Abstract
There is currently accumulating evidence that endogenous estrogens play a critical role in the development of breast cancer. Estrogens and their metabolites have been studied in both pre- and postmenopausal women with more consistent results shown in the latter population, in part because of large hormonal variations during the menstrual cycle and far fewer studies having been performed in premenopausal women. In this review we describe in detail estrogen metabolism and associated genetic variations, and provide a critical review of the current literature regarding the role of estrogens and their metabolites in breast cancer risk.
Keywords: Breast cancer, Sex hormones, Estrogen, Estrogen metabolites, Postmenopausal women, Premenopausal women
1. Introduction
Breast cancer remains an overwhelming health burden, with an estimated 232,670 new breast cancer cases and 40,000 deaths among women living in the U.S in 2014 [1]. Age is the strongest risk factor for breast cancer. Unlike many cancers that increase beginning at the end of the fifth decade of life, breast cancer begins to rise in the third decade of life, most likely due to the effects of ovarian hormones on breast tissue [2–4]. More than 2/3 of all new cases occur after the age of 55 and women older than 65 have a relative risk greater than 4.0 when compared with those younger than 65.
To date, many additional risk factors for breast cancer have been identified. Some risk factors are non-modifiable, such as age, BRCA1 and BRCA2 gene mutations, family history, reproductive history, and high-dose radiation to the chest. Others are potentially modifiable, such as high endogenous estrogens, hormone therapy, obesity (for postmenopausal breast cancer) and alcohol consumption [2, 3]. There is some controversy regarding whether or not the risk factor of high mammographic density is modifiable [5–9].
Since a number of these known risk factors are related to endogenous estrogen levels, the effect of estrogens on breast carcinogenesis has drawn a great deal of attention in the last two decades, with evidence suggesting that estrogens play a causal role in the etiology of breast cancer [10]. In this review, we will discuss the metabolism of estrogens and will present a detailed analysis of published data evaluating the role of circulating and urinary estrogens and their metabolites in human breast cancer.
2. Estrogen Metabolism
All steroid hormones originate from C27 cholesterol (Figure 1). The main source of cholesterol required for the synthesis of steroid hormones (steroidogenesis) is LDL-cholesterol [11]. Cholesterol is metabolized down a number of enzymatic pathways and is converted to the 21-, 19-, and 18-carbon steroid hormones, respectively.
Figure 1. Pathways of steroid hormone synthesis in humans.
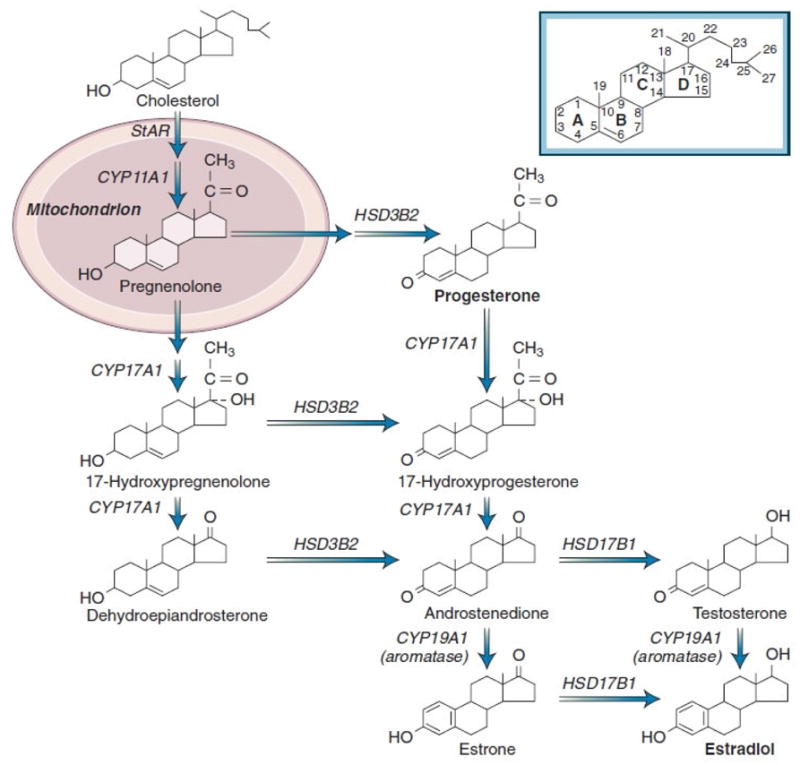
Abbreviations: StAR, steroidogenic acute regulatory protein; CYP11A1, side-chain cleavage of P450; CYP17A1, 17-hydroxylase/17,20-lyase; HSD3B2, 3β-hydroxysteroid dehydrogenase-Δ5,4 isomerase type 2; ; CYP19A1, aromatase; HSD17B1, 17β-hydroxysteroid dehydrogenase type 1 [13].
The first step in ovarian steroidogenesis is the movement of cholesterol into the mitochondrion. This step is regulated by the steroidogenic acute regulatory protein (StAR) encoded by the STAR gene [12]. The next step involves the conversion of cholesterol to preg-nenolone, catalyzed by the mitochondrial side-chain cleavage enzyme complex. Pregnenolone acts as a precursor for all steroid hormones. It is metabolized by different enzymes, and under the action of 17-hyroxylase/17, 20-lyase enzyme, a product of the CYP17A1 gene is converted to progesterone or androstendione. Androstendione, in turn, is further metabolized to other androgens or estrogens.
Estrogens are among very few aromatic molecules in humans. They are all C18 steroids and consist of one benzene ring, a phenolic hydroxyl group at C3, and a hydroxyl group (17β-estradiol) or a ketone group (estrone) at C17 (Figure 1). The main estrogens circulating in the human body are estradiol and estrone, as well as 16-hydroxyestradiol (estriol). Although estriol is usually the major estrogen in pregnant women [13], and is the most abundant estrogen in the urine of all women, estradiol is the most biologically active estrogen, primarily secreted by ovarian granulosa cells located next to theca cells and regulated by follicle-stimulating hormone (FSH). Estrone is reversibly converted to estradiol through the action of 17β-hydroxysteroid dehydrogenase enzyme [14]. Androstenedione, the most important product of the theca cells during the follicular phase of the menstrual cycle, is not biologically active; however, it acts as a precursor for both estrone and testosterone in the ovaries and peripheral tissues. [15]. Testosterone, in turn, is converted to estradiol by the action of aromatase enzyme in the peripheral tissues (Figure 1).
In premenopausal women, estradiol synthesized in the ovaries is the most important estrogen, while in postmenopausal women, estrone synthesized in peripheral tissues is predominant. Aromatase (CYP19), encoded by the CYP19A1 gene, is the rate-limiting enzyme in catalyzing the conversion of androgens to estrogens [16, 17]. Given the importance of this enzyme, blocking aromatase activity is an important pharmacological tool used for the treatment of estrogen-dependent diseases such as breast cancer, endometriosis, and endometrial cancer.
Estradiol and estrone are metabolized by three competitive pathways involving irreversible hydroxylations catalyzed by the NADPH-dependent cytochrome P450 (CYP) enzymes including CYP1A1, CYP1B1, and CYP1A2 (Figure 2). Estrone and estradiol are hydroxylated at positions C2, C4 and C16 and are converted to catechol estrogens (2-hydroxyestrone, 4-hydroxyestrone, 2-hydroxyestradiol, and 4-hydroxyestradiol), and 16α-hydroxyestrone. Estriol is produced by the hydroxylation of estradiol or 16α-hydroxyestrone. Catechol estrogens are further metabolized (methylated) to methoxyestrogens (2-methoxyestrone, 4-methoxyestrone, 2-methoxyestradiol and 4-methoxyestradiol) by the catechol-O-methyltransferase (COMT) enzyme (Figures 2 and 3).
Figure 2. Endogenous estrogen metabolism in human.
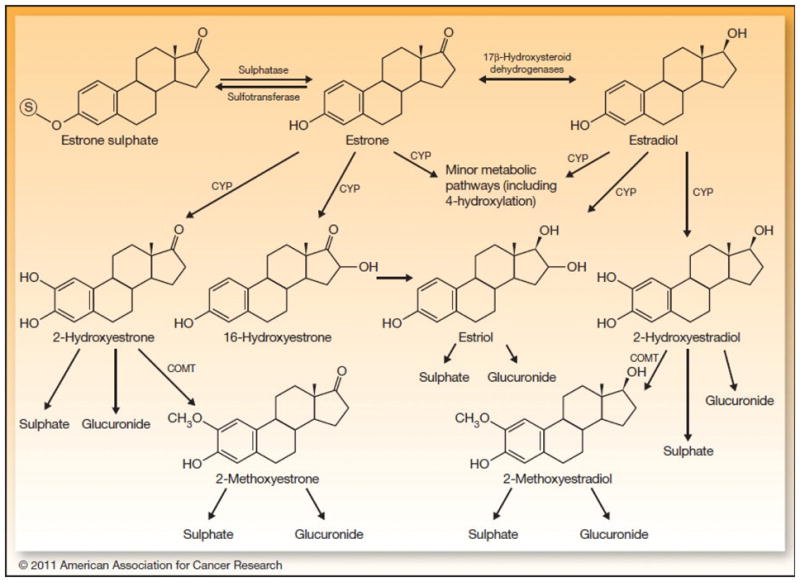
The parent estrogens estrone and estradiol are reversibly inter-converted, catalyzed by the 17β-hydroxysteroid dehydrogenase enzyme. They are also converted to catechol estrogens including 2-hydroxestrogens, 4-hydroxestrogens, 16-hydroxestrone, or estriol through the action of CYP enzymes. Catechol estrogens, in turn, are metabolized to 2-methoxyestrogens and 4-methoxyestrogens. Estrone, catechol estrogens, and methoxyestrogens can be conjugated to glucuronic acid and sulfate.
Abbreviations: COMT, catechol-O-methyltransferase; CYP, cytochrome P-450 enzyme [114].
Figure 3. Estradiol metabolism and DNA adduct formation.
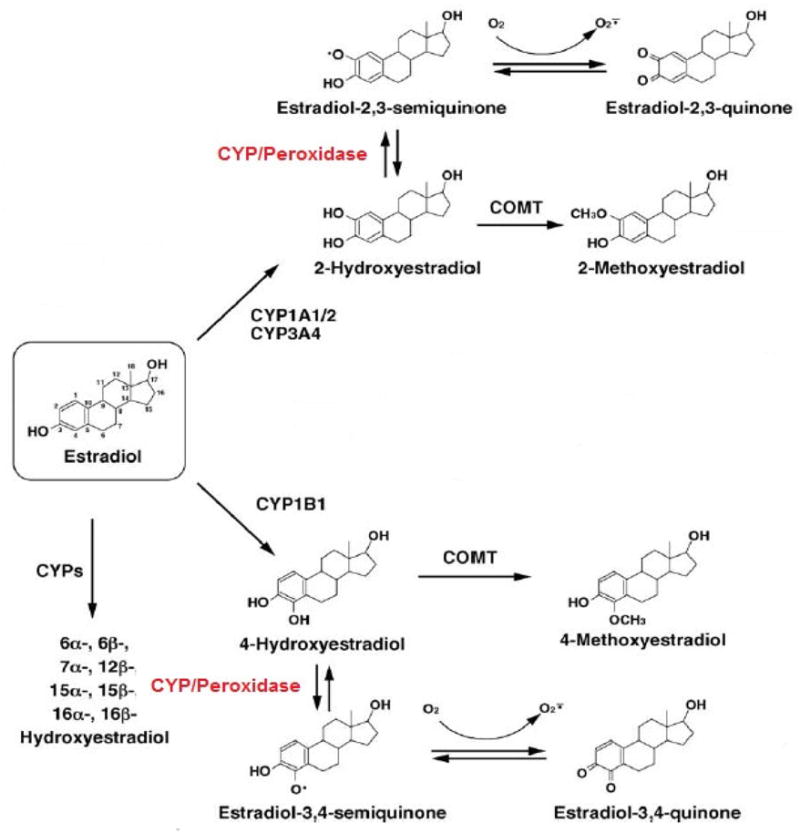
Estradiol catechol estrogens, including 2-hydroxyestradiol and 4-hydroxyestradiol, can go through reductive-oxidative cycling and produce mutagenic free radicals. These reactions are catalyzed by CYP and peroxidase enzymes. Estrogen semiquinones and quinones are reactive and carcinogenic intermediate metabolites of redox cycling pathways and can cause DNA damage.
