173 (16.6%) uninfected, 33,730 (22.0%) HCV, 333 (15.9%) HIV, and 728 (20.3%) HIV/HCV; missing SBP in 850 (0.5%) uninfected, 990 (0.7%) HCV, 2 (0.1%) HIV, and 7 (0.2%) HIV/HCV; and missing smoking status in 12,142 (7.7%) uninfected, 11,308 (7.4%) HCV, 144 (6.9%) HIV, and 266 (7.4%) HIV/HCV. Baseline characteristics and FRS for the four groups are compared in Table 1.
Table 1.
Characteristics and Framingham Risk Score of the Cohort
| Characteristica | Uninfected N=121,380 | HCV-monoinfected N=111,436 | HIV-monoinfected N=1616 | HIV/HCV-coinfected N=2637 | p-valueb |
|---|---|---|---|---|---|
| Age (years) | 52.4 (8.8) | 52.7 (8.7) | 49.1 (7.5) | 50.0 (7.2) | <0.001 |
| Race/ethnicity | |||||
| White | 66,699 (55.0) | 62,659 (56.2) | 601 (37.2) | 759 (28.8) | <0.001 |
| Black | 33,723 (27.8) | 30,039 (27.0) | 830 (51.4) | 1,555 (59.0) | |
| Hispanic | 7376 (6.1) | 6365 (5.7) | 94 (5.8) | 187 (7.1) | |
| Other/unknown | 13,582 (11.2) | 12,373 (11.1) | 91 (5.6) | 136 (5.2) | |
| CD4 T cell countc | NA | NA | 345 (136–546) | 327 (135–527) | 0.46 |
| HIV RNA (copies/ml)d | |||||
| Undetectable (<500) | NA | NA | 158/767 (20.6) | 296/1,449 (20.4) | 0.92 |
| Median (IQR) | 10,300 (923–61,107) | 10,300 (777–71,258) | 0.87 | ||
| On antiretroviral therapy | 1,095 (67.8) | 1,644 (62.3) | <0.001 | ||
| PI | 682 (42.2) | 1,034 (39.2) | 0.054 | ||
| NRTI | NA | NA | 1,118 (69.2) | 1,692 (64.2) | <0.001 |
| NNRTI | 688 (42.6) | 945 (35.8) | <0.001 | ||
| BMIe | 28.9 (5.6) | 27.6 (5.3) | 25.5 (4.5) | 24.8 (4.3) | <0.001 |
| Systolic blood pressure (mmHg) | 133 (15.2) | 134 (15.9) | 128 (14.2) | 129 (15.8) | <0.001 |
| On antihypertensive medication | 73,669 (60.7) | 62,718 (56.3) | 857 (53.0) | 1,332 (50.5) | <0.001 |
| ALT (U/liter)f | 29 (21–42) | 42 (26–72) | 29 (20–43) | 41 (26–66) | <0.001 |
| Chronic kidney disease | <0.001 | ||||
| Stage 0–2 | 110,124 (90.7) | 101,005 (90.6) | 1,379 (85.3) | 2,233 (84.7) | |
| Stage 3–5 | 11,256 (9.3) | 10,431 (9.4) | 237 (14.7) | 404 (15.3) | |
| History of drug abuse | 14,677 (12.1) | 27,532 (24.7) | 297 (18.4) | 1,011 (38.3) | <0.001 |
| History of alcohol abuse | 20,231 (16.7) | 28,257 (25.4) | 249 (15.4) | 720 (27.3) | <0.001 |
| Total cholesterol (mg/dl) | 195 (170–224) | 177 (153–205) | 183 (156–215) | 163 (138–191) | <0.001 |
| HDL-C (mg/dl) | 43 (36–53) | 43 (35–53) | 39 (33–50) | 39 (31–50) | <0.001 |
| LDL-C (mg/dl)f | 119 (95–144) | 105 (83–130) | 106 (84–133) | 91 (68–115) | <0.001 |
| Triglycerides (mg/dl)e | 130 (88–199) | 112 (78–166) | 145 (99–232) | 131 (92–199) | <0.001 |
| On lipid-lowering medication | 36,796 (30.3) | 17,908 (16.1) | 379 (23.5) | 277 (10.5) | <0.001 |
| Smoking status | |||||
| Current | 60,364 (49.7) | 73,537 (67.0) | 887 (54.9) | 1,792 (68.0) | <0.001 |
| Former or never | 61,016 (50.3) | 37,899 (34.0) | 729 (45.1) | 845 (32.0) | |
| Framingham risk score | |||||
| Points | 13 (10–15) | 13 (10–14) | 11 (9–14) | 11 (9–13) | <0.001 |
| Risk % | 12 (6–20) | 12 (6–16) | 8 (5–16) | 8 (5–12) | <0.001 |
Data presented as n (%) or mean (SD) or median (IQR).
