Abstract
An evaluation of CD4 T cell responses to candidate Chlamydia trachomatis vaccine antigens was conducted in an adolescent female cohort exposed through natural infection to explore antigen immunogenicity and correlation with protection from reinfection. The frequency of peripheral blood CD4 T cell IFN-γ and IL-17 responses to three candidate vaccine antigens, polymorphic membrane protein G (PmpG), F (PmpF), and major outer membrane protein (MOMP), were determined by ELISPOT; responses to chlamydial heat shock protein 60 (HSP60) and to elementary bodies (EB) were included for comparison. Responses of Infected (n = 8), Seropositive/Uninfected (n = 13), and Seronegative/Uninfected (n = 18) participants were compared. The median CD4 IFN-γ response to EB was significantly increased in Infected (P = 0.003) and Seropositive/Uninfected (P = 0.002) versus Seronegative/Uninfected female subjects. Higher rates of positive IFN-γ responders to EB, PmpF, and MOMP were detected in Seropositive/Uninfected versus Seronegative/Uninfected participants (P = 0.021). IL-17 responses were generally low. A positive IFN-γ response to any of the antigens tested was associated with a trend toward a reduced risk of reinfection, although not statistically significant. Among this adolescent cohort, chlamydial-specific CD4 IFN-γ but not IL-17 responses were detected in acutely and previously infected participants and a positive CD4 IFN-γ response was associated with a non-significant reduced risk of reinfection.
Keywords: Immunity, Antigen, Genital, Pelvic inflammatory disease, Th1, Th17
1. Introduction
Chlamydia trachomatis is the most common bacterial sexually transmitted infection (STI). It is estimated that more than four million chlamydial infections occur each year in sexually active adolescents and adults in the United States. C. trachomatis cervical infection is asymptomatic in 70–90% of cases, but untreated infections can ascend to the upper genital tract resulting in pelvic inflammatory disease (PID), and chronic morbidities of pelvic pain, ectopic pregnancy, and tubal infertility (Peipert, 2003). Asymptomatic infection and inadequate immunity to reinfection in previously exposed individuals are likely responsible for its high prevalence.
Extensive animal modeling suggests that partial immunity develops after infection and that protection is dependent on the trafficking of Chlamydia-specific CD4 IFN-γ-producing T cells (Th1 cells) to the genital tract, with Chlamydia-specific antibodies playing a secondary but contributory role (Igietseme and Rank, 1991; Morrison and Morrison, 2005; Morrison et al., 2000; Perry et al., 1997; Ramsey and Rank, 1991; Rank et al., 1989). Data from the female mouse model of genital infection suggest that repeated infections abbreviated by antibiotic administration elicit partial immunity that does not prevent infection, but lowers the bacterial burden sufficiently that oviduct damage is avoided (Riley et al., 2012; Su et al., 2000).
Evidence exists for partial or sterilizing immunity in humans, mediated by analogous responses (Arno et al., 1990, 1994; Brunham et al., 1983; Cohen et al., 2005). A study of female sex workers identified younger age and fewer years in prostitution as risk factors for incident infection. In the same cohort, IFN-γ responses to chlamydial HSP60 but not to EB were significantly associated with reduced risk of incident infection (Cohen et al., 2005). Development of effective chlamydial vaccines may require the incorporation of multiple antigens combined with Th1-inducing adjuvants to elicit a protective CD4 T cell response. Immunodominant antigens recognized by mice and humans have been identified as potential components (Cong et al., 2007; Crane et al., 2006; Finco et al., 2011;Karunakaran et al., 2008; Yu et al., 2009, 2012). Chlamydial PmpF and PmpG are immunodominant outer membrane antigens that elicit protection in the murine chlamydial genital tract model equivalent to or better than vaccination with recombinant MOMP (Yu et al., 2009, 2012), an outer membrane structural protein, which makes up to 60% of the total outer membrane protein content. MOMP and PmpG are antibody targets, and T cell lines generated from multiple Chlamydia-infected human donors recognized these proteins (Coler et al., 2009). Although data indicate that CD4 Th1 cells are essential for protective immunity against Chlamydia, recent murine studies suggest that IL-17-producing CD4 T cells (Th17 cells) might contribute to protection after vaccination (Yu et al., 2010). Cervical mononuclear cells and peripheral blood mononuclear cells (PBMCs) from women infected with C. trachomatis secrete IL-17 in response to in vitro stimulation with inactivated EBs (Jha et al., 2011). Thus, chlamydial-specific Th17 cells induced by natural infection may promote resistance to reinfection.
In the Chlamydia Adolescent Response Evaluation (CARE) study, using a sensitive enzyme-linked immunosorbent spot (ELISPOT) assay, we sought to determine whether or not CD4 T cell IFN-γ and IL-17 responses against candidate chlamydial vaccine antigens could be detected in the peripheral blood of sexually active adolescent females and whether these responses predicted protection from rein-fection.
2. Materials and methods
2.1. Study population
The Institutional Review Board for human subject research at the University of Pittsburgh approved the study protocol. We established a cohort of 42 female adolescents at the Adolescent Medicine Clinic at the Children’s Hospital of Pittsburgh to investigate CD4 T cell responses to C. trachomatis in at-risk teenagers. We recruited subjects randomly from among 13- to 21-year-old adolescents presenting for a reproductive health care visit. After obtaining informed consent, demographic and clinical data were collected and general physical and pelvic examinations were performed. At this initial visit, cervical specimens were obtained for PCR detection of C. trachomatis and Neisseria gonorrhoeae (Roche AMPLICOR® CT/NG Test), a vaginal swab was collected for wet mount examination, serum was obtained for detection of C. trachomatis antibodies and for human immunodeficiency virus type I (HIV-1) and syphilis testing. Urine collected for pregnancy testing was stored for subsequent chlamydial OmpA typing by PCR. A heparinized blood sample was collected for the ELISPOT assay. Incident C. trachomatis infection was evaluated at ~6 weeks, 3, 6, and 9 months post-enrollment via urine PCR (Roche AMPLICOR® CT/NG Test). Additional demographic and clinical data were collected at follow-up visits, but examinations were performed only if indicated by the presence of symptoms. Of the 42 subjects, 20 (48%) completed all four follow-up visits, 9 (21%) completed three, 6 (14%) completed two, and 7 (17%) were seen at least once at follow-up. All subjects who tested positive for chlamydial infection at any visit were treated with 1 g of azithromycin administered orally, and other STIs were treated as per Centers for Disease Control and Prevention guidelines (Geisler, 2011).