Abbreviations: CYP, cytochrome P-450 enzyme; COMT, catechol-O-methyltransferase [17].
In addition to methylation, parent estrogens and catechol estrogens are also conjugated with glucuronic acid and sulfate by hepatic phase II enzymes including UDP-glucuronosyltransferases and sulfotransferases, respectively. Conjugation is considered a detoxification reaction by which hormones either become water soluble and are excreted in the urine or feces, or turn into a more lipophilic moiety with elevated half-lives (Figure 2) [18–20].
2-hydroxylation pathway
Quantitatively, the 2-hydroxylation pathway is the major metabolic pathway compared to the 4- and16-hydroxylation pathways. The cytochrome P-450 enzymes, including CYP1A1 and CYP1B1, are major phase I enzymes mainly expressed in breast and liver tissues [21]. These enzymes, along with CYP1A2, catalyze the C2 hydroxylation of parent estrogens to their respective catechol estrogens [22]. Two-hydroxylated estrogens possess low binding affinity for the estrogen receptor (ER) [23, 24]. These metabolites demonstrate reduced hormonal potency when compared with estradiol, and both non-estrogenic and anti-estrogenic activities have been attributed to them. There is some evidence from cell culture studies in ER+ human MCF-7 breast cancer cells suggesting that 2-hydroxyestrone and 2-hydroxyestradiol inhibit cell growth and proliferation [25, 26]. In addition, 2-hydroxy metabolites have been associated with normal cell differentiation and apoptosis [27, 28]. Taken together, these findings have led some researchers to classify 2-hydroxyestrone as a “good estrogen” [29]. The lack of tumorigenic activity of 2-hydroxy metabolites has been attributed to a few mechanisms including a high rate of clearance, more rapid rate of O-methylation by the COMT enzyme, lower hormonal potency in estrogen target tissues, and methylated products of 2-hydroxyestradiol such as 2-methoxyestradiol, which suppress tumor cell proliferation and angiogenesis [23]. At the same time, it has been shown that 2-hydroxyestrogens can damage DNA and generate free radicals as they go through redox cycling or when COMT is inhibited [30, 31]. It is important to note that high inter-individual variability in 2-hydroxylation has been shown in human liver samples, possibly explaining the high variability of metabolite levels in individuals [32].
Methoxyestrogens, including 2-methoxyestradiol, have been shown to inhibit carcinogenesis by suppressing cell proliferation and estrogen oxidation due to effects on microtubule stabilization [33–35]. Lottering et al. [36] investigated the effects of 17-β estradiol and its metabolites on cell cycle in MCF-7 cells and reported that 2-methoxyestradiol acts as a cytostatin and inhibits mitosis.
4-hydroxylation pathway
CYP3A4/3A5 has been shown to be the primary enzyme in the 4 hydroxylation of estradiol in human liver microsomes [32]. 4-hydroxylated catechol estrogens possess carcinogenic potential due to their ability to cause DNA damage by forming depurinating adducts, which in turn, generate mutations with subsequent oxidative damage and initiation of breast cancer [37]. In microsomal preparations of human mammary fibroadenoma and adenocarcinoma, formation of 4-hydroxyestradiol was four times higher than 2-hydroxyestradiol formation, indicating that the ratio of 4-/2-hydroxyestradiol may be used as a biomarker for detection of malignant breast tumors [38]. In addition, it has been shown that the ratios of quinone-estrogen DNA adducts to their parent or conjugated catechol estrogens were significantly higher in women with breast cancer or at high risk of breast cancer compared with control women [39]. On the other hand, it has been suggested that the 4-methoxyestrogens prevent oxidative metabolism of estradiol [35] and oxidative DNA damage [40]. These findings are in agreement with a more recent study in which inhibition of COMT enzyme activity was associated with higher levels of depurinating 4-hydroxyestrone (estradiol)-1-N3Adenine and 4-hydroxyestrone (estradiol)-1-N7Guanine adducts in MCF-10F cells [41].
16-hydroxylation pathway
16α-hydroxyestrone is the most important metabolite of the 16-hydroxylation pathway. 16α-hydroxyestrone is a potential tumor initiator, which promotes unscheduled DNA synthesis and anchorage independent growth in mouse mammary epithelial cells [42–44]. Animal studies have shown that urinary concentrations of 16α-hydroxyestrone are associated with increased proliferation of mammary cells [43, 44], Ras oncogene expression [45], and mammary tumor incidence [46]. Osborne et al. investigated the extent of estradiol 16α-hydroxylation in relation to the risk of developing breast cancer in human breast tissue. They reported that 16α-hydroxyestrone levels were eight-fold higher in cancerous mammary terminal duct lobular units than nearby mammary fat tissue, suggesting that 16α-hydroxyestrone production may play an important role in breast cancer induction [47].
There are currently substantial data suggesting a link between concentrations of individual metabolites or the ratio of specific metabolites and breast cancer risk in humans; this will be discussed in further details later in this review.
3. Role of Genetic Variation in Estrogen Metabolism
It has been postulated that genetic polymorphisms in genes encoding enzymes involved in estrogen metabolism pathways and the genes encoding the ERs are associated with breast cancer risk. Polymorphic variations in genes encoding COMT, CYP1A1, CYP1B1, estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), CYP17A1, and CYP19A1 have received extensive attention within the last decade.
COMT is a phase II enzyme that inactivates catechol estrogens by conjugating them into nongenotoxic methoxyestrogens [48]. COMT also prevents biotransformation of catechol estrogens to quinone-DNA adducts and development of reactive oxygen species (ROS) capable of damaging cellular macromolecules such as DNA, lipids, and proteins [49–51]. COMT, located on chromosome 22q11 [52], is polymorphic; a single G to A transition at codon 158 of COMT (single nucleotide polymorphism (SNP) rs4680) results in a 3- to 4-fold decrease in enzymatic activity (GG vs. AA genotype). Individuals with heterozygous genotype (A/G) show intermediate levels of COMT activity [53–54].
Given the role of COMT in the conversion of catechol estrogens to methoxyestrogens, genetic variations in this enzyme may influence the risk of breast cancer as a result of significant changes in catechol estrogen and methoxyestrogen levels [55]. It has been hypothesized that women possessing the low activity COMT genotype (AA or Met/Met) might be at greater risk of breast cancer due to higher concentrations of catechol estrogen intermediates [56–58]. Unexpectedly, results from the most recent meta-analysis [59] of 56 studies including 34,358 breast cancer cases and 45,429 controls show no evidence of significant associations between the COMT Val158Met polymorphism and breast cancer risk in any genetic model (comparing recessive and dominant models with each other). Although these findings did not change in subgroup analyses by ethnicity, source of controls, or menopausal status, they must be interpreted with caution because of the large heterogeneity between studies and lack of data for adjustment of other covariates such as age, body mass index (BMI), lifestyle, and environmental factors.
In addition to the hydroxylation of parent estrogens to catechol estrogens, CYP along with peroxidase enzymes catalyze the oxidation of catechol estrogens to estrogen semiquinones and quinones, which are carcinogenic metabolites of estrogens (Figure 3) [60]. It has been hypothesized that polymorphic variations in CYP1A1 and CYP1B1genes are linked with increased risk of breast cancer. In a case-control study, Taioli et al. [61] demonstrated that African-American breast cancer cases with the Msp1 homozygous variant polymorphism in CYP1A1 had an odds ratio (OR) of 8.4 (95% confidence interval: 1.7–41.7) compared with controls. This association was not observed in Caucasian women. In contrast, Miyoshi [62] reported an inverse association between the Msp1 (6235(T/C)) and breast cancer risk in Japanese women (OR= 0.60; 95% CI, 0.41–0.88). Taken together, these findings are mixed and more research is needed to clarify this discrepancy.
SNPs in CYP1B1genes have also been investigated in relation to breast cancer risk [63–66]. In a population-based case-control study conducted by Reding et al. [67] of 891 breast cancer cases and 878 controls, women homozygous with the T allele in CYP1B1*2 (Ser119; rs1056827) were compared with women homozygous with the G allele. Those homozygous for the CYP1B1*2 Ser allele (T/T) had a 1.69 times higher risk of breast cancer (95% CI, 1.17–2.46); however, results from this study were not in agreement with an earlier meta-analysis [68] that showed no overall associations of breast cancer risk with CYP1B1 polymorphisms. This inconsistency may be due to selection bias or the result of chance in Redwing’s study, or limitations of meta-analysis, such as variability in populations and publication bias.
In a meta-analysis of more than 10,000 mostly Caucasian breast cancer cases, multiple potentially functional SNPs in ERα including rs2234693, rs9340799, rs1801132, rs3798577, and rs2228480 have been studied. The only SNP that showed borderline significant association with reduced risk of breast cancer was rs2234693 (CC genotype vs. TT; OR=0.92; 95 % CI, 0.86–0.99; P =0.08 for heterogeneity test) [69]. Polymorphisms in the ERβ gene and breast cancer risk have also been studied in a recent systematic review [70] that reported that rs2987983, and rs4986938 SNPS are significantly associated with overall breast cancer risk
Most recently, Chattopadhyay et al. [71] reported significant associations between SNPs in ERα (rs2234693), ERβ (rs2987983), CYP17A1 (rs743572) and CYP19A1 (rs700519) and breast cancer risk in a case control study performed in North India. The study included 360 cases with corresponding controls matched on age, sex, ethnicity, and geographical location. For the ERα genetic polymorphism, the CC genotype was the reference genotype for all comparisons. The CT and CT+TT genotypes were positively associated with postmenopausal status (P=0.018 and P=0.017; respectively) and histological grade I and II cases (P=0.022 and P=0.008; respectively), and negatively associated with advanced clinical stage (III+IV) (P=0.008 and P=0.021; respectively). However, in each case the significance was lost after conducting Bonferroni corrections for multiple comparisons. For the other SNPs, a number of associations remained statistically significant after performing Bonferroni corrections and adjusting for age. For ERβ, the TC+CC genotypes were inversely correlated with premenopausal status when compared with the TT genotype (OR=0.31; 95 % CI, 0.15–0.62; P value=0.001). For CYP17A1, the TC+CC genotypes were positively associated with ER− status compared with TT genotype (OR=2.77; 95 % CI, 1.52–5.04; P value =0.001). Finally, a SNP in CYP19A1 or the aromatase gene (rs700519) was linked with an increased risk among postmenopausal women (CT+TT vs. CC; OR=2.72; 95 % CI, 1.47–5.10; P value=0.001).