Continuous variables compared by Kruskal–Wallis rank test and categorical variables by Chi-squared test.
Missing values in 27–29%.
Missing values in 45–53%.
Missing values in 0.9–2%.
Missing values in 5–9%.
HCV, hepatitis C virus; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; BMI, body mass index; ALT, alanine aminotransferase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
There were significant differences in demographic, behavioral, and clinical characteristics by HCV and HIV status. The HCV groups more often had a history of drug- and alcohol-related diagnoses, were current smokers, and had lower total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglyceride levels compared to the respective uninfected and HIV-infected groups. The HCV group was less often on lipid-lowering treatment and had higher ALT levels. The HIV groups were slightly younger than the uninfected and HCV-monoinfected groups and had a higher proportion with black race and stage 3–5 CKD, lower BMI, and lower HDL. Systolic blood pressure was similar between the HCV-monoinfected and HCV-uninfected groups, and lowest in the HIV groups; antihypertensive treatment was slightly more common in the uninfected group than the HCV-monoinfected group, and least common in the HIV groups.
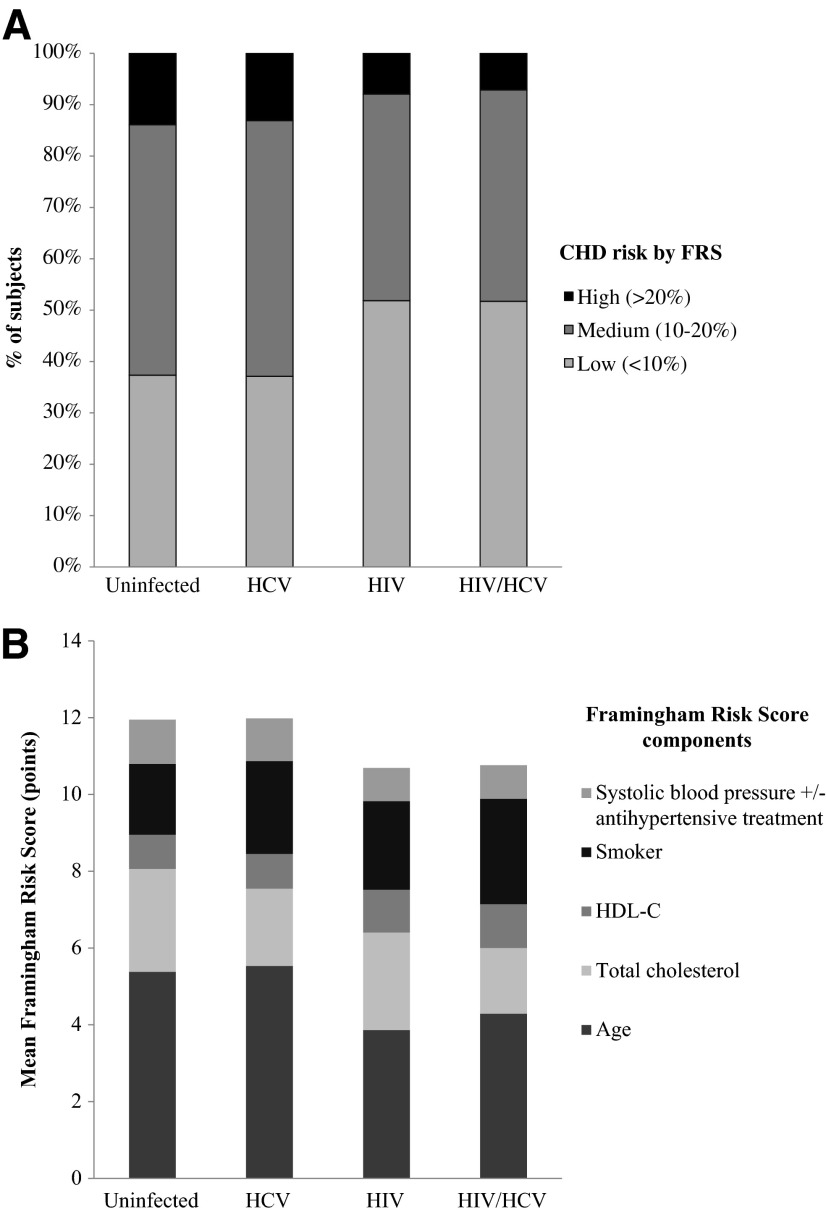
For the primary comparison of FRS in HCV-infected and HCV-uninfected persons, which included HIV-infected persons, the median FRS (in risk points) was not different between the two groups [13 (interquartile range, IQR 10–14) vs. 13 (10–14), p=0.192]. Similar results were found when HIV-infected persons were excluded from the analysis [13(10–14) vs. 13 (10–15), p=0.659]. The FRS was lower in the HIV and HIV/HCV groups compared with the uninfected and HCV-monoinfected groups (Table 1). Figure 1 demonstrates the differences in CHD risk class distribution as classified by FRS (high, medium, and low 10-year predicted risk). Figure 1 also depicts the differences in contribution of the FRS components between the four groups, with the biggest difference between the groups being from age, as well as total cholesterol and smoking status. After adjusting for age and potential confounders of race/ethnicity, BMI, CKD stage, drug and alcohol abuse, and HIV infection, HCV infection was associated with lower FRS (in risk points), although the association was very modest (β=−0.095, p<0.001). The association between HIV and lower FRS persisted, but was attenuated compared to unadjusted analyses (β=−0.282, p<0.001).
FIG. 1.
Predicted 10-year coronary heart disease (CHD) risk by Framingham Risk Score (FRS). (A) Distribution of CHD risk categories and (B) relative contribution of CHD risk factors to FRS by HIV and HCV status. HCV, hepatitis C virus; HDL-C, high-density lipoprotein cholesterol.
