Abstract
Background
Isolated limb infusion (ILI) with melphalan is a minimally invasive, effective treatment for in transit melanoma. We hypothesized that burden of disease (BOD) would correlate to treatment response.
Methods
We retrospectively analyzed a prospectively collected database from two academic centers. BOD was stratified as high or low (less than 10 lesions, none > 2cm). Response rates were measured 3 months post-ILI. Multivariable analysis (MV) was used to evaluate the association between the response rate and BOD. Kaplan-Meier methods with log-rank tests and multivariable Cox proportional hazard models were used to analyze overall survival (OS) and progression free survival (PFS)
Results
Sixty (38%) patients had low and 100 (62%) high BOD. Patients with low BOD had an overall response rate (ORR) of 73%, and 50% CR; compared to an ORR of 47% and 24% CR in patients with high BOD (p= 0.002). MV analysis of preoperative, intraoperative, and postoperative parameters showed no significant impact on 3-month response. Patients with a CR at 3 months demonstrated improved PFS over the remainder of the cohort, but OS was equal. Low BOD patients had an increased median PFS of 6.9 vs 3.8 months (p= 0.047), and a non-statistically significantly increased median OS, 38.4 vs. 30.9 months (p=0.146).
Conclusions
Lower BOD is associated with an increased ORR and CR rate with statistically significantly improved PFS in patients undergoing ILI for in transit extremity melanoma. BOD provides useful prognostic information for patient counseling and serves as a marker to stratify patient risk groups.
Introduction
Melanoma is increasing in incidence faster than any other malignancy in the United States, with over 70,000 new cases annually, making it a significant health concern.1 Most melanomas are detected early and are associated with a good prognosis.2 An unusual pattern of spread that is unique to melanoma is that of regional in transit metastases, thought to represent the growth of tumor deposits in dermal or subcutaneous lymphatic channels, which occurs in 2-10% of melanomas and can be present without evidence of distant disease.3
In extremity melanomas this situation represents a unique therapeutic opportunity in that the circulation of the limb can be isolated from the rest of the body through the techniques of hyperthermic isolated limb perfusion (HILP) and isolated limb infusion (ILI), allowing the delivery of high doses of chemotherapy to only the tissues of the affected limb.4-6 Several different groups have reported single and multi-institution studies ILI with melphalan (ILI-M), with overall response rates ranging from 53-84%, and complete responses occurring in 25-38% of patients.7-10
Because the efficacy of ILI is extremely variable, recent studies have sought to identify factors that would predict an individual patient's response to treatment, but so far these answers have remained elusive.11 Lidksy et al, looked at intraoperative, perioperative, patient and disease related factors in patients with intransit disease of the extremities undergoing either first time ILI or HILP. Burden of disease (BOD) was not readily defined and the authors concluded that no patient-related clinical, pathological or technical factors proved to be a significant predictor of progressive disease. 11 Steinman et al also published a small series in 2013 looking at BOD in patients undergoing ILI. In that series ILI was performed in 62 patients over 12 years with mixed histologies included. In the current study, we analyzed a large database of patients treated similarly in terms of technique of ILI for in transit melanoma. We proposed that, BOD might be a predictor of response to ILI.
Methods
Separate prospectively collected databases of patients undergoing ILI at Duke University, Durham, NC and at Moffitt Cancer Center, Tampa, FL were reviewed after IRB approval for the study. The patients were selected for study inclusion based on the following criteria: 1) First time ILI-M for in transit extremity melanoma, 2) Measurable BOD noted and recorded pre-operatively, 3) 3-month follow-up data available.
Definition of Burden of Disease
Burden of disease was defined as follows
Low BOD: less than 10 distinct lesions, none greater than 2cm in maximal dimension
High BOD: more than 10 distinct lesions, or any single lesion greater than 2cm in maximal dimension. We chose 10 lesions, or any lesion larger than 2 cm, as the cut off due to our previous observations that patients with a smaller number of lesions in general and smaller tumors appeared to do better after ILI.