Studies assessing different SNPs in the CYP19A1 and CYP17A1 genes in relation to breast cancer have yielded inconclusive results. Talbott et al. [72] have demonstrated that polymorphic variations in the CYP 19 gene in rs1008805 (A/G) SNP with at least one G allele, but not rs730154 (C/T) SNP, is linked with higher risk of premenopausal breast cancer (OR = 1.72; 95% CI, 1.20–2.49). There was no association between postmenopausal breast cancer and rs1008805 SNP. Yet Reding and other investigators [73] from the Women’s Contraceptive And Reproductive Experiences (CARE) study observed no substantial association with breast cancer risk for neither SNPs in CYP19A1, nor for CYP17A1, ERα, COMT, CYP1A1, or CYP1B1 genes in more than 1,600 White and Black cases. Similar results have been noted from the Shanghai Breast Cancer Study for 19 SNPs in CYP19A1gene [74]. Polymorphic variations in the CYP17A1 gene in relation breast cancer have been also considerably evaluated, but findings are mixed and no firm conclusion can be drawn at this time [75–78].
Further studies with more homogenous populations and larger sample sizes are required to substantiate the role of ERs and estrogen-metabolizing gene polymorphisms in breast cancer risk pathogenesis.
4. Estrogens and Breast Cancer Risk
There is increasing evidence from epidemiological, animal, and in vitro studies that endogenous estrogens are involved in breast carcinogenesis [79]. Evidence suggesting a hormonal role in breast cancer development began with an early observation that bilateral oophorectomy significantly reduces breast-cancer risk, and that risk reduction is greater if the ovaries are removed earlier in life [80]. In addition, some of the well-established risk factors for breast cancer, including early onset of menarche (<12 years), late menopause (>55 years), nulliparity or having child late in life, are related to lifetime exposure of breast tissue to sex hormones. Given that approximately 2/3 of breast tumors are ER positive (ER+) [81] and responsive to circulating estrogens, and that almost all ER negative (ER−) cases are resistant to endocrine therapy, it is important to elucidate the specific mechanisms by which estrogens are related to elevated breast cancer risk. We will discuss the association of estrogens with breast cancer risk in the following sections separately based on menopausal status. Assessing the role of androgens and progesterone in breast cancer is beyond the scope of this review and will not be discussed here.
4.A. Postmenopausal Women
4.A.1. Circulating primary hormones
In postmenopausal women, increased circulating concentrations of estradiol, estrone, estrone-sulfate, and androstendione have been associated with higher breast cancer risk, whereas higher levels of sex hormone binding globulin (SHBG) have been associated with lower risk [82–89]. Key et al. [90], in a pooled analysis of 9 prospective studies of 663 women who developed breast cancer and were not on any exogenous sex hormones, showed that risk of breast cancer significantly increases with higher levels of total estradiol, free estradiol, estrone, estrone-sulfate, androstenedione, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), and testosterone. The relative risk (RR) and 95% CI for the highest quintile versus the lowest quintile of estradiol levels was 2.0 and 1.47–2.71, respectively (Table 1). Since this analysis was published, a few more prospective and case-control studies have been reported that have found similar results [91–95]. It should be noted that the majority of populations studied were general populations with average breast cancer risk who were not taking any exogenous sex hormones. However, when breast cancer risk category (low versus high based on Gail or Rosner and Colditz risk model scores) [96, 97] was taken into consideration, no difference in the association between sex hormones and breast cancer was observed in low versus high breast cancer risk subjects [94, 98]. These findings should be evaluated in further studies.
Table 1.
Risk estimates for estrogens and breast cancer risk in postmenopausal women in studies of over 300 cases only.
| First author, year | Hormone | Cases/controls | Design/Biospecimen | RR (95% CI) for the top vs. bottom hormone category level | Ptrend |
|---|---|---|---|---|---|
| Key, 2002*† [90] | Total estradiol | 656/1709 | Pooled analysis/blood | 2·00 (1·47–2.71) | <0.001 |
| Free estradiol | 478/980 | 2·58 (1·76–3.78) | <0.001 | ||
| Estrone | 469/1188 | 2·19 (1·48–3.22) | <0.001 | ||
| Estrone-sulfate | 310/651 | 2·00 (1·26–3.16) | <0.001 | ||
| SHBG | 373/1160 | 0.66 (0.43–1.00) | 0.041 | ||
| Androstenedione | 375/1000 | 2·15 (1·44–3.21) | <0.001 | ||
| DHEA | 231/423 | 2·04 (1·21–3.45) | 0.018 | ||
| DHEAS | 578/1501 | 1.75 (1·26–2.43) | 0.002 | ||
| Testosterone | 585/1574 | 2.22 (1·59–3.10) | <0.001 | ||
| Missmer, 2004†† [92] | Estradiol | Nested case-control study within the Nurses’ Health Study/blood | 2.1 (1.5–3.2) | <0.001 | |
| Free estradiol | 1.9 (1.2–2.9) | <0.001 | |||
| Estrone | 1.7 (1.1–2.6) | <0.001 | |||
| Estrone-sulfate | 2.4 (1.6–3.8) | <0.001 | |||
| SHBG | 322/643 | 0.8 (0.6–1.3) | 0.14 | ||
| Androstenedione | 1.5 (1.0–2.3) | 0.04 | |||
| DHEA | 1.4 (0.9–2.2) | 0.02 | |||
| DHEAS | 1.7 (1.1–2.7) | 0.003 | |||
| Testosterone | 1.6 (1.0–2.4) | <0.001 | |||
|
|
|||||
| Manson, 2013††† [99] | Treatment intervention | # of BC events in treatment/placebo | Design | HR (95% CI) | P value |
| CEE (Premarin) | 168/216 | RCT | 0.79 (0.65–0.97) | 0.02 | |
| CEE+MPA( Prempro) | 434/323 | 1.28 (1.11–1.48) | <0.001 | ||
Abbreviations: SHBG, sex hormone binding globulin; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; RR, relative risk; HR, hazard ratio; CI, confidence interval; CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate; RCT, randomized controlled trial; BC, breast cancer.
From individual studies cited in references [82–89], [124], [127], and [140–142]. Reported relative risks are based on quintile comparisons.
Cases and controls are matched on different factors such as age, date of blood collection, etc.
Cases and controls are matched on age and date and time of blood collection. Reported relative risks are based on quartile comparisons.
With a median cumulative follow-up of 13 years. Median intervention was 7.2 year for CEE trial and 5.6 years for CEE+MPA trial.
Surprisingly, the results from the Women’s Health Initiative (WHI) are in disagreement with those from observational studies. Manson and colleagues [99] have recently published updated data from the WHI with 13 years of cumulative follow-up. Briefly, WHI randomized 27,347 postmenopausal women 50–79 years to take either conjugated equine estrogens alone (CEE) for 7.2 years or CEE plus medroxyprogesterone acetate (MPA) for 5.6 years. Compared with the placebo group, the CEE group showed a 21% reduced risk of invasive breast cancer (95% CI, 0.65–0.97) while the CEE+MPA group showed a 28% increased risk (95% CI, 1.11–1.48) (Table 1). As suggested by Chlebowski and others [100, 101] the discrepancy between observational studies and the WHI may be due to methodological issues. For example, in non-research setting, women using hormones usually have more screening mammograms than non-hormone users, and consequently breast cancer is detected earlier; however, in the WHI study, all participants were required to receive screening mammograms at baseline and annually. In addition, there were a relatively small number of breast cancer cases in the estrogen–alone group (n=168 for the intervention group and n=216 for the control group).
There are a number of limitations of observational studies, including the collection of a single blood sample. It has been suggested that one blood sample with a long period of follow up time, which is characteristic of epidemiological studies, may not be a good predictor of breast cancer risk. However, Zhang et al. in a nested case–control analysis within the Nurses’ Health Study, showed that one single measurement of blood reproductive hormones is sufficient to predict ER+/PR+ breast cancer in postmenopausal women 16–20 years following blood draw. In addition, when two blood measurements, collected 10 years apart, were compared, the intra-class correlation coefficients were found to be 0.69 (95 % CI, 0.61–0.75) for estradiol, and 0.74 (95 % CI, 0.67–0.80) for SHBG, indicating that hormone levels are well correlated over long period of time [102].
Due to scarce available data, it is unclear if the relationship between circulating sex hormones and breast cancer differs according to receptor status. In a recent short review by Key, results from four studies were compared, and it was reported that estradiol was directly linked with ER+ breast cancer in postmenopausal women. However, since the number of ER− breast cancer cases was very small, no firm conclusion could be established [103].
A number of known breast cancer risk factors have been proposed to influence risk via effects on estrogens. Obesity, defined as BMI> 30 kg/m2, raises the risk of postmenopausal breast cancer, and this has been attributed to the higher circulating levels of estrogens synthesized in the adipose tissue of obese women. At the same time, an inverse association between obesity and SHBG blood levels has been reported, which in turn, contributes to higher concentrations of free estradiol (bioavailable fraction) in the circulation.
The magnitude of the associations of estrogens with a number of breast cancer risk factors including obesity, reproductive, demographic, and life style factors has been investigated by the Endogenous Hormones and Breast Cancer Collaborative Group in several studies. In a pooled analysis of eight prospective studies in postmenopausal women, adjusting data for free estradiol concentrations attenuated breast cancer risk by 17% for each 5 kg/m2 increase in BMI, resulting in a loss of statistical significance for the association between BMI and breast cancer risk [104]. In another cross-sectional analysis of 13 prospective studies by the same group, estrogen and androgen levels were positively associated with obesity, smoking (15+ cigarettes daily) and alcohol consumption (20+g alcohol daily), and inversely linked with age. By contrast, SHBG concentrations were greater in older women and lower in obese women and those consuming alcohol [105].
Mammographic density, a known risk factor for breast cancer development, is a measure of the amount of fibroglandular tissue that appears on a mammogram [106]. It has been hypothesized that sex steroid effects on breast cancer are mediated through mammographic density [107, 108]; however, available data do not consistently support the hormonal basis for mammographic density mostly because of the confounding influence of BMI [109–111].
It has long been of concern that circulating estrogen may not be an appropriate surrogate for breast tissue levels. Some studies have shown that estrogen concentrations in normal or breast tumor tissue are greater than in the circulation [112–113]. It has been suggested that inhibition of local estrogen aromatization in tumor tissue may be an appropriate breast cancer prevention strategy. This notion has recently been rejected by Lønning et al. [114] who proposed a model in which plasma-to-tissue equilibration explains the high estrogen levels in breast tissue. According to this model, malignant breast tumors are constantly exposed to circulating estrogens through ER binding or active uptake of estrogens. Therefore, they propose that systemic suppression of estrogen production may be superior to targeting local aromatase enzyme in hormone responsive breast cancer. Given the strong positive association observed between blood sex hormones and breast cancer in postmenopausal women, circulating estrogens seem to be an appropriate marker of tissue exposure, as suggested by Hankinson and Eliassen [115].
