Abstract
Aims
Coronary computed tomographic angiography (CCTA) has become an important tool for non-invasive diagnosis of coronary artery disease (CAD). Coronary dominance can be assessed by CCTA; however, the predictive value of coronary dominance is controversially discussed. The aim of this study was to evaluate the prevalence and prognosis of coronary dominance in a large prospective, international multicentre cohort of patients undergoing CCTA.
Methods and results
The study population consisted of 6382 patients with or without CAD (47% females, 53% males, mean age 56.9 ± 12.3 years) who underwent CCTA and were followed over a period of 60 months. Right or left coronary dominance was determined. Right dominance was present in 91% (n = 5817) and left in 9% (n = 565) of the study population. At the end of follow-up, outcome in patients with obstructive CAD (>50% luminal stenosis) and right dominance was similar compared with patients with left dominance [hazard ratio (HR) 0.46, 95% CI 0.16–1.32, P = 0.15]. Furthermore, no differences were observed for the type of coronary dominance in patients with non-obstructive CAD (HR 0.95, 95% CI 0.41–2.21, P = 0.8962) or normal coronary arteries (HR 1.04, 95% CI 0.68–1.59, P = 0.9). Subgroup analysis in patients with left main disease revealed an elevated hazard of the combined endpoint for left dominance (HR 6.45, 95% CI 1.66–25.0, P = 0.007), but not for right dominance.
Conclusion
In our study population, survival after 5 years of follow-up did not differ significantly between patients with left or right coronary dominance. Thus, assessment of coronary vessel dominance by CCTA may not enhance risk stratification in patients with normal coronary arteries or obstructive CAD, but may add prognostic information for specific subpopulations.
Keywords: Coronary dominance, Coronary computed tomographic angiography, Predictive value
Introduction
Coronary computed tomographic angiography (CCTA) has recently been introduced as a highly accurate1–4 and prognostically robust5–8 non-invasive imaging modality for the assessment of coronary artery disease (CAD). The CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) registry enrolled ≥20 000 patients from 12 centres across North America, Europe, and Asia with suspected CAD who underwent a ≥64-detector row CCTA, and is the first prospective database evaluating the prognostic role of CCTA.9 Coronary artery dominance is determined according to the coronary artery that emits the posterior descending artery. Right dominance is the most prevalent pattern of coronary circulation and is found in 72–90% of individuals, while prevalence of left dominance is reported to be 8–33%, whereas co-dominance has ∼3–7% of population prevalence.10 The relatively low prevalence of left dominance in the general population and the decreasing prevalence of a left dominant or co-dominant coronary system with age have raised the question whether this variant may reflect a biological disadvantage relative to right dominance, and recent studies have hypothesized that left dominance may represent less well-balanced circulation with more myocardium at risk in acute coronary syndromes (ACSs).11 Indeed, a previous study of 27 289 patients undergoing cardiac catheterization for ACS demonstrated that left dominance was associated with an increased hazard of death during a 3.5-year follow-up,12 and a US registry reported that left dominance and co-dominance were associated with increased in-hospital mortality in 207 926 patients undergoing percutaneous coronary intervention (PCI) for ACS.13 However, this work has been based on conventional angiograms. Since it is often difficult to delineate the course of coronary arteries by angiography because it only provides a two-dimensional view of a three-dimensional structure, the present study analysed coronary dominance and outcome by multidetector coronary CCTA that not only provides information about the presence and degree of coronary stenosis, but also allows to see the origin and course of coronary arteries by a three-dimensional display of anatomy thereby permitting the determination of coronary artery variations.14–17 Although coronary vessel dominance is easily assessed on coronary CCTA, there is sparse information about the prognostic value of coronary vessel dominance in patients referred for CCTA. Therefore, the goal of the present study was to assess the prevalence and prognosis of coronary dominance in a large prospective, international multicentre cohort of patients undergoing CCTA.
Methods
Study design, patients, and outcome measures
This study represents 6382 patients from the CONFIRM registry. Briefly, CONFIRM enrolled consecutive adults >18 years of age between 2005 and 2009 who underwent ≥64-detector row CCTA for suspected CAD at 12 centres in six countries (Canada, Germany, Italy, Korea, Switzerland, and the USA). Details of the CONFIRM registry design and data elements have been published.9,18–20 Patients with no CAD, non-obstructive, obstructive, and severe obstructive CAD where coronary dominance had been assessed were included in the present analysis. Patients with a balanced coronary artery system were excluded from the analysis, because of the low number of patients in this group. Cases with missing data on dominance were excluded from analysis; therefore, 6382 remaining individuals with and without CAD were included for the final analyses. The primary clinical endpoint of the study was a composite of all-cause mortality, non-fatal myocardial infarction (MI), and early and late coronary revascularizations. Non-fatal MI was defined as evidence of myocardial necrosis consistent with myocardial ischaemia, as detected by changes in cardiac biomarkers together with symptoms of ischaemia, ECG changes, or imaging evidence, according to the ESC/ACCF/AHA/WHF consensus document on the universal definition of non-fatal MI.21 Notably, in the CONFIRM registry, post-CCTA treatment regimens were not mandated and our database did not include information on previous or post-CCTA functional testing results. The study complies with the Declaration of Helsinki, and patient consent or a waiver of informed consent (as per recommendations of each institutional review board) was obtained at each site in keeping with site-specific regulations.
Data acquisition, image reconstruction, and CCTA analysis
CCTA scanners used in the CONFIRM registry and data acquisition for CCTA have been described in detail previously.9 Image interpretation was uniformly performed at each site according to the Society of Cardiovascular Computed Tomography guidelines22 by at least one highly experienced imager who was level III equivalent and/or board certified in cardiovascular computed tomography. Dominance was determined independently at each participating site. The coronary artery system was classified as a right dominant if the right coronary artery (RCA), as a left dominant if the left circumflex coronary artery (LCx), or as a co-dominant if RCA and LCx gave rise to the posterior descending artery. Each site performed per-segment analysis for individual coronary artery segments by using a 16-segment model. A CAD was defined as the presence of any plaque. Coronary atherosclerotic lesions were quantified for lumen diameter stenosis by visual estimation and graded as none (0% luminal stenosis), mild (1–49%), moderate (50–69%), or severe (>70%). A coronary lesion compromising the lumen by >50% was defined as obstructive. Vessels were classified into four arterial territories: left main artery (LM), left anterior descending artery (LAD), LCx, and RCA. Obstructive CAD in the diagonal branches, obtuse marginal branches, and posterolateral branches was considered as part of the LAD, LCx, and RCA system, respectively. The posterior descending artery was considered as part of the RCA or LCx system, depending on the coronary artery dominance. A >50% stenosis in the LM was considered obstructive in all models. Individuals manifesting obstructive CAD were further categorized as having one-, two-, and three-vessel disease or left main disease. For the purposes of the study analysis, a left main coronary stenosis of ≥50% was considered equivalent to three-vessel CAD.
Statistical analysis
SPSS version 12.0 and 17.0 (SPSS, Inc., Chicago, IL, USA) and SAS version 9.2 (SAS Institute, Cary, NC, USA) were used for all statistical analyses. Categorical variables are presented as frequencies and continuous variables as mean ± SD. Variables were compared with χ2 statistic for categorical variables and by Student's unpaired t-test, Wilcoxon/Mann–Whitney non-parametric test, or median comparison test where appropriate for continuous variables. The Kaplan–Meier method and the log-rank A Cox proportional hazards analysis were used to compare cumulative event-free survival by dominance in patients without significant CAD on CCTA and in those with significant CAD on CCTA. The primary outcome variable was a composite endpoint of all-cause mortality, non-fatal MI, and revascularization. Multivariable analyses were calculated with the multivariabe Cox regression model for prediction of the combined endpoint (with 95% confidence intervals). According to univariate significance and baseline differences between groups, risk factors such as age, male gender, hypertension, dyslipidaemia, diabetes, and smoking were included in the multivariate model. Furthermore, the prognostic value of severity of stenosis and significant stenosis location were determined for patients with a right dominant coronary artery system and patients with a left dominant coronary artery system. A two-tailed P-value of 0.05 was considered statistically significant.
Results
Study cohort
The CONFIRM registry screened 27 125 CCTA patients at 12 participating centres in six countries. Patients were followed for a median of 2.1 years (interquartile range 1.5–3.1 years). A total of 956 (3.5%) patients were lost to follow-up; for 20 743 patients, coronary artery dominance pattern had not been evaluated due to different reasons including technical reasons, extensive atherosclerosis, presence of occluding thrombi with large filling defects distally, or prior CABG. Thus, the final study population comprised 6382 patients (47% females, 53% males, mean age 56.9 ± 12.3 years) with or without CAD remained for the present analysis and was included in the study. Table 1 depicts baseline characteristics of the patient population, categorized by coronary vessel dominance. Left coronary dominance (LCD) patients tend to have a higher BMI (27.8 ± 5.4 vs. 27.2 ± 5.3, P = 0.0288), and were more often male (62 vs. 38%, P < 0.0001) and asymptomatic (24 vs. 37%, P = 0.0003) than patients with right coronary dominance (RCD).
Table 1.
Baseline characteristics of the study population by dominance
| Patient characteristics | Total (n = 6382) | Right dominant (n = 5817) | Left dominant (n = 565) | P-value |
|---|---|---|---|---|
| Male gender | 3343 (53%) | 2995 (51%) | 348 (62%) | <0.0001 |
| BMI | 27.3 ± 5.3 | 27.2 ± 5.3 | 27.8 ± 5.4 | 0.0288 |
| Age (years) | 56.9 ± 12.3 | 56.9 ± 12.2 | 56.6 ± 13.0 | 0.1726 |
| Hypertension | 3287 (52%) | 2970 (52%) | 317 (56%) | 0.0453 |
| Diabetes | 757 (12%) | 680 (12%) | 77 (14%) | 0.1799 |
| Dyslipidaemia | 3373 (54%) | 3093 (54%) | 280 (50%) | 0.0742 |
| Current smoking | 1231 (19%) | 1111 (19%) | 120 (21%) | 0.246 |
| Family history of CAD | 2036 (33%) | 1839 (33%) | 197 (36%) | 0.2314 |
| Symptoms | ||||
| Asymptomatic | 2114 (34%) | 1913 (24%) | 201 (37%) | 0.0003 |
| Non-Cardiac | 977 (16%) | 868 (15%) | 109 (20%) | |
| Atypical | 2230 (36%) | 2077 (37%) | 153 (28%) | |
| Typical | 825 (13%) | 747 (13%) | 78 (14%) | |
| Dyspnoea | 1793 (31%) | 1611 (31%) | 182 (35%) | 0.0383 |
| Diamond and Forrester pre-test probability | 32.9 ± 29.2 | 33.0 ± 29.1 | 32.5 ± 29.8 | 0.69 |
| Morise score | 11.9 ± 4.3 | 11.8 ± 4.3 | 12.0 ± 4.2 | 0.56 |
Data are presented as n (%) and mean ± SD. Patients with a balanced coronary artery system were excluded from the analysis.