2.2. C. trachomatis antigens
Gradient purified C. trachomatis EBs were inactivated by X-ray irradiation (O’Connell et al., 2011). Recombinant chlamydial antigens were generously provided by Dr. Karuna Karunakaran. Each antigen (MOMP, PmpG, PmpF, and HSP60) was purified as a His-tagged fusion protein from Escherichia coli BL21 (Stephens et al., 1998) in either full-length, MOMP and HSP60, or truncated form, PmpG and PmpF, amino acids 25–512 and 26–585 respectively.
2.3. OmpA typing
Cells pelleted from urine samples were processed with QuickExtract (Epicenter) to yield a template for PCR according to the manufacturer’s specifications. Chlamydial DNA was detected using nested primer sets directed against ompA (Bom et al., 2011). Individual PCR products obtained were sequenced on both strands and a single consensus sequence generated. Serovar assignments were made based on the strongest homology to ompA sequences of known serovar present in the GenBank database.
2.4. IFN-γ and IL-17 ELISPOT assays
Mononuclear cells were isolated from peripheral blood samples by density gradient centrifugation Mononuclear cells were enriched for CD4+ CD8− cells by partial depletion of CD8+ cells using anti-CD8+ magnetic microbeads (Miltenyi) following the manufacturer’s protocol. Flow cytometry analysis revealed that 70–80% of the mononuclear cells were positive for CD3 and αβTCR by flow cytometry, and CD3+ cells were >85% positive for CD4 (data not shown). CD4-enriched mononuclear cells were used in human Ready-SET-Go IFN-γ and IL-17 A ELISPOT assays according to the manufacturer’s instructions (eBioscience). Cells (2.5 × 105) were cultured in triplicate in complete medium (RPMI, 10% FBS, 2 mM β-mercaptoethanol, 1 mM sodium pyruvate, 1 mM non-essential amino acids) in the presence of EB, HSP60, PmpG, PmpF, MOMP (all at the pre-determined optimal concentration of 1 µg/mL), or positive control stimulant (concanavalin-A) for 18 h at 37°C. Cells cultured in media alone served as negative controls. Spots were enumerated on a S5 Core Analyzer using ImmunoSpot Version 5.0 (Cellular Technologies Ltd.).
2.5. Serum antibodies to Chlamydia
The MIF serology assay that detects Chlamydia species and C. trachomatis serovar-specific IgM and IgG was performed at the University of Washington (Wang and Grayston, 1974). Elementary bodies from the 14 major serovars of C. trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci 6BC were used individually; titers ≥ 1:16 were considered positive.
2.6. Statistical analyses
Based on chlamydial infection, serum antibody status at enrollment and medical records indicating past chlamydial infection (PCR+), subjects were categorized into three groups: Active Infection (Group 1; n = 8; 19%); seropositive for antibody to C. trachomatis or documented prior infection and currently uninfected, grouped as Seropositive/Uninfected (Group 2; n = 14; 33%); currently uninfected, seronegative and no documented prior infection, grouped as Seronegative/Uninfected (Group 3; n = 20; 48%). The clinical data on 39 subjects with available laboratory data were summarized by outcome group as frequencies and percentages. Fisher’s exact test was used to assess statistical significance.
ELISPOT data (IFN-γ and IL-17) for each subject were analyzed as follows: the net count for each antigen was calculated as the mean of three replicates for a given antigen minus the mean expression level of three media replicates. The net count was multiplied by 4 to express all results as spot-forming cells (SFC) per million. If the net count was ≥25 SFC/million and the mean response for antigen was at least three-fold higher than media, then the response was designated as positive (Ondondo et al., 2009).
For any given antigen response, the distribution of net counts was compared (across the three outcome groups) using a nonparametric Kruskal–Wallis test; Wilcoxon rank-sum tests were then conducted to determine which groups were different after a significant Kruskal–Wallis test. The number of positive antigen responses was also calculated for each subject and each antigen; differences in the numbers of positive responses were then compared using Fisher’s exact test, and exact binomial tests were then conducted for cases with a significant overall Fisher’s exact test. For each of these analyses, nonparametric statistics were utilized since the distributions of net counts were not always reflective of normality. For 21 subjects who were either infected at enrollment or who had been previously infected, we evaluated whether an IFN-γ response to a single antigen or EB was associated with protection from reinfection over a 9-month follow-up period using a mixed logistic regression analysis that accounts for multiple follow-ups on the same subject.
3. Results
3.1. Cohort characteristics
Forty-two female subjects were enrolled; clinical data are reported on 39 subjects for whom ELISPOT results were available. The mean age of the subjects was 17.3 years; range 13–21 years. Thirty subjects were black (77%), seven were white (18%) and two were bi-racial (5%). At enrollment, seven of the eight actively infected subjects and 11 uninfected subjects were seropositive to C. trachomatis (IgG ≥ 1:16 by MIF assay). Two seronegative, uninfected subjects had previously documented histories of chlamydial infection (PCR+). The remaining 18 subjects were seronegative and their medical records did not report prior infection. Twenty of the 42 subjects were seropositive to C. pneumoniae.