4.A.2. Urinary and circulating estrogen metabolites
The role of estrogen metabolites in breast cancer has been the subject of discussion for the last three decades; however, compared to circulating primary estrogens, few studies have investigated the association between breast cancer and individual estrogen metabolites, their pathways or ratios. Among catechol estrogens, 16α-hydroxyestrone and 4-hydroxy metabolites are relatively more estrogenic and have genotoxic potential while 2-hydroxy metabolites are considered to have little estrogenic activity or antiestrogenic properties [29].
Findings from small observational studies [25, 116, 117] have led to the hypothesis that a lower ratio of urinary 2-hydroxyestrone to 16α-hydroxyestrone (2/16-hydroxyestrone) is a breast cancer risk factor. The most recent combined analysis [118] reviewed the results of 5 trials comprised of 385 invasive breast cancer cases and 723 controls. All metabolites were analyzed utilizing an enzyme linked immunosorbent assay (ELISA), and odds ratios adjusted for known breast cancer risk factors were calculated. Results comparing the women in the lowest tertiles with those in the highest tertiles did not reveal any significant association for either the 2/16-hydroxyestrone ratio (OR=1.02; 95% CI, 0.71–1.48) (Table 2) or any individual metabolites (OR for 2-hydroxyestrone =0.93; 95% CI, 0.67–1.30, and OR for 16α-hydroxyestrone =1.01; 95% CI, 0.73–1.41). Additionally, when data were stratified by ER status, no significant difference in the relative risk was found. The pattern of results was similar in a systematic review by Obi et al. [119] in which 6 prospective and 3 retrospective studies including 1189 breast cancer cases and 1888 matched controls were reviewed. Women in the top category of either urinary or circulating 2/16-hydroxyestrone did not differ in risk of breast cancer compared to those in the bottom category (Table 2). Risk associations were not changed when ER subtype was also taken into account.
Table 2.
Risk estimates for estrogen metabolite pathways or ratios and breast cancer risk in postmenopausal women in studies of over 300 cases only.
| First author, year | Estrogen metabolite pathways or ratios* | Cases/controls | Design/Biospecimen | Risk estimates† (95% CI) for the top vs. bottom hormone category level | Ptrend |
|---|---|---|---|---|---|
| Dallal, 2013a [118] | 2-OHE1/16α-OHE1 Ratio | 385/723 | Combined analysis/urine | 1.02 (0.71–1.48) | 0.9 |
| Obi, 2011b,c [119] | 2-OHE1/16α-OHE1 Ratio | 1189/1888 | Systematic review/urine& blood | 0.75 (0.35–1.62) to 1.31 (0.53–3.18) | N/A |
| Dallal, 2014d [121] | 2-OHE1/16α-OHE1 Ratio | Case-cohort study within the B~FIT/blood | 0.88 (0.59–1.32) | 0.8 | |
| 2-pathway/16-pathway | 407/496 | 0.6 (0.40–0.90) | 0.002 | ||
| 2-pathway/parent estrogens | 0.69 (0.46–1.05) | 0.01 |
Abbreviations: N/A, not available; 2-OHE1, 2-hydroxyestrone; 16α-OHE1, 16-alpha-hydroxyestrone; CI, confidence interval; PLCO, the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; B~FIT, the Breast and Bone Follow-up to the Fracture Intervention Trial.
Parent estrogens include estrone and estradiol.
2-hydroxylation pathway: 2-hydroxyestrone, 2-methoxyestrone, 2-hydroxyestradiol, 2-methoxyestradiol, and 2-hydroxyestrone-3-methyl ether.
16-hydroxylation pathway: 16a-hydroxyestrone, estriol, 17-epiestriol, 16-ketoestradiol, and 16-epiestriol.
Risk estimates for [118] and [119] refrences are odds ratios, but risk estimates for [121] reference are hazard ratios.
From individual studies cited in references [117], [137], and [143–145]. Reported odds ratio is based on tertile comparison.
Range of odds ratios with respective CIs. Reported odds ratios are based on tertile and quintile comparisons, respectively.
Reported hazard ratios are based on quintile comparisons.
Recently, Fuhrman et al. [120] examined the associations of circulating levels of 15 estrogens and estrogen metabolites individually and grouped by pathway, as well as metabolic pathway ratios, with breast cancer risk in a prospective case-control study nested within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Participants were 277 invasive breast cancer cases and 423 matched controls, all postmenopausal women not using exogenous hormones at the time of the blood draw. In contrast to previous studies, an assay with high sensitivity and specificity, liquid chromatography–tandem mass spectrometry (LC-MS/MS), was utilized to measure circulating levels of hormones and their metabolites. The study demonstrated positive associations between breast cancer risk and unconjugated estradiol levels (HR = 2.07; 95% CI, 1.22–3.51; P trend=0.01) and the ratio of 4-hydroxylation pathway catechol estrogens to 4-hydroxylation pathway methylated catechol estrogens (HR = 1.76; 95% CI, 1.06–2.93; P trend=0.02) comparing the highest to the lowest quintile. Interestingly, the ratio of 2-hydroxylation pathway to parent estrogens was found to be inversely associated with breast cancer risk (HR Q5 vs. Q1 = 0.54; 95% CI, 0.32–0.90; P trend=0.003) (Table 2).
Since the publication of the Fuhrman et al. results, another two studies have been conducted by the same lab and core investigators with inconsistent findings. Dallal et al. [121] reproduced similar results in a prospective case-cohort study within the Breast and Bone Follow-up to the Fracture Intervention Trial (B~FIT), of 407 cases and 496 controls. In this study, high circulating levels of estradiol were associated with elevated breast cancer risk (HRtop vs. bottom quintile=1.86; 95% CI, 1.19–2.90; Ptrend=0.04). Additionally, increased ratios of the 2-hydroxylation pathway to parent estrogens, and 2:16-hydroxylation pathways were associated with lower risk (HR=0.69; 95% CI, 0.46–1.05; Ptrend=0.01; and, HR=0.60; 95% CI, 0.40–0.90; Ptrend=0.002, respectively) (Table 2). Surprisingly, the ratio of 4-hydroxylation pathway to parent estrogens was reported to be inversely related to breast cancer risk (HRtop vs. bottom quintile =0.61; 95% CI, 0.40–0.93; Ptrend=0.004), which is not in agreement with the findings from previous studies. In contrast to Fuhrman and Dallal studies, no statistically significant results were observed in another nested case-control study by the same lab using blood samples from the Columbia Missouri Serum Bank [122]. The reasons for the discrepancies among these results are not quite clear, however, the investigators believe they might be due to differences in COMT polymorphisms or simply due to chance alone.
The association between circulating endogenous estrogens and breast cancer risk in postmenopausal women has been conclusively established, and compelling evidence exists to support a causal relationship. Epidemiological studies have consistently shown a 2–3 fold increase in breast cancer risk in women with elevated blood estradiol levels. On the other hand, findings from estrogen metabolite investigations are mixed. This inconsistency in estrogen metabolite results may be due to methodological differences in participant characteristics, study design and follow-up length (for some studies insufficient), number of cases (some studies under-powered), and high inter-individual variation in serum and urinary concentrations of estrogen metabolites, or limitations associated with estrogen metabolite measurement. Of particular note are differences in assay methodologies. Until recently, the leading methodology for measurement of estrogen metabolites was ELISA, a method that has limited specificity and sensitivity. This is of particular importance for analysis of samples from postmenopausal women, whose levels are extremely low. Recently, some groups have used liquid chromatography–tandem mass spectrometry (LC–MS/MS), which has much higher sensitivity and specificity.
4. B. Premenopausal Women
4. B.1. Circulating primary hormones
Much less research has been performed on the effects of endogenous estrogens on breast cancer risk in premenopausal women than in postmenopausal women. Thus, the role of estrogens in breast carcinogenesis in this population is not thoroughly understood and remains relatively unclear. This is likely due to the much smaller number of breast cancer cases in premenopausal women. Another potential reason may be the large inter- and intra-individual variations in sex hormone concentration during the menstrual cycle. To the best of our knowledge, only nine prospective studies [123–132] have evaluated the associations between serum estrogens and breast cancer risk in premenopausal women.
Hankinson and Eliassen reviewed the results of seven of these studies in 2010 [133]. Briefly, three cohort studies [123, 124, 127] failed to show significant associations between breast cancer risk and estradiol, estrone, or estrone-sulfate, possibly due to the fact they were small studies with less than 50 cases, or had not adjusted for the timing of the menstrual cycle at the blood draw. Although the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort [128], Guernsey cohort [126], and Rosenberg studies [125] had larger sample sizes with 285, 62 and 79 cases respectively and accounted for the phase of menstrual cycle, no statistically significant results were found for associations between breast cancer risk and estrone, estradiol, or SHBG.
On the other hand, early follicular blood samples from breast cancer cases (n=197) in the Nurses Health Study II (NHSII) demonstrated a significantly elevated breast cancer risk in women with higher total and free estradiol (RRQ4 vs. Q1= 2.1; 95% CI, 1.1–4.1; and, RRQ4 vs. Q1= 2.4; 95% CI, 1.3–4.5; respectively). Importantly, the magnitude of the effect estimate was more pronounced among the ER+/PR+ cases compared with all breast cancer cases (RRQ4 vs. Q1= 2.7; 95% CI, 1.2–6.0 for follicular total estradiol) consistent with the classical role of ERs in stimulating higher cell proliferation and mutagenesis [134]. On the other hand, no evidence of a relationship between breast cancer risk and estrone, estrone-sulfate, or SHBG was seen [130].
In a 2011 meta-analysis of those seven nested case–control studies with a total of 693 cases and 1609 controls, only a weak relationship between circulating estradiol and breast cancer risk was reported (OR for a doubling of estradiol=1.10;95% CI, 0.96–1.27) (Table 3) [135].
Table 3.
Risk estimates for estrogens and their metabolites and breast cancer risk in premenopausal women: results from meta-analysis, systematic review, or individual studies of over 300 cases only.
| First author, year, reference | Hormone | Cases/controls | Design/Biospecimen | Odds ratio (95% CI) for the top vs. bottom hormone category level | Ptrend |
|---|---|---|---|---|---|
| Fortner, 2013a [132] | Estradiol | Nested case-control/blood | |||
| Follicular | 462/909 | 1.0 (0.7 to 1.5) | 0.76 | ||
| Luteal | 479/959 | 0.9 (0.6 to 1.4) | 0.65 | ||
| Free estradiol | |||||
| Follicular | 447/886 | 0.8 (0.5 to 1.2) | 0.48 | ||
| Luteal | 469/944 | 1.0 (0.7 to 1.5) | 0.99 | ||
| Estrone | |||||
| Follicular | 469/920 | 1.0 (0.7 to 1.4) | 0.62 | ||
| Luteal | 500/1005 | 0.9 (0.7 to 1.3) | 0.89 | ||
| SHBG | 624/1246 | 1.2 (0.8 to 1.6) | 0.23 | ||
| Key, 2013b,c [136] | Total estradiol | 600/1375 | Pooled analysis/blood | 1·19 (1·06–1·35) | 0·0042 |
| Free estradiol | 587/1341 | 1·17 (1·03–1·33) | 0·014 | ||
| Estrone | 477/933 | 1·27 (1·05–1·54) | 0·014 | ||
| SHBG | 767/1699 | 1·07 (0·94–1·23) | 0.29 | ||
| Androstenedione | 569/1177 | 1·30 (1·10–1·55) | 0·0026 | ||
| Walker, 2011d [135] | Estradiol | 693/1609 | Meta-analysis/blood | 1.10 (0.96–1.27) | N/A |
| Dallal, 2013e [118] | 2-OHE1/16α-OHE1 Ratio | 183/543 | Combined analysis/urine | 0.74 (0.45–1.23) | 0.25 |
| Obi, 2011f,g [119] | 2-OHE1/16α-OHE1 Ratio | 682/1027 | Systematic review/urine | 0.5 (0.25–1.01) to 0.75 (0.35–1.62) | 0.05, N/A |
| Arslan, 2009 h [138] | 2-OHE1/16α-OHE1 Ratio | 377/377 | Nested case-control/blood | 1.13 (0.68–1.87) | 0.51 |
Abbreviations: N/A, not available; SHBG, sex hormone binding globulin; 2-OHE1, 2-hydroxyestrone; 16α-OHE1, 16-alpha-hydroxyestrone; OR, odds ratio; CI, confidence interval.