BMI, body mass index; CAD, coronary artery disease.
CCTA findings
Right dominance was present in 91% (n = 5817) and left dominance in 9% (n = 565) of the study population. Normal coronary arteries were found by CCTA in 3361 (53%) patients, non-obstructive CAD in 1787 (28%), obstructive CAD in 457 (7%), and severe obstructive in 776 (12%; Table 2). Patients with left dominance tend to have a lower Agatston score than those with right dominance (420.0 in the right dominance and 363.0 in the left dominance, P < 0.0001, median comparison test; Table 2). In our study cohort, 648 (10%) patients had one-vessel disease, 351 (10%) had two-vessel disease, and 222 (3%) were diagnosed with three-vessel disease. The severity of CAD and stenosis location on CCTA differed significantly among patients with a left dominant and right dominant coronary artery system: patients with left dominance tend to have more non-obstructive CAD (35 vs. 27%, P < 0.0001) and significant stenosis in the left anterior descending or circumflex artery (19 vs. 14%, P = 0.0067 and 10 vs. 7%, P = 0.0203, respectively), whereas patients with right dominance tend to have more often normal coronary arteries (54 vs. 43%, P < 00001) or obstructive CAD in the RCA (10 vs. 5%, P < 0.0001; Table 2).
Table 2.
CCTA results: prevalence of coronary dominance in the study population
| CCTA findings | Total (N = 6382) | Right dominant (n = 5817) | Left dominant (n = 565) | P-value |
|---|---|---|---|---|
| Agatston score (mean ± SD)a | 141.3 ± 415 | 141.1 ± 420.0 | 144.2 ± 363.0 | <0.001b |
| Number of obstructive vessels | ||||
| None/normal | 3361 (53%) | 3119 (54%) | 242 (43%) | <0.0001 |
| Non-obstructive | 1788 (28%) | 1591 (27%) | 197 (35%) | |
| One-vessel CAD | 636 (10%) | 563 (10%) | 73 (13%) | |
| Two-vessel CAD | 325 (5%) | 289 (5%) | 36 (6%) | |
| Three-vessel/LM CAD | 272 (4%) | 255 (4%) | 17 (3%) | |
| Level of obstructive CAD | ||||
| Normal | 3361 (53%) | 3119 (54%) | 242 (43%) | <0.0001 |
| Non-obstructive (1–49%) | 1788 (28%) | 1591 (27%) | 197 (35%) | |
| Obstructive CAD (50–70%) | 457 (7%) | 408 (7%) | 49 (9%) | |
| Severe obstructive CAD (>70%) | 776 (12%) | 699 (12%) | 77 (14%) | |
| Left main >50% | 85 (1%) | 76 (1%) | 9 (2%) | 0.5423 |
| LAD >50% | 939 (15%) | 834 (14%) | 105 (19%) | 0.0067 |
| LCx >50% | 465 (7%) | 410 (7%) | 55 (10%) | 0.0203 |
| RCA >50% | 602 (9%) | 576 (10%) | 26 (5%) | <0.0001 |
Data are presented as mean (±SD) or n (%). Patients with a balanced coronary artery system were excluded from the analysis.
CCTA, coronary computed tomographic angiography; CAD, coronary artery disease; LCx, left circumflex artery; RCA, right coronary artery; LM, left main artery; LAD, left anterior descending artery.
aMissing in 1309 patients.
bP value given for median Agatston score (non-normal distribution of data).
Event and survival rate
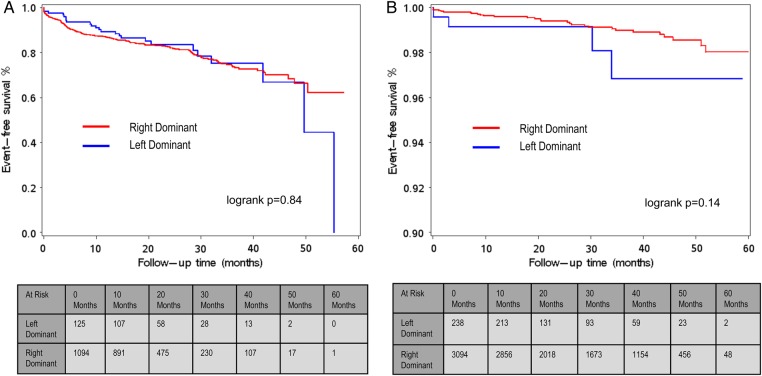
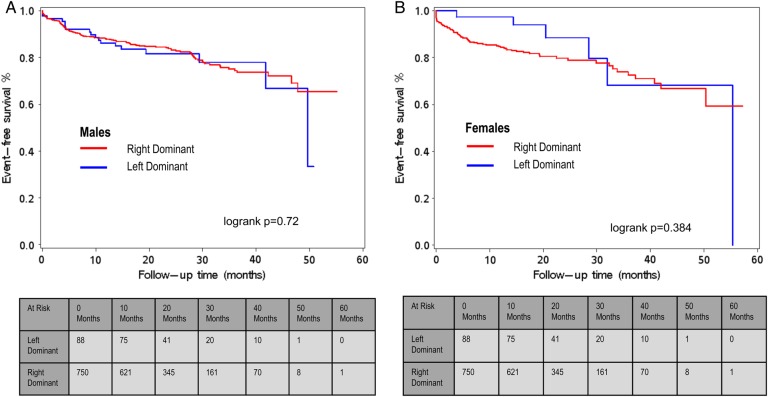
During a follow-up of 60 months, the composite endpoint occurred in 321 (5.0%) patients. All-cause mortality was reported in 100 (1.6%) patients, non-fatal MI occurred in 131 (2.1%), and 120 patients (1.9%) underwent revascularization. When comparing event-free survival during 5 years of follow-up in patients with normal coronary arteries according to coronary vessel dominance, survival rates for the cumulative incidence of all-cause mortality, non-fatal MI, and coronary revascularization did not significantly differ between patients with LCD or RCD (log-rank P = 0.14, Figure 1B), with low cumulative event rates of 1.7 and 0.9%, respectively. Similar results were obtained when a separate analysis for each endpoint in patients with normal coronary arteries was conducted (log-rank P = 0.41 for all-cause mortality, log-rank P = 0.13 for MI, and P = 0.73 for coronary revascularization, data not shown). Likewise, in patients with significant CAD (>50% stenosis), no significant difference was observed in event-free survival between left dominant and right dominant coronary artery systems, with cumulative event rates of 18.8 and 19.1% after 5 years of follow-up for a right- and left dominant coronary artery system, respectively (log-rank P = 0.84, Figure 1A). These results remained the same when a separate analysis for each endpoint in patients with significant CAD was conducted (log-rank P = 0.069 for all-cause mortality, log-rank P = 0.63 for MI, and P = 0.76 for coronary revascularization, data not shown) or when patients with obstructive CAD (stenosis 50–70%; log-rank P = 0.60, data not shown) or severe obstructive CAD (stenosis >70%; log-rank 0.92, data not shown) were analysed separately. When stratified for sex, patients with LCD and RCD showed similar survival rates for the incidence of all-cause mortality, non-fatal MI, and coronary revascularization (log-rank P = 0.72 for males and log-rank P = 0.3842 for females; Figure 2A and B).
