Abstract
Purpose
To evaluate the efficacy of vitrectomy with vancomycin for the treatment of experimental Bacillus cereus endophthalmitis.
Methods
Endophthalmitis was initiated in rabbits via intravitreal injection of 100 CFU B. cereus. Treatment groups included included 25-gauge transconjunctival sutureless vitrectomy with intravitreal vancomycin (1 mg) or vancomycin alone. Groups were treated at 4 h, 5 h, or 6 h postinfection. At 48 h (for 4 h and 5 h groups) or 36 h (for the 6 h group) postinfection, eyes were analyzed by electroretinography, histology, and inflammatory cell counts.
Results
Treatment with vitrectomy/vancomycin at 4 h resulted in significantly greater retinal function compared to that of vancomycin alone. Intraocular inflammation following treatment at 4 h was minimal for both treatment groups. Treatment with vitrectomy/vancomycin or vancomycin alone at 5 h or 6 h postinfection resulted in similar levels of retinal function loss (i.e. >90%) and significant intraocular inflammation.
Conclusions
These results demonstrate that vitrectomy may be of therapeutic benefit in the treatment of B. cereus endophthalmitis, but only during the early stages of infection.
Keywords: Bacillus, infection, eye, vancomycin, vitrectomy
INTRODUCTION
Bacterial endophthalmitis is an infection that can result from contamination of the posterior segment following eye surgery, a penetrating eye injury, or from septic spread of infection into the eye from the bloodstream (1, 2). The incidence of post-traumatic endophthalmitis varies from 3 to 17% following a penetrating injury (3, 4) Bacillus cereus is a common bacterial cause of post-traumatic bacterial endophthalmitis. Patients with B. cereus endophthalmitis often present with severe ocular pain, periorbital swelling, proptosis of the globe, polymorphonuclear leukocytosis, and a fever (5–7). Only 9% of B. cereus endophthalmitis patients retain 20/70 vision or better, and nearly half require enucleation or evisceration of the infected eye (6). B. cereus is susceptible to commonly-used antibiotics aminoglycosides, fluoroquinolones, and vancomycin (8–10). However, the regularity of treament failures necessitates improvements in therapy of this blinding infection.
Vitrectomy is widely used to remove intravitreal contents following ocular injuries, inflammation, and other diseases. Microincision vitrectomy surgery, such as 23-and 25-gauge vitrectomy, is often described as being minimally invasive (11, 12). The main concept of sutureless vitrectomy is to decrease postoperative inflammation, potentially resulting in early recovery and improvement of patient comfort. In rabbits, inflammation induced by 25-gauge vitrectomy was less than that induced by 20- or 23-gauge vitrectomy (13). Vancomycin is a commonly administered intravitreal antibiotic used for treating intraocular infections, with reported 100% effectiveness against the most common Gram-positive ocular pathogens, including B. cereus (8–10). In an experimental B. cereus endophthalmitis model, vancomycin sterilized infected eyes when intravitreally administered as late as 6 h postinfection (14, 15). However, significant vision was lost if vancomycin was administered after 4 h postinfection in this model, indicating that early treatment was critical for salvaging vision. We recently reported potential vancomycin-based anti-inflammatory activity in this model (15). Vitrectomy and injection of intravitreal vancomycin sterilized the vitreous cavity following experimental staphylococcal endophthalmitis, resulting in minimal inflammation (16). Previous efficacy studies suggested that vancomycin (17–19) can be effective against experimental B. cereus endophthalmitis, but time courses of these infections may not have been clinically similar to that seen in rapid human infections.
The majority of recent studies analyzing the potential benefits of vitrectomy for the treatment of endophthalmitis have utilized experimental fungal infection models (20–22). For bacterial or fungal endophthalmitis, vitrectomy can be utilized as an effective strategy to remove not only dead organisms and cellular debris, but also damaging toxins and other inflammogenic factors that may exacerbate infection (23). We therefore hypothesized that additional surgical measures to remove offending toxic contents in the vitreous may improve the visual outcome of infection, which is important if treatment is delayed. To this end, we analyzed the therapeutic effectiveness of vitrectomy and intravitreal vancomycin with that of vancomycin alone in a well-established B. cereus endophthalmitis rabbit model (14, 15) to determine whether vitrectomy and antibiotics offered an improved therapeutic benefit over that of antibiotics alone.
SUBJECTS AND METHODS
Subjects and Drugs
Specific pathogen-free New Zealand White rabbits (male, 2–4 kg, Myrtle’s Rabbitry, Thompsons Station, TN) were used in this study and were maintained in accordance with institutional guidelines and the Association for Research in Vision and Ophthalmology Statement on the Use of Laboratory Animals in Ophthalmic Research (online). Prior to intravitreal injection and retinal function analysis (electroretinography [ERG]), rabbits were anesthetized with an intramuscular injection of ketamine (Ketamine HCl Injection, Bioniche Pharma, Lake Forest, IL; 35 mg/kg of body weight) and xylaxine (Rompun™; Bayer Corp., Shawnee Mission, KS; 5mg/kg of body weight). Proparacaine HCl (Ophthetic™; Allergan, Hormigueros, Puerto Rico; 0.5%) was used to topically anesthetize the eyes prior to paracentesis and intravitreal injection. Vancomycin (1 mg final concentration in 100 μL PBS, Hospira, Lake Forest IL) was administered by intravitreal injection or immediately following vitrectomy. Vancomycin (1 mg) was previously demonstrated to be effective against B. cereus and non-toxic in this model (14, 15).
