Abstract
Introduction
Venous thromboembolism (VTE) has been identified as a major public health issue. Postbariatric body contouring surgery represents a major challenge for VTE prophylaxis due to the presence of multiple risk factors and broad areas of dissection that potentially increase the risk of postoperative bleeding.
Aim
To define current VTE prophylaxis practices among surgeons of the American Society of Plastic Surgeons, performing postbariatric body contouring surgery in the United States.
Material and Methods
A total of 4081 surveys were sent to registered members of the American Society of Plastic Surgeons by e-mail. We received 596 (14.6%) responses.
Results
A total of 596 surgeons returned completed surveys, with 83% of respondents in private practice and 17% in academic practice. Deep venous thrombosis (DVT) was reported by 40% surgeons, pulmonary embolism (PE) by 34%, and 7% had at least 1 patient having died of a postoperative PE. About 39% to 48% participant surgeons reported providing no chemoprophylaxis to their postbariatric body contouring patients. The most common reason for not using routine prophylaxis was the concern for bleeding (84%), followed by lack of evidence specific to plastic surgery practice (50%). Academic surgeons were more likely to provide chemoprophylaxis when compared with those in nonacademic practice (P < 0.05).
Conclusion
For postbariatric body contouring surgery, DVT has occurred in over one-third of plastic surgeons’ practices with 7% of surgeons reporting a patient death from PE. A substantial proportion of surgeons performing postbariatric body contouring are not using chemoprophylaxis due to bleeding risk and perceived lack of evidence. VTE prophylaxis in postbariatric body contouring remains a topic that deserves further study.
Keywords: VTE, DVT, PE, body contouring, postbariatric, national survey, ASPS
The current changes by the Centers for Medicare and Medicaid Services’ (CMS) reimbursement policies have underscored the importance of “never events,” complications deemed potentially preventable for which CMS will not pay if acquired in-hospital.1 The concept's aim is to provide an additional, financial incentive for physicians and hospitals to improve patient care and safety. Venous thromboembolism (VTE) has been cited as “reasonably preventable” and is actively being considered for addition to this list of “never events.”1 The United States Surgeon General recently published a “Call to Action” for deep venous thrombosis (DVT) and pulmonary embolism (PE) prevention. This document emphasized the importance of physician and public education on the scope and severity of the VTE problem, as well as promoting ongoing research to develop evidence-based guidelines for VTE prevention.2
The American College of Chest Physicians (ACCP) recently published their updated guidelines for VTE prophylaxis in medical and surgical patients. Though exhaustively researched and comprehensive, not 1 of the 728 references in the document's references was taken from a plastic surgery journal. This is secondary to the lack of high-level evidence evaluating VTE prophylaxis in plastic surgery patients.3 The plastic surgery literature currently lacks high-level evidence for appropriate means of VTE prophylaxis specific to our patient population, though several large retrospective case series and cohort studies have recently been published.4 – 8 Due to the presence of multiple risk factors, including age, obesity, prolonged operative time, multiple site surgery, and relative inability to ambulate after surgery, one of plastic surgery's highest risk patient populations for VTE are those undergoing postbariatric body contouring surgery. Although a descriptive paper on VTE incidence and prevention in postbariatric body contouring patients has recently been published,6 the current practice patterns for prophylaxis among the American Society of Plastic Surgeons (ASPS) members remains unknown.
We investigated current practice patterns for VTE prophylaxis among members of the ASPS, using a web-based survey. We focused on postbariatric body contouring surgeries (specifically circumferential lower body lift, abdominoplasty, or panniculectomy and combined procedures) due to the increasing number of these cases performed in the last 5 years9–11 and to the high risk of VTE in this patient population.
MATERIALS AND METHODS
Approval for the study was obtained from the University of Pittsburgh Institutional Review Board.
An online survey (see Supplemental Digital Content, http://links.lww.com/SAP/A7) was created using a commercial internet survey tool that was specific to patients undergoing body contouring after massive weight loss. The survey was designed to query 4 areas of clinical practice: respondent surgeon's personal demographic information, surgeon knowledge of VTE risk factors, current utilization of pharmacological and mechanical methods for VTE prophylaxis, and surgeon experience with VTE in their patients.