Statistical Analysis
Demographic and clinical variables were summarized, and Pearson exact Chi-square tests or Van der Waerden normal scores tests were used to test the difference between BOD groups. Response rates were calculated for all patients combined and by BOD status. Normal scores tests and Fisher's exact tests were used to determine if there is difference between response status and continuous or categorical variables respectively. Univariable and multivariable logistic regression were employed to generate odds ratios of BOD for predicting overall response (CR/PR), while adjusting for potential confounding variables. Kaplan-Meier plots along with log-rank tests were used to display the probabilities of overall survival (OS), progression free survival (PFS) and time to progression (TTP).
Operative Technique
The ILI procedures were performed as we have previously described in the literature.12 Briefly, each limb infusion involved percutaneous placement of arterial and venous catheters in the affected limb. Actinomycin-D (100 μg/L) and melphalan (7.5 mg/L for LE and 10 mg/L for UE) were dosed based on limb volume, and further corrected for patient ideal body weight. After the limb was warmed to ≥37 degrees Celsius, chemotherapy was circulated for 30 min and then the limb was washed out with saline before tourniquet release. Typically the ILI was performed within 2-3 weeks of the diagnosis or referral to our centers for in transit disease management. There was no difference in ILI technique or follow up for the patients at either center.
Postoperative Follow-up
Patients were monitored with daily physical examination and twice daily assessment of creatine kinase (CK) levels and discharged home once the CK peaked. Response to treatment was assessed at 3 months and patients were assigned to response groups (complete response (CR), partial response (PR), stable disease (SD), progression of disease (PD) according to RECIST criteria guidelines modified for cutaneous lesions.13 The patients were seen every 3 months for year 1 and then every 3-6 months thereafter with physical examination and full body PET/CT imaging.14 Overall response rate (ORR) was defined as the percentage of patients with CR or PR. PFS was defined as the time from ILI until evidence of any disease progression or death. TTP was defined as the time from ILI until PD was noted within the treatment field.
Results
Cohort
Between December 2003 and February 2013, 160 patients were identified that met inclusion criteria. Eighty-five patients underwent ILI at Duke University Hospital; 75 at Moffitt Cancer Center. One hundred patients (62.5%) had high BOD and 60 (32.5%) had low BOD. Fifty-seven percent of the patients were female, with age that ranged from 29-89 years (mean 67 years). Mean Breslow depth of the primary melanoma was 3.4mm overall (3.2mm low BOD; 3.4 mm high BOD, p=ns). All patients were stage IIIb or IIIc at time of ILI.
Procedural data
80% of the ILIs performed were in the lower extremity. Papavarine (60 mg) was used in the circuit in all but 25%. As shown in table 1, there were no significant differences seen between the high and low BOD groups in regard to preoperative, intraoperative, and postoperative factors including papaverine use, intra-operative blood gas values (pH, base excess, and PaO2), ischemia time, post-operative peak CPK values, or Wieberdink toxicity scores.15
Table 1. Demographic and peri-operative variables.
| Variable | levels | Overall | Low BOD (n = 60) | High BOD (n = 100) | p-value* |
|---|---|---|---|---|---|
| Age | Mean (sd) | 67.4 (12.7) | 67 (12.5) | 67.7 (12.9) | 0.619 |
| range | (29, 89) | (29, 89) | (34, 88) | ||
| Sex | F | 91 (56.9) | 42 (70.0) | 49 (49.0) | 0.013 |
| M | 69 (43.1) | 18 (30.0) | 51 (51.0) | ||
| Breslow depth (mm) | Mean (sd) | 3.4 (2.5) | 3.2 (2.7) | 3.5 (2.4) | 0.382 |