Reported odds ratio is based on quintile comparisons.
ORs for a doubling in concentrations of hormones.
From individual studies cited in references [117], [137], and [143]. Reported odds ratio is based on tertile comparison.
Range of ORs with respective CIs. Reported odds ratios are based on tertile comparisons.
Reported odds ratios is based on quartile comparisons.
Note: All studies adjusted or matched for different covariates affecting breast cancer risk, but not necessarily for phase of menstrual cycle. Studies with less than 300 cases are part of meta-analysis or systematic review studies reported above.
Following this meta-analysis, results of three studies and a new systematic review have been published. Dorgan et al. [129] reported no association between total or bioavailable estradiol or SHBG and breast cancer risk, in a prospective case-control study of 98 breast cancer cases nested in the Columbia, MO, Serum Bank cohort matched with 168 controls on factors such as the day of blood draw and menstrual cycle phase. Similarly, data from 104 cases of the Italian Hormones and Diet in the Etiology of Breast Tumors (ORDET) cohort did not suggest any associations with risk when the highest tertiles of estradiol and SHBG levels were compared with the lowest tertiles [131]. The Nurses’ Health Study II has recently published updated data regarding the relationship of plasma sex hormones to breast cancer risk [132]. Since the last data were published in 2006, more than 400 additional cases were identified, bringing the total number of cases to 634. Estrogens were measured by radioimmunoassay or LC–MS/MS, and SHBG was measured by chemiluminescence immunoassay. Overall, after adjusting for known breast cancer risk factors, no significant associations were reported between breast cancer risk and early follicular or mid-luteal total estradiol, free estradiol, estrone, or SHBG. There was weak evidence indicating that mid-luteal estradiol was positively related with ER+/PR+ breast cancer (ORQ5 vs. Q1= 1.7; 95% CI, 1.0–2.9; Ptrend = 0.02).
Interestingly, results from the most recent pooled analysis of data by the Endogenous Hormones and Breast Cancer Collaborative Group [136] were not consistent with the findings of the previous studies. This inconsistency may be due to the exclusion of data from four of the previously discussed studies [123, 125, 127, 131]. Participants included in the analyses were premenopausal women below 50 years of age who were not using any exogenous sex hormones at the time of blood collection. Cases and controls were matched for age, menstrual cycle day, and blood draw date, and an odds ratio associated with a doubling in hormone levels was calculated separately for each hormone. Data from 600 women with incident breast cancer and 1375 controls were used to calculate the odds ratio for breast cancer in relation to serum estradiol. The odds ratio for circulating estrone was estimated based on data from 477 cases and 933 controls. Findings showed that a doubling in concentrations of estradiol and estrone was associated with 19% and 27% elevated risk of breast cancer, respectively (OR for estradiol= 1.19; 95% CI, 1.06–1.35; Ptrend=0.004; OR for estrone= 1.27; 95% CI, 1.05–1.54; Ptrend=0.01) (Table 3). SHBG was not related to breast cancer risk. These risk estimates were not changed significantly after adjustment for hormonal breast cancer risk factors. When data were evaluated based on the tumor ER subtype, no significant differences in results were observed between ER+ or ER− cases, although odds ratios were generally larger among the ER+ subjects.
Overall, observational data regarding the associations of circulation estrogen concentrations with breast cancer risk in premenopausal are not as strong as postmenopausal women. Therefore, no conclusions can be drawn until larger well-designed studies with enhanced analytical methods are conducted.
4.B.2. Urinary and circulating estrogen metabolites
Four prospective studies have investigated the relationships between breast cancer incidence and concentrations of individual estrogen metabolites, their ratios or metabolic pathways in premenopausal women. The ratio of 2/16-hydroxyestrone has been studied in either urine or blood samples with inconsistent results. Muti et al. [137] reported that relative to the lowest quintile, cases in the highest quintile of luteal phase urinary 2/16-hydroxyestrone had a 45% reduction in breast cancer risk (n =67 cases; adjusted RR= 0.55; 95% CI, 0.23–1.32). Additionally, both 2-hydroxyestrone and 16α-hydroxyestrone were positively but non-significantly linked with risk. Consistent with these results was a study conducted by Meilahn et al. [117] in which 60 breast cancer cases were matched to 184 controls on age, baseline visit date, and menstrual cycle phase. Results showed that women in the top tertile of the 2/16-hydroxyestrone ratio had a lower breast cancer risk compared to those in the bottom tertile (OR= 0.75; 95% CI, 0.35–1.62; P value= 0.46).
The New York University Women’s Health Study [138] is the only trial that has reported circulating levels of the 2/16-hydroxyestrone ratio. In this study, 377 cases were matched with 377 controls on day and phase of menstrual cycle. Results revealed no significant associations between breast cancer risk and 2-hydroxyestrone or 16α-hydroxyestrone, or their ratio (OR for 2-hydroxyestrone: 16α-hydroxyestrone =1.13; 95% CI, 0.68–1.87; Ptrend=0.5). Along the same lines, in the subgroup analysis based on ER status when comparing the highest quartile with the lowest quartile among ER+ cases, the 2/16-hydroxyestrone ratio was associated with more than doubling of breast cancer risk (OR=2.15; 95% CI, 0.88–5.27; Ptrend=0.09).
Contrary to the previous studies that had analyzed 2-hydroxyestrone, 16α-hydroxyestrone, and their ratio with the ELISA method, the NHSII quantitated a comprehensive list of 15 urinary estrogens and estrogen metabolites utilizing the gold standard method, LC-MS/MS [139]. Mid-luteal urine samples were collected by 247 cases and 485 matched controls. Findings indicated that 2- and 4-hydroxylation pathway estrogen metabolites, but not 16-hydroxylation pathway, were inversely associated with risk, but results did not reach statistical significance. Likewise, the 2/16-hydroxyestrone ratio was not linked to risk (RRQ4 vs. Q1=0.90; 95% CI, 0.57–1.41; Ptrend = 0.86). The only estrogen metabolite positively associated with risk was 17-epiestriol, which is a metabolite in the 16-hydroxylation pathway (RRQ4 vs. Q1= 1.74; 95% CI, 1.08–2.81; Ptrend = 0.01). Interestingly, when comparing the highest quartile with the lowest quartile, elevated urinary concentrations of estrone and estradiol were associated with 48% and 49% reductions in risk, respectively (RR for estrone = 0.52; 95% CI, 0.30–0.88; RR for estradiol = 0.51; 95% CI, 0.30–0.86). Additionally, elevated ratio of 16-pathway estrogen metabolites to parent estrogen metabolites was associated with higher risk (RRQ4 vs. Q1= 1.61; 95% CI, 0.99–2.62; Ptrend = 0.04), and parent estrogen metabolites to non–parent estrogen metabolites ratio was negatively linked to risk (RRQ4 vs. Q1= 0.58; 95% CI, 0.35–0.96; Ptrend = 0.03). It is difficult to interpret these findings since the directions of the associations for the primary estrogens in the urine are not in agreement with that observed in circulating primary estrogens. Also, due to a lack of research done in this area, sufficient data do not exist for comparison. One possible explanation for these results is that higher levels of primary estrogens are excreted into urine prior to finding any chance to convert into genotoxic metabolites. Also, one may speculate that urine is not as relevant as blood to breast tissue exposure.
Recently, two systematic reviews and combined analyses have examined the relationships between circulating or urinary 2-hydroxyestrone, 16α-hydroxyestrone, and their ratio with breast cancer risk. Obi et al. [119], in a study of 682 premenopausal cases and 1027 matched controls, concluded that the urinary 2/16-hydroxyestrone ratio, but not circulating levels, is non-significantly associated with lower risk of breast cancer (range of ORs=0.5–0.75; 95% CI, 0.25–1.01 and 0.35–1.62; respectively) (Table 3). Similarly, Dallal et al. [118], in a combined analysis of 726 women (n=183 cases) demonstrated that elevated urinary 2/16-hydroxyestrone is suggestive of lower breast cancer risk (ORtop tertile vs. low tertile= 0.74; 95% CI, 0.45–1.23) (Table 3). Additionally, data from the same analysis showed that higher urinary 2/16-hydroxyestrone is indicative of decreased risk of breast cancer for ER− cases (ORtop tertile vs. low tertile= 0.33; 95% CI, 0.13–0.84). This latter finding is based on small number of 31 cases; therefore, it may be due to chance and needs more research to be confirmed. A summary of data for both circulating and urinary estrogens and their metabolites is presented in Table 3.
5. Conclusions
There is currently convincing evidence that endogenous estrogens are associated with breast cancer in postmenopausal women; however, this relationship has not been firmly established in premenopausal women, possibly due to the large variations in hormone levels during the menstrual cycle, the small number of studies that have been performed, and the small number of cases. Of recent interest are genetic polymorphisms in the enzymes involved in estrogen metabolism that may modify breast cancer risk in relation to sex hormones.
The role of estrogen metabolites in the etiology of breast cancer has been studied, but available data are mixed and no firm conclusion can be drawn in either pre- or postmenopausal women. Developments in mass spectrometry have greatly enhanced the sensitivity and specificity in the quantification of estrogens and estrogen metabolites in both blood and urine.
Further studies with particular attention to factors such as hormone receptor subtype, controlling for hormones variations during menstrual cycle in premenopausal women, are needed to improve our understanding of the importance of estrogen to breast cancer risk. Taken together, there are not sufficient data to confirm the role of estrogen metabolites as predictors of breast cancer, but it can be concluded at this point that any intervention which leads to reduced circulating levels of primary estrogens have the potential to lower the risk of breast cancer in postmenopausal women.