These risk factors were based on the recommendations published in 2004 by Davidson et al.12 They assigned unit values from 1 to 5 to the various risk factors. The higher the risk factor value, the higher the risk for VTE. Finally, they classified the risk of VTE from low to high, based on the additional risk factor values that a patient may accrue (Table 1).
TABLE 1.
Level of Risk Assignment Based on the Cumulative Number of Risk Factor Units (Georgetown Risk Assessment Model)12
| Total Risk Factor Units | Level of Risk for VTE |
|---|---|
| 1 factor | Low risk |
| 2 factors | Moderate |
| 3-4 factors | High |
| Greater than 4 | Highest |
VTE indicates venous thromboembolism.
The survey was accessible only one time from a distinct computer terminal, thus preventing the same surgeon from completing the survey on more than one occasion.
A link to the secure, web-based survey was distributed to active ASPS members with published e-mail addresses in November 2008. Four reminders at 2 to 3 week intervals were sent over the course of 10 weeks prior to survey closure. Any survey returned with incomplete data was excluded from final data analysis.
Data were stored and analyzed using the Microsoft Excel statistical package. Reported practice patterns were compared with published, evidence-based guidelines for VTE prophylaxis. Descriptive statistics were produced, and comparisons among categorical data were performed using χ2 analysis. Significance was established prior to the study's beginning as P < 0.05.
RESULTS
Demographics
We identified a total of 4455 active members with email addresses published in the ASPS database. From the submitted e-mail messages, 296 accounts returned messages as nondelivered, 20 participants were not able to open the link, and 58 returned an auto-response. A total of 374 e-mails were returned or undeliverable making the number of surveys sent 4081. We obtained a total of 596 responses for a response rate of 14.6%. About 493 (83%) of respondents identified themselves as in nonacademic practice and 103 (17%) were in academic practice.
VTE Experience
Forty percent of respondents reported a postoperative DVT and 34% reported a postoperative PE in at least 1 patient during their careers. Seven percent of respondents have had at least 1 patient die of pulmonary embolus after postbariatric body contouring surgery. No significant differences were found when comparing the reported experience with DVT or PE among academic or private practice surgeons.
Knowledge of VTE Risk Factors
Surgeons were presented with a list of common VTE risk factors, as determined by the Davidson et al risk assessment model.12 They were then asked “Which of the following risk factors—when considered alone—would persuade you to use pharmacologic (eg, heparin, Lovenox) DVT prophylaxis?” Table 1 compares surgeon's reported utilization of chemoprophylaxis with the Davidson et al recommended prophylaxis. When present in isolation, the most frequent risk factors triggering the use of chemoprophylaxis were (in descending order): prior personal history of VTE (90%), obesity (52%), lower extremity fractures (50%), malignancy (47%), and procedures greater than 4 hours (46%). Table 2 shows the distribution of factor units per risk factor for VTE (as proposed by the Davidson group).13 Contrary to our expectations, the risk factors with the highest risk factor units for VTE, have a low grade of concern to out responders for the use of chemoprophylaxis.
TABLE 2.
Percentage of Surgeons (N = 596) Who Would Provide Chemoprophylaxis in Response to Isolated Risk Factors
| Risk Factors and Numeric Score From Davison 2004 | Percentage of Surgeons Providing Chemoprophylaxis |
|---|---|
| 1 factor | |
| Age >40 | 15 |
| Surgical time >45 min | 11 |
| Obesity | 52 |
| Estrogen/OCP use | 35 |
| Pregnancy | 19 |
| 2 factors | |
| Surgical time >4h | 46 |
| Malignancy | 47 |
| Age >60 | 19 |
| 3 factors | |
| Personal DVT or PE | 90 |
| Family history DVT or PE | 41 |
| 5 factors | |
| Recent spinal cord injury | 34 |
| Recent multi-system trauma | 43 |
| Lower extremity fracture | 50 |
| Recent stroke | 23 |
The risk factors were subdivided on the risk factor units that Davidson et al assigned. The higher the risk factor unit, the higher the risk to favor VTE.