Experimental Endophthalmitis
Rabbit eyes were intravitreally infected with B. cereus as previously described (14, 15, 24, 25). Briefly, an overnight culture of B. cereus strain ATCC 14579 (American Type Tissue Culture, Manassas, VA) was subcultured into brain heart infusion media (BHI; Difco Laboratories, Detroit, MI), and serially diluted to 100 CFU/0.1 ml for intravitreal injections. Contralateral eyes served as a non-injection control. The MIC of B. cereus ATCC 14579 for vancomycin was 1.95 μg/mL, as reported previously (14).
Vitrectomy/Vancomycin Therapy
The treatment regimens chosen for this study sought to mimic an elapsed time when a patient suffering from a penetrating injury may reasonably expect to receive treatment. Previous studies have demonstrated that at and prior to 4 h, intravitreal administration of vancomycin can reduce inflammation and salvage significant vision (14, 15). We sought to determine whether vitrectomy further reduced the inflammation and vision loss associated with delayed treatment.
A total of 44 NZW rabbits were randomized to 7 treatment groups. At 4 h, 5 h, or 6 h postinfection, one eye of each rabbit underwent either 25-gauge transconjunctival sutureless vitrectomy (TSV-25 Millennium System; Bausch & Lomb Inc., Rochester NY) with intravitreal instillation of vancomycin or intravitreal instillation of vancomycin alone. A non-infected vitrectomy/vancomycin group was included as a control.
Vitrectomy was performed as follows. After general and topical anesthesia, a standard 3-ports pars plana vitrectomy was performed with removal of all visible vitreous gel and inflammatory debris. One-step transconjunctival cannula insertion was achieved using a beveled trochar at 2.0 mm from the corneoscleral limbus. Three incisions were made and the infusion cannula was inserted into the inferotemporal cannula. Core vitrectomies removed vitreous using the Millennium 25-gauge high speed vitrector for 10 min by a combination of cutting (1500 cuts/min) and suction (400 mmHg), while continually supplying BSS irrigating solution at an ocular fluid pressure of 30 mmHg. Upon completion of the surgery, the vitreous was infused through the cannula with 0.1 ml of 1.0% vancomycin. The surgical time required from opening to closure was an average of 20 min. Surgery was completed by removal of the entry site cannulas without scleral suturing. An experienced vitreoretinal surgeon performed all vitrectomies and was masked to the identity of the groups. There were no complications during surgery.
At 12, 24, and 48 h (for eyes treated at 4 h or 5 h) or 12, 24, and 36 h (for eyes treated at 6 h) postinfection, eyes were analyzed as described below.
Analysis of Therapeutic Efficacy
Retinal Function Analysis
Retinal function was measured and recorded (UTAS3000; LKC Technologies, Inc., Gaithersburg, MD) for both eyes of each rabbit by scotopic electroretinography (ERG) as previously described (14, 24–27). Prior to ERG, eyes were dilated with phenylephrine HCl (AK-Dilate®; Akorn, Inc., Buffalo Grove, IL) and dark adapted for 10 min. A-wave amplitudes were measured from the pre-stimulus baseline to the A-wave trough, while B-wave amplitudes were measured from the trough of the A-wave to the peak of the B-wave. The following equations were used to calculate the percentage of retinal function retained (experimental = infected; absolute control = uninjected): (i) 100 − {[1-(experimental A-wave amplitude/absolute control A-wave amplitude)] × 100} or (ii) 100 − {[1-(experimental B-wave amplitude/absolute control B-wave amplitude)] × 100} (14, 24–27).
Biomicroscopy and Histology
An operating biomicroscope (Zeiss S7; Zeiss Inc, Thornwood, NY) was used to visualize and photograph rabbit eyes. Changes in anterior and posterior segment inflammation and retinal architecture were scored in a masked independent fashion based on a scale from 0 (no change) to 4+ (significant inflammation and retinal architecture damage) (28). Eyes used for histology were enucleated, fixed in 10% formalin for 24 h, placed in 70% alcohol for 48 h, paraffin sectioned, and stained with hematoxylin and eosin by standard procedures.
Bacterial Quantitation
Viable bacteria in aspirated vitreous were quantified, as described previously (14, 24–27). Briefly, eyes were harvested after euthanasia, vitreal contents were aspirated and homogenized, aspirates were serially diluted in PBS, and aliquots were plated out in triplicate on BHI agar for quantitation.
PMN Quantitation
Quantitation of infiltrating PMN into the aqueous humor is a direct measure of the progression of intraocular inflammation throughout the eye (25–27). Prior to harvest, eyes underwent paracentesis, aqueous samples were loaded onto a hemocytometer, and PMN were manually counted following trypan blue staining.