At enrollment, six subjects complained of lower abdominal pain and cervical and/or adnexal tenderness consistent with PID. Five of these subjects were actively infected with C. trachomatis, and the remaining subject was uninfected, Chlamydia seronegative, and negative for other STIs. Within the cohort, two subjects were infected with N. gonorrhoeae, one in the Active Infection and one in the Seropositive/Uninfected group. Four subjects uninfectedat enrollment, two in the Seropositive/Uninfected group and two in the Seronegative/Uninfected group, tested positive for C. trachomatis infection in the following 9 months; none had clinical symptoms. Two subjects, initially positive, also tested positive for C. trachomatis of the same serovar, one 6 weeks and one 3 months post-treatment; both were asymptomatic. Sequencing of ompA PCR products amplified from subjects’ samples indicated that they had been infected with strains corresponding to the following serovars: D (3), E (4), F (3), Ia (1), and J (1).
Table 1 compares clinical and behavioral data for 39 participants. Overall, history of prior PID differed significantly among the three groups (P = 0.045). In pairwise comparisons, women in the Active Infection group were more likely to report a prior history of PID than women in the Seropositive/Uninfected group (P = 0.042). Two of the 39 subjects had more than one episode of chlamydial infection documented prior to enrollment (one with Active Infection and one Seropositive/Uninfected status). No associations between infection and condom use (~half of sexual encounters), or exposure to combined hormonal contraceptives or depot medroxyprogesterone acetate injections were detected. Of those reporting condom use, 9 of the 23 (39%) used it ≤2 times out of 10; and only 3 (13%) used it every time in the 4 weeks prior to interview.
Table 1.
Clinical and behavioral data frequencies (%) compared among groups categorized by Chlamydia trachomatis infection and antibody status.
| Variable | Antibody/infection status | P valuea,b | |||
| Active Infection (n = 8) | Seropositive/Uninfected (n = 13) | Seronegative/Uninfected (n = 18) | Total (n = 39) | ||
| Used condom when last had sex | |||||
| No | 5 (62.50) | 7 (53.85) | 9 (50.00) | 21 (53.85) | 0.916 |
| Yes | 3 (37.50) | 6 (46.15) | 9 (50.00) | 18 (46.15) | |
| Used condom during last 4 weeksc | |||||
| No | 2 (25.00) | 6 (50.00) | 6 (35.29) | 14 (37.84) | 0.570 |
| Yes | 6 (75.00) | 6 (50.00) | 11 (64.71) | 23 (62.16) | |
| Prior STI | |||||
| No | 6 (75.00) | 10 (76.92) | 12 (66.67) | 28 (71.79) | 0.898 |
| Yes | 2 (25.00) | 3 (23.08) | 6 (33.33) | 11 (28.21) | |
| Prior cervicitis | |||||
| No | 5 (62.50) | 12 (92.31) | 16 (88.89) | 33 (84.62) | 0.257 |
| Yes | 3 (37.50) | 1 (7.69) | 2 (11.11) | 6 (15.38) | |
| Prior PID | |||||
| No | 5 (62.50) | 13 (100.00) | 13 (72.22) | 31 (79.49) | 0.045d |
| Yes | 3 (37.50) | 0 (0.00) | 5 (27.78) | 8 (20.51) | |
| Combined hormonal contraception | |||||
| No | 4 (50.00) | 5 (38.46) | 7 (38.89) | 16 (41.03) | 0.835 |
| Yes | 4 (50.00) | 8 (61.54) | 11 (61.11) | 23 (58.97) | |
| Medroxyprogesterone | |||||
| No | 2 (25.00) | 9 (69.23) | 11 (61.11) | 22 (56.41) | 0.140 |
| Yes | 6 (75.00) | 4 (30.77) | 7 (38.89) | 17 (56.41) | |
An overall P value for each condition was calculated using Fisher’s exact test.
P values for individual comparisons were calculated using an exact binomial test when the overall Fisher’s exact test was significant.
Two subjects without data on condom use.
For Prior PID, individual P values were P = 0.042 for Active Infection versus Seropositive/Uninfected, P = 0.667 for Active Infection versus Seronegative/Uninfected, and P = 0.058 for Seropositive/Uninfected versus Seronegative/Uninfected.