Footnotes
Conflict of Interest
None of the authors has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts & Figures 2013–2014. Atlanta: American Cancer Society, Inc; 2013. [accessed 11.20.13]. < http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-040951.pdf>. [Google Scholar]
- 3.Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas. 2001;38:103–116. doi: 10.1016/s0378-5122(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: 2013. [Google Scholar]
- 5.Steiner E, Klubert D, Knutson D. Assessing breast cancer risk in women. Am Fam Physician. 20081;78:361–6. [PubMed] [Google Scholar]
- 6.Thomson CA. Diet and breast cancer: understanding risks and benefits. Nutr Clin Pract. 2012;27:636–50. doi: 10.1177/0884533612454302. [DOI] [PubMed] [Google Scholar]
- 7.Petracci E, Decarli A, Schairer C, Pfeiffer RM, Pee D, Masala G, Palli D, Gail MH. J Natl Cancer Inst. 2011;103:1037–48. doi: 10.1093/jnci/djr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 20042;230:9–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 9.Ursin G, Qureshi SA. Mammographic density – a useful biomarker for breast cancer risk in epidemiologic studies. Norsk Epidemiologi. 2009;19:59–68. [Google Scholar]
- 10.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–68. [PubMed] [Google Scholar]
- 11.Carr BR, MacDonald PC, Simpson ER. The role of lipoproteins in the regulation of progesterone secretion by the human corpus luteum. Fertil Steril. 19823;38:03–311. doi: 10.1016/s0015-0282(16)46511-8. [DOI] [PubMed] [Google Scholar]
- 12.Miller WL, Strauss JF., 3rd Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol. 19991;69:31–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 13.Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 12. Philadelphia, PA: Saunders press; 2011. pp. 599–602. [Google Scholar]
- 14.Ryan J. Biological aromatization of steroids. J Biol Chem. 1959;234:268–272. [PubMed] [Google Scholar]
- 15.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatases: a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 16.Dotsch KJ, Dorr HG, Wildt L. Exposure to endogenous estrogens during lifetime. In: Metzler M, editor. The Handbook of Environmental Chemistry Vol 3, Part L Endocrine Disruptors, Part I. Springer-Verlag; Berlin Heidelberg: 2001. pp. 83–99. [Google Scholar]
- 17.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Lakhani NJ, Venitz J, Figg WD, Sparreboom A. Pharmacogenetics of estrogen metabolism and transport in relation to cancer. Curr Drug Metab. 2003;4:505–13. doi: 10.2174/1389200033489244. [DOI] [PubMed] [Google Scholar]
- 19.Raftogianis R, Creveling C, Weinshilboum R, Weisz J. Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000;27:113–124. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- 20.Hobkirk R. Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles. Can J Biochem Cell Biol. 19851;63:127–1144. doi: 10.1139/o85-141. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, Fasco MJ, Figge HL, Keyomarsi K, Kaminsky LS. Expression of cytochromes P450 in human breast tissue and tumors. Drug Metab Dispos. 19968;24:99–905. [PubMed] [Google Scholar]
- 22.Cribb AE, Knight MJ, Dryer D, Guernsey J, Hender K, Tesch M, Saleh TM. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidemiol Biomarkers Prev. 2006;15:551–558. doi: 10.1158/1055-9965.EPI-05-0801. [DOI] [PubMed] [Google Scholar]
- 23.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 24.van Aswegen CH, Purdy RH, Wittliff JL. Binding of 2-hydroxyestradiol and 4-hydroxyestrdiol to estrogen receptor human breast cancers. J Steroid Biochem. 1989;32:485–492. doi: 10.1016/0022-4731(89)90380-4. [DOI] [PubMed] [Google Scholar]
- 25.Schneider J, Huh MM, Bradlow HL, Fishman J. Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J Biol Chem. 1984;259:4840–5. [PubMed] [Google Scholar]
- 26.Gupta M, McDougal A, Safe S. Estrogenic and antiestrogenic activities of 16alpha- and 2-hydroxy metabolites of 17 beta-estradiol in MCF-7 and T47D human breast cancer cells. J Steroid Biochem Mol Biol. 1998;67:413–419. doi: 10.1016/s0960-0760(98)00135-6. [DOI] [PubMed] [Google Scholar]
- 27.Telang NT, Katdare M, Bradlow HL, Osborne MP. Estradiol metabolism: an endocrine biomarker for modulation of human mammary carcinogenesis. Environ Health Perspect. 1997;105:559–564. doi: 10.1289/ehp.97105s3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandewalle B, Lefebvre J. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Mol Cell Endocrinol. 1989;61:239–246. doi: 10.1016/0303-7207(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 29.Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-hydroxyestrone: the ‘good’ estrogen. J Endocrinol. 1996;150:S259–S265. [PubMed] [Google Scholar]
- 30.Liehr JG, Roy D. Free radical generation by redox cycling of estrogens. Free Radical Biol Med. 1990;8:415–423. doi: 10.1016/0891-5849(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 31.Lavigne JA, Goodman JE, Fonong T, Odwin S, He P, Roberts DW, Yager JD. The effects of catechol-Omethyltransferase inhibition on estrogen metabolite and oxidative DNA damage levels in estradiol-treated MCF-7 cells. Cancer Res. 2001;61:7488–94. [PubMed] [Google Scholar]
- 32.Kerlan V, Dreano Y, Bercovici JP, Beaune PH, Floch HH, Berthou E. Nature of cytochrome P450 involved in the 2-/4-hydroxylations of estradiol human liver microsomes. Biocbem Pharmacol. 19921;44:745–1756. doi: 10.1016/0006-2952(92)90068-t. [DOI] [PubMed] [Google Scholar]
- 33.Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23:165–72. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- 34.Cushman M, He HM, Katzenellenbogen JA, Lin CM, Hamel E. Synthesis, antitubulin and antimitotic activity, and cytotoxicity of analogs of 2-methoxyestradiol, an endogenous mammalian metabolite of estradiol that inhibits tubulin polymerization by binding to the colchicine binding site. J Med Chem. 1995;38:2041–9. doi: 10.1021/jm00012a003. [DOI] [PubMed] [Google Scholar]
- 35.Dawling S, Roodi N, Parl FF. Methoxyestrogens exert feedback inhibition on cytochrome P450 1A1and 1B1. Cancer Res. 2003;63:3127–3132. [PubMed] [Google Scholar]
- 36.Lottering ML, Haag M, Seegers JC. Effects of 17 beta-estradiol metabolites on cell cycle events in MCF-7 cells. Cancer Res. 1992;52:5926–32. [PubMed] [Google Scholar]
- 37.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liehr JG, Ricci MJ. 4-hydroxylation of estrogens as marker of human mammary tumors. Proc Natl Acad Sci USA. 1996;93:3294–6. doi: 10.1073/pnas.93.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaikwad NW, Yang L, Muti P, Meza JL, Pruthi S, Ingle JN, Rogan EG, Cavalieri EL. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int J Cancer. 2008;122:1949–57. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavigne JA, Goodman JE, Fonong T, Odwin S, He P, Roberts DW, Yager JD. The effects of catechol-O-methyltransferase inhibition on estrogen metabolite and oxidative DNA damage levels in estradiol-treated MCF-7 cells. Cancer Res. 20017;61:488–7494. [PubMed] [Google Scholar]
- 41.Lu F, Zahid M, Saeed M, Cavalieri EL, Rogan EG. Estrogen metabolism and formation of estrogen-DNA adducts in estradiol-treated MCF-10F cells. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction and catechol-O-methyltransferase inhibition. J Steroid Biochem Mol Biol. 20071;105:50–8. doi: 10.1016/j.jsbmb.2006.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradlow HL, Hershcopf RE, Fishman JF. Oestradiol 16 alpha-hydroxylase: a risk marker for breast cancer. Cancer Surv. 1986;5:573–583. [PubMed] [Google Scholar]
- 43.Telang NT, Suto A, Wong GY, Osborne MP, Bradlow HL. Induction by estrogen metabolite 16alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992;84:634–638. doi: 10.1093/jnci/84.8.634. [DOI] [PubMed] [Google Scholar]
- 44.Suto A, Bradlow HL, Wong GY, Osborne MP, Telang NT. Experimental down regulation of intermediate biomarkers of carcinogenesis in mouse mammary epithelial cells. Breast Cancer Res Treat. 1993;27:193–202. doi: 10.1007/BF00665689. [DOI] [PubMed] [Google Scholar]
- 45.Suto A, Bradlow HL, Wong GY, Osborne MP, Telang NT. Persistent estrogen responsiveness of ras oncogene-transformed mouse mammary epithelial cells. Steroids. 1992;57:262–268. doi: 10.1016/0039-128x(92)90058-h. [DOI] [PubMed] [Google Scholar]
- 46.Bradlow HL, Hershcopf RJ, Martucci CP, Fishman J. Estradiol 16 alpha-hydroxylation in the mouse correlates with mammary tumor incidence and presence of murine mammary tumor virus: a possible model for the hormonal etiology of breast cancer in humans. Proc Natl Acad Sci USA. 1985;82:6295–6299. doi: 10.1073/pnas.82.18.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osborne MP, Bradlow HL, Wong GY, Telang NT. Upregulation of estradiol C16 alpha-hydroxylation in human breast tissue: a potential biomarker of breast cancer risk. J Natl Cancer Inst. 1993;85:1917–20. doi: 10.1093/jnci/85.23.1917. [DOI] [PubMed] [Google Scholar]
- 48.Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 19751;27:35–206. [PubMed] [Google Scholar]
- 49.Stack DE, Byun J, Gross ML, Rogan EG, Cavalieri EL. Molecular characteristics of catechol estrogen quinones in reactions with deoxyribonucleosides. Chem Res Toxicol. 1996;9:851–9. doi: 10.1021/tx960002q. [DOI] [PubMed] [Google Scholar]
- 50.Gaikwad NW, Yang L, Muti P, Meza JL, Pruthi S, Ingle JN, Rogan EG, Cavalieri EL. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int J Cancer. 20081;122:949–57. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grossman MH, Emanuel BS, Budarf ML. Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1——q11.2. Genomics. 1992;12:822–825. doi: 10.1016/0888-7543(92)90316-k. [DOI] [PubMed] [Google Scholar]
- 53.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics:description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens:comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- 55.Yim DS, Parkb SK, Yoo KY, Yoon KS, Chung HH, Kang HL, Ahn SH, Noh DY, Choe KJ, Jang IJ, Shin SG, Strickland PT, Hirvonen A, Kang D. Relationship between the Val158Met polymorphism of catechol Omethyl transferase and breast cancer. Pharmacogenetics. 2001;11:279–286. doi: 10.1097/00008571-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Lavigne JA, Helzlsouer KJ, Huang HY, Strickland PT, Bell DA, Selmin O, Watson MA, Hoffman S, Comstock GW, Yager JD. An association between the allele coding for a low activity variant of catechol-Omethyltransferase and the risk for breast cancer. Cancer Res. 1997;57:5493–5497. [PubMed] [Google Scholar]
- 57.Thompson PA, Shields PG, Freudenheim JL, Stone A, Vena JE, Marshall JR, Graham S, Laughlin R, Nemoto T, Kadlubar FF, Ambrosone CB. Genetic polymorphisms in catechol-O-methyltransferase, menopausal status, and breast cancer risk. Cancer Res. 1998;58:2107–2110. [PubMed] [Google Scholar]
- 58.Mitrunen K, Jourenkova N, Kataja V, Eskelinen M, Kosma VM, Benhamou S, Kang D, Vainio H, Uusitupa M, Hirvonen A. Polymorphic catechol-Omethyltransferase gene and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:635–640. [PubMed] [Google Scholar]