| Extremity | Upper | 32 (20.0) | 9 (15.0) | 23 (23.0) | 0.312 |
| Lower | 128 (80.0) | 51 (85.0) | 77 (77.0) | ||
| Clinical Site | Duke | 85 (53.1) | 34 (56.7) | 51 (51.0) | 0.539 |
| Moffitt | 75 (46.9) | 26 (43.3) | 49 (49.0) | ||
| Papaverine | No | 41 (25.6) | 16 (26.7) | 25 (25.0) | 0.846 |
| Yes | 119 (74.4) | 44 (73.3) | 75 (75.0) | ||
| Mean Blood Gas values from circuit after 30 minutes of circulation (SD) | Base Excess | -9.6 (3.8) | -9.8 (3.2) | -9.4 (4.2) | 0.529 |
| PaO2 | 10.1 (7.2) | 10 (7.9) | 10.2 (6.8) | 0.577 | |
| pH | 7.2 (0.1) | 7.2 (0.1) | 7.2 (0.1) | 0.759 | |
| Ischemia time (min) | Mean (sd) | 64.4 (16.5) | 64.2 (14.7) | 64.6 (17.5) | 0.879 |
| Post-op CPK peak values | Mean (sd) | 1577 (2640.3) | 2180 (3505.2) | 1218 (1883.5) | 0.068 |
| Wieberdink Peak Score | 1 | 20 (12.5) | 4 (6.7) | 16 (16.0) | 0.236 |
| 2 | 85 (53.1) | 34 (56.7) | 51 (51.0) | ||
| 3 | 43 (26.9) | 19 (31.7) | 24 (24.0) | ||
| 4 | 8 (5.0) | 2 (3.3) | 6 (6.0) | ||
| unavailable | 4 (2.5) | 1 (1.7) | 3 (3.0) |
Exact Chi-square test with monte carlo simulation BOD- Burden of Disease, CPK-Creatine Phosphokinase
Response to Treatment
Response to treatment was assessed at 3 months using RECIST criteria modified for cutaneous lesions, and was stratified by high or low burden of disease. As shown in table 2, those with low BOD demonstrated an ORR of 73%, with 50% of those responses being CRs, as opposed to an ORR of 47% (24% CRs) in patients with high BOD (p=0.002). PRs were similar between groups, both at 23%. A minority of patients in both groups had SD (3% and 14% in the low and high BOD groups, respectively). Thirty-nine percent of the patients in the high BOD group developed PD by 3 months, as opposed to 23% of the low BOD group. These results were statistically significant (p=0.002).
Table 2. Response to ILI at 3 months by Burden of Disease patients (High vs. Low): N (%).
| Complete Response | Partial Response | Stable Disease | Progressive Disease | p-value* | ||
|---|---|---|---|---|---|---|
| Burden of disease | High (n=100) | 24 (24.0) | 23 (23.0) | 14 (14.0) | 39 (39.0) | 0.002 |
| Low (n=60) | 30 (50.0) | 14 (23.3) | 2 (3.3) | 14 (23.3) |
Fisher Exact test.
Univariable and Multivariable Analysis
Univariable analysis was performed using all the demographic and peri-operative variables shown in Table 1, comparing responders (CR+PR) to non-responders (SD+PD). The only variable which demonstrated a statistically significant association to the 3-month clinical response was the treating institution (Duke vs. Moffitt). (data not shown)
All variables were analyzed using multivariable analysis with logistic regression to quantify the observed influence of BOD on response at 3 months, while adjusting for confounding variables. Again, the only significant associations noted were low versus high BOD and the institution. The final logistic regression model showed low BOD patients were 3.5 times more likely than high BOD patients to have a response to treatment at three months, after adjusting for the clinical site (OR=3.51, 95%CI:1.7-7.24, p<0.001).
Progression Free Survival (PFS)
Following ILI, patients at both institutions were followed at regular intervals with cross sectional imaging (PET and/or CT scans on an every 3-6 month basis) and physical exam, with a median follow up time of 17.3 months (range: 0.5-70). As shown in figure 1a, median PFS for low BOD patients was significantly higher, at 6.9 months, versus 3.8 months for high BOD patients (p= 0.047). We also looked at PFS as a function of response (CR, PR, SD) at 3 months (figure 1b). Median PFS for complete responders was 12 months, whereas patients with PR and SD had median PFS of 5.1 and 5.9 months, respectively (p<0.001). Patients with a CR had an increased median TTP of 25 months, versus 11months for those with a PR, and 9 months for SD (fig 1c).
Figure 1.