OCP indicates oral contraceptive pills; DVT, deep venous thrombosis; PE, pulmonary embolism.
Pharmacological and Mechanical VTE Prophylaxis
Seventy-two percent of the respondents reported utilization of existing guidelines as aids in making VTE prophylaxis decisions. Among those surgeons using guidelines, 44% reported using guidelines provided to them by their institutions, 29% use the Davidson et al risk assessment model,12 23% use the 2008 American College of Chest Physicians guidelines (ACCP),3 and 4% use the Caprini Risk Assessment Model.14
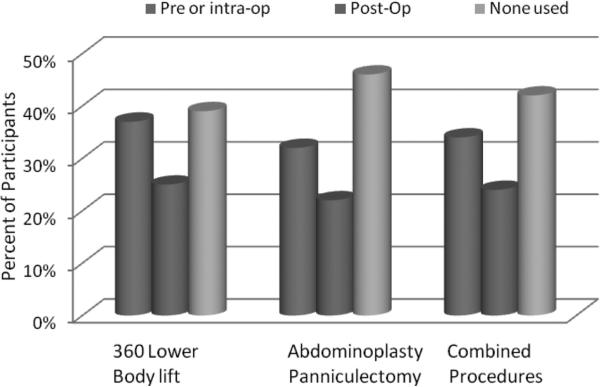
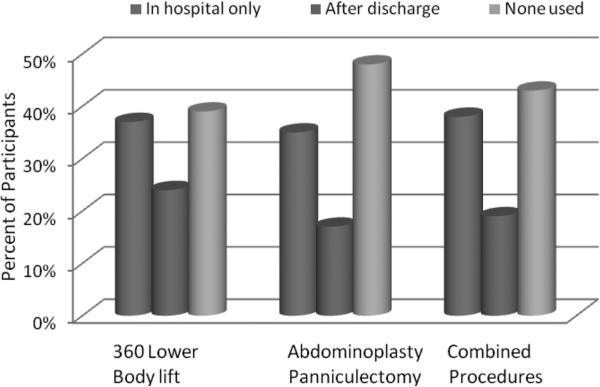
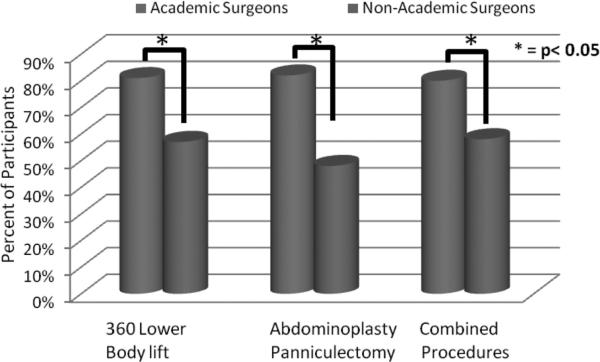
We queried surgeons regarding practice patterns for chemoprophylaxis utilization for 3 distinct postbariatric body contouring procedures: circumferential trunk lift, abdominoplasty/panniculectomy, and combined body contouring operations. Specific inquiry was made regarding when chemoprophylaxis was initiated (pre/intraoperatively, postoperatively, or none used) and the duration of chemoprophylaxis continuation (in hospital only, after discharged from hospital, or none used). Between 32% and 37% of surgeons reported providing initial chemoprophylaxis in the preoperative holding area or intraoperatively (Fig. 1, blue columns), as compared with 22% to 25% who initially provided chemoprophylaxis postoperatively (Fig. 1, red columns). About 35% to 38% of respondents maintain chemoprophylaxis during the hospital stay (Fig. 2, blue columns). A smaller proportion of surgeons (17%–24%) reported providing patients with chemoprophylaxis after discharge (Fig. 2, red columns). A large proportion of those responding (39%–48%) reported providing no chemoprophylaxis to their postbariatric body contouring patients according to the type of surgical procedure performed (360 degrees trunk lift: 39%, abdominoplasty or panniculectomy: 48%, combined procedures: 43%) (Fig. 2, green columns). Academic surgeons were significantly more likely to provide chemoprophylaxis when compared with surgeons in nonacademic practice for all 3 surgery types (P < 0.05) (Fig. 3).
FIGURE 1.
Timing of chemoprophylaxis initiation for post-bariatric body contouring (N = 596).
FIGURE 2.
Duration of chemoprophylaxis continuation for postbariatric body contouring (N = 596).
FIGURE 3.
Chemoprophylaxis utilization between academic and nonacademic surgeons in postbariatric body contouring surgery (N = 596). All differences between academic and nonacademic surgeons are significant (P < 0.05).
Among the subgroup of surgeons not providing chemoprophylaxis to their postbariatric body contouring patients, risk of reoperative hematoma (84%) and lack of evidence specific to plastic surgery (50%) were most commonly cited. Cost (6%) and patient discomfort (4%) were less likely to contribute. Two percent of respondents reported routine VTE screening of asymptomatic patients with lower extremity venous duplex, d-dimer or PE protocol computed tomography prior to discharge.
DISCUSSION
VTE prophylaxis remains a challenging topic in plastic surgery. Postbariatric patients represent a cohort with increased risk factors for VTE in addition to increased risks for complications secondary to the use of VTE prophylactic medications. The purpose of our survey was to evaluate the current knowledge, current approach, and complications related to this topic by members of the ASPS. After 4 reminders via e-mail, we were able to obtain over 500 responses to our survey focused on bariatric patients. Unfortunately our response rate was only 14%, which is similar to other large, recently published surveys in the plastic surgery literature.15–17 This low response rate may introduce a selection bias to our study. Perhaps only those physicians particularly interested in this topic answered the survey, the deviation from accepted VTE prophylaxis protocols may have be even larger than we have estimated. Another limitation of the study is the influence by recall bias. However, it is highly likely that the recollection of unfavorable complications such as DVT, PE and mortality secondary to these complications are well recall by the responders.
Massive weight loss patients undergoing body contouring surgery are at increased risk for VTE due to elevated BMI, presence of pulmonary comorbidities, extended operative time, multiple-site surgery, and decreased ability to ambulate postoperatively.18 BMI over 35,6,19 concomitant liposuction6 and resection weight over 1500 g6,19 may be independent risk factors for VTE in body contouring surgery. Additionally, the majority of body contouring surgery is performed under general anesthesia,15 a known risk factor for VTE in elective reconstructive and cosmetic surgery.20,21 Despite these risk factors, 40% of surgeons performing abdominoplasty with liposuction do not use VTE prophylaxis, based on 2007 survey results.16 By comparison, 48% of surgeons responding to our survey do not administer chemoprophylaxis for patients undergoing abdominoplasty or panniculectomy.
Large series of body contouring patients support VTE incidence of 0.58% to 5.5% for abdominoplasty combined with concomitant procedures.6,16,22,23 Those undergoing circumferential abdominoplasty have significantly elevated DVT incidence (7.7%) when compared with any other body contouring procedures, though the risk of PE does not appear to be increased.6 Patients undergoing breast and upper body contouring procedures have a 2.9% incidence of VTE.6
Perioperative heparin, in addition to nonoperative measures, is recommended for those massive weight loss patients undergoing body contouring procedures with recognizable risk factors.6,24,25 Small case series of patients undergoing abdominoplasty with concomitant procedures supports a 2.5% to 2.9% PE incidence with administration of perioperative heparin prophylaxis.23,26 Perioperative LMWH administration has been associated with a significantly decreased DVT incidence in circumferential abdominoplasty patients.6 Body contouring patients receiving perioperative LMWH until discharge are known to have overall decreased VTE incidence when compared with patients not receiving pharmacologic prophylaxis (4.38% vs. 5.88%), though the difference did not reach statistical significance.6 Although data supporting significant risk reduction for VTE in circumferential abdominoplasty patients receiving LMWH prophylaxis were published in Plastic and Reconstructive Surgery6 4 months prior to our survey, 39% of surgeons responding to our survey currently use no chemoprophylaxis for this surgery; 50% of these surgeons cite lack of evidence as a contributing factor.
The Davidson et al Risk Assessment Model12 has been retrospectively validated in a large series of body contouring patients; the algorithm placed 89% of patients with perioperative VTE in the highest risk category. Perioperative risk stratification would be improved with the addition of 3 additional factors, namely hormone replacement therapy, body mass index greater than 30, and undergoing circumferential abdominoplasty, as independent risk factors for VTE.6
Seventy-two percent of respondents to our survey report utilization of guidelines when making VTE prophylaxis decisions. However, those reporting guideline adherence have been shown to provide inadequate levels of prophylaxis. A recent survey on practice patterns for VTE prophylaxis in autogenous breast reconstruction showed that among a subgroup of surgeons reporting strict adherence to ACCP guidelines, only 38% actually provided the ACCP's minimum acceptable level of prophylaxis.17
In a cohort of highest risk plastic surgery patients, defined by a Caprini score14 greater than 4, the overall rate of VTE was 7.5%. Administration of chemoprophylaxis was shown to significantly decrease VTE rate (1.7% vs. 14.6%) without significantly altering the rate of reoperative hematoma. Compliance with postdischarge low molecular weight heparin chemoprophylaxis in a small subgroup was 93% (13/14).8 Data on efficacy of postdischarge chemoprophylaxis is not available for plastic surgery patients. However, large series of patients undergoing major operations for abdominal and pelvic cancers have demonstrated significant VTE risk reduction with extended (7 vs. 28 day) courses of chemoprophylaxis.27
Our study demonstrates variability in surgeon's ability to identify common VTE risk factors. Additionally, these results reveal the heterogeneity and lack of consensus in our specialty regarding appropriate VTE prophylaxis. To address these issues, the ASPS could create data-driven CME modules to provide surgeon education on VTE risk factors, diagnosis, and treatment in postbariatric body contouring patients. Additionally, recognition of VTE risk factors would be improved if surgeons routinely used existing VTE risk stratification modules such as those published by Caprini14 or Davison et al.12
The 2 major barriers to chemoprophylaxis administration appear to be the perceived increased risk of reoperative hematoma and lack of evidence in plastic surgery patients, cited by 84% and 50% of those not providing chemoprophylaxis, respectively. In a large series of highest risk plastic surgery patients (n = 173 surgeries in 120 patients) with Caprini score greater than 4, receipt of chemoprophylaxis with unfractionated heparin, LMWH or Aspirin did not significantly increase hematoma rate compared with patients undergoing mechanical prophylaxis only (8.9% vs. 6.3%; P not significant), though the authors did not specify rate of reoperative hematoma.8 Conversely, a series of 347 postbariatric body contouring patients published from UT Southwestern6 showed significantly increased hematoma rate in patients chemoprophylaxed with enoxaparin, compared with patients undergoing only mechanical prophylaxis (7.3% vs. 0.5%). Although the rate of reoperative hematoma was not specified, there was an increased transfusion requirement associated with low molecular weight heparin administration compared with the mechanically prophylaxed group (6.6% vs. 0.9%). The data on reoperative hematoma risk remains unclear and this represents both a major barrier to surgeon administration of chemoprophylaxis and an important direction for future research.
The Davidson et al risk assessment model recommends that LMWH prophylaxis be provided when a total additive score of 5 factors is reached. More recently, the same group published guidelines for VTE prophylaxis in plastic surgery patients. These recommendations were based on the latest ACCP guidelines. Unfortunately, this is an extrapolation of other patient populations different than plastic surgery. In their new guidelines, they recommended the use of intermittent compression devices (IPC) in all patients undergoing surgery greater than 1 hour. They proposed the use of chemoprophylaxis with enoxaparin 30 mg BID or 40 mg Q day within the first 12 hours post operative, in patients with cumulative risk factors greater than 3. Finally, patients with high risk of bleeding and high risk for VTE, should be managed with nonpharmacological methods for VTE prophylaxis.13
Survey Limitations
Our survey was only distributed to ASPS members with published email addresses and thus, by proxy, to those with access to the internet. As only board certified surgeons were included, our data do not reflect practice patterns in nonplastic surgeons performing postbariatric body contouring or board eligible plastic surgeons who are not yet board certified. Recent ASPS data demonstrates that 12.5% of the ASPS membership practices in an academic setting (K. Hume, personnel communication, 2009). Compared with this percentage, academic surgeons were slightly overrepresented in our data set, comprising 17% of the respondent pool. Additionally, recall bias, in which surgeons who had negative experiences with VTE would be more likely to share their experience and complete the survey, may have altered the reported rates of VTE.
