Abstract
Existing analgesics are not efficacious in treating all patients with chronic pain and have harmful side effects when used long-term. A deeper understanding of pain signaling and sensitization could lead to the development of more efficacious analgesics. Nociceptor sensitization occurs under conditions of inflammation and nerve injury where diverse chemicals are released and signal through receptors to reduce the activation threshold of ion channels, leading to an overall increase in neuronal excitability [98; 28]. Drugs that inhibit specific receptors have so far been unsuccessful in alleviating pain, possibly because they do not simultaneously target the diverse receptors that contribute to nociceptor sensitization. Hence, focus has shifted towards targeting downstream convergence points of nociceptive signaling [98]. Lipid mediators, including phosphatidylinositol 4,5-bisphosphate (PIP2), are attractive targets as these molecules are required for signaling downstream of G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs). Furthermore, PIP2 regulates the activity of various ion channels [80]. Thus, PIP2 sits at a critical convergence point for multiple receptors, ion channels and signaling pathways that promote and maintain chronic pain. Decreasing the amount of PIP2 in neurons was recently shown to attenuate pronociceptive signaling and could provide a novel approach for treating pain. Here, we review the lipid kinases that are known to regulate pain signaling and sensitization and speculate on which additional lipid kinases might regulate signaling in nociceptive neurons.
Keywords: lipid kinase, PIP2, phosphatidylinositol 4-phosphate 5-kinase 1C, PIP5K1C, PIP5KIγ, phosphoinositide 3-kinase, PI3K, Phosphotidylinositol 4-kinase, PI4K, TRPV1, pronociceptive receptor, NGF
1. Introduction
Chronic pain affects approximately 100 million American adults, making it more prevalent than diabetes, cancer, and heart disease combined. In addition to being in a state of discomfort, patients suffering from chronic pain are plagued by depression, loss of sleep, and an inability to complete daily tasks, all of which lead to a significant decrease in overall quality of life [14]. Unfortunately, non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen (paracetamol), and opioid-based analgesics such as morphine—the current first-line therapeutics for pain—have harmful side effects while only providing partial relief [14]. The complexity of nociception, defined as detection of noxious stimuli, and subsequent pain processing, creates many challenges for analgesic drug discovery [28]. Current therapeutic inadequacies highlight the need to identify new molecular targets for analgesic drug development. In order to identify new therapeutic targets, the molecules and mechanisms associated with peripheral nociceptive signaling and sensitization need to be further elucidated [98].
Pain-producing heat, mechanical or chemical stimuli activate receptors, including Transient Receptor Potential (TRP) channels, which depolarizes pain-sensing neurons, also known as nociceptors [28]. Depolarization leads to action potential firing via the activation and interplay of voltage-gated sodium and potassium channels. The generated signal is then relayed from the periphery to the spinal cord via slowly conducting unmyelinated small-diameter neurons (C-fibers) and more-rapidly conducting myelinated neurons (Aδ-fibers) [28]. Sensation carried by Aδ-fibers is robust, pricking and more accurate of the location of pain whereas C-fibers are thought to convey the sensation/perception of throbbing or burning pain, with relatively poor somatotopic localization [36].
Sensory inputs from Aδ-fibers synapse at lamina I whereas C-fibers synapse at lamina II of the dorsal horn, a region where some input integration and processing occurs [43]. The lateral thalamus, which has been implicated in sensory and discriminative aspects of pain, receives inputs from neurons in the dorsal horn via the lateral spinothalamic tract while medial thalamus and limbic structures receive inputs via the medial spinothalamic tract and spinobrachial tract and are believed to mediate the emotional and aversive components of pain [43]. Activity evoked by noxious stimuli can be modulated at the peripheral, spinal and supraspinal levels, which can significantly alter pain perception [57].
Under normal physiological conditions, nociceptors function as a defense mechanism to promote avoidance of painful, tissue-damaging stimuli [73]. This type of pain is called nociceptive or physiological pain [41; 28]. In contrast, persistent or chronic pain is normally uncoupled from a noxious stimulus and can be exacerbated by various mechanisms such as peripheral and central sensitization [43; 97]. Sensitization is characterized by a reduction in detection threshold and an increase in response to noxious stimuli which mediates two common symptoms of pain in humans, allodynia in which typically innocuous stimuli become painful and hyperalgesia in which a painful stimulus becomes more painful, respectively [97; 73; 6]. Sensitization of nociceptors occurs most commonly after inflammation and nerve injury [73; 6] and contributes to the two most common forms of chronic pain in humans, inflammatory and neuropathic pain, respectively [97]. Central sensitization reflects an amplification of pain signals in the central nervous system and takes place at the level of the dorsal spinal cord, in spinal neurons that are postsynaptic to nociceptive neurons, while peripheral sensitization occurs in DRG neurons and their axon terminals [97]. Central sensitization is often preceded by peripheral sensