Abstract
Purpose
HPV status and smoking history stratifies patients into 3 distinct risk groups for survival following definitive chemoradiotherapy. Local-regional recurrences are common patterns of failure across all 3 risk -groups. SBRT ± cetuximab has emerged as a promising salvage strategy for unresectable locally-recurrent, previously-irradiated head-and-neck cancer (rHNC) relative to conventional re-irradiation ± chemotherapy. However the influence of HPV and smoking remains unknown in the setting of re-irradiation.
Methods/Materials
Patients (n=30) with rHNC of the oropharynx salvaged with SBRT ± cetuximab from August 2002 through August 2013 were retrospectively reviewed; HPV status was determined based on p16 staining of primary pathology.
Results
At a median follow-up of 10 months for surviving patients, the mean overall survival for all patients was 12.6 months. HPV positivity was a significant predictor of overall survival (13.6 vs 6.88 months, p=0.024), while smoking status did not significantly impact overall survival (p = 0.707).
Conclusion
HPV status remains a significant predictor of overall survival in the re-irradiation setting with HPV positive rHNC demonstrating superior overall survival following salvage SBRT ± cetuximab.
Keywords: oropharyngeal, human papilloma virus, radiosurgery, stereotactic body radiotherapy, recurrence
Introduction
Changes in oropharyngeal squamous cell carcinoma (OPSCC) over the past two decades has been marked by changing patient demographics such as decreased rates of smoking and the emergence of human papilloma virus (HPV). HPV is responsible for an oncologic epidemic1: over 60% of OPSCC was estimated to be secondary to HPV in the 2010s versus 16% in the 1980s.2 OPSCC associated with HPV positivity has a distinct, favorable, prognosis following primary chemoradiotherapy; HPV positivity is the single strongest prognostic factor for OPSCC. 3,4 Similarly, smoking status is known to be an independent risk factor for the development of OPSCC; HPV positivity in the setting of at least 10 pack-year smoking history behaves prognostically as an intermediate risk group.4 Recently, a retrospective analysis from 2 contemporary RTOG trials examining cisplatin-based chemoradiotherapy and/or cetuximab (RTOG 0129 and 0522) showed that HPV status remains a strong prognostic factor in patients that fail primary chemoradiotherapy with a 2-year overall survival of 55% for HPV+ versus 28% for HPV-, (p<0.001); HPV status was also a significant predictive factor in patients treated with and without salvage surgery.5,6
At our institution, the preferred salvage re-irradiation regimen for patients with unresectable, locally-recurrent, previously-irradiated head-and-neck cancer is stereotactic body radiotherapy (SBRT). Initial phase I dose escalation, showed the feasibility and safety of 44Gy in 5 fractions without any grade 3+ toxicity and a 76% overall response rate.7 Matched pair-analysis further supported the potential efficacy of SBRT + cetuximab in the re-irradiation setting, for which the safety of this regimen has been validated in both a phase II trial from our institution as well as in recently reported French multi-institutional data.8,9 Herein, we present a secondary analysis of patients treated at our institution with salvage SBRT ± cetuximab (including patients treated on two prospective clinical trials, UPCI 04-144 and 06-093) examining the impact of HPV status in the re-irradiation setting. We hypothesize that HPV status will remain a significant predictor of overall survival following re-irradiation with SBRT. Secondarily, we will examine the influence of HPV positivity on failure patterns and treatment characteristics such as toxicity.
Material and Methods
All patients treated at The UPMC CancerCenter with rOPSCC salvaged with SBRT ± cetuximab from August 2002 and August 2013 were retrospectively reviewed. Patients excluded from this analysis included those with non-oropharyngeal primaries, those treated with SBRT as a planned boost after definitive radiation therapy, patients who had not received prior irradiation, patients who did not complete >50% of prescribed treatment, and patients with non-squamous cell histologies. Patients were referred to SBRT with or without cetuximab after having been deemed unresectable by a multidisciplinary tumor board. Most patients were determined to be surgically unresectable secondary to the extent of disease precluding reconstruction; less commonly, patients were medically inoperable secondary to comorbidity and/or general deconditioning. Original pathology reports of all primary lesions were reviewed where available. HPV status was determined by immunohistochemistry (IHC) using an antibody against p16. A positive test was defined as intermediate/strong nuclear and cytoplasmic staining in ≥ 70% of cells. All patients had no chemotherapy, radiation therapy, or ablative surgery at least 1 month prior to SBRT; and underwent formal restaging evaluation to rule our distant metastases (usually via PET/CT) within 1-month prior to SBRT.