Antibiotic Penetration into the Eye
Antibiotic diffusion assays were used to quantify the concentration of antibiotics in the vitreous and aqueous humor, as previously described (14). Indicator strains (105 CFU/ml of S. aureus or K. pneumoniae) were inoculated onto BHI agar for vancomycin or gatifloxacin bioassays, respectively. Sterile filter discs loaded with 10 μl of aqueous humor, vitreous, or antibiotic standards were placed onto the inoculated agar. Standards and samples were prepared and analyzed in triplicate. Assays were incubated at 37°C for 24 h, and zones of inhibition were measured to the nearest 0.1mm. A standard curve of zone of inhibition size versus log10 concentration was plotted and used to determine antibiotic concentrations, the slope of which was determined from a best-fit curve by least-square means method.
Statistics
For the PMN quantitation and antibiotic penetration assays, all values represent the mean ± standard error of the mean (SEM) for ≥ 4 replicate samples per time point. All other reported values represent the mean ± SEM for ≥ 5 eyes per time point. Two-tailed, two-sample Student’s t-tests were used to statistically compare groups. A P-value of ≤ 0.05 was considered significant.
RESULTS
Bacterial Killing
All eyes treated with vancomycin, with or without vitrectomy, were sterile at 12 h postinfection. These results are consistent with previous reports of vancomycin treatment in this infection model (14, 15)
Retinal Function
Retinal function retained following each treatment is summarized in Figure 1. In uninfected eyes, vitrectomy with vancomycin resulted in an approximate 40% decrease in A-wave amplitude and 35% decrease in B-wave amplitude at 48 h post-surgery.
Figure 1.
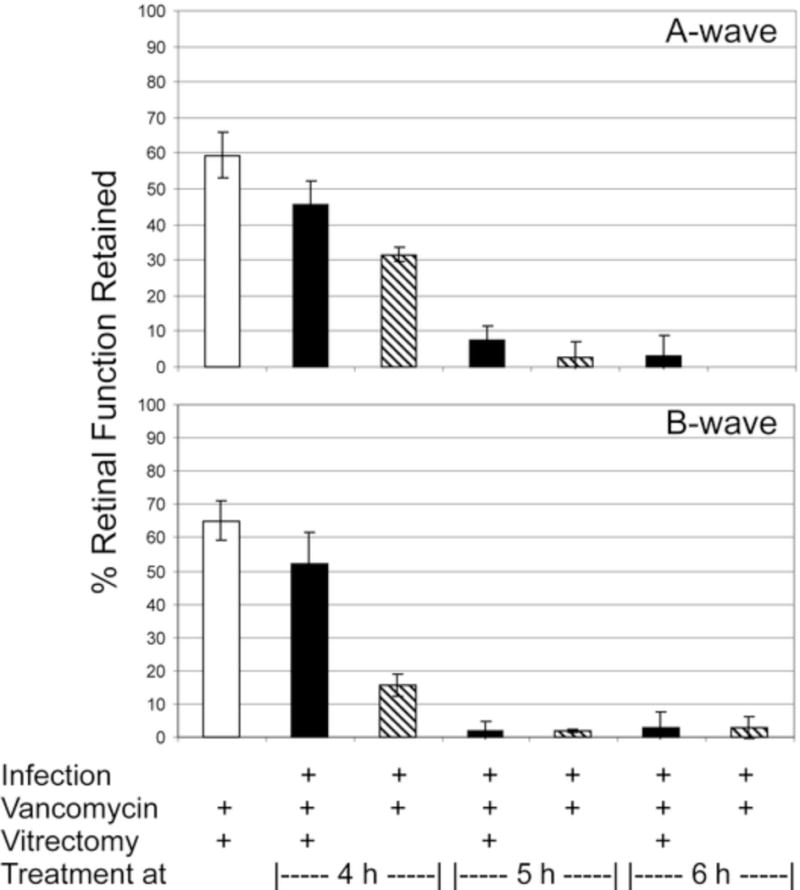
Retinal function analysis following treatment of experimental B. cereus endophthalmitis with vitrectomy and vancomycin. Eyes were infected with B. cereus and treated with vancomycin (1%) and vitrectomy or vancomycin alone at various times postinfection. The control group included uninfected eyes treated with vitrectomy and vancomycin. Eyes were analyzed by electroretinography at 36 h or 48 h postinfection postinfection. Values represent the mean ± SEM of N≥5 eyes per group.
Treatment at 4 h postinfection with vitrectomy and vancomycin resulted in a 45% reduction in A-wave amplitude and a 50% reduction in B-wave amplitude at 48 h. These values are less than but similar to those of the uninfected vitrectomy/vancomycin-treated group (P=0.07 A-wave, P=0.13 B-wave). These values are significantly greater than that of the vancomycin alone group, which resulted in70% and 85% reductions in A-wave and B-wave amplitudes, respectively (P=0.02 A-wave, P=0.004 B-wave). Five-h or 6-h treatment resulted in significant loss of vision (i.e. >95% loss) regardless of whether vitrectomy was part of the vancomycin treatment regimen. These results demonstrate that vitrectomy can reduce the vision loss associated with experimental B. cereus endophthalmitis, but only if treatment is begun no later than 4-h postinfection in this model.