3.2. Magnitude of ELISPOT responses for IFN-γ and IL-17
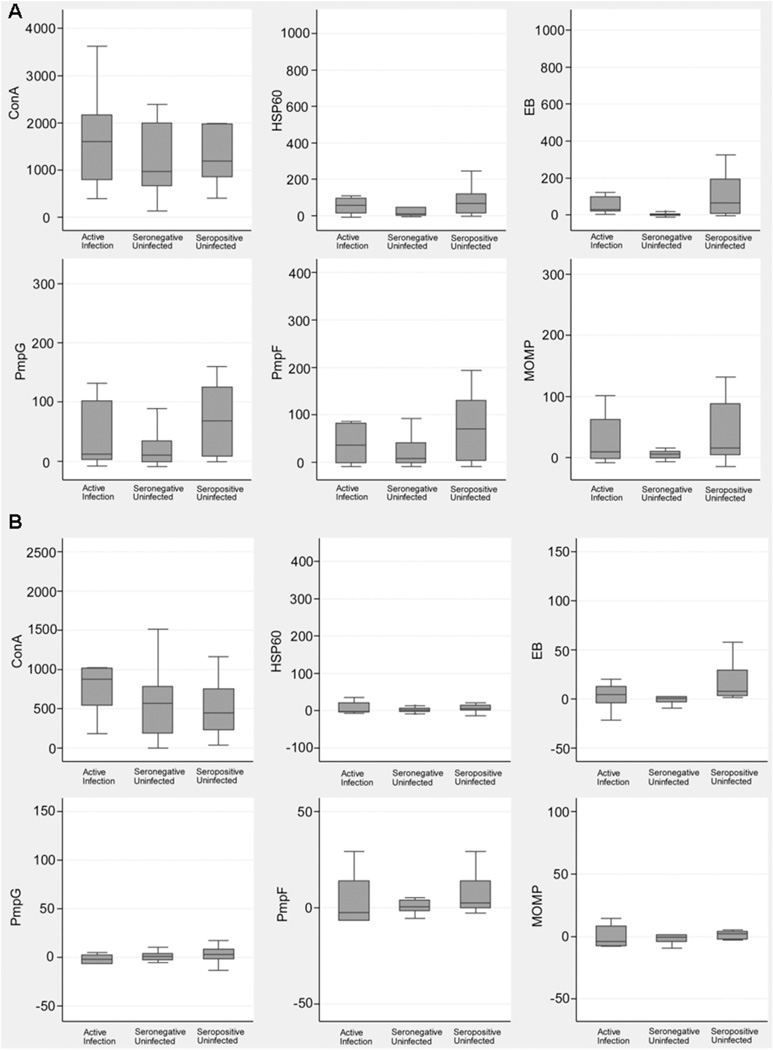
Summary statistics are displayed for each antigen and each outcome group to summarize differences (Fig. 1A and B; Table 2). For IFN-γ, although antigen-specific responses exhibit substantial overlap between the groups, the Seronegative/Uninfected group’s responses had lower median values and reduced interquartile ranges, especially for EB, but also for MOMP (Fig. 1A and Table 2).For IL-17, the responses among the groups overlapped, although a lower median and interquartile range response was observed for EB in the Seronegative/Uninfected group (Fig. 1B and Table 2). The magnitude of IL-17 responses was generally much lower than for IFN-γ. Negative values reflect the extremely low number of IL-17-producing cells detected. The only differences among the subject groups that achieved statistical significance were for EB (P = 0.001 for IFN-γ and P = 0.02 for IL-17; Table 2). For the IFN-γ response to EB, both the Active Infection and the Seropositive/Uninfected groups’ responses were significantly higher than that of the Seronegative/Uninfected group (P = 0.003 and 0.002 respectively). For IL-17, the Seropositive/Uninfected group’s response to EB was significantly higher than that of the Seronegative/Uninfected group (P = 0.003), but the other groups were not significantly different. Thus, compared with recombinant antigens, whole EBs elicited greater responses in exposed adolescents and were more specific, eliciting the lowest responses in Seronegative/Uninfected subjects.
Fig. 1.
Magnitude and variability of peripheral blood CD4 T cell IFN-γ (A) and IL-17 (B) responses among teenage female subjects grouped according to chlamydial infection and serum antibody status. Data are for 39 and 37 subjects for IFN-γ and IL-17, respectively. The box plots represent a graphical display of the minimum to maximum mean net count of spot-forming cells/million. The box represents the middle 50% of the count and the line through the box is the median count value; whiskers are at the 75th percentiles. Concanavalin A (ConA) is the positive control.
Table 2.
Summary of the net counts (spot-forming cells/million) at enrollment for IFN-γ and IL-17 by infection and seropositivity status.
| IFN-γ | |||||||
| Antigen | Active Infection (n = 8) | Seropositive/Uninfected (n = 13) | Seronegative/Uninfected (n = 18) | P valuea | |||
| Median | 1st, 3rd quartile | Median | 1st, 3rd quartile | Median | 1st, 3rd quartile | ||
| Con A | 1606.7 | 796.0, 2164.7 | 1192.0 | 858.7, 1982.7 | 973.3 | 657.3, 2000.0 | 0.55 |
| HSP60 | 55.3 | 13.7, 94.7 | 68.0 | 14.7, 120.0 | 10.0 | 1.3, 46.7 | 0.26 |
| EB | 28.7 | 22.3, 100.0 | 65.3 | 9.3, 193.3 | 2.0 | −2.7, 6.7 | 0.001b |
| PmpG | 12.0 | 2.3, 102.0 | 68.0 | 8.0, 125.3 | 10.0 | −1.3, 34.7 | 0.17 |
| PmpF | 36.0 | −1.0, 82.7 | 70.0 | 4.0, 130.7 | 8.0 | −1.3, 41.3 | 0.17 |
| MOMP | 9.3 | −2.3, 62.7 | 16.0 | 4.0, 88.0 | 6.0 | −1.3, 10.7 | 0.35 |
| IL-17 | |||||||
| Antigen | Active Infection (n = 8) | Seropositive/Uninfected (n = 12) | Seronegative/Uninfected (n = 18) | P valuea | |||
| Median | 1st, 3rd quartile | Median | 1st, 3rd quartile | Median | 1st, 3rd quartile | ||
| Con A | 877.0 | 542.7, 1016.7 | 450.0 | 232.0, 752.7 | 567.3 | 186.7, 784.0 | 0.30 |
| HSP60 | −2.3 | −4.0, 20.7 | 5.3 | 0.0, 14.7 | 1.24 | −2.7, 6.7 | 0.54 |
| EB | 4.7 | −4.0, 12.7 | 8.0 | 1.53, 29.3 | 1.14 | −2.7, 2.7 | 0.02c |
| PmpG | −1.7 | −6.7 2.7, | 3.3 | 0.81, 8.3 | 1.12 | −2.7, 4.0 | 0.48 |
| PmpF | −2.3 | −6.7, 14.0 | 2.7 | 1.02, 14.0 | 1.16 | −1.3, 4.0 | 0.26 |
| MOMP | −4.0 | −7.3, 8.3 | 2.0 | 0.78, 4.0 | 0.92 | −4.0, 1.3 | 0.42 |
P values were calculated using the Kruskal–Wallis test.