SBRT techniques for target delineation, patient setup, and treatment/delivery have been previously described 7,8,10,11 Briefly, SBRT planning was CT-based or PET/CT-based with custom thermoplastic mask for immobilization delivered using one of several treatment platforms including Cyberknife™ (Accuracy, Inc., Sunnyvale, CA), Trilogy™, and TrueBeam™ (Varian Medical Systems Inc., Palo Alto, CA). SBRT consisted of 40-50Gy in 5 fractions depending on treatment volume ≥25cc, delivered on alternating days over 1-2 weeks. Initially in our dose-escalation experience, planning target volume (PTV) equaled gross tumor volume (GTV) with no expansion margin, however based on recent patterns of failure outcomes analysis, we now incorporate a maximum 5mm GTV to PTV expansion depending on treatment volume, prior treatment, and proximity to surrounding critical structures.10-12 Organs-at-risk included the spinal cord in all cases and brainstem as well as the parotids, pharyngeal constrictor muscles, mandible and oral cavity depending on treatment site. Dose limit to the spinal cord was set at 8Gy with SBRT. Building on promising single-institution and Phase II data, SBRT was combined with concurrent cetuximab administered at 400mg/m2 on day -7 then 250mg/m2 on day 0 and +8 in select patient including patients treated on our prospective Phase II study SBRT + concurrent cetuximab (UPCI 06-093).10--12
The following primary endpoints were assessed post-SBRT stratified by HPV status and smoking history: re-irradiation interval (measured from the time of initial diagnosis to the initiation of SBRT), locoregional control (LRC, defined as failure within any head-and-neck site including regional nodal failure), overall survival (OS, measured from the date of initiation of SBRT to the date of death or last follow-up) and physician recorded toxicities. Using the Kaplan-Meier method for tumor control and survival, a log-rank test was used to compare the difference in time from diagnosis to initiation of SBRT and OS rates by HPV status and smoking history between groups. SPSS software package version 21.0 was used for statistical computation (SPSS Inc, Chicago, IL).
Results
Results: All patients with rOPSCC
Sixty-nine patients (51 males, 18 females; mean age 64.42 +/- 10.15 years) with recurrent, previously-irradiated oropharyngeal squamous cell carcinoma (OPSCC), who were treated with SBRT (Cyberknife = 37, Trilogy-IMRS = 12, Truebeam = 20) were included in this study. The median follow-up of all patients was 9.71 months (<1 month-53 months). The median follow-up for patients who remained alive at last follow-up (n=15) was 10.1 months (<1 month-40 months). Patient, tumor, and treatment characteristics are summarized in Table 1.
Table 1. Baseline Patient and Treatment Characteristics.
| Characteristics | N (%) |
|---|---|
| Age, years (mean ± standard deviation) | 64.4 ± 10.2 |
| Sex | |
| Male | 18 (26%) |
| Female | 51 (74%) |
| Smoking Status (n=66, 96%) | |
| Never – less than 10 pack years | 15 (22%) |
| Greater than 10 pack year | 51 (74%) |
| HPV status (n=30, 43%) | |
| Positive | 17 (57%) |
| Negative | 13 (45%) |
| Primary Site in Oropharynx | |
| Tonsil | 26 (38%) |
| Base of Tongue | 32 (46%) |
| Other or NOS | 11 (16%) |
| Prior Treatment | |
| Prior Full Dose Radiotherapy | 69 (100%) |
| Prior Surgery | 18 (26%) |
| Prior Chemotherapy | 44 (64%) |
| Recurrence Treatment Site | |
| Base of Tongue | 23 (33%) |
| Cervical Lymph Nodes | 10 (15%) |
| Base of Skull | 6 (9%) |
| Other* | 30 (43%) |
| Second Primary¥ | 14 (20%) |
| SBRT Treatment Volume, cc, mean (range) | 45 (2.5-345.1) |
| SBRT Dose, Gy, mean (range) | 40.9 Gy (15-50) |
Other sites: tonsil, hypopharynx, nasopharynx, parotid, other oropharynx.