Figure 1.
(A) Event-free survival (Kaplan–Meier curve) from major adverse events (all-cause mortality, non-fatal MI, and coronary revascularization) with follow-up extending to 5 years in patients with RCD and LCD stratified for the presence of obstructive CAD (>50%) on CCTA. Patients with a balanced coronary artery system were excluded from the analysis, because of the low number of patients in this group. (B) Event-free survival (Kaplan–Meier curve) from major adverse events (all-cause mortality, non-fatal MI, and coronary revascularization) in patients with RCD and LCD stratified for normal coronary arteries. Patients with a balanced coronary artery system were excluded from the analysis, because of the low number of patients in this group.
Figure 2.
(A) Males: event-free survival (Kaplan–Meier curve) from major adverse events (all-cause mortality, non-fatal MI, and coronary revascularization) with follow-up extending to 5 years stratified by coronary dominance in patients with obstructive CAD (>50%) on CCTA. (B) Females: event-free survival (Kaplan–Meier curve) from major adverse events (all-cause mortality, non-fatal MI, and coronary revascularization) with follow-up extending to 5 years stratified by coronary dominance in patients with obstructive CAD (>50%) on CCTA. Patients with a balanced coronary artery system were excluded from the analysis.
Prognostic value of coronary dominance
Uni- and multivariable proportional hazards models confirmed that obstructive and severe obstructive CAD in both coronary variations were predictors of all-cause mortality, non-fatal MI, and revascularization, and had an incremental value over clinical variables (Table 3). In patients with non-obstructive CAD, a right dominant system was identified as a significant predictor of the combined endpoint when compared with patients without coronary artery atherosclerosis [hazard ratio (HR) 4.78, 95% CI 3.01–7.59, P < 0.0001, Table 3] and remained a significant predictor after correction for baseline risk factors (P < 0.0001), whereas left dominance did not predict any events in this subpopulation (HR 2.79, 95% CI 0.77–10.1, P = 0.1172, Table 3). When female and male patients were analysed separately, results remained the same (P < 0.0001 for females with RCD patients and non-obstructive CAD and P < 0.0001 for males with RCD patients and non-obstructive CAD, data not shown).
Table 3.
Uni- and multivariate analyses adjusted by Framingham risk factors including age, sex, hypertension, diabetes mellitus, current smoking, and dyslipidaemia
| Univariate |
Multivariate (CAD RF adjusted) |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Left dominant | ||||||
| None/normal | 1 | Reference | Reference | 1 | Reference | Reference |
| Non-obstructive | 2.79 | 0.77–10.1 | 0.1172 | 1.67 | 0.34–8.27 | 0.5278 |
| Obstructive (50–70%) | 10.31 | 2.88–36.8 | 0.0003 | 9.75 | 2.10–45.2 | 0.0036 |
| Severe obstructive (>70%) | 20.67 | 6.81–62.8 | <0.0001 | 18.16 | 4.19–78.8 | 0.0001 |
| Right dominant | ||||||
| None/normal | 1 | Reference | Reference | 1 | Reference | Reference |
| Non-obstructive | 4.78 | 3.01–7.59 | <0.0001 | 3.39 | 2.06–5.59 | <0.0001 |
| Obstructive (50–70%) | 23.38 | 14.9–36.7 | <0.0001 | 15.08 | 9.12–24.9 | <0.0001 |
| Severe obstructive (>70%) | 35.58 | 23.7–53.4 | <0.0001 | 22.83 | 14.2–36.8 | <0.0001 |
HRs of CAD (non-obstructive: <50% stenosis, obstructive: >50%stenosis, severe obstructive: >70%stenosis) for the composite outcome of all-cause mortality, non-fatal MI, and coronary revascularization in LCD and RCD compared with normal coronary arteries on CCTA. Patients with a balanced coronary artery system were excluded from the analysis.