For the pairwise comparisons of IFN-γ EB between groups, the Active Infection and Seropositive/Uninfected groups were both significantly different from the Seronegative/Uninfected group (P = 0.003, and P = 0.002 respectively), but the seropositive groups were not significantly different (P = 0.66).
For the pairwise comparisons of IL-17 EB between groups, the Seropositive/Uninfected group was significantly different from the Seronegative/Uninfected group (P = 0.003), but the Active Infection group was not significantly different from the Seronegative/Uninfected group (P = 0.60), or the Seropositive/Uninfected group (P = 0.19).
3.3. Rate of positive ELISPOT responses for IFN-γ and IL-17
Comparisons of the rate of positive CD4 T cell IFN-γ and IL-17 antigen-specific responses are displayed in Table 3. Significant differences were observed among the subject groups between the rates of positive IFN-γ responses to EB, PmpF, and MOMP. The Active Infection and Seropositive/Uninfected groups had higher percentages of positive responders to EB than the Seronegative/Uninfected group, but the difference was significant only for the Seropositive/Uninfected group (P = 0.021). For PmpF and MOMP, significance was driven largely by differences between the Seropositive/Uninfected and Seronegative/Uninfected groups. Over 35% of the Seronegative/Uninfected subjects exhibited positive IFN-γ responses to HSP60 and PmpG, suggesting that these responses might be nonspecific. For IL-17, very few participants exhibited positive responses to any antigen and there were no significant differences among the groups.
Table 3.
Rate (%) of CD4 T cell IFNγ and IL-17 antigen-specific positive responses among groups categorized by Chlamydia trachomatis infection and antibody status.
| IFNγ | ||||||
| Antigen | Positive responsea | Active Infection (n = 8) |
Seropositive/ Uninfected (n = 13) |
Seronegative/ Uninfected (n = 18) |
Total | P valueb |
| HSP60 | No | 4 (50.00) | 5 (38.46) | 11(61.11) | 20 (51.28) | 0.451 |
| Yes | 4 (50.00) | 8 (61.54) | 7 (38.89) | 19 (48.72) | ||
| EB | No | 3 (37.50) | 5 (38.46) | 15 (83.33) | 23 (58.97) | 0.019c |
| Yes | 5 (62.50) | 8 (61.54) | 3 (16.67) | 16 (41.03) | ||
| PmpG | No | 6 (75.00) | 5 (38.46) | 11 (61.11) | 22 (56.41) | 0.253 |
| Yes | 2 (25.00) | 8 (61.54) | 7 (38.89) | 17 (43.59) | ||
| PmpF | No | 5 (62.50) | 4 (30.77) | 14 (77.78) | 23 (58.97) | 0.032d |
| Yes | 3 (37.50) | 9 (69.23) | 4 (22.22) | 16 (41.03) | ||
| MOMP | No | 6 (75.00) | 7 (53.85) | 17 (94.44) | 30 (76.92) | 0.024e |
| Yes | 2 (25.00) | 6 (46.15) | 1 (5.56) | 9 (23.08) | ||
| IL-17 | ||||||
| Antigen | Positive responsea | Active Infection (n = 8) |
Seropositive/ Uninfected (n = 12) |
Seronegative/ Uninfected (n = 18) |
Total | P valueb |
| HSP60 | No | 7 (87.50) | 10 (76.92) | 16 (88.89) | 33 (84.62) | 0.844 |
| Yes | 1 (12.50) | 3 (23.08) | 2 (11.11) | 6 (15.38) | ||
| EB | No | 6 (75.00) | 10 (76.92) | 16 (88.89) | 32 (82.05) | 0.639 |
| Yes | 2 (25.00) | 3 (23.08) | 2 (11.11) | 7 (17.95) | ||
| PmpG | No | 7 (87.50) | 11 (84.62) | 16 (88.89) | 34 (87.18) | 1.000 |
| Yes | 1 (12.50) | 2 (15.38) | 2 (11.11) | 5 (12.82) | ||
| PmpF | No | 6 (75.00) | 10 (76.92) | 16 (88.89) | 32 (82.05) | 0.639 |
| Yes | 2 (25.00) | 3 (23.08) | 2 (11.11) | 7 (17.95) | ||
| MOMP | No | 6 (75.00) | 11 (84.62) | 16 (88.89) | 33 (84.62) | 0.737 |
| Yes | 2 (25.00) | 2 (15.38) | 2 (11.11) | 6 (15.38) | ||
A positive response was defined by a net count ≥25 and at least three-fold higher than the media response.
P values were calculated using Fisher’s exact test.
For the pairwise comparisons of IFN-γ EB, the Seropositive/Uninfected group and the Seronegative/Uninfected group were significantly different (P = 0.021), but neither of the other two comparisons was significant (P = 1.00 for Active Infection versus Seropositive/Uninfected and P = 0.060 for Active Infection versus Seronegative/Uninfected).
For the pairwise comparisons of IFN-γ PmpF, the Seropositive/Uninfected group and the Seronegative/Uninfected group were significantly different (P = 0.013), but neither of the other two comparisons was significant (P = 0.203 for Active Infection versus Seropositive/Uninfected and P = 0.635 for Active Infection versus Seronegative/Uninfected).
For the pairwise comparisons of IFN-γ MOMP, the Seropositive/Uninfected group and the Seronegative/Uninfected group were again significantly different (P = 0.012), but neither of the other two comparisons was significant (P = 0.399 for Active Infection versus Seropositive/Uninfected and P = 0.215 for Active Infection versus Seronegative/Uninfected).