Biomicroscopy and Histology
Representative biomicroscopy and histology data is presented in Figure 2. Prior to surgery, infected eyes at 4, 5, and 6 h postinfection demonstrated mild iritis and mild to moderate vitritis (scores of 1+ to 2+). Uninfected eyes were normal. Immediately after surgery, these observations were unchanged except for minimal conjunctival injection caused by speculum placement.
Figure 2.
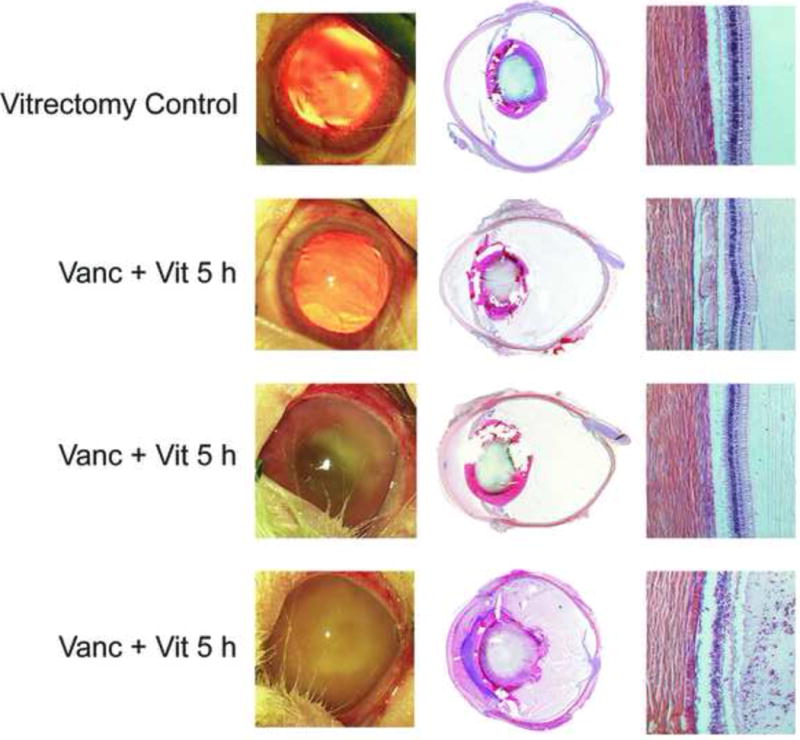
Photography and histology of eyes following treatment of experimental B. cereus endophthalmitis with vitrectomy and vancomycin (1%) at various times postinfection. The control group included uninfected eyes treated with vitrectomy + vancomycin. Eyes were photographed, then harvested for histology and hematoxylin and eosin staining. Figures are representative of N=3 eyes per group. Vanc = vancomycin, Vit = vitrectomy. Magnification of retina sections, 100×.
Uninfected eyes treated with vitrectomy and vancomycin demonstrated mild iritis and vitritis (scores of 1+) at 48 h postinfection. The histology data corroborate this observation, with fibrin and cellular infiltrate seen in the posterior segment and intact retinas. Infected eyes treated with vitrectomy and vancomycin appeared similar to that of uninfected treated eyes at 4 h, with mild iritis and mild to moderate vitritis (scores of 1+ to 2+). The histology data demonstrated significant infiltrate into the anterior and posterior segments, but retinas remained relatively intact. Vitrectomy/vancomycin treatment at 6 h resulted in eyes with moderate cell and flare and vitritis (scores of 3+), with the occasional hypopyon. The histology results of the vitrectomy/vancomycin 6-h treatment directly corroborate these findings. Biomicroscopy and histology of vancomycin-treated eyes at 4 and 6-h postinfection has been reported elsewhere (14, 15), with similarities in biomicroscopy scores and histology findings between the two treatment groups at each time point. As with the 6-h treatment groups, eyes treated with vitrectomy/vancomycin or vancomycin alone at 5 h postinfection were similar in both the rate of evolution and severity of endophthalmitis signs. These findings were corroborated by histology data.
Inflammation
Intraocular inflammation was estimated by counting PMN in harvested aqueous. The results are summarized in Figure 3. Vitrectomy/vancomycin treatment of uninfected eyes resulted in infiltration of PMN detected at 48 h post-surgery. The numbers of PMN in this group were similar to that of infected eyes treated with vitrectomy/vancomycin at 4 h postinfection (P=0.22). The numbers of PMN in infected eyes treated with vitrectomy/vancomycin were significantly less than that of infected eyes treated with vancomycin alone at 4 h postinfection (P=0.02). The numbers of PMN recovered from infected eyes treated at 5 h or 6 h postinfection with vitrectomy/vancomycin or vancomycin alone were similar (P≥0.08). Recall that eyes treated at 5 h were recovered at 48 h postinfection, while eyes treated at 6 h were recovered at 36 h postinfection. These results further corroborate that either type of treatment after 4 h postinfection leads to significant inflammation in this model.