CAD, coronary artery disease; RF, risk factor; HR, hazard ratio; CI, confidence interval.
We further assessed the difference in a prognostic value between left and right coronary vessel dominance in patients with obstructive CAD for the composite endpoint of all-cause mortality, non-fatal MI, and coronary revascularization: Cox regression model analysis showed that the difference in the risk estimate of obstructive CAD between patients with a right dominant and those with a left dominant coronary artery system was statistically not significant (HR 1.04, 95% CI 0.68–1.59, P = 0.8461, right vs. left dominant, Table 4). Similarly, in patients with normal coronary arteries or non-obstructive CAD, no difference in the predictive value between the two coronary dominance pattern was found (HR 0.46, 95% CI 0.16–1.32, P = 0.1496 and HR 0.95, 95% CI 0.41–2.21, P = 0.8962, right vs. left dominant, respectively, Table 4).
Table 4.
Uni- and multivariate analyses adjusted by Framingham risk factors including age, sex, hypertension, diabetes mellitus, current smoking, and dyslipidaemia
| Univariate |
Multivariate (CAD RF adjusted) |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Normal coronary artery | ||||||
| Right dominant (vs. left dominant) | 0.46 | 0.16–1.32 | 0.1496 | NS | NS | NS |
| Non-obstructive CAD | ||||||
| Right dominant (vs. left dominant) | 0.95 | 0.41–2.21 | 0.8962 | NS | NS | NS |
| Obstructive CAD | ||||||
| Right dominant (vs. left dominant) | 1.04 | 0.68–1.59 | 0.8461 | NS | NS | NS |
HRs of RCD vs. LCD for the composite outcome of all-cause mortality, non-fatal MI, and coronary revascularization according to the extend of CAD (non-obstructive: <50% stenosis, obstructive: >50% stenosis) on CCTA. Patients with a balanced coronary artery system were excluded from the analysis.
CAD, coronary artery disease; RF, risk factor; HR, hazard ratio; CI, confidence interval.
Furthermore, significant CAD in one vessel was also identified as a predictor for the combined endpoint with a HR of 16.92 (95% CI 5.5–52.1, P < 0.0001 vs. normal coronary arteries) in the left dominant system and a HR of 24.43 (95% CI 15.9–37.5, P < 0.0001 vs. normal coronary arteries) in the right dominant system. Consequently, in both uni- and multivariable models accounting for individual Framingham risk factors, the risk was dose-dependently increased when more vessels were affected (data not shown).
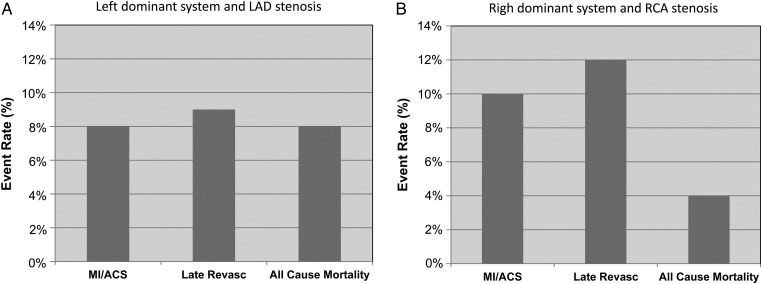
Prognostic value of significant stenosis location
After stratification according to stenosis location, the rate of cumulative event for LCD patients with significant LAD stenosis was 8% for non-fatal MI, 9% for coronary revascularization, and 8% for all-cause mortality (Figure 3A), whereas in patients with right dominance and significant RCA stenosis event rates for non-fatal MI, coronary revascularization, and all-cause mortality were 10, 12, and 4%, respectively (Figure 3B). A significant stenosis in the left coronary system (LAD and LCx) was observed in 1489 patients and was associated with an increased risk of the combined endpoint all-cause mortality, non-fatal MI, and coronary revascularization for left dominance (HR 7.01 for LAD and 3.83 for LCx) as well as for right dominance (HR 10.12 for LAD and 8.29 for LCx, Table 5, lower panel). However, significant left main disease was observed in 85 patients and the presence of LM disease conferred an increased HR for the combined adverse event by 6.45 after multivariable adjustment (95% CI 1.66–25.0, P = 0.007) in patients with left dominance. In right dominance, however, LM disease was not significantly associated with the composite prognosis endpoint (HR 1.35, 95% CI 0.73–2.51, P = 0.3456 after adjustment for CAD and risk factors; Table 5, lower panel). A significant lesion in the right system was associated with an increased risk of the composite endpoint in left dominance (HR 3.49, 95% CI 1.29–9.47, P = 0.0141) as well as in right dominance (HR 5.7, 95% CI 4.27–7.59, P < 0.0001, Table 5, lower panel).
Figure 3.
(A) Risk estimates for all-cause mortality, non-fatal MI, and coronary revascularization in patients with significant LAD stenosis and a left dominant system. Patients with a balanced coronary artery system were excluded from the analysis. (B) Risk estimates for all-cause mortality, non-fatal MI, and coronary revascularization in patients with significant RCA stenosis and a right dominant system.
Table 5.
Upper and lower panels: Uni- and multivariate analyses adjusted by Framingham risk factors including age, sex, hypertension, diabetes mellitus, current smoking, and dyslipidaemia
| Univariate |
Multivariate (CAD RF adjusted) |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Upper panel | ||||||
| Left dominant | ||||||
| None/normal | 1 | Reference | Reference | 1 | Reference | Reference |
| Non-obstructive | 2.76 | 0.77–9.92 | 0.1209 | 1.75 | 0.35–8.71 | 0.4975 |
| One-vessel disease | 16.92 | 5.50–52.1 | <0.0001 | 16.27 | 3.81–69.4 | 0.0002 |
| Right dominant | ||||||
| None/normal | 1 | Reference | Reference | 1 | Reference | Reference |
| Non-obstructive | 4.8 | 3.02–7.63 | <0.0001 | 3.5 | 2.12–5.77 | <0.0001 |
| One-vessel disease | 24.43 | 15.9–37.5 | <0.0001 | 15.91 | 9.79–25.8 | <0.0001 |
| Left dominant | ||||||
| LM >50% | 9.02 | 2.71–30.0 | 0.0003 | 6.45 | 1.66–25.0 | 0.007 |
| RCA >50% | 5.34 | 2.19–13.0 | 0.0002 | 3.49 | 1.29–9.47 | 0.0141 |
| Right dominant | ||||||
| LM >50% | 4.13 | 2.37–7.22 | <0.0001 | 1.35 | 0.73–2.51 | 0.3456 |
| RCA >50% | 10.33 | 8.16–13.1 | <0.0001 | 5.7 | 4.27–7.59 | <0.0001 |
HRs of CAD according to (upper panel) the amount of diseased vessels (one-, two-, and three-vessel disease) and (lower panel) the stenosis location for the composite outcome of all-cause mortality, non-fatal MI, and coronary revascularization in LCD or RCD compared with normal coronary arteries on CCTA.
LM, left main artery; RCA, right coronary artery; CAD, coronary artery disease; RF, risk factor; HR, hazard ratio; CI, confidence interval.
Discussion
In this prospective multicentre study, we systematically evaluated the prognostic value of coronary dominance assessed by CCTA in a large cohort of patients. When comparing event-free survival in patients with normal coronary arteries or obstructive CAD according to coronary vessel dominance, survival rates for the cumulative incidence of all-cause mortality, non-fatal MI, and coronary revascularization after 5 years of follow-up did not differ significantly between patients with LCD or RCD.
In our study, right dominance was present in 91% and left dominance in 9%, which is not significantly different from values given in the literature, varying from 8.2 to 15% for left dominance and from 72 to 90% for right dominance.10–12,23,24 Left dominance was observed more often in males (62%) compared with females (38%), while previous retrospective studies indicate that there is no difference in coronary dominance with regard to gender.23,25–27 However, these differences may arise due to different selection of patients, e.g. the inclusion of low-to-intermediate risk patients at an advanced age in the present study.
In contrast to our findings, two previous retrospective angiographic studies using cardiac catheterization databases in patients with ACS have shown that left dominance was associated with modestly increased odds of death during a 3.5-year follow-up (HR 1.13; 1.00–1.28) or in-hospital mortality (HR 1.19; 1.06–1.34) following PCI, respectively.12,13 Nevertheless, those studies were retrospective analyses done on conventional angiograms and the study population consisting of high-risk ACS patients and patients with prior coronary artery bypass graft differed substantially from our study population. In a recent prospective study of 1425 patients referred for CCTA, non-fatal MI and all-cause mortality were increased (HR 3.15) in patients with left dominance during a 2-year follow-up period.28 However, potential selection bias due to smaller patient numbers in this study cannot be excluded, and no differences in prognosis for different coronary dominance patterns were observed when coronary revascularization was included in the combined primary endpoint. Taken together, it seems that left dominance may have different prognostic values regarding short- and long-term mortality in patients with ACS compared with patients with stable CAD, thereby, emphasizing the importance of angiographic interventions in left dominance patients with ACS. However, prospective studies in patients with ACS are needed to confirm this.
At present, little is known about the prognostic value of stenosis location in relation to coronary vessel dominance, and only one recent study in 1425 patients referred for CCTA demonstrated that a stenosis in the left coronary system was associated with an increased risk of events, while a stenosis in the RCA did not statistically significant predict events.28 Our analysis among subgroups with left main disease showed an elevated hazard of the combined endpoint for left dominance that was statistically significant while a stenosis in the left main did not predict events in right dominance. This finding is consistent with previous observations in patients undergoing PCI for ACS.13 Coronary vessel dominance has influence on the relative contribution of the different coronary arteries to the total left ventricular blood flow29 and in most individuals with LCD, the RCA is usually small and often fails to reach the acute margin of the heart. Thus, a proximal stenosis of the left coronary artery may result in more extensive ischaemia and worse consequences in a left dominant system than in a right dominant system. In addition, the potential to rapidly form collaterals might be diminished in patients with a left dominant coronary artery system due to the fact that the RCA is not sufficient to perfuse the myocardium.30 However, to date, the underlying pathophysiology has not been investigated and further research is needed to assess the effect modification by culprit lesion site or coronary collateral formation in patients with left main disease and left coronary system.
The relationship between coronary vessel dominance and the extent of CAD remains uncertain as different studies showed opposing results. Indeed, one previous study has shown that LCD was associated with a higher incidence of atherosclerosis,31 whereas others showed more extensive CAD in patients with a right dominant coronary artery system12,23 or did not detect differences in the extent of CAD between LCD or RCD.26,28 However, this discrepancy can most likely be explained by a potential selection bias due to small study populations, and the differences in modalities used for the assessment of CAD in these studies. In the present study, we observed a higher incidence of CAD (obstructive and non-obstructive) in left dominance patients, whereas the prevalence of normal coronary arteries was more frequent in right dominance. However, no difference in predisposition to three-vessel disease was seen between LCD or RCD which strongly supports the hypothesis that dominance pattern does not predict outcomes in patients with CAD.