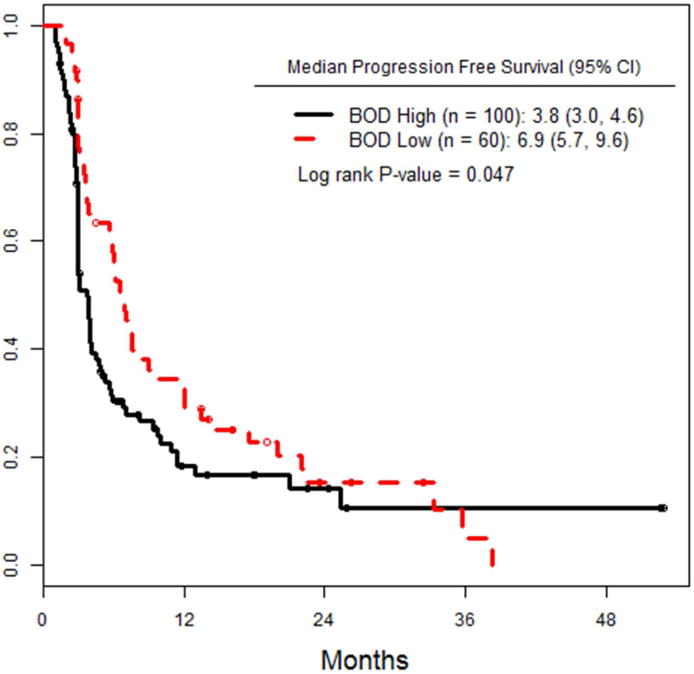
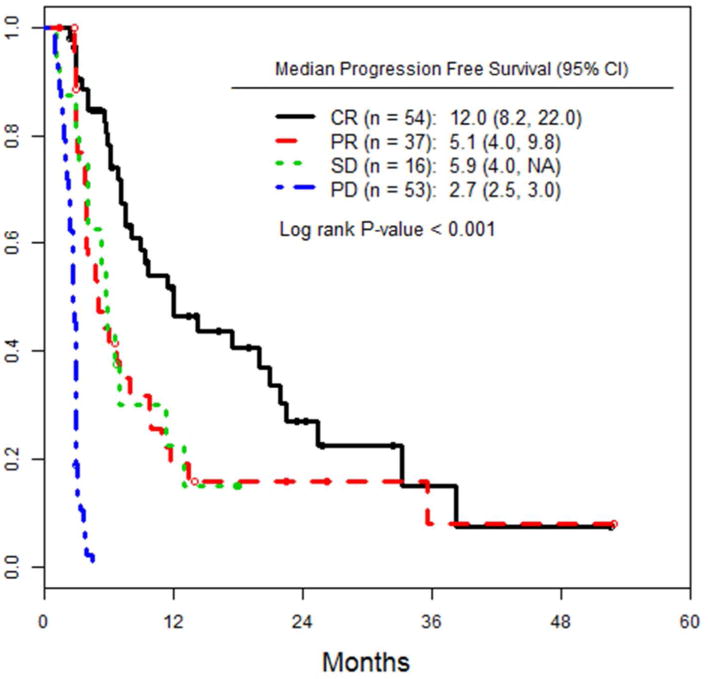
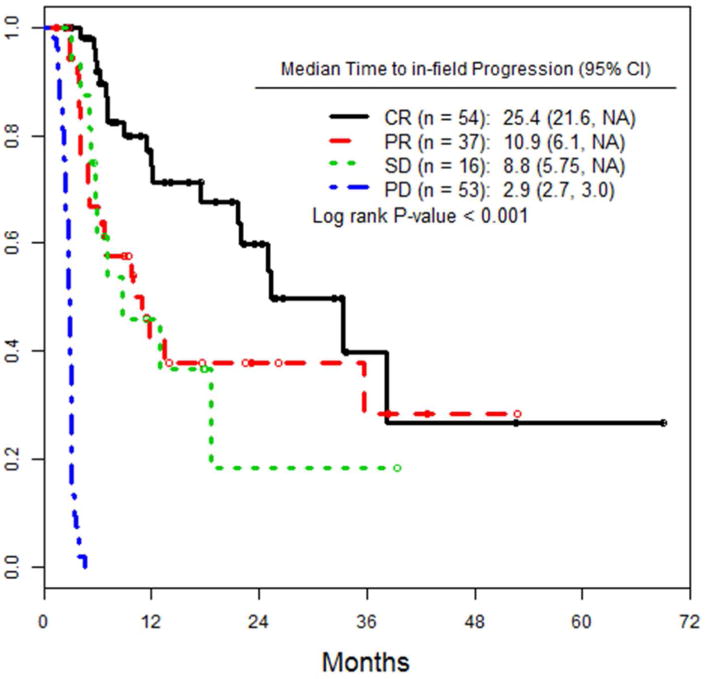
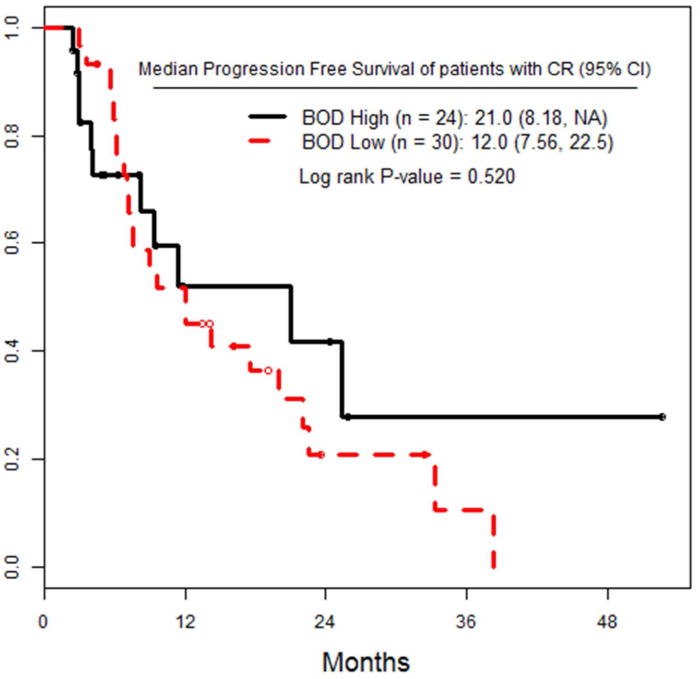
a) PFS of the entire cohort, stratified by high versus low pre-infusion BOD status. b) PFS of the entire cohort, stratified by RECIST response at 3 months after ILI. c) TTP in all patients, stratified by RECIST response at 3 months after ILI. d) PFS of the subset of patients who achieved a CR, stratified by pre-infusion BOD status.
Patients who attained a CR were also stratified by high or low BOD and analyzed for PFS. There were 24 CR patients with high BOD, and 30 CR patients with low BOD. There was no difference in PFS between these two subgroups (p= 0.52).
Overall Survival (OS)
Median OS for low BOD patients was 38.4 months, compared to 30.9 months for high BOD patients (p=0.146), (figure 2a). When OS was analyzed by response to treatment at 3 months (CR, PR, SD, PD), there were no significant differences seen between the groups in this study (figure 2b). 52 (32.5%) patients died during course of follow up. 19/60 (31.7%) low BOD and 33/100 (33%) high BOD (p=ns).
Figure 2.
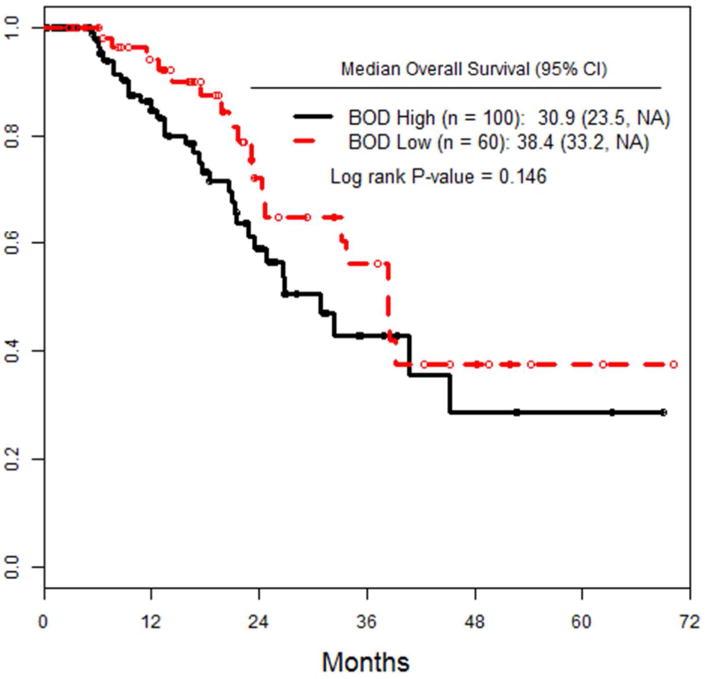
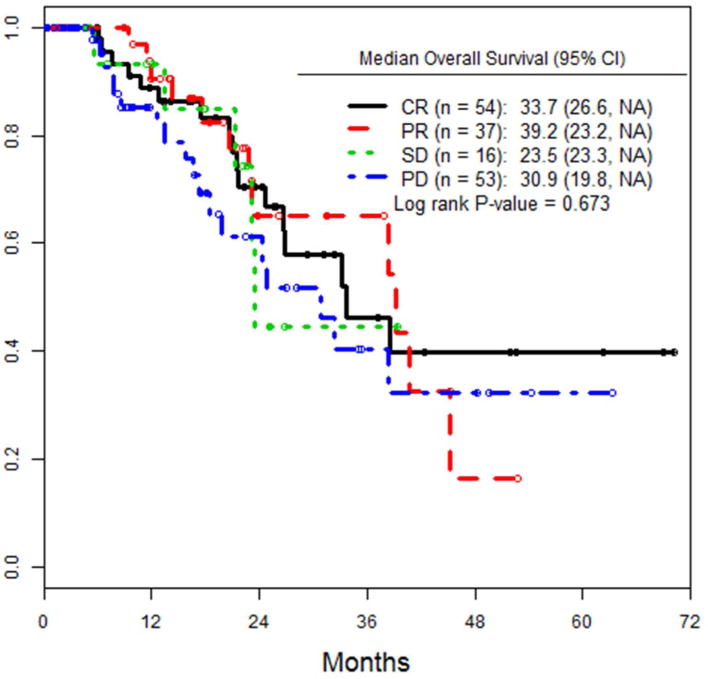
a) OS of all patients, stratified by pre-infusion BOD status. b) OS of the entire cohort, stratified by RECIST response at 3 months after ILI.