Figure 3.
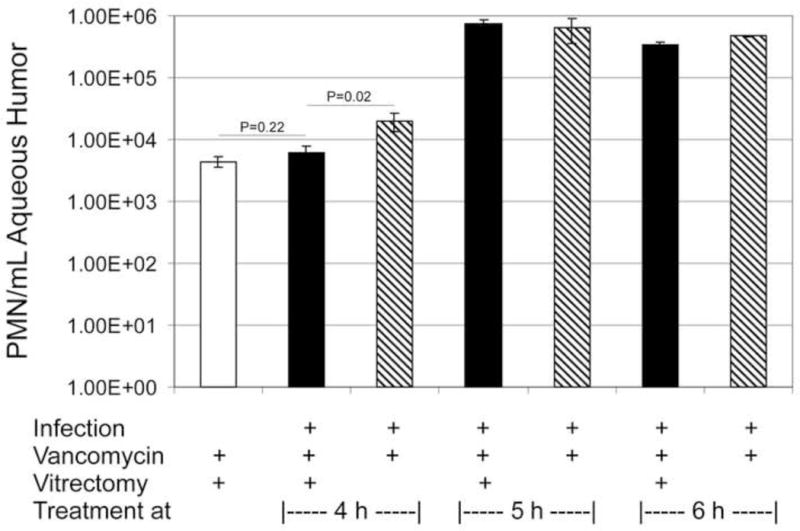
PMN infiltration into the anterior segment following treatment of experimental B. cereus endophthalmitis with vitrectomy and vancomycin. PMN were quantified from aqueous humor samples at 48 h postinfection for eyes treated and 4 and 5 h or at 36 h postinfection for eyes treated at 6 h postinfection. The values represent the mean ± SEM of N≥3 eyes per group.
Intraocular Antibiotic Levels
Significant bactericidal levels must be achieved in an infected eye following antibiotic treatment to prevent the infection from progressing. In vitrectomy-treated infected eyes, intraocular contents were removed and replaced with balanced salt solution containing vancomycin, and vancomycin levels were later quantified (Figure 4). No vancomycin was detected in the aqueous humor at 48 h postinfection regardless of the time of treatment or whether vitrectomy was involved in the regimen. Vancomycin was detected in the vitreous of infected eyes treated at 4 h or 5 h postinfection, but not in the vitreous of infected eyes treated at 6 h postinfection. Although there was a trend toward decreased vancomycin levels in eyes treated at 5 h postinfection, these values were not significantly different from those following treatment at 4 h postinfection (P≥0.08). Vancomycin levels were also similar at these time points regardless of whether vitrectomy was involved in the regimen (P≥0.3). When vancomycin was detected, these concentrations were well above the MIC for this particular B. cereus strain.
Figure 4.
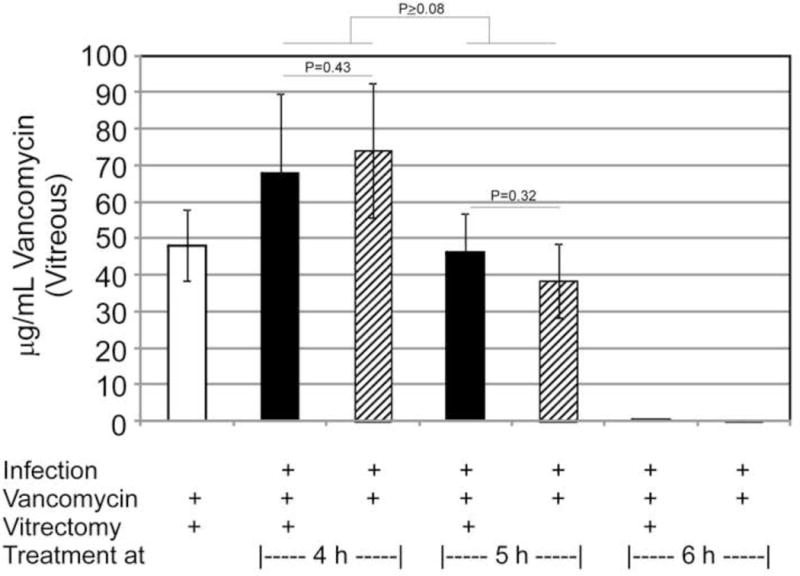
Ocular vancomycin concentrations following treatment of experimental B. cereus endophthalmitis with vitrectomy and vancomycin. Aqueous and vitreous were harvested at 48 h postinfection for eyes treated and 4 and 5 h or at 36 h postinfection for eyes treated at 6 h postinfection. Vancomycin concentrations were quantified using a standard bioassay. The values represent the mean ± SEM of N≥3 eyes per group. Vancomycin was not detected in any aqueous samples, so only vitreous results are shown.