In this age- and race-matched cohort, contrary to our expectations, Framingham risk scores for 10-year CHD risk were similar between HCV-infected and HCV-uninfected persons. This may be explained in part by systolic blood pressure not being lower in HCV-infected patients as we expected, perhaps due to our selection of a relatively low-risk population (we excluded diabetics and known CVD). The lower total cholesterol levels in HCV-infected patients did lead to fewer points contributed to FRS, but this was offset by higher smoking rates in those HCV-infected. In multiple regression analysis adjusting for potential confounders, HCV infection was associated with lower FRS, but the effect size was small and may not significantly change CHD risk estimation; further investigations prospectively testing FRS or other risk scores are necessary. The very large sample size likely contributed to our ability to identify such a small effect size.
Utilizing the FRS for routine clinical assessment of CHD risk would suggest that CHD risk is unaffected by HCV status. However, there are a number of known CHD risk factors not captured by the FRS that were more prevalent in the HCV-infected groups, including drug and alcohol-related diagnoses. The phenomenon of lower cholesterol levels in HCV-infected persons is well established,22–24 but whether such reduced cholesterol levels are indeed protective or instead are masking cardiovascular risk is unknown. In fact, in one HCV-monoinfected cohort, treatment and clearance of HCV led to increased cholesterol levels, some reaching indication for lipid-lowering therapy,25 and in other chronic diseases such as heart failure, lower cholesterol is associated with a worse prognosis.26 In addition to the observation that HCV infection is associated with an increased risk of cardiovascular events and coronary disease, HCV infection is associated with several potential mechanisms for CVD, including insulin resistance, hepatic steatosis, and chronic immune activation.27–36
Given the higher prevalence of non-FRS CHD risk factors, the potential that HCV-associated low cholesterol levels may in fact not be protective, and the potential independent contribution of HCV infection by the above mechanisms to CHD, the FRS may underestimate CHD risk in HCV-infected persons and a low FRS should not be reassuring. Furthermore, despite the known increased risk of CHD with HIV infection, FRS was lower in the HIV groups, unexplained fully by younger age or other confounders and suggesting, as in other studies,37 that the FRS may underestimate the risk of CHD events in HIV-infected persons. One limitation of our analysis is that HIV and HCV classification was based on available laboratories collected in routine clinical practice, and thus some subjects may have been misclassified. However, VA recommendations include systematic HIV and HCV screening; thus, it is unlikely that a significant number of subjects was misclassified.