3.4. Antigen-specific response and infection during follow-up
Logistic regression was performed to calculate the odds ratios (OR) and 95% confidence intervals (CI) for incident infection during follow-up among subjects in the Active Infection and Seropositive/Uninfected groups according to the presence or absence of a positive response to antigen or EB at enrollment. Since medical records data and negative serum antibody status suggested that the Seronegative/Uninfected subjects might be infection naïve, and therefore might have had a lower inherent infection risk owing to lack of exposure to an infected partner, we restricted our analysis to participants with the highest risk of infection. Of these 21 subjects, 76% completed two or more follow-up visits and contributed 54 follow-up PCR tests with four positives detected, two in the Active Infection and two in the Seropositive/Uninfected groups. A positive response to any antigen or to EB was associated with a nonsignificant reduced risk of infection (Table 4). The greatest reductions in risk were observed with a positive response to HSP60 (OR = 0.24, 95% CI: 0.07–0.79, P = 0.25) or PmpG (OR = 0.33, 95% CI: 0.10–1.09, P = 0.37). A positive IFN-γ response to either HSP60 or PmpG was associated with a reduced risk that approached statistical significance (OR = 0.17, 95% CI: 0.014–2.10, P = 0.11). Although the reinfection rate was high (19%), this prediction model may not be robust because of the small sample size.
Table 4.
Associations between a positive CD4 T cell IFNγ response to EB or antigen and incident infection during follow-up among 21 adolescent females previously infected with C. trachomatis.
| Antigen | Odds ratio (95% CI) | P value |
|---|---|---|
| EB | 0.67 (0.24–1.88) | 0.70 |
| HSP60 | 0.24 (0.07–0.79) | 0.25 |
| PmpG | 0.33 (0.10–1.09) | 0.37 |
| PmpF | 0.79 (0.28–2.22) | 0.82 |
| MOMP | 0.54 (0.16–1.79) | 0.62 |
| HSP60 or PmpG | 0.17 (0.01–2.10) | 0.11 |
| HSP60 or MOMP | 0.19 (0.02–2.29) | 0.16 |
Mixed-effects logistic regression model was used to derive odds ratios, 95% confidence intervals, and P values.
4. Discussion
The primary aim of this study was to determine if CD4 T cell IFN-γ and IL-17 responses specific to putative chlamydial vaccine antigens could be detected in the peripheral blood of female teens at high risk of C. trachomatis. Although our cohort was small (42 subjects), we accomplished 85% of 168 follow-up visits in a 15-month period. The rate of chlamydial infection was 19% in our subject cohort at enrollment, and the rate of PID among those positive for Chlamydia at enrollment was 5 out of 8 or 63%, reflecting our recruitment of subjects from a clinic where girls presented for reproductive health care related to STI exposure and/or genitourinary symptoms. A repeat PCR positivity rate of 25% in those infected at enrollment and 15% in the Seropositive/Uninfected group supports current recommendations to test for Chlamydia reinfection 3 months after treatment and to screen even asymptomatic, sexually active adolescent girls for Chlamydia annually (Workowski and Berman, 2011). These data combined with the low rates of condom use reported illustrate the need for ongoing promotion of safer sex practices among adolescents. The detection of two of the same serovar repeat infections among the eight participants infected at enrollment highlights the importance of aggressive partner notification and treatment, including expedited partner therapy. Failed treatment is possible in one participant who tested positive 6 weeks post-treatment. However, as with the participant who had a negative PCR test at 6 weeks and returned positive at 6 months, reinfection was more likely the explanation.
In this small adolescent cohort study, although the frequencies of peripheral blood CD4 IFN-γ SFCs specific for HSP60, PmpG, PmpF, and MOMP were higher in actively or previously infected participants compared with Seronegative/Uninfected participants, the differences in median net counts for these antigens were not significant among the groups. In contrast, the frequencies of CD4 IFN-γ SFCs specific to EB were significantly higher in both groups of exposed participants compared with subjects who were naïve for chlamydial infection. Since the median IFN-γ responses to all of the recombinant antigens except for MOMP were similar to or higher than the median response to EB, this implies that these antigens are stimulatory to CD4 Th1 cells in adolescent females sustaining chlamydial infection. However, the higher 3rd quartile IFN-γ responses for HSP60, PmpG, and PmpF, compared with EB and MOMP observed in the Seronegative/Uninfected group, indicate that nonspecific responses to these antigens may occur.
IL-17 responses were rare and smaller than IFN-γ responses. However, the detection of IL-17 responses to EB in Seropositive/Uninfected subjects that were significantly higher than those of Seronegative/Uninfected subjects (P = 0.003) indicated that EB-specific Th17 cells were induced in a subset of exposed female teenagers. The low median response to MOMP for IFN-γ and IL-17 and the relatively low number of positive responders to MOMP may reflect our use of recombinant MOMP representing a single serovar. However, the majority of mapped MOMP T cell epitopes are located in conserved regions of the protein (Ortiz et al., 1996). The two Active Infection subjects with positive IFN-γ and IL-17 responses to MOMP were infected with D and E, both of the serogroup B complex.
The probability of detecting an EB-positive response within a small patient cohort is likely increased because the bacterial preparation contains multiple antigens. This is supported by the detection of a higher rate of subjects with a positive IFN-γ response to EB among the Active Infection and Seropositive/Uninfected groups compared with the Seronegative/Uninfected group.
Interestingly, positive IFN-γ responses to HSP60 and PmpG were detected in over 35% of the Seronegative/Uninfected subjects and positive IFN-γ responses to PmpF were detected in ~20% of these subjects. Significant homology exists between C. trachomatis HSP60 and human HSP60 (Cerrone et al., 1991; Yi et al., 1993), which may contribute to CD4+ T cell cross-reactivity. Eighty percent of the Seronegative/Uninfected subjects with positive responses to PmpG and PmpF were seropositive to C. pneumoniae, which may have contributed to CD4+ T cell cross reactivity arising from homologous epitopes in PmpG and PmpF from this species (Kalman et al., 1999; Voigt et al., 2012).