DISCUSSION
Vitrectomy is designed to remove potentially harmful contents and pathogens from the inside of the eye in an effort to minimize inflammation and salvage vision during many types of ocular infections. This study demonstrates the efficacy of vitrectomy and vancomycin over that of vancomycin alone for the salvaging vision and limiting inflammation in experimental B. cereus endophthalmitis. However, vitrectomy and vancomycin were more effective than vancomycin alone only when treatment was initiated prior to 4 h postinfection. After that time, all treatments were relatively ineffective, resulting in significant inflammation and vision loss.
In this model, 4 h postinfection is the critical time within which intravitreal treatment must be initiated to salvage useful vision. B. cereus endophthalmitis is unique in its rapid course and invariably devastating outcome. The experimental rabbit model used herein reproducibly mimicks that course. B. cereus synthesizes multiple toxins in the eye during experimental infection (23), and we have demonstrated that toxins are involved in vision loss in this disease (24, 25). Which toxins are involved, the kinetics of their synthesis, and the specific activities on the retina are all open questions. However, considering the in vitro and in vivo findings of the importance of quorum sensing to B. cereus toxin production and virulence (25, 29–32), one can envision a scenario in which B. cereus reaches a threshold quorum in the eye between 2 and 4 h postinfection, begins to synthesize toxins, the retina is affected, and vision loss occurs. Once treatment is begun, the eye is sterilized, toxins are no longer produced, and the retinal damage and vision loss is limited to that already done.
In uninfected control eyes, vitrectomy with vancomycin caused inflammation and retinal function loss. Vitrectomy has been associated with blood-retinal barrier breakdown and other physiological changes (33–35). Inoue et al. (13) recently reported that vitreal protein concentrations decreased from 1 to 7 days following 20-, 23-, or 25-gauge vitrectomy, indicating that surgically-induced inflammation began to resolve within a week in this model. Wallenten et al. (36) demonstrated significant reductions in retinal function that were detected up to 28 days post-vitrectomy. In that study, upregulation of retinal GFAP, an indicator of vitrectomy-related retinal trauma, was also detected in vitrectomy-treated rabbit eyes. The majority of recent studies on retinal function following vitrectomy have been short-term toxicity or clearance experiments (37–39). Intravitreal injection of PBS, bacterial media, or vancomycin alone can cause a transient posterior segment inflammation that, in the rabbit eye, resolves within 4 h (data not shown). Therefore, any changes observed in uninfected eyes treated with vitrectomy and vancomycin are likely attributed to vitrectomy alone.
In comparing our results in this study with that of our previous therapeutic studies, we noted that retinal function loss was greater, eyes appeared to be clinically worse, and antibiotic concentrations were less in comparable treatment groups. The difference between this study and the previous two therapeutic studies (14, 15) was the animal. All three studies used New Zealand White rabbits of similar age. However, the present study used specific pathogen-free rabbits, while the previous studies used rabbits raised in conventional conditions. It is therefore possible that the difference in pathogenicity and clinical outcome seen in this study lie in the overall immune status of the rabbit. An animal raised in a pathogen-free environment may be more susceptible to infections with organisms that have not previously been encountered and may not be able to quickly mount the degree of immune response needed to fight the infection (40). This may account for the elevated severity of infection at an earlier time point in the present study compared to our previous studies. Although our results draw clear comparisons among efficacies of the treatment regimens tested, careful interpretation of potential model-to-model variations must be considered when comparing the effectiveness of a particular experimental regimen. Nevertheless, these results reinforces the critical necessity for early antibiotic treatment of B. cereus endophthalmitis and indicates that timely vitrectomy in addition to antibiotics may result in a better therapeutic outcome than the use of antibiotics alone.
Acknowledgments
The authors would like to acknowledge Mark Dittmar (Dean A. McGee Eye Institute, Oklahoma City OK) for his assistance in animal husbandry and experiments and Paula Pierce (Excalibur Pathology, Moore OK) for histology expertise.
This study was funded by the Department of Defense Congressionally Directed Medical Research Program (W81XWH-07-1-0280 to MCC). Our research is also supported in part by NIH Grants P30EY12191 (NIH CORE grant to Robert E. Anderson, OUHSC), Research Resources Grant P20RR17703 (NCRR COBRE grant to Robert E. Anderson, OUHSC), and an unrestricted grant to the Dean A. McGee Eye Institute from Research to Prevent Blindness.
Footnotes
The authors have no proprietary interest in any of the commercial products used in these studies.
LITERATURE CITED
- 1.Callegan MC, Gilmore MS, Gregory M, Ramadan RT, Wiskur BJ, Moyer AL, Hunt JJ, Novosad BD. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interactions. Prog Retin Eye Res. 2007;26:189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory M, Callegan MC, Gilmore MS. Role of bacterial and host factors in infectious endophthalmitis. Chem Immunol Allergy. 2007;92:266–275. doi: 10.1159/000099277. [DOI] [PubMed] [Google Scholar]
- 3.Meredith TA. Posttraumatic endophthalmitis. Arch Ophthalmol. 1999;117:520–521. doi: 10.1001/archopht.117.4.520. [DOI] [PubMed] [Google Scholar]
- 4.Thompson ST, Parver LM, Enger CL, Meiler WF, Ligget PE. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. Ophthalmology. 1993;100:1468–1474. doi: 10.1016/s0161-6420(93)31454-5. [DOI] [PubMed] [Google Scholar]
- 5.Davey RJ, Tauber WB. Posttraumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev Infect Dis. 1987;9:110–123. doi: 10.1093/clinids/9.1.110. [DOI] [PubMed] [Google Scholar]
- 6.David DB, Kirkby GR, Noble BA. Bacillus cereus endophthalmitis. Br J Ophthalmol. 1994;78:577–580. doi: 10.1136/bjo.78.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Day DM, Smith RS, Gregg CR, Turnbull PC, Head WS, Ives JA, Ho PC. The problem of Bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/s0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- 8.Callegan MC, Cochran DC, Kane ST, Ramadan RT, Chodosh J, McLean C, Stroman DW. Virulence factor profiles and antimicrobial susceptibilities of ocular Bacillus isolates. Curr Eye Res. 2006;31:693–702. doi: 10.1080/02713680600850963. [DOI] [PubMed] [Google Scholar]
- 9.Chhabra S, Kunimoto DY, Kazi L, Regillo CD, Ho AC, Belmont J, Maguire J, Vander J, Brown GC. Endophthalmitis after open globe injury: microbiologic spectrum and susceptibilities of isolates. Am J Ophthalmol. 2007;142:852–854. doi: 10.1016/j.ajo.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Miller JJ, Scott IU, Flynn HW, Jr, Smiddy WE, Murray TG, Berrocal A, Miller D. Endophthalmitis caused by Bacillus species. Am J Ophthalmol. 2008;145:883–888. doi: 10.1016/j.ajo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Fujii GY, De Juan E, Jr, Humayun MS, Chang TS, Pieramici DJ, Barnes A, Kent D. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology. 2002;109:1814–1820. doi: 10.1016/s0161-6420(02)01119-3. [DOI] [PubMed] [Google Scholar]
- 12.Fine HF, Iranmanesh R, Iturralde D, Spaide RF. Outcomes of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment disease. Ophthalmology. 2007;114:1197–1200. doi: 10.1016/j.ophtha.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Inoue Y, Kadonosono K, Yamakawa T, Uchio E, Watanabe Y, Yanagi Y, Tamaki Y, Araie M. Surgically-induced inflammation with 20-, 23-, and 25-gauge vitrectomy systems: an experimental study. Retina. 2009;29:477–480. doi: 10.1097/IAE.0b013e31819a6004. [DOI] [PubMed] [Google Scholar]
- 14.Wiskur BJ, Robinson ML, Farrand AJ, Novosad BD, Callegan MC. Toward improving therapeutic regimens for Bacillus endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49:1480–1487. doi: 10.1167/iovs.07-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiskur BJ, Woods DC, Wheatley NR, Callegan MC. Updates on improvements for therapy of severe bacterial endophthalmitis. Proc Int Soc Ophth Pharmacol Ther. 2010 In press. [Google Scholar]
- 16.Aguilar HE, Meredith TA, Drews C, Grossniklaus H, Sawant AD, Gardner S. Comparative treatment of experimental Staphylococcus aureus endophthalmitis. Am J Ophthalmol. 1996;121:310–317. doi: 10.1016/s0002-9394(14)70280-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Kwok AK, Cheung BM. The efficacy of intravitreal vancomycin and dexamethasone in the treatment of experimental Bacillus cereus endophthalmitis. Curr Eye Res. 2008;33:761–768. doi: 10.1080/02713680802344690. [DOI] [PubMed] [Google Scholar]
- 18.Liu SM, Way T, Rodrigues M, Steidl SM. Effects of intravitreal corticosteroids in the treatment of Bacillus cereus endophthalmitis. Arch Ophthalmol. 2000;118:803–806. doi: 10.1001/archopht.118.6.803. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Kwok AK, Cheung BM. The efficacy of intravitreal vancomycin and dexamethasone in the treatment of experimental bacillus cereus endophthalmitis. Curr Eye Res. 2008;33:761–768. doi: 10.1080/02713680802344690. [DOI] [PubMed] [Google Scholar]
- 20.Cheng CK, Yang CH, Hsueh PR, Liu CM, Lu HY. Vitrectomy with fluconazole infusion: retinal toxicity, pharmacokinetics, and efficacy in the treatment of experimental candidal endophthalmitis. J Ocul Pharmacol Ther. 2004;20:430–438. doi: 10.1089/jop.2004.20.430. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Dong XG, Liu AM, Sun SY, Xie LX, Wang SG. A pharmacodynamics study of an intravitreal amphotericin B drug delivery system for the treatment of experimental Aspergillus fumigatus endophthalmitis. Zhonghua Yan Ke Za Zhi. 2007;43:546–553. [PubMed] [Google Scholar]