Reduced sensitivity of the Framingham risk score may lead to a gap in optimizing cardiovascular outcomes in the aging HCV- and HIV-infected populations and opportunities to reduce CVD mortality may be missed. No studies have evaluated the predictive value of the Framingham risk score in HCV-infected populations, despite its widespread use in the general population and these potential limitations. Furthermore, the newest CVD risk tool endorsed by the American College of Cardiology and American Heart Association uses similar risk factors as the Framingham risk score for risk estimation, and may be subject to the same limitations.38 New or modified risk assessment tools may need to be derived, validated, and applied to the clinical care of HCV-infected persons.
Acknowledgments
This project was supported by the UCLA AIDS Institute and Center for AIDS Research (NIH P30AI028697). This material is the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System and the central data repositories maintained by the VA Information Resource Center, including the National Patient Care Database, Decisions Support System Database, and Pharmacy Benefits Management Database. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
These data were presented in part at the 20th Conference on Retroviruses and Opportunistic Infections (CROI), Atlanta, GA, March 3–6, 2013 and at the 21st CROI, Boston, MA, March 3–6, 2014.
Author Disclosure Statement
A.A.B. has received research grants (to the institution) from Gilead and Abbvie.
References
- 1.Currier JS: Update on cardiovascular complications in HIV infection. Top HIV Med 2009;17(3):98–103 [PubMed] [Google Scholar]
- 2.Hemkens LG. and Bucher HC: HIV infection and cardiovascular disease. Eur Heart J 2014;35(21):1373–1381 [DOI] [PubMed] [Google Scholar]
- 3.Price JC. and Thio CL: Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol 2010;8(12):1002–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branch AD, Van Natta ML, Vachon ML, et al. : Mortality in hepatitis C virus-infected patients with a diagnosis of AIDS in the era of combination antiretroviral therapy. Clin Infect Dis 2012;55(1):137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenguer J, Alejos B, Hernando V, et al. : Trends in mortality according to hepatitis C virus serostatus in the era of combination antiretroviral therapy. AIDS 2012;26(17):2241–2246 [DOI] [PubMed] [Google Scholar]
- 6.Ishizaka N, Ishizaka Y, Takahashi E, et al. : Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet 2002;359(9301):133–135 [DOI] [PubMed] [Google Scholar]
- 7.Butt AA, Xiaoqiang W, Budoff M, et al. : Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 2009;49(2):225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alyan O, Kacmaz F, Ozdemir O, et al. : Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J 2008;72(12):1960–1965 [DOI] [PubMed] [Google Scholar]
- 9.Petta S, Macaluso FS, and Craxi A: Cardiovascular diseases and HCV infection: A simple association or more? Gut 2014;63(3):369–375 [DOI] [PubMed] [Google Scholar]
- 10.Adinolfi LE, Restivo L, Zampino R, et al. : Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis 2012;221(2):496–502 [DOI] [PubMed] [Google Scholar]
- 11.Sosner P, Wangermez M, Chagneau-Derrode C, et al. : Atherosclerosis risk in HIV-infected patients: The influence of hepatitis C virus co-infection. Atherosclerosis 2012;222(1):274–277 [DOI] [PubMed] [Google Scholar]
- 12.Bedimo R, Westfall AO, Mugavero M, et al. : Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med 2010;11(7):462–468 [DOI] [PubMed] [Google Scholar]
- 13.Greenland P, Alpert JS, Beller GA, et al. : 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: Executive summary: A report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation 2010;122(25):2748–2764 [DOI] [PubMed] [Google Scholar]
- 14.D'Agostino RB, Sr., Grundy S, Sullivan LM, et al. : Validation of the Framingham coronary heart disease prediction scores: Results of a multiple ethnic groups investigation. JAMA 2001;286(2):180–187 [DOI] [PubMed] [Google Scholar]
- 15.D'Agostino RB Sr: Cardiovascular risk estimation in 2012: Lessons learned and applicability to the HIV population. J Infect Dis 2012;205(Suppl 3):S362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt AA, Justice AC, Skanderson M, et al. : Rate and predictors of treatment prescription for hepatitis C. Gut 2007;56(3):385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butt AA, Justice AC, Skanderson M, et al. : Rates and predictors of hepatitis C virus treatment in HCV-HIV-coinfected subjects. Aliment Pharmacol Ther 2006;24(4):585–591 [DOI] [PubMed] [Google Scholar]