Discussion
While definitive explanations for variable responses probably exist inside the genes of individual melanoma cancer cells, the holy grail of personalized molecular therapeutics has yet to bear much tangible fruit. In this study, we sought to further clarify the role of BOD as a predictor of patient responses to ILI-M.
Lidsky et al. recently published a report in which BOD was examined as one of several prognostic factors after ILI, however BOD was not well defined in that study, and no statistically significant impact was found when analyzing the relationship between BOD or any other pre-, intra-, postoperative or tumor or patient related factor and response. Steinman et al. reported their experience with 62 patients undergoing an ILI for melanoma, Merkel cell carcinoma or sarcoma with 58 undergoing ILI for melanoma.16 They found a 48% CR rate for low BOD patients (similar to our findings) although their overall CR rate was lower than the current study at 25%. That study also showed an improved survival advantage for patients with low BOD undergoing ILI. 16 The limitations of the Steinman study included inclusion of different histologies and variable dosages of melphalan and actinomycin given, as well as 25 of the 62 patients were also retrospectively reviewed, (like the current study design where retrospective collection is also a limitation). 16
Our cohort numbered 160 stage III patients undergoing initial ILI for melanoma. The largest cohort presented to date is that of the Melanoma Institute of Australia (MIA), which included 185 patients undergoing initial ILI for melanoma.8 However the MIA data included 15 patients with stage II and 6 with stage 1 disease which might impact the response rates and outcomes reported.
We further examined the outcomes of patients who achieved a CR confirming findings of previous studies showing that patients with a CR demonstrate a longer RFS (figure 1c), but we did not see this translate to an increased OS (figure 2b). This is in contrast to the data reported by the MIA, where OS of 53 months was seen in CR patients, vs. 27 months for those with PR. OS was also significantly improved in the Steinman study in those patients with a CR. 16 Since 2010, there have been dramatic changes in the systemic therapy for metastatic melanoma with immunotherapies (ipilimumab (IPI) and anti PD-1 antibodies) and targeted therapy with BRAF and MEK inhibition changing the landscape of melanoma treatment.17,18 These treatments have had a major impact on survival in metastatic melanoma19 and theoretically may contribute to the lack of difference in overall survival seen in the current study if those who progressed were treated with such therapies on trial or as standard of care (data not available).
We also examined PFS in the patients who had a CR, comparing patients with low versus high BOD, and found no difference between them (figure 1d). This speaks to the biology of the tumor being the most significant determinant of outcome. It might be that a complete response to chemotherapy, whether regional or systemic, presages a better outcome, regardless of the initial BOD, though patients with low BOD were more likely to achieve a CR.
To gain insight on the potential impact that BOD could have in melanoma treatment and prognosis, consider the role of BOD in peritoneal malignancies. In ovarian cancer, primary peritoneal mesothelioma, as well as intraperitoneally disseminated gastrointestinal malignancies, the assessment of peritoneal BOD is rigorous and standardized, according to the peritoneal carcinomatosis index (PCI). In numerous publications on this topic it has been shown that patients with PCI scores > 20 (high BOD) have significantly worse long term survival. 20,21
The definition of BOD for in transit melanoma has not been standardized. We used 2cm as the cutoff point to distinguish high from low BOD, while several previous studies have defined high BOD as having any single lesion >3cm.16 Further refinement of this definition and development of a standardized in transit melanoma BOD score is an important next step for the future of research and therapy in this field. An ideal way to try to standardize the definition would have to be done in a working group or collaborative group of investigators specifically treating in transit melanoma with perfusion type modalities and prospectively collect burden of disease as well as treatment and outcomes based data in a registry and then analyze the data to determine the best definition of BOD. Disease features to incorporate in such a score might include the number and location of lesions (intra-muscular vs. subcutaneous vs. dermal), presence of ulceration in the primary tumor, as well as presence of lesions proximal to the elbow or knee.
Given the findings that patients with high BOD have lower rates of OR, CR, and shortened PFS, we ask if these patients should be managed according to a different algorithm than low BOD patients? Chai et al. touched on this topic while discussing repeat ILI and HILP.22 They proposed that in the setting of in transit disease and regional adenopathy an HILP might be a reasonable first choice over ILI. Most of the additional morbidity of HILP over ILI is due to the surgical exposure of the vessels and studies have shown an incrementally improved ORR for HILP over ILI.23 If no lymphadenectomy is required, potential alternatives to avoid the surgical morbidity of HILP are planned double ILI, or participation in clinical trials combining ILI with systemic treatment.
Incorporating a standardized in transit BOD scoring system would also be helpful in the analysis of ongoing and future investigational trials. A few promising current studies evaluating systemic IPI combined with ILI are in progress. The MSKCC group presented preliminary results at the 2014 ASCO meeting of 18 patients who underwent ILI-M, followed 1-3 weeks later by 4 doses of IPI, 3 weeks apart. ORR was 89% with 65% CRs at 3 months, and PFS was 57% at one year.24
A limitation of our study was its retrospective design, but the strengths are that the patients were prospectively followed and the ILI was performed with the same technique and patients were seen and evaluated at regular intervals to assess response. The relative rarity of ILIs even at our institutions, which are among the highest volume in the world, makes randomized prospective studies difficult to accomplish. As ILI grows in familiarity and as new and more effective chemotherapy agents are approved for use, it may become possible to organized larger scale, prospective, randomized trials to provide higher quality evidence regarding the efficacy of the procedure.
Table 3. Multivariable Analysis of Overall Response (CR/PR).
| Variable | Adjusted Odds Ratio (95%CI) | p-value* |
|---|---|---|
| Low vs. High BOD | 3.51 (1.70, 7.24) | < 0.001 |
| Moffitt vs. Duke | 2.75 (1.39, 5.44) | 0.004 |
Wald's test, n = 160
Synopsis.
Patients undergoing isolated limb infusion for in transit extremity melanoma were stratified by burden of disease. Low burden of disease patients had higher rates of complete response and improved disease free survival.
Acknowledgments
none
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Dec 20;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Annals of surgical oncology. 2005 Aug;12(8):587–596. doi: 10.1245/ASO.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Minor DR, Allen RE, Alberts D, Peng YM, Tardelli G, Hutchinson J. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985 Jun 1;55(11):2638–2644. doi: 10.1002/1097-0142(19850601)55:11<2638::aid-cncr2820551118>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Klaase JM, Kroon BB, van Geel AN, Eggermont AM, Franklin HR, Hart AA. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery. 1994 Jan;115(1):39–45. [PubMed] [Google Scholar]
- 6.Kapteijn BA, Klaase JM, van Geel AN, Eggermont AM, Kroon BB. Results of regional isolated perfusion for locally inoperable melanoma of the limbs. Melanoma research. 1994 Apr;4(2):135–138. doi: 10.1097/00008390-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Brady MS, Brown K, Patel A, Fisher C, Marx W. Isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft-tissue sarcoma of the extremity: final report of a phase II clinical trial. Melanoma research. 2009 Apr;19(2):106–111. doi: 10.1097/CMR.0b013e32832985e3. [DOI] [PubMed] [Google Scholar]
- 8.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Annals of surgical oncology. 2008 Nov;15(11):3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 9.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. Journal of the American College of Surgeons. 2009 May;208(5):706–715. doi: 10.1016/j.jamcollsurg.2008.12.019. discussion 715-707. [DOI] [PubMed] [Google Scholar]
- 10.Wong J, Chen YA, Fisher KJ, Zager JS. Isolated limb infusion in a series of over 100 infusions: a single-center experience. Annals of surgical oncology. 2013 Apr;20(4):1121–1127. doi: 10.1245/s10434-012-2782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lidsky ME, Turley RS, Beasley GM, Sharma K, Tyler DS. Predicting disease progression after regional therapy for in-transit melanoma. JAMA surgery. 2013 Jun;148(6):493–498. doi: 10.1001/jamasurg.2013.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santillan AA, Delman KA, Beasley GM, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Annals of surgical oncology. 2009 Sep;16(9):2570–2578. doi: 10.1245/s10434-009-0563-9. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer. 2009 Jan;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Beasley GM, Parsons C, Broadwater G, et al. A multicenter prospective evaluation of the clinical utility of F-18 FDG-PET/CT in patients with AJCC stage IIIB or IIIC extremity melanoma. Annals of surgery. 2012 Aug;256(2):350–356. doi: 10.1097/SLA.0b013e318256d1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. European journal of cancer & clinical oncology. 1982 Oct;18(10):905–910. doi: 10.1016/0277-5379(82)90235-8. [DOI] [PubMed] [Google Scholar]
- 16.Steinman J, Ariyan C, Rafferty B, Brady MS. Factors associated with response, survival, and limb salvage in patients undergoing isolated limb infusion. Journal of surgical oncology. 2014 Apr;109(5):405–409. doi: 10.1002/jso.23519. [DOI] [PubMed] [Google Scholar]
- 17.Ascierto PA, Simeone E, Sileni VC, et al. Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: data from the Italian cohort of the ipilimumab expanded access program. Cancer investigation. 2014 May;32(4):144–149. doi: 10.3109/07357907.2014.885984. [DOI] [PubMed] [Google Scholar]
- 18.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 Oct 1;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 19.Gyorki DE, Spillane J, Speakman D, Shackleton M, Henderson MA. Current management of advanced melanoma: a transformed landscape. ANZ journal of surgery. 2014 May 20; doi: 10.1111/ans.12673. [DOI] [PubMed] [Google Scholar]
- 20.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. Journal of the American College of Surgeons. 2006 Dec;203(6):878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Annals of surgery. 1995 Feb;221(2):124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai CY, Deneve JL, Beasley GM, et al. A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Annals of surgical oncology. 2012 May;19(5):1637–1643. doi: 10.1245/s10434-011-2151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymond AK, Beasley GM, Broadwater G, et al. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. Journal of the American College of Surgeons. 2011 Aug;213(2):306–316. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ariyan CLR, Panageas K, et al. Safety and Clinical Activity of Combining Systemic Ipilimumab with Isolated Limb Infusion in Patients with In-transit Melanoma. Journal of Clinical Oncology. 2014;32:5s. Abstract; Poster presentation at 50th ASCO meeting May 29th-June 2nd 2014. [Google Scholar]