- 22.Koçak N, Kaynak S, Kirdar S, Irmak O, Bahar IH. Comparison of different antifungal treatment regimens for experimental Candida endophthalmitis in rabbit models. Mikrobiyol Bul. 2009;43:619–626. [PubMed] [Google Scholar]
- 23.Pollack JS, Beecher DJ, Pulido JS, Lee Wong AC. Failure of intravitreal dexamethasone to diminish inflammation or retinal toxicity in an experimental model of Bacillus cereus endophthalmitis. Curr Eye Res. 2004;29:253–259. doi: 10.1080/02713680490516701. [DOI] [PubMed] [Google Scholar]
- 24.Callegan MC, Kane ST, Cochran DC, Novosad B, Gilmore MS, Gominet M, Lereclus D. Bacillus endophthalmitis: roles of bacterial toxins and motility during infection. Invest Ophthalmol Vis Sci. 2005;46:3233–3238. doi: 10.1167/iovs.05-0410. [DOI] [PubMed] [Google Scholar]
- 25.Callegan MC, Kane ST, Cochran DC, Gilmore MS, Gominet M, Lereclus D. Relationship of plcR-regulated factors to Bacillus endophthalmitis virulence. Infect Immun. 2003;71:3116–3124. doi: 10.1128/IAI.71.6.3116-3124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callegan MC, Jett BD, Hancock LE, Gilmore MS. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect Immun. 1999;67:3357–3366. doi: 10.1128/iai.67.7.3357-3366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callegan MC, Cochrane DC, Kane ST, Gilmore MS, Gominet M, Lereclus D. Contribution of membrane-damaging toxins to Bacillus endophthalmitis pathogenesis. Infect Immun. 2002;70:5381–5389. doi: 10.1128/IAI.70.10.5381-5389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31:955–965. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 29.Declerck N, Bouillaut L, Chaix D, Rugani N, Slamti L, Hoh F, Lereclus D, Arold ST. Structure of PlcR: Insights into virulence regulation and evolution of quorum sensing in Gram-positive bacteria. Proc Natl Acad Sci U S A. 2007;104:18490–18495. doi: 10.1073/pnas.0704501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gohar M, Faegri K, Perchat S, Ravnum S, Økstad OA, Gominet M, Kolstø AB, Lereclus D. The PlcR virulence regulon of Bacillus cereus. PLoS One. 2008;3:e2793. doi: 10.1371/journal.pone.0002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsueh YH, Somers EB, Lereclus D, Wong AC. Biofilm formation by Bacillus cereus is influenced by PlcR, a pleiotropic regulator. Appl Environ Microbiol. 2006;72:5089–5092. doi: 10.1128/AEM.00573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramarao N, Lereclus D. Adhesion and cytotoxicity of Bacillus cereus and Bacillus thuringiensis to epithelial cells are FlhA and PlcR dependent, respectively. Microbes Infect. 2006;8:1483–1491. doi: 10.1016/j.micinf.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Garner WH, Scheib S, Berkowitz BA, Suzuki M, Wilson CA, Graff G. The effect of partial vitrectomy on blood-ocular barrier function in the rabbit. Curr Eye Res. 2001;23:372–381. doi: 10.1076/ceyr.23.5.372.5439. [DOI] [PubMed] [Google Scholar]
- 34.Stefánsson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247:147–163. doi: 10.1007/s00417-008-0980-7. [DOI] [PubMed] [Google Scholar]
- 35.Sparrow JR, Chang S, Vinals AF. Evaluation of the blood-aqueous barrier after vitreous replacement with perfluoropropane gas and liquid silicone. Retina. 1992;12:370–375. doi: 10.1097/00006982-199212040-00015. [DOI] [PubMed] [Google Scholar]
- 36.Wallentén KG, Andréasson S, Ghosh F. Retinal function after vitrectomy. Retina. 2008;28:558–563. doi: 10.1097/IAE.0b013e31815e9890. [DOI] [PubMed] [Google Scholar]
- 37.Lee SS, Ghosn C, Yu Z, Zacharias L, Kao H, Lanni C, Abdelfattah N, Kuppermann B, Csaky KG, D’Argenio DZ, Burke JA, Hughes P, Robinson MR. Vitreous VEGF Clearance is Increased following Vitrectomy. Invest Ophthalmol Vis Sci. 2009 Dec 17; doi: 10.1167/iovs.09-3582. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Shimoda Y, Hamano R, Ishihara K, Shimoda N, Hagimura N, Akiyama H, Kishi S, Kaneko A. Effects of intraocular irrigation with melphalan on rabbit retinas during vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2008;246:501–508. doi: 10.1007/s00417-007-0685-3. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita T, Sakamoto T, Yamakiri K, Miura M, Enaida H, Ueno A, Atsumi I, Matsuhisa K, Sakamoto Y, Kida T, Ishibashi T. Polylactic acid for visualizing the vitreous body during vitrectomy. Invest Ophthalmol Vis Sci. 2007;48:3277–3282. doi: 10.1167/iovs.06-1020. [DOI] [PubMed] [Google Scholar]
- 40.Gordon HA, Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971;35:390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