- 59.Qin X, Peng Q, Qin A, Chen Z, Lin L, Deng Y, Xie L, Xu J, Li H, Li T, Li S, Zhao J. Association of COMT Val158Met polymorphism and breast cancer risk: an updated meta-analysis. Diagnostic Pathology. 2012;7:136. doi: 10.1186/1746-1596-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavalieri EL, Rogan EG. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J Steroid Biochem Mol Biol. 2011;125:169–80. doi: 10.1016/j.jsbmb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taioli E, Bradlow HL, Garbers SV, Sepkovic DW, Osborne MP, Trachman J, Ganguly S, Garte SJ. Role of estradiol metabolism and CYP1A1 polymorphisms in breast cancer risk. Cancer Detect Prev. 1999;23:232–237. doi: 10.1046/j.1525-1500.1999.09912.x. [DOI] [PubMed] [Google Scholar]
- 62.Miyoshi Y, Takahashi Y, Egawa C, Noguchi S. Breast cancer risk associated with CYP1A1genetic polymorphisms in Japanese women. Breast J. 2002;8:209–21. doi: 10.1046/j.1524-4741.2002.08404.x. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe J, Shimada T, Gillam EM, Ikuta T, Suemasu K, Higashi Y, Gotoh O, Kawajiri K. Association of CYP1B1genetic polymorphism with incidence to breast and lung cancer. Pharmacogenetics. 2000;10:25–33. doi: 10.1097/00008571-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Zheng W, Xie DW, Jin F, Cheng JR, Dai Q, Wen WQ, Shu XO, Gao YT. Genetic polymorphism of cytochrome P450-1B1 and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:147–50. [PubMed] [Google Scholar]
- 65.Rylander-Rudqvist T, Wedren S, Granath F, Humphreys K, Ahlberg S, Weiderpass E, Oscarson M, Ingelman-Sundberg M, Persson I. Cytochrome P450 1B1 gene polymorphisms and postmenopausal breast cancer risk. Carcinogenesis. 2003;24:1533–9. doi: 10.1093/carcin/bgg114. [DOI] [PubMed] [Google Scholar]
- 66.de Jong MM, Nolte IM, te Meerman GJ, van der Graaf WT, Oosterwijk JC, Kleibeuker JH, Schaapveld M, de Vries EG. Genes other than BRCA1 and BRCA2 involved in breast cancer susceptibility. J Med Genet. 2002;39:225–42. doi: 10.1136/jmg.39.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reding KW, Weiss NS, Chen C, Li CI, Carlson CS, Wilkerson HW, Farin FM, Thummel KE, Daling JR, Malone KE. Genetic polymorphisms in the catechol estrogen metabolism pathway and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1461–7. doi: 10.1158/1055-9965.EPI-08-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen W, Cai Q, Shu XO, Cheng JR, Parl F, Pierce L, Gao YT, Zheng W. Cytochrome P450 1B1 and catechol-O-methyltransferase genetic polymorphisms and breast cancer risk in Chinese women: results from the Shanghai breast cancer study and a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:329–35. doi: 10.1158/1055-9965.EPI-04-0392. [DOI] [PubMed] [Google Scholar]
- 69.Li N, Dong J, Hu Z, Shen H, Dai M. Potentially functional polymorphisms in ESR1 and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 20101;121:77–84. doi: 10.1007/s10549-009-0532-9. [DOI] [PubMed] [Google Scholar]
- 70.Yu KD, Rao NY, Chen AX, Fan L, Yang C, Shao ZM. A systematic review of the relationship between polymorphic sites in the estrogen receptor-beta (ESR2) gene and breast cancer risk. Breast Cancer Res Treat. 20113;126:7–45. doi: 10.1007/s10549-010-0891-2. [DOI] [PubMed] [Google Scholar]
- 71.Chattopadhyay S, Siddiqui S, Akhtar MS, Najm MZ, Deo SV, Shukla NK, Husain SA. Genetic polymorphisms of ESR1, ESR2, CYP17A1, and CYP19A1 and the risk of breast cancer: a case control study from North India. Tumour Biol. 2014 doi: 10.1007/s13277-013-1594-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 72.Talbott KE, Gammon MD, Kibriya MG, Chen Y, Teitelbaum SL, Long CM, Gurvich I, Santella RM, Ahsan H. A CYP19 (aromatase) polymorphism is associated with increased premenopausal breast cancer risk. Breast Cancer Res Treat. 2008;111:481–7. doi: 10.1007/s10549-007-9794-2. [DOI] [PubMed] [Google Scholar]
- 73.Reding KW, Chen C, Lowe K, Doody DR, Carlson CS, Chen CT, Houck J, Weiss LK, Marchbanks PA, Bernstein L, Spirtas R, McDonald JA, Strom BL, Burkman RT, Simon MS, Liff JM, Daling JR, Malone KE. Estrogen-related genes and their contribution to racial differences in breast cancer risk. Cancer Causes Control. 2012;23:671–81. doi: 10.1007/s10552-012-9925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai Q, Kataoka N, Li C, Wen W, Smith JR, Gao YT, Shu XO, Zheng W. Haplotype analyses of CYP19A1 gene variants and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2008;17:27–32. doi: 10.1158/1055-9965.EPI-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feigelson HS, Coetzee GA, Kolonel LN, Ross RK, Henderson BE. A polymorphism in the CYP17 gene increases the risk of breast cancer. Cancer Res. 19971;57:063–5. [PubMed] [Google Scholar]
- 76.Chen Y, Gammon MD, Teitelbaum SL, Britton JA, Terry MB, Shantakumar S, Eng SM, Wang Q, Gurvich I, Neugut AI, Santella RM, Ahsan H. Estrogen-biosynthesis gene CYP17 and its interactions with reproductive, hormonal and lifestyle factors in breast cancer risk: results from the Long Island Breast Cancer Study Project. Carcinogenesis. 2008;29:766–71. doi: 10.1093/carcin/bgn042. [DOI] [PubMed] [Google Scholar]
- 77.Helzlsouer KJ, Huang HY, Strickland PT, Hoffman S, Alberg AJ, Comstock GW, Bell DA. Association between CYP17 polymorphisms and the development of breast cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:945–9. [PubMed] [Google Scholar]
- 78.Setiawan VW, Schumacher FR, Haiman CA, Stram DO, Albanes D, Altshuler D, Berglund G, Buring J, Calle EE, Clavel-Chapelon F, Cox DG, Gaziano JM, Hankinson SE, Hayes RB, Henderson BE, Hirschhorn J, Hoover R, Hunter DJ, Kaaks R, Kolonel LN, Kraft P, Ma J, Le Marchand L, Linseisen J, Lund E, Navarro C, Overvad K, Palli D, Peeters PH, Pike MC, Riboli E, Stampfer MJ, Thun MJ, Travis R, Trichopoulos D, Yeager M, Ziegler RG, Spencer Feigelson H, Chanock SJ. CYP17 genetic variation and risk of breast and prostate cancer from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium (BPC3) Cancer Epidemiol Biomarkers Prev. 2007;16:2237–46. doi: 10.1158/1055-9965.EPI-07-0589. [DOI] [PubMed] [Google Scholar]
- 79.Parl FF. Estrogens, estrogen receptor and breast cancer. IOS Press; Amsterdam: 2000. Estrogen receptor expression in breast cancer; pp. 135–204. [Google Scholar]
- 80.Trichopoulos D, MacMahon B, Cole P. Menopause and breast cancer risk. J Natl Cancer Inst. 1972;48:605–13. [PubMed] [Google Scholar]
- 81.Roodi N, Bailey LR, Kao WY, Verrier CS, Yee CJ, Dupont WD, Parl FF. Estrogen receptor gene analysis in estrogen receptor-positive and receptor-negative primary breast cancer. J Natl Cancer Inst. 1995;87:446–51. doi: 10.1093/jnci/87.6.446. [DOI] [PubMed] [Google Scholar]
- 82.Gordon GB, Bush TL, Helzlsouer KJ, Miller SR, Comstock GW. Relationship of serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing postmenopausal breast cancer. Cancer Res. 1990;50:3859–62. [PubMed] [Google Scholar]
- 83.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, Strax P, Pasternack BS. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87:190–7. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 84.Dorgan JF, Longcope C, Stephenson HE, Jr, Falk RT, Miller R, Franz C, Kahle L, Campbell WS, Tangrea JA, Schatzkin A. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:533–9. [PubMed] [Google Scholar]
- 85.Berrino F, Muti P, Micheli A, Bolelli G, Krogh V, Sciajno R, Pisani P, Panico S, Secreto G. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst. 1996;88:291–6. doi: 10.1093/jnci/88.5.291. [DOI] [PubMed] [Google Scholar]
- 86.Dorgan JF, Stanczyk FZ, Longcope C, Stephenson HE, Jr, Chang L, Miller R, Franz C, Falk RT, Kahle L. Relationship of serum dehydroepiandrosterone (DHEA), DHEA sulfate, and 5-androstene-3 beta, 17 beta-diol to risk of breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers prev. 1997;6:177–81. [PubMed] [Google Scholar]
- 87.Zeleniuch-Jacquotte AB, Bruning PF, Bonfrer JM, Koenig KL, Shore RE, Kim MY, Pasternack BS, Toniolo P. Relation of serum levels of testosterone and dehydroepiandrosterone sulfate to risk of breast cancer in postmenopausal women. Am J Epidemiol. 1997;145:1030–8. doi: 10.1093/oxfordjournals.aje.a009059. [DOI] [PubMed] [Google Scholar]
- 88.Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–9. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 89.Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1999;130:270–7. doi: 10.7326/0003-4819-130-4_part_1-199902160-00004. [DOI] [PubMed] [Google Scholar]
- 90.Key T, Appleby P, Barnes I, Reeves G Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 91.Manjer J, Johansson R, Berglund G, Janzon L, Kaaks R, Agren A, Lenner P. Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden) Cancer Causes Control. 2003;14:599–607. doi: 10.1023/a:1025671317220. [DOI] [PubMed] [Google Scholar]
- 92.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 93.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, Dossus L, Lukanova A, Bingham S, Khaw KT, Allen NE, Bueno-de-Mesquita HB, van Gils CH, Grobbee D, Boeing H, Lahmann PH, Nagel G, Chang-Claude J, Clavel-Chapelon F, Fournier A, Thiébaut A, González CA, Quirós JR, Tormo MJ, Ardanaz E, Amiano P, Krogh V, Palli D, Panico S, Tumino R, Vineis P, Trichopoulou A, Kalapothaki V, Trichopoulos D, Ferrari P, Norat T, Saracci R, Riboli E. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 94.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer: Does the association vary by a woman’s predicted breast cancer risk? J Clin Oncol. 2006;24:1823–1830. doi: 10.1200/JCO.2005.03.7432. [DOI] [PubMed] [Google Scholar]
- 95.Baglietto L, Severi G, English DR, Krishnan K, Hopper JL, McLean C, Morris HA, Tilley WD, Giles GG. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:492–502. doi: 10.1158/1055-9965.EPI-09-0532. [DOI] [PubMed] [Google Scholar]
- 96.Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, Wieand HS. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J NatlCancer Inst. 1999;91:1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 97.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152:950–964. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 98.Beattie MS, Costantino JP, Cummings SR, Wickerham DL, Vogel VG, Dowsett M, Folkerd EJ, Willett WC, Wolmark N, Hankinson SE. Endogenous Sex Hormones, Risk of Breast Cancer, and Response to Tamoxifen: An Ancillary Study in the NSABP Breast Cancer Prevention Trial (P-1) J Natl Cancer Inst. 2006;18:110–5. doi: 10.1093/jnci/djj011. [DOI] [PubMed] [Google Scholar]
- 99.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–68. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chlebowski RT, Anderson GL. Changing concepts: Menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012;104:517–27. doi: 10.1093/jnci/djs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Joffe MM, Byrne C, Colditz GA. Postmenopausal hormone use, screening and breast cancer characterization and control of a bias. Epidemiology. 2001;12:429–438. doi: 10.1097/00001648-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat. 2013;137:883–92. doi: 10.1007/s10549-012-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Key TJ. Endogenous oestrogens and breast cancer risk in premenopausal and postmenopausal women. Steroids. 2011;76:812–5. doi: 10.1016/j.steroids.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 104.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Jr, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, HankinsonE SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Longcope C Endogenous Hormones Breast Cancer Collaborative Group. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–26. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 105.Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, Rollison DE, Dorgan JF, Brinton LA, Overvad K, Kaaks R, Trichopoulou A, Clavel-Chapelon F, Panico S, Duell EJ, Peeters PH, Rinaldi S, Fentiman IS, Dowsett M, Manjer J, Lenner P, Hallmans G, Baglietto L, English DR, Giles GG, Hopper JL, Severi G, Morris HA, Hankinson SE, Tworoger SS, Koenig K, Zeleniuch-Jacquotte A, Arslan AA, Toniolo P, Shore RE, Krogh V, Micheli A, Berrino F, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Lui LY, Cummings SR, Gunter MJ, Rohan TE, Strickler HD Endogenous Hormones and Breast Cancer Collaborative Group. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–22. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 107.Söderqvist G. Effects of sex steroids on proliferation in normal mammary tissue. Ann Med. 1998;30:511–24. [PubMed] [Google Scholar]
- 108.Henderson BE, Ross RK, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1988;48:246–53. [PubMed] [Google Scholar]
- 109.Verheus M, Peeters PH, van Noord PA, van der Schouw YT, Grobbee DE, van Gils CH. No relationship between circulating levels of sex steroids and mammographic breast density: the Prospect-EPIC cohort. Breast Cancer Res. 2007;9:R53. doi: 10.1186/bcr1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:2641–7. doi: 10.1158/1055-9965.EPI-05-0558. [DOI] [PubMed] [Google Scholar]
- 111.Greendale GA, Palla SL, Ursin G, Laughlin GA, Crandall C, Pike MC, Reboussin BA. The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Am J Epidemiol. 2005;162:826–34. doi: 10.1093/aje/kwi286. [DOI] [PubMed] [Google Scholar]
- 112.van Landeghem AA, Poortman J, Nabuurs M, Thijssen JH. Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res. 1985;45:2900–2906. [PubMed] [Google Scholar]
- 113.Lønning PE, Helle H, Duong NK, Ekse D, Aas T, Geisler J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol. 2009;117:31–41. doi: 10.1016/j.jsbmb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 114.Lønning PE, Haynes BP, Straume AH, Dunbier A, Helle H, Knappskog S, Dowsett Mitch. Exploring breast cancer estrogen disposition: the basis for endocrine manipulation. Clin Cancer Res. 2011;17:4948–58. doi: 10.1158/1078-0432.CCR-11-0043. [DOI] [PubMed] [Google Scholar]
- 115.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kabat GC, Chang CJ, Sparano JA, Sepkovie DW, Hu XP, Khalil A, Rosenblatt R, Bradlow HL. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1997;6:505–9. [PubMed] [Google Scholar]
- 117.Meilahn EN, De Stavola B, Allen DS, Fentiman I, Bradlow HL, Sepkovic DW, Kuller LH. Do urinary oestrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br J Cancer. 1998;78:1250–5. doi: 10.1038/bjc.1998.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dallal CM, Stone RA, Cauley JA, Ness RB, Vogel VG, Fentiman IS, Fowke JH, Krogh V, Loft S, Meilahn EN, Muti P, Olsen A, Overvad K, Sieri S, Tjønneland A, Ursin G, Wellejus A, Taioli E. Urinary estrogen metabolites and breast cancer: a combined analysis of individual level data. Int J Biol Markers. 2013;23:3–16. doi: 10.5301/JBM.2012.9353. [DOI] [PubMed] [Google Scholar]
- 119.Obi N, Vrieling A, Heinz J, Chang-Claude J. Estrogen metabolite ratio: Is the 2-hydroxyestrone to 16α-hydroxyestrone ratio predictive for breast cancer? Int J Womens Health. 2011;8:37–51. doi: 10.2147/IJWH.S7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, Buys SS, Isaacs C, Keefer LK, Veenstra TD, Berg CD, Hoover RN, Ziegler RG. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104:326–39. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV, Cauley JA, Hue TF, Lacroix A, Falk RT, Pfeiffer R, Fuhrman BJ, Veenstra TD, Xu X, Brinton LA. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B~FIT. Carcinogenesis. 2014;35:346–355. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, Gierach GL. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15:R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wysowski DK, Comstock GW, Helsing KJ, Lau HL. Sex hormone levels in serum in relation to the development of breast cancer. Am J Epidemiol. 1987;125:791–99. doi: 10.1093/oxfordjournals.aje.a114596. [DOI] [PubMed] [Google Scholar]
- 124.Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev. 1994;18:79–85. [PubMed] [Google Scholar]
- 125.Rosenberg CR, Pasternack BS, Shore RE, Koenig KL, Toniolo PG. Premenopausal estradiol levels and the risk of breast cancer: a new method of controlling for day of the menstrual cycle. Am J Epidemiol. 1994;140:518–25. doi: 10.1093/oxfordjournals.aje.a117278. [DOI] [PubMed] [Google Scholar]
- 126.Thomas HV, Key TJ, Allen DS, Moore JW, Dowsett M, Fentiman IS, Wang DY. A prospective study of endogenous serum hormone concentrations and breast cancer risk in premenopausal women on the island of Guernsey. Br J Cancer. 1997;75:1075–79. doi: 10.1038/bjc.1997.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiol Biomarkers Prev. 2000;9:575–79. [PubMed] [Google Scholar]
- 128.Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, Clavel-Chapelon F, Fournier A, van Gils CH, Gonzalez CA, Gurrea AB, Critselis E, Khaw KT, Krogh V, Lahmann PH, Nagel G, Olsen A, Onland-Moret NC, Overvad K, Palli D, Panico S, Peeters P, Quirós JR, Roddam A, Thiebaut A, Tjønneland A, Chirlaque MD, Trichopoulou A, Trichopoulos D, Tumino R, Vineis P, Norat T, Ferrari P, Slimani N, Riboli E. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97:755–65. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 129.Dorgan JF, Stanczyk FZ, Kahle LL, Brinton LA. Prospective case-control study of premenopausal serum estradiol and testosterone levels and breast cancer risk. Breast Cancer Res. 2010;12:R98. doi: 10.1186/bcr2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–15. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 131.Schernhammer ES, Sperati F, Razavi P, Agnoli C, Sieri S, Berrino F, Krogh V, Abbagnato CA, Grioni S, Blandino G, Schunemann HJ, Muti P. Endogenous sex steroids in premenopausal women and risk of breast cancer: the ORDET cohort. Breast Cancer Res. 2013;15:R46. doi: 10.1186/bcr3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fortner RT, Eliassen AH, Spiegelman D, Willett WC, Barbieri RL, Hankinson SE. Premenopausal endogenous steroid hormones and breast cancer risk: results from the Nurses’ Health Study II. Breast Cancer Res. 2013;15:R19. doi: 10.1186/bcr3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hankinson SE, Eliassen AH. Circulating sex steroids and breast cancer risk in premenopausal women. Horm Cancer. 2010;1:2–10. doi: 10.1007/s12672-009-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Russo J, Russo IH. Genotoxicity of steroidal estrogens. Trends Endocrinol Metab. 2004;15:211–214. doi: 10.1016/j.tem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 135.Walker K, Bratton DJ, Frost C. Premenopausal endogenous oestrogen levels and breast cancer risk: a meta-analysis. Br J Cancer. 20111;105:451–7. doi: 10.1038/bjc.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Endogenous Hormones and Breast Cancer Collaborative Group. Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoffman-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009–19. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muti P, Bradlow HL, Micheli A, Krogh V, Freudenheim JL, Schunemann HJ, Stanulla M, Yang J, Sepkovic DW, Trevisan M, Berrino F. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11:635–40. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 138.Arslan AA, Shore RE, Afanasyeva Y, Koenig KL, Toniolo P, Zeleniuch-Jacquotte A. Circulating estrogen metabolites and risk for breast cancer in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18:2273–9. doi: 10.1158/1055-9965.EPI-09-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eliassen AH, Spiegelman D, Xu X, Keefer LK, Veenstra TD, Barbieri RL, Willett WC, Hankinson SE, Ziegler RG. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res. 2012;72:696–706. doi: 10.1158/0008-5472.CAN-11-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomas HV, Key TJ, Allen DS, Moore JW, Dowsett M, Fentiman IS, Wang DY. A prospective study of endogenous serum hormone concentrations and breast cancer risk in post-menopausal women on the island of Guernsey. Br J Cancer. 1997;76:401–5. doi: 10.1038/bjc.1997.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barrett-Connor E, Friedlander NJ, Khaw KT. Dehydroepiandrosterone sulfate and breast cancer risk. Cancer Res. 1990;50:6571–4. [PubMed] [Google Scholar]
- 142.Garland CF, Friedlander NJ, Barrett-Connor E, Khaw KT. Sex hormones and postmenopausal breast cancer: a prospective study in an adult community. Am J Epidemiol. 1992;135:1220–30. doi: 10.1093/oxfordjournals.aje.a116228. [DOI] [PubMed] [Google Scholar]
- 143.Fowke JH, Qi D, Bradlow HL, Shu XO, Gao YT, Cheng JR, Jin F, Zheng W. Urinary estrogen metabolites and breast cancer: differential pattern of risk found with pre-versus post-treatment collection. Steroids. 2003;68:65–72. doi: 10.1016/s0039-128x(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 144.Wellejus A, Olsen A, Tjonneland A, Thomsen BL, Overvad K, Loft S. Urinary hydroxyestrogens and breast cancer risk among postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2005;14:2137–42. doi: 10.1158/1055-9965.EPI-04-0934. [DOI] [PubMed] [Google Scholar]
- 145.Ursin G, London S, Stanczyk FZ, Gentzschein E, Paganini-Hill A, Ross RK, Pike MC. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91:1067–72. doi: 10.1093/jnci/91.12.1067. [DOI] [PubMed] [Google Scholar]
- 146.Kabat GC, O’Leary ES, Gammon MD, Sepkovic DW, Teitelbaum SL, Britton JA, Terry MB, Neugut AI, Bradlow HL. Estrogen metabolism and breast cancer. Epidemiology. 2006;17:80–8. doi: 10.1097/01.ede.0000190543.40801.75. [DOI] [PubMed] [Google Scholar]
- 147.Cauley JA, Zmuda JM, Danielson ME, Ljung BM, Bauer DC, Cummings SR, Kuller LH. Estrogen metabolites and the risk of breast cancer in older women. Epidemiology. 2003;14:740–744. doi: 10.1097/01.ede.0000091607.77374.74. [DOI] [PubMed] [Google Scholar]
- 148.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2029–2035. doi: 10.1158/1055-9965.EPI-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]