Fourteen patients (20.3%) had been previously diagnosed and treated with squamous cell carcinoma of the head and neck and presented with OPSCC as a second primary lesion. Of these, 5 patients were treated for laryngeal squamous cell carcinoma (SCC), 3 patients were treated for oral cavity SCC, and 3 were treated for OPSCC. Thirteen (92.9%) of the patients with second primary tumors were smokers, for the remaining 1 patient smoking status was unknown.
Smoking history was available for 95.7% of patients (n=66). The majority of patients were either current or former smokers by history (n=51, 73.9%). Of these, 13 patients (18.8%) continued to smoke through last follow up visit, 34 patients quit smoking after the diagnosis of OPSCC (49.3%), and post-diagnosis smoking data was either unavailable or conflicting for 22 patients (31.9%).
Overall, 33 patients (47.8%) received concurrent cetuximab with SBRT including patients on our prospective institutional protocol UPCI 06-093. There was no difference in cetuximab use between smoking (50%) and nonsmoking (50%) groups.
The average re-irradiation interval was 41.1 months (1-271 months). Neither smoking history (p=0.354; 29.0 months vs. 46.5 months) nor HPV positive status (p=0.709; 32.1 months vs. 39.7 months) were associated with a difference in re-irradiation interval.
The most common sites of failure following re-irradiation with SBRT were metastatic disease (n=18; 26.1%) and persistent local disease (n=17; 24.6%). The mean time to recurrence was 7.13 months (<1 month-27.97 months, SD 5.99). Disease failure following SBRT was most often treated with palliative care only (n=28, 63.64%), followed by salvage chemotherapy (n=13, 29.5%), additional SBRT (n=7; 15.91) and surgical salvage (n=2; 4.5%).
The mean overall survival after the initiation of SBRT was 12.6 months (range: <1 – 40 months), with 1- and 2- year actuarial overall survival rates of 30.6% and 13.3%, respectively. Smoking status did not predict for improved OS (p = 0.707).
Overall, treatment was well-tolerated with no grade 5 treatment-related toxicities. The incidence of grade 1 and 2 toxicities was 88.4%, including mucositis, pain, dysphagia, dysguesia, and rash related to concurrent cetuximab. Two patients were admitted to the hospital during treatment for dehydration. One patient was unable to complete treatment secondary to pain. Treatment site complications such as bleeding and persistent wounds were reported in 5 patients.
Results: Patients with rOPSCC by HPV status
HPV data from the primary lesion was available for 30 patients (43% of overall cohort). The characteristics of these patients are summarized in Table 2. Of these, 17 were HPV positive by p16 immunohistochemistry testing (56.7%). Patients without HPV testing were more likely to have failed primary treatment at an outside institution prior to presenting to our tertiary care center for salvage options (data not shown).
Table 2. Characteristics of Patients by HPV Status.
| Characteristics | HPV +, n=17 N (%) | HPV -, n=13 N (%) |
|---|---|---|
| Age, years (mean) | 56.10 | 61.84 |
| Sex | ||
| Male | 16 (94.1%) | 6 (46.2%) |
| Female | 1 (5.9%) | 7 (53.8%) |
| Smoking Status | 1 unknown | |
| Never – less than 10 pack years | 10 (58.8%) | 2 (16.7%) |
| Greater than 10 pack year | 7 (41.2%) | 10 (83.3) |
| Primary Site in Oropharynx | ||
| Tonsil | 12 (70.6%) | 3 (23.1%) |
| Base of Tongue | 5 (29.4%) | 4 (30.8%) |
| Soft Palate | 0 | 6 (46.2%) |
| Prior Treatment | ||
| Prior Definitive Radiotherapy | 14 (82.4%) | 12 (92.3%) |
| Prior Surgery | 3 (17.6%) | 1 (7.7%) |
| SBRT Treatment Volume, cc, mean (sd) | 58.86 (86.01) | 41.47 (30.47) |
| Second Primary | 0 | 4 (30.8%) |
With regards to potential oncologic variables, seven of the 17 patients known to be HPV positive had a significant smoking history. HPV negative (90.9%) patients were more likely to have received concurrent cetuximab with SBRT than HPV positive (50 %) patients (p = 0.038). These data, along with toxicity data, are summarized in Table 3.
Table 3. SBRT Outcomes in Patients with Known HPV Status.
| Patient | HPV Status | Failure site(s) | Toxicity (grade) | Cancer Status | Smoking history | Concurrent cetuximab |
|---|---|---|---|---|---|---|
| 1 | Negative | Lung | Hyperpigmentation1 | AWD | Yes | Yes |
| 2 | Negative | Local | None | AWD | Unknown | Unknown |
| 3 | Negative | Neck | Mucositis2, Dysgeusia1 | DOD | Yes | Yes |
| 4 | Negative | No change | None | DOD | No | Yes |
| 5 | Negative | Local | Unknown | DOD | Yes | Yes |
| 6 | Negative | No change | Dehydration2, Anemia2 | DOD | Yes | Yes |
| 7 | Negative | No change | Pain1 | DOD | Yes | Yes |
| 8 | Negative | Local | Pain1, Dysphagia2 | DOD | Yes | Yes |
| 9 | Negative | Local | Odynophagia3, Fatigue2 | DOD | Yes | Yes |
| 10 | Negative | Local, Neck, Lung | Odynophagia2, Dehydration3 | DOD | Yes | Yes |
| 11 | Negative | Local | Rash1, Dysphagia1 | DOD | Yes | Yes |
| 12 | Negative | Neck | Dysphagia1 | DOD | Yes | Yes |
| 13 | Negative | Local | Pain1 | DOD | Unknown | No |
| 14 | Positive | Maxilla, Lung | Xerostomia1 Mucositis2 | AWD | Unknown | No |
| 15 | Positive | Lung | Dysphagia1 | AWD | No | No |
| 16 | Positive | Mandible, Lung | Mucositis1 | AWD | No | Unknown |
| 17 | Positive | Local | Pain1 | AWD | No | Yes |
| 18 | Positive | No change | Dysgeusia2, Xerostomia2 | DOD | No | Yes |
| 19 | Positive | No change | Edema2 | DOD | No | Yes |
| 20 | Positive | Local, Neck | Unknown | DOD | Yes | No |
| 21 | Positive | Neck, Lung | None | DOD | Yes | No |
| 22 | Positive | Local | Mucositis1, Rash1 | DOD | Yes | Yes |
| 23 | Positive | Local, Neck, Lung | Mucositis2 | DOD | Yes | No |
| 24 | Positive | Lung | Mucositis1, Rash1 | DOD | Yes | No |
| 25 | Positive | Local | Pain1, Dysphagia2 | DOD | Yes | Yes |
| 26 | Positive | Lung | Unknown | DOD | No | Yes |
| 27 | Positive | Liver | Neck pain1, Arthralgia1 | DOD | No | No |
| 28 | Positive | Liver, Spleen | Non-healing wound of right neck3 | DOD | Yes | No |
| 29 | Positive | No change | Unknown | DOD | Yes | Unknown |
| 30 | Positive | Lung, Spine, Hip | Mucositis2 | DOD | No | Yes |
Failure patterns of patients with known HPV status are summarized in Table 3. HPV positive patients received an average of 2.29 salvage therapies; while HPV negative patients underwent an average of 2.0 salvage treatments. Eleven of 17 (65%) HPV positive patients eventually developed distant metastases, compared to only two (15%) HPV negative patients had documented distant metastatic disease during the follow up period.
HPV positivity (OS = 13.63 months) was a significant predictor for improved overall survival versus patients with HPV negative (OS = 6.88 months) tumors (p = 0.024) (see Figure 1).
Figure 1. The Impact of HPV Status and Smoking History Following Re-irradiation with SBRT for Locally-Recurrent Previously-Irradiated Squamous Cell Carcinoma of the Oropharynx.
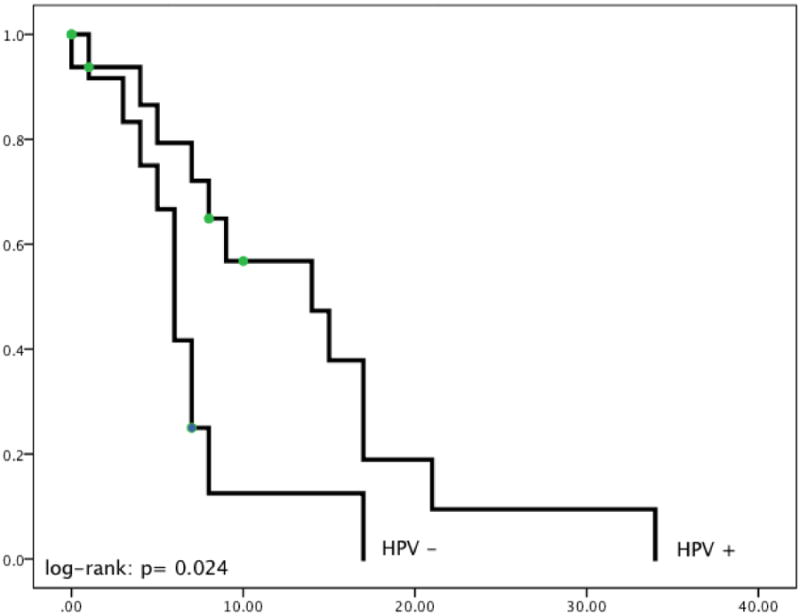
HPV positivity (OS = 13.63 months) did predict for better overall survival versus patients with HPV negative (OS = 6.88 months) tumors (P = 0.024). Smoking status did not predict for improved OS (P = 0.707) (data not shown).
Discussion
The results from this study present important prognostic information for patients and oncologists, highlighting that the favorable influence of HPV status is maintained in the re-irradiation setting of SBRT ± cetuximab. In our cohort, patients with HPV positive OPSCC had double the median overall survival in the unresectable setting when treated with re-irradiation; this is similar to overall survival for salvage surgery following failed definitive chemoradiotherapy on RTOG 0129 and 0522.5,6 In our patients, improved overall survival in HPV positive patients was found despite their being less likely to receive concurrent cetuximab with SBRT as compared to HPV negative patients (90% vs 50 %, p=0.038). There were no other significant imbalances in smoking history or re-irradiation interval. The initial oncogenic insult in virally-mediated tumors is distinct from the traditional carcinogens of smoking and alcohol. It is postulated that the sequestration and degradation of certain cell cycle proteins induced by incorporation of HPV DNA into the cell are more apt to treatment with current regimens. Furthermore, DNA repair mechanisms may be compromised by the virus, yielding an increased response to radiation with a propensity towards apoptosis.13-16 Indeed, HPV positive tumors have been shown to have differential response rates to primary irradiation with HPV positive tumors having a larger volume of tumor response during radiation.17
Immune surveillance has also shown to be a modifier of overall survival in HPV positive patients.18 The virus is thought to induce a host response targeting tumor clearance.19,20 In-vitro resistance to radiation and cisplatin was demonstrated to resolve in an in-vivo, immunocompetent mouse model. This was subsequently abrogated in immunocompromised mice enhanced with adenovirus vector vaccine of E6 & E7 proteins.21 While the correlates in humans are not well-developed, these murine studies may in part predict the continued advantage in overall survival seen in patients treated with SBRT for recurrent HPV positive OPSCC. Additionally, active study in the area of vaccination for active disease has shown benefit of generating an immune response by exposure to non-oncogenic E6 and E7 proteins.22
In this cohort, HPV positive patients developed distant metastases more often than patients with HPV negative tumors. Distant recurrence in the HPV positive population is thought to occur at longer intervals than HPV negative population, with incidences of distant disease occurring up to 5 years versus stabilizing at 2 years, respectively. One theory is the HPV negative tumors are more likely to have fatal locoregional recurrence or tumor sequelae such as aspiration, thereby not manifesting the burden of distant disease. Differences in field cancerization likely contribute to these disparate rates of failure as supported by HPV positive patients having low rates of locoregional recurrence, a low incidence of second primary tumors, and a paucity of HPV-related lesions in the healthy population. A retrospective review of 20 patients treated surgically was conducted to include 97 resection margins in a recent study by Rietbergen et al. The specimens were analyzed for tumor and presence of transcriptionally active HPV by detection of HPV16-E6-mRNA. All negative resection margins were found to be negative for HPV16-E6-mRNA, suggesting the absence of field effect.23