Interestingly, in patients with non-obstructive CAD, a right dominance system was identified as a significant predictor of the combined endpoint, whereas left dominance did not predict any events in this subpopulation. The possibility that intermediate lesions may carry an increased risk in right dominant circulations is of particular importance since it would challenge the current paradigm of non-intervention for these non-obstructive lesions. However, there was no statistically significant difference in univariate analysis in this subgroup when right dominance was compared with left dominance. Yet, our study was likely statistically underpowered to detect effect modification between left and right dominance in this subgroup with non-obstructive CAD.
As with any study, certain design limitations are inherent. Of note is the low prevalence of left and co-dominant coronary circulation in the general population. While our study was sufficiently powered to detect an effect size in LCD, we did not include patients with co-dominant circulation in our analysis, since our study was underpowered to detect statistical effect modification in this subgroup. Secondly, as with any observational, open-label registry, potential heterogeneity between sites, interobserver and intersite variability in CCTA diagnosis, and different post-CCTA treatment patterns cannot be excluded. Thirdly, in the CONFIRM registry, CAD was defined using CCTA and not using invasive coronary angiography or other imaging modalities; therefore, the possibility of false-positive or false-negative CCTA findings exists despite the performance of CCTA by international experts. Finally, information regarding the coronary dominance pattern was not uniformly available for our study cohort, since not all CONFIRM sites collected this information. Thus, the final study comprised only 23.5% of the entire CONFIRM population and, as such, may have the potential for selection bias which may limit the generalizability of the data. However, our study population is the largest, presently available prospective CCTA cohort evaluating the predictive value of coronary dominance and may therefore provide solid data and good evidence regarding the prognostic information of coronary dominance.
In conclusion, our findings suggest that that the assessment of coronary vessel dominance by CCTA may not enhance the risk stratification beyond the assessment of the degree of stenosis in patients with normal coronary arteries or obstructive CAD referred for CCTA, but may add prognostic information for specific subpopulations such as patients with left main disease or non-obstructive CAD.
Conflict of interest: J.K.M. received modest speakers' bureau and medical advisory board compensation, and significant research support from GE Healthcare. M.A.-M. received support from the American Heart Association, BCBS Foundation of Michigan, and Astellas. B.J.W.C. received research and fellowship support from GE Healthcare, research support from Pfizer and AstraZeneca, and educational support from TeraRecon. J.H. received a research grant from Siemens Medical Systems. J.L. has received research support and serves on the speakers bureau for GE Healthcare. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding
T.C.V. has received speaker's honoraria from Boehringer-Ingelheim, Ingelheim, Germany. S.A. has received grant support from Siemens Healthcare, Erlangen, Germany, and Bayer Schering Pharma AG, Berlin, Germany, and has served as a consultant for Servier. M.J.B. has received speaker's honoraria from GE Healthcare, Milwaukee, WI, USA. F.C. has received grant support from GE Healthcare, has served on the Speakers' Bureau of Bracco and as a consultant for Servier, and speaker's honoraria from Bracco Diagnostics, Milan, Italy. T.Q.C. is on the speaker's bureau of GE Healthcare. K.C. has received grant support from Bayer Pharma AG, Berlin, Germany, and Blue Cross Blue Shield Blue Care Michigan. B.J.W.C. has received research support from GE Healthcare; Pfizer, Inc., New York, NY, USA and AstraZeneca, Wilmington, DE, USA, and also has received educational support from TeraRecon, Foster City, CA, USA. J.H. has received research grant support from Siemens Healthcare. P.A.K. has received research support from GE Healthcare and grant support from the Swiss National Science Foundation, Bern, Switzerland. E.M. has received grant support from GE Healthcare and is a consultant for Servier, Neuilly-sur-Seine, France. G.R. has received grant support from Siemens Healthcare, Blue Cross Blue Shield Blue Care Michigan, and Bayer Pharma AG. J.K.M. has received speaker's honoraria and research support from and serves on the medical advisory board of GE Healthcare. C.G. has received grant support from Novartis, Switzerland, and the Swiss National Science Foundation. The views expressed here are those of the investigators only and are not to be construed as those of the US Department of the Army or Department of Defense. Funding to pay the Open Access publication charges for this article was provided by the Department of Nuclear Medicine, University Hospital Zurich and University of Zurich, Switzerland.