- 18.Butt AA, Khan UA, McGinnis KA, et al. : Co-morbid medical and psychiatric illness and substance abuse in HCV-infected and uninfected veterans. J Viral Hepat 2007;14(12):890–896 [DOI] [PubMed] [Google Scholar]
- 19.Fultz SL, Skanderson M, Mole LA, et al. : Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 2006;44(8 Suppl 2):S25–30 [DOI] [PubMed] [Google Scholar]
- 20.McGinnis KA, Skanderson M, Levin FL, et al. : Comparison of two VA laboratory data repositories indicates that missing data vary despite originating from the same source. Med Care 2009;47(1):121–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285(19):2486–2497 [DOI] [PubMed] [Google Scholar]
- 22.Floris-Moore M, Howard AA, Lo Y, et al. : Hepatitis C infection is associated with lower lipids and high-sensitivity C-reactive protein in HIV-infected men. AIDS Patient Care STDS 2007;21(7):479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrester JE, McGovern BH, Rhee MS, et al. : The individual and combined influence of HIV and hepatitis C virus on dyslipidaemia in a high-risk Hispanic population. HIV Med 2009;10(9):555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polgreen PM, Fultz SL, Justice AC, et al. : Association of hypocholesterolaemia with hepatitis C virus infection in HIV-infected people. HIV Med 2004;5(3):144–150 [DOI] [PubMed] [Google Scholar]
- 25.Corey KE, Kane E, Munroe C, Barlow LL, et al. : Hepatitis C virus infection and its clearance alter circulating lipids: Implications for long-term follow-up. Hepatology 2009;50(4):1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwich TB, Hamilton MA, Maclellan WR, et al. : Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail 2002;8(4):216–224 [DOI] [PubMed] [Google Scholar]
- 27.Duong M, Petit JM, Piroth L, et al. : Association between insulin resistance and hepatitis C virus chronic infection in HIV-hepatitis C virus-coinfected patients undergoing antiretroviral therapy. J Acquir Immune Defic Syndr 2001;27(3):245–250 [DOI] [PubMed] [Google Scholar]
- 28.Machado MV, Oliveira AG, and Cortez-Pinto H: Hepatic steatosis in patients coinfected with human immunodeficiency virus/hepatitis C virus: A meta-analysis of the risk factors. Hepatology 2010;52(1):71–78 [DOI] [PubMed] [Google Scholar]
- 29.Machado MV. and Cortez-Pinto H: Insulin resistance and steatosis in chronic hepatitis C. Ann Hepatol 2009;8(Suppl 1):S67–75 [PubMed] [Google Scholar]
- 30.Conjeevaram HS, Wahed AS, Afdhal N, et al. : Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology 2011;140(2):469–477 [DOI] [PubMed] [Google Scholar]
- 31.Younossi ZM, Stepanova M, Nader F, et al. : Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther 2013;37(6):647–652 [DOI] [PubMed] [Google Scholar]
- 32.Kovacs A, Al-Harthi L, Christensen S, et al. : CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis 2008;197(10):1402–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez VD, Falconer K, Blom KG, et al. : High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol 2009;83(21):11407–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loria P, Marchesini G, Nascimbeni F, et al. : Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis 2014;232(1):99–109 [DOI] [PubMed] [Google Scholar]
- 35.Dai CY, Yeh ML, Huang CF, et al. : Chronic hepatitis C infection is associated with insulin resistance and lipid profiles. J Gastroenterol Hepatol 2015;30(5):879–884 [DOI] [PubMed] [Google Scholar]
- 36.Miyajima I, Kawaguchi T, Fukami A, et al. : Chronic HCV infection was associated with severe insulin resistance and mild atherosclerosis: A population-based study in an HCV hyperendemic area. J Gastroenterol 2013;48(1):93–100 [DOI] [PubMed] [Google Scholar]
- 37.Law MG, Friis-Moller N, El-Sadr WM, et al. : The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: Comparison with observed events in the D:A:D study. HIV Med 2006;7(4):218–230 [DOI] [PubMed] [Google Scholar]
- 38.Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. : 2013 ACC/AHA Guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25_PA):2935–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]