We used the sensitive ELISPOT method to detect antigen-specific cytokine-producing CD4+ T cells in the peripheral blood. Although several days of antigen stimulation may be required to detect central memory T cells, overnight incubation should be sufficient to detect effector memory T cells. Nevertheless, we failed to detect EB-specific IFN-γ responses in 37.5% of subjects in the Active Infection group and 38.5% of subjects in the Seropositive/Uninfected group. Negative responders were detected despite a high rate of documented prior chlamydial infection both in the Active Infection (50%) and Seropositive/Uninfected (70%) groups. Cohen et al. also found a high rate of negative IFN-γ responders to EB (60%) among a cohort of sex workers (Cohen et al., 2005). These data suggest that Chlamydia-specific effector memory CD4+ Th1 cells might remain undetectable in the peripheral blood of a subset of female subjects despite repeated infection. The low immunogenicity of this mucosal infection likely contributes to the high risk of repeated chlamydial infection observed in clinical studies (Batteiger et al., 2010; Geisler, 2011).
Rates of positive IL-17 responses were low, with 75% of subjects in the Active Infection group and 77% of the subjects in the Seropositive/Uninfected group having negative CD4+ T cell IL-17 responses to EB. These data contrast with a study conducted in New Delhi, India (Jha et al., 2011). There, IL-17 levels detected in supernatants of PBMCs stimulated with EB for 5 days were significantly higher in C. trachomatis-infected women than in a group of uninfected, seronegative women. While murine vaccine studies suggest a role for Th17 cells in protection after vaccination (Yu et al., 2010), the Th17 response may also play a tissue-damaging role through enhanced recruitment of neutrophils during infection (Scurlock et al., 2011). Additional studies are needed to determine the protective versus pathological role of chlamydial-specific Th17 cells during human genital tract infection.
In a prior study of adult female sex workers PBMC IFN-γ responses to recombinant chlamydial HSP60 with protective immunity to C. trachomatis, but responses to EB were not associated with protection (Cohen et al., 2005). Although positive responders among Seronegative/Uninfected subjects suggested some lack of specificity of IFN-γ responses to HSP60 and PmpG, a positive response to these antigens was associated with a 76% or 66% reduced risk of infection during follow-up respectively. Subjects with a positive response to either HSP60 or PmpG had an 83% reduction in risk. However, these reductions in risk were nonsignificant, likely because of our small sample size.
Strengths of our study included the recruitment of an at-risk sexually active adolescent female cohort with well-documented medical records and a high follow-up rate, and the use of the serovar- and species-specific MIF assay to indicate prior chlamydial exposure. Limitations included the small size of the cohort and the use of a limited panel of antigens for testing. Data confounders include the restricted age group, the high incidence of PID among subjects with active infection, and the potential contribution of non-CD4+ T cells to the cytokine responses detected by ELISPOT. Finally, systemic responses may not be reflective of protective responses at the cervical mucosa. The use of longer incubation times and agonistic antibodies to costimulatory molecules may enhance ELISPOT sensitivity. Future studies are needed in larger adolescent cohorts to determine peripheral blood and mucosal chlamydial antigen-specific responses that predict resistance to reinfection. Additionally, evaluations of the protective nature of these responses against the development of upper tract disease are warranted because it appears that sterilizing immunity may require multiple natural infections.
Acknowledgements
This work was supported by the NIH-NIAID via grants R01 AI054624 and U19 AI084024 to T.D., and by a fellowship research grant from the Children’s Hospital of Pittsburgh to R.B. We are thankful to Drs. Robert Brunham and Karuna Karunakaran for the recombinant chlamydial antigens used in the study, to Ms. Debra Bass for assistance with patient questionnaires and to Bridgett Begg for technical assistance. We are grateful to the medical staff of the Adolescent Medicine clinic of Children’s Hospital of Pittsburgh and to the adolescent participants.
References
- Arno J, Ricker V, Batteiger B, Katz B, Caine V, Jones R. Interferon-gamma in endocervical secretions of women infected with Chlamydia trachomatis. J. Infect. Dis. 1990;162:1385–1389. doi: 10.1093/infdis/162.6.1385. [DOI] [PubMed] [Google Scholar]
- Arno J, Katz B, McBride R, Carty G, Batteiger B, Caine V, et al. Age and clinical immunity to infections with Chlamydia trachomatis. Sex. Transm. Dis. 1994;21:47–52. doi: 10.1097/00007435-199401000-00010. [DOI] [PubMed] [Google Scholar]
- Batteiger B, Tu W, Ofner S, Van Der Pol B, Stothard D, Orr D, et al. Repeated Chlamydia trachomatis genital infections in adolescent women. J. Infect. Dis. 2010;201:42–51. doi: 10.1086/648734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bom R, Christerson L, Schim van der Loeff M, Coutinho R, Herrmann B, Bruisten S, et al. Evaluation of high-resolution typing methods for Chlamydia trachomatis in samples from heterosexual couples. J. Clin. Microbiol. 2011;49:2844–2853. doi: 10.1128/JCM.00128-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R, Kuo C, Cles L, Holmes K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect. Immun. 1983;39:1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrone M, Ma J, Stephens R. Cloning and sequence of the gene for heat shock protein 60 from Chlamydia trachomatis and immunological reactivity of the protein. Infect. Immun. 1991;59:79–90. doi: 10.1128/iai.59.1.79-90.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Koochesfahani K, Meier A, Shen C, Karunakaran K, Ondondo B, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon-gamma. J. Infect. Dis. 2005;192:591–599. doi: 10.1086/432070. [DOI] [PubMed] [Google Scholar]