References
- 1.Janne d’Othee B, Siebert U, Cury R, Jadvar H, Dunn EJ, Hoffmann U. A systematic review on diagnostic accuracy of CT-based detection of significant coronary artery disease. Eur J Radiol 2008;65:449–61. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32. [DOI] [PubMed] [Google Scholar]
- 3.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–36. [DOI] [PubMed] [Google Scholar]
- 4.Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44. [DOI] [PubMed] [Google Scholar]
- 5.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol 2011;57:1237–47. [DOI] [PubMed] [Google Scholar]
- 6.Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med 2010;152:167–77. [DOI] [PubMed] [Google Scholar]
- 7.Meijboom WB, van Mieghem CA, Mollet NR, Pugliese F, Weustink AC, van Pelt N, et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol 2007;50:1469–75. [DOI] [PubMed] [Google Scholar]
- 8.Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography a process in evolution. J Am Coll Cardiol 2010;55:957–65. [DOI] [PubMed] [Google Scholar]
- 9.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 10.Allwork SP. The applied anatomy of the arterial blood supply to the heart in man. J Anat 1987;153:1–16. [PMC free article] [PubMed] [Google Scholar]
- 11.Knaapen M, Koch AH, Koch C, Koch KT, Li X, van Rooij PC, et al. Prevalence of left and balanced coronary arterial dominance decreases with increasing age of patients at autopsy. A postmortem coronary angiograms study. Cardiovasc Pathol 2013;22:49–53. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg A, Southern DA, Galbraith PD, Traboulsi M, Knudtson ML, Ghali WA. Coronary dominance and prognosis of patients with acute coronary syndrome. Am Heart J 2007;154:1116–22. [DOI] [PubMed] [Google Scholar]
- 13.Parikh NI, Honeycutt EF, Roe MT, Neely M, Rosenthal EJ, Mittleman MA, et al. Left and codominant coronary artery circulations are associated with higher in-hospital mortality among patients undergoing percutaneous coronary intervention for acute coronary syndromes: report from the National Cardiovascular Database Cath Percutaneous Coronary Intervention (CathPCI) Registry. Circ Cardiovasc Qual Outcomes 2012;5:775–82. [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Aschoff AJ, Brambs HJ, Hoffmann MH. Multislice CT imaging of anomalous coronary arteries. Eur Radiol 2004;14:2172–81. [DOI] [PubMed] [Google Scholar]
- 15.van Ooijen PM, Dorgelo J, Zijlstra F, Oudkerk M. Detection, visualization and evaluation of anomalous coronary anatomy on 16-slice multidetector-row CT. Eur Radiol 2004;14:2163–71. [DOI] [PubMed] [Google Scholar]
- 16.Cademartiri F, La Grutta L, Malago R, Alberghina F, Meijboom WB, Pugliese F, et al. Prevalence of anatomical variants and coronary anomalies in 543 consecutive patients studied with 64-slice CT coronary angiography. Eur Radiol 2008;18:781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosar P, Ergun E, Ozturk C, Kosar U. Anatomic variations and anomalies of the coronary arteries: 64-slice CT angiographic appearance. Diagn Interv Radiol 2009;15:275–83. [DOI] [PubMed] [Google Scholar]
- 18.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah MH, Berman DS, et al. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr 2011;5:84–92. [DOI] [PubMed] [Google Scholar]
- 19.Villines TC, Hulten EA, Shaw LJ, Goyal M, Dunning A, Achenbach S, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol 2011;58:2533–40. [DOI] [PubMed] [Google Scholar]
- 20.Small GR, Yam Y, Chen L, Ahmed O, Al-Mallah M, Berman DS, et al. Prognostic assessment of coronary artery bypass patients with 64-slice computed tomography angiography: anatomical information is incremental to clinical risk prediction. J Am Coll Cardiol 2011;58:2389–95. [DOI] [PubMed] [Google Scholar]
- 21.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J 2007;28:2525–38. [DOI] [PubMed] [Google Scholar]
- 22.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr 2009;3:122–36. [DOI] [PubMed] [Google Scholar]
- 23.Vasheghani-Farahani A, Kassaian SE, Yaminisharif A, Davoodi G, Salarifar M, Amirzadegan A, et al. The association between coronary arterial dominancy and extent of coronary artery disease in angiography and paraclinical studies. Clin Anat 2008;21:519–23. [DOI] [PubMed] [Google Scholar]
- 24.Abuchaim DC, Spera CA, Faraco DL, Ribas Filho JM, Malafaia O. Coronary dominance patterns in the human heart investigated by corrosion casting. Rev Bras Cir Cardiovasc 2009;24:514–8. [DOI] [PubMed] [Google Scholar]
- 25.Virmani R, Chun PK, Robinowitz M, Goldstein RE, McAllister HA., Jr Length of left main coronary artery. Lack of correlation to coronary artery dominance and bicuspid aortic valve: an autopsy study of 54 cases. Arch Pathol Lab Med 1984;108:638–41. [PubMed] [Google Scholar]
- 26.Balci B, Yilmaz O. Atherosclerotic involvement in patients with left or right dominant coronary circulation. Kardiol Pol 2004;60:564–6. [PubMed] [Google Scholar]
- 27.Kaimkhani ZA, Ali MM, Faruqi AM. Pattern of coronary arterial distribution and its relation to coronary artery diameter. J Ayub Med Coll Abbottabad 2005;17:40–3. [PubMed] [Google Scholar]
- 28.Veltman CE, de Graaf FR, Schuijf JD, van Werkhoven JM, Jukema JW, Kaufmann PA, et al. Prognostic value of coronary vessel dominance in relation to significant coronary artery disease determined with non-invasive computed tomography coronary angiography. Eur Heart J 2012;33:1367–77. [DOI] [PubMed] [Google Scholar]
- 29.Ilia R, Rosenshtein G, Weinstein J, Cafri C, Abu-Ful A, Gueron M. Left anterior descending artery length in left and right coronary artery dominance. Coron Artery Dis 2001;12:77–8. [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson MC. A study of the artrial arteries in man. J Anat 1978;125:39–54. [PMC free article] [PubMed] [Google Scholar]
- 31.Falci R, Guimarães MH, Santos APS, Cabral RH, Jatene FB, Prates NEVB. Estudo comparativo do padrão de circulação coronariana entre peças anatômicas e pacientes cirúrgicos. Rev Hosp Clin Fac Med Univ São Paulo 1996;51:224–7. [PubMed] [Google Scholar]