Most recurrent HPV positive patients were intermediate risk as characterized by smoking history greater than 10 pack years.4 Secondary to small sample size and limited availability of initial tumor blocks for p16 staining, meaningful statistics to compare low-, intermediate-, and high- risk groups defined by both smoking status and HPV status were not permissible. Power calculations to design an analogous perspective study to answer the effect of smoking and HPV status as variables are cumbersome, given the need to account for three patient groups and the somewhat unknown magnitude of change that is considered clinically significant. It is worthwhile, however, to illustrate the burden of smoking history in this unfortunate group of HPV positive patients with recurrent disease. This is in concordance with previously published literature. 24-26 Whether the less-favorable prognosis experienced by the intermediate-risk group is secondary to differing tumor biology, differing response to therapy, or smoking-induced discrepancies to the effects of radiation specifically is not fully understood.25 One promising explanation is related to EGFR status. High EGFR protein expression or copy number translates into worse oncologic outcomes in preclinical and clinical data. Interestingly, there is a growing body of literature which illustrates an inverse relationship between EGFR expression and HPV positivity, as reviewed by Mirghani, et al.27 Active smoking is suggested to increase EGFR expression in tumor cells by a hypoxia-induced mechanism, proposing a possible rationale for the worse oncologic outcomes in smokers which is not abrogated by HPV positivity.28
Re-irradiation with SBRT with or without cetuximab was well-tolerated. There were no severe complications or Grade 5 toxicities. At our institution, the SBRT protocol is predicated on outpatient management, short treatment times, and a low burden of treatment-related toxicity. Prior long-term prospective quality of life evaluation has shown long-term sustained improvements in patient reported quality of life which transcended age, re-irradiation interval, tumor volume, and use of cetuximab; however including quality of life measures and reporting patient satisfaction with SBRT will be an ever-increasing aspect of ongoing study.29
Conclusion
Despite changes in the tumor biology and primary treatment of OPSCC over the past thirty years, locoregional recurrent disease still exists in this population where HPV positivity may be associated with a more favorable response to salvage re-irradiation. SBRT with or without cetuximab is an increasingly accepted option for salvage treatment and appears to offer improved overall survival in HPV positive versus HPV negative patients.
Highlights.
The influence of HPV status and smoking remains unknown in the setting of re-irradiation.
69 patients with recurrent OPSCC salvaged with SBRT were reviewed.
Smoking status did not significantly impact overall survival.
HPV positive tumors demonstrated superior overall survival following salvage SBRT ± cetuximab.
Footnotes
Disclosures: none
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariyawardana A, Johnson NW. Trends of lip, oral cavity and oropharyngeal cancers in Australia 1982-2008: overall good news but with rising rates in the oropharynx. BMC Cancer. 2013;13:333. doi: 10.1186/1471-2407-13-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human Papillomavirus (HPV) and Overall Survival (OS) After Progression of Oropharyngeal Squamous Cell Carcinoma (OPSCC) Int J Radiat Oncol Biol Phys. 2014;88(2):466. [Google Scholar]
- 6.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human Papillomavirus and Overall Survival After Progression of Oropharyngeal Squamous Cell Carcinoma. JCO. 2014 doi: 10.1200/JCO.2014.55.1937. JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heron DE, Ferris RL, Karamouzis M, et al. Stereotactic body radiotherapy for recurrent squamous cell carcinoma of the head and neck: results of a phase I dose-escalation trial. Int J Radiat Oncol Biol Phys. 2009;75(5):1493–1500. doi: 10.1016/j.ijrobp.2008.12.075. [DOI] [PubMed] [Google Scholar]
- 8.Heron DE, Rwigema JC, Gibson MK, et al. Concurrent cetuximab with stereotactic body radiotherapy for recurrent squamous cell carcinoma of the head and neck: a single institution matched case-control study. Am J Clin Oncol. 2001;34(2):165–72. doi: 10.1097/COC.0b013e3181dbb73e. [DOI] [PubMed] [Google Scholar]
- 9.Lartiqau EF, Tresch E, Thariat J, et al. Multi institutional phase II study of concomitant stereotactic reirradiation and cetuximab for recurrent head and neck cancer. Radiother Oncol. 2013;109(2):281–5. doi: 10.1016/j.radonc.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Vargo JA, Wegner Heron DE, et al. Stereotactic body radiation therapy for locally recurrent, previously irradiated nonsquamous cell cancers of the head and neck. Head Neck. 2012;34(8):1153–1161. doi: 10.1002/hed.21889. [DOI] [PubMed] [Google Scholar]
- 11.Vargo JA, Heron DE, Ferris RL, et al. Examining tumor control and toxicity following stereotactic body radiotherapy in locally-recurrent, previously-irradiated head-and-neck cancers: implications of treatment duration and tumor volume. Head Neck. 2013;24038398 doi: 10.1002/hed.23462. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Heron DE, Clump DA, et al. Target delineation in stereotactic body radiation therapy for recurrent head and neck cancer: a retrospective analysis of the impact of margins and automated PET-CT segmentation. Radiother Oncol. 2013;106(1):90–95. doi: 10.1016/j.radonc.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26(19):3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lassen P. The role of human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol. 2010;95(3):371–380. doi: 10.1016/j.radonc.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Kimple RJ, Smith MA, Blitzer GC, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73(15):4791–4800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen AM, Li J, Beckett LA, et al. Differential response rates to irradiation among patients with human papillomavirus positive and negative oropharyngeal cancer. Laryngoscope. 2013;123:152–157. doi: 10.1002/lary.23570. [DOI] [PubMed] [Google Scholar]
- 18.Benson E, Li R, Eisele D, Fakrhy C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2013 doi: 10.1016/j.oraloncology.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppock JD, Wieking BG, Molinolo AA, et al. Improve clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition. Neoplasia. 2013;15(6):620–630. doi: 10.1593/neo.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams R, Lee DW, Elzey BD, et al. Preclinical models of HPV+ and HPV- HNSCC in mice: an immune clearance of HPV+ HNSCC. Head Neck. 2009;31(7):911–918. doi: 10.1002/hed.21040. [DOI] [PubMed] [Google Scholar]
- 21.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(11):1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 22.Wieking BG, Vermeer DW, Spanos WC, et al. A non-oncogenic HPV 16 E6/E7 vaccine enhances treatment of HPV expressing tumors. Cancer Gene Ther. 2012;19(10):667–674. doi: 10.1038/cgt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rietbergen MM, Braakhuis BJM, Moukhtari N, et al. No evidence for active human papillomavirus (HPV) in fields surrounding HPV-positive oropharyngeal tumors. J Oral Pathol Med. 2013 doi: 10.1111/jop.12123. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression of patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smoker and nonsmokers with HPV16-associated tonsillar carcinomas. Int J Cancer. 2008;122(12):2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 27.Mirghani H, Amen F, Moreau F, et al. Oropharyngeal cancers: relationship between epidermal growth factor receptor alterations and human papillomavirus status. Eur J Cancer. 2014;50(6):1100–11. doi: 10.1016/j.ejca.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 29.Vargo JA, Heron DE, Ferris RL, et al. Prospective evaluation of patient-reported quality-of-life outcomes following SBRT+/- cetuximab for locally-recurrent, previously-irradiated head and neck cancer. Radiother Oncol. 2012;104(1):91. doi: 10.1016/j.radonc.2012.04.020. [DOI] [PubMed] [Google Scholar]