- Coler R, Bhatia A, Maisonneuve J, Probst P, Barth B, Ovendale P, et al. Identification and characterization of novel recombinant vaccine antigens for immunization against genital Chlamydia trachomatis. FEMS Immunol. Med. Microbiol. 2009;55:258–270. doi: 10.1111/j.1574-695X.2008.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Jupelli M, Guentzel M, Zhong G, Murthy A, Arulanandam B. Intranasal immunization with chlamydial protease-like activity factor and cpg deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine. 2007;25:3773–3780. doi: 10.1016/j.vaccine.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane D, Carlson J, Fischer E, Bavoil P, Hsia R, Tan C, et al. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1894–1899. doi: 10.1073/pnas.0508983103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco O, Frigimelica E, Buricchi F, Petracca R, Galli G, Faenzi E, et al. Approach to discover T- and B-cell antigens of intracellular pathogens applied to the design of Chlamydia trachomatis vaccines. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9969–9974. doi: 10.1073/pnas.1101756108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler W. Diagnosis and management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases treatment guidelines. Clin. Infect. Dis. 2011;53(Suppl. 3):S92–S98. doi: 10.1093/cid/cir698. [DOI] [PubMed] [Google Scholar]
- Igietseme J, Rank R. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect. Immun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R, Srivastava P, Salhan S, Finckh A, Gabay C, Mittal A, et al. Spontaneous secretion of interleukin-17 and -22 by human cervical cells in Chlamydia trachomatis infection. Microbes Infect. 2011;13:167–178. doi: 10.1016/j.micinf.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Kalman S, Mitchell W, Marathe R, Lammel C, Fan J, Hyman R, et al. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 1999;4:385–389. doi: 10.1038/7716. [DOI] [PubMed] [Google Scholar]
- Karunakaran K, Rey-Ladino J, Stoynov N, Berg K, Shen C, Jiang X, et al. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J. Immunol. 2008;180:2459–2465. doi: 10.4049/jimmunol.180.4.2459. [DOI] [PubMed] [Google Scholar]
- Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 2005;175:7536–7542. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SG, Su H, Caldwell H, Morrison R. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect. Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell C, AbdelRahman Y, Green E, Darville H, Saira K, Smith B, et al. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect. Immun. 2011;79:1044–1056. doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondondo B, Brunham R, Harrison W, Kinyari T, Sheth P, Mugo N, et al. Frequency and magnitude of Chlamydia trachomatis elementary body- and heat shock protein 60-stimulated interferon gamma responses in peripheral blood mononuclear cells and endome-trial biopsy samples from women with high exposure to infection. J. Infect. Dis. 2009;199:1771–1779. doi: 10.1086/599095. [DOI] [PubMed] [Google Scholar]
- Ortiz L, Demick K, Petersen J, Polka M, Rudersdorf R, Van der Pol B, et al. Chlamydia trachomatis major outer membrane protein (momp) epitopes that activate HLA class ii-restricted T cells from infected humans. J. Immunol. 1996;157:4554–4567. [PubMed] [Google Scholar]
- Peipert JF. Clinical practice genital chlamydial infections. N. Engl. J. Med. 2003;349:2424–2430. doi: 10.1056/NEJMcp030542. [DOI] [PubMed] [Google Scholar]
- Perry L, Feilzer K, Caldwell H. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through ifn-gamma-dependent and-independent pathways. J. Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- Ramsey K, Rank R. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect. Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank RG, Soderberg L, Sanders M, Batteiger B. Role of cell-mediated immunity in the resolution of secondary chlamydial genital infection in guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect. Immun. 1989;57:706–710. doi: 10.1128/iai.57.3.706-710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M, Zurenski M, Frazer L, O’Connell C, Andrews C, Jr, Mintus M, et al. The recall response induced by genital challenge with Chlamydia muridarum protects the oviduct from pathology but not from reinfection. Infect. Immun. 2012;80:2194–2203. doi: 10.1128/IAI.00169-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurlock A, Frazer L, Andrews C, Jr, O’Connell C, Foote I, Bailey S, et al. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect. Immun. 2011;79:1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- Su H, Messer R, Whitmire W, Hughes S, Caldwell H. Subclinical chlamydial infection of the female mouse genital tract generates a potent protective immune response: implications for development of live attenuated chlamydial vaccine strains. Infect. Immun. 2000;68:192–196. doi: 10.1128/iai.68.1.192-196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt A, Schofl G, Saluz H. The Chlamydia psittaci genome: a comparative analysis of intracellular pathogens. PLoS ONE. 2012;7:e35097. doi: 10.1371/journal.pone.0035097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Grayston J. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J. Infect. Dis. 1974;130:388–397. doi: 10.1093/infdis/130.4.388. [DOI] [PubMed] [Google Scholar]
- Workowski K, Berman S. Centers for disease control and prevention sexually transmitted disease treatment guidelines. Clin. Infect. Dis. 2011;53(Suppl. 3):S59–S63. doi: 10.1093/cid/cir694. [DOI] [PubMed] [Google Scholar]
- Yi Y, Zhong G, Brunham R. Continuous B-cell epitopes in Chlamydia trachomatis heat shock protein 60. Infect. Immun. 1993;61:1117–1120. doi: 10.1128/iai.61.3.1117-1120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Jiang X, Shen C, Karunakaran K, Brunham R. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J. Immunol. 2009;182:1602–1608. doi: 10.4049/jimmunol.182.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Jiang X, Shen C, Karunakaran K, Jiang J, Rosin N, et al. Chlamydia muridarum T-cell antigens formulated with the adjuvant dda/tdb induce immunity against infection that correlates with a high frequency of interferon-gamma)/tumor necrosis factor alpha and interferon-gamma/interleukin-17 double-positive CD4+ T cells. Infect. Immun. 2010;78:2272–2282. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Karunakaran K, Jiang X, Shen C, Andersen P, Brunham R. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect. Immun. 2012;80:1510–1518. doi: 10.1128/IAI.06338-11. [DOI] [PMC free article] [PubMed] [Google Scholar]