The response rate to our survey of 14.6% is low, but comparable with other survey-based studies of ASPS members recently published.15–17 When designing our survey, low response rate was recognized as a significant limitation; to compensate, our survey was initially sent to over 4000 ASPS members. Our data set, reflective of practice patterns for 596 plastic surgeons, represents the opinion of nearly 2 times the number of surgeons as other recently published surveys with similar response rates.16 Possible solutions to the low response rate could be addressed by providing CME credits by the ASPS to those participants in key topics for the development of our specialty.
CONCLUSION
Among 596 plastic surgeons responding to our survey, greater than one-third report a postbariatric body contouring patient with a venous thromboembolic event after surgery; 7% of surgeons report a patient death from postoperative pulmonary embolus. Nearly half of surgeons currently use no chemoprophylaxis when performing major body contouring procedures. The majority cite risk of reoperative hematoma and lack of evidence specific to plastic surgery patients as reasons. As additional large, preferably prospective, studies are published, we hope that data-driven guidelines for VTE prophylaxis specific to massive weight loss patients undergoing body contouring surgery will be created.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.annalsplasticsurgery.com).
REFERENCES
- 1.Centers for Medicare and Medicaid Services (CMS) Medicare and Medicaid Move Aggressively to Encourage Greater Patient Safety in Hospitals and Reduce Never Events. Centers for Medicare and Medicaid Services (CMS); Baltimore, MD: 2008. [Google Scholar]
- 2.Galson SK. The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. US Department of Health and Human Services; Rockville, MD: 2008. [Google Scholar]
- 3.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(suppl 6):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 4.Pannucci CJ, Chang EY, Wilkins EG. Venous thromboembolic disease in autogenous breast reconstruction. Ann Plast Surg. 2009;63:34–38. doi: 10.1097/SAP.0b013e318188bedf. [DOI] [PubMed] [Google Scholar]
- 5.Liao EC, Taghinia AH, Nguyen LP, et al. Incidence of hematoma complication with heparin venous thrombosis prophylaxis after TRAM flap breast reconstruction. Plast Reconstr Surg. 2008;121:1101–1107. doi: 10.1097/01.prs.0000302454.43201.83. [DOI] [PubMed] [Google Scholar]
- 6.Hatef DA, Kenkel JM, Nguyen MQ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122:269–279. doi: 10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 7.Kim EK, Eom JS, Ahn SH, et al. The efficacy of prophylactic low-molecular-weight heparin to prevent pulmonary thromboembolism in immediate breast reconstruction using the TRAM flap. Plast Reconstr Surg. 2009;123:9–12. doi: 10.1097/PRS.0b013e3181904be7. [DOI] [PubMed] [Google Scholar]
- 8.Seruya M, Venturi ML, Iorio ML, et al. Efficacy and safety of venous thromboembolism prophylaxis in highest risk plastic surgery patients. Plast Reconstr Surg. 2008;122:1701–1708. doi: 10.1097/PRS.0b013e31818dbffd. [DOI] [PubMed] [Google Scholar]
- 9.American Society of Plastic Surgeons 2007 Statistics . American Society of Plastic Surgeons; Arlington Heights, IL: 2007. [Google Scholar]
- 10.2000/2006/2007 National Plastic Surgery Statistics . Cosmetic and Reconstructive Procedure Trends. American Society of Plastic Surgeons (ASPS); Arlington Heights, IL: 2008. [Google Scholar]
- 11.Bariatric Surgery Statistics. American Society for Bariatric Surgery; Gainesville, FL: 2008. [Google Scholar]
- 12.Davison SP, Venturi ML, Attinger CE, et al. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114:43E–51E. doi: 10.1097/01.prs.0000131276.48992.ee. [DOI] [PubMed] [Google Scholar]
- 13.Venturi ML, Davison SP, Caprini JA. Prevention of venous thromboembolism in the plastic surgery patient: current guidelines and recommendations. Aesthet Surg J. 2009;29:421–428. doi: 10.1016/j.asj.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Matarasso A, Swift RW, Rankin M. Abdominoplasty and abdominal contour surgery: a national plastic surgery survey. Plast Reconstr Surg. 2006;117:1797–1808. doi: 10.1097/01.prs.0000209918.55752.f3. [DOI] [PubMed] [Google Scholar]
- 16.Broughton G II, Rios JL, Rohrich RJ, et al. Deep venous thrombosis prophylaxis practice and treatment strategies among plastic surgeons: survey results. Plast Reconstr Surg. 2007;119:157–174. doi: 10.1097/01.prs.0000240810.52392.51. [DOI] [PubMed] [Google Scholar]
- 17.Pannucci CJ, Oppenheimer AJ, Wilkins EG. Practice patterns in venous thromboembolism prophylaxis: a survey of 606 reconstructive breast surgeons. Ann Plast Surg. 2010;64:732–737. doi: 10.1097/SAP.0b013e3181ba57a0. [DOI] [PubMed] [Google Scholar]
- 18.Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38(2 suppl 5):12–19. doi: 10.1016/s0037-1963(01)90094-0. [DOI] [PubMed] [Google Scholar]
- 19.Shermak MA, Chang DC, Heller J. Factors impacting thromboembolism after bariatric body contouring surgery. Plast Reconstr Surg. 2007;119:1590–1596. doi: 10.1097/01.prs.0000256070.37066.7e. discussion 1597–1598. [DOI] [PubMed] [Google Scholar]
- 20.Reinisch JF, Bresnick SD, Walker JW, et al. Deep venous thrombosis and pulmonary embolus after face lift: a study of incidence and prophylaxis. Plast Reconstr Surg. 2001;107:1570–1575. doi: 10.1097/00006534-200105000-00044. discussion 1576–1577. [DOI] [PubMed] [Google Scholar]
- 21.Lehnhardt M, Homann HH, Daigeler A, et al. Major and lethal complications of liposuction: a review of 72 cases in Germany between 1998 and 2002. Plast Reconstr Surg. 2008;121:396e–403e. doi: 10.1097/PRS.0b013e318170817a. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CE., III Reduction of lipoplasty risks and mortality: an ASAPS survey. Aesthet Surg J. 2001;21:120–127. doi: 10.1067/maj.2001.115166. [DOI] [PubMed] [Google Scholar]
- 23.Gravante G, Araco A, Sorge R, et al. Pulmonary embolism after combined abdominoplasty and flank liposuction: a correlation with the amount of fat removed. Ann Plast Surg. 2008;60:604–608. doi: 10.1097/SAP.0b013e3181344470. [DOI] [PubMed] [Google Scholar]
- 24.Young VL, Watson ME. The need for venous thromboembolism (VTE) prophylaxis in plastic surgery. Aesthet Surg J. 2006;26:157–175. doi: 10.1016/j.asj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Rohrich RJ, Rios JL. Venous thromboembolism in cosmetic plastic surgery: maximizing patient safety. Plast Reconstr Surg. 2003;112:871–872. doi: 10.1097/01.PRS.0000067916.54634.43. [DOI] [PubMed] [Google Scholar]
- 26.Shermak MA. Hernia repair and abdominoplasty in gastric bypass patients. Plast Reconstr Surg. 2006;117:1145–1150. doi: 10.1097/01.prs.0000204587.10550.21. discussion 1151–1152. [DOI] [PubMed] [Google Scholar]
- 27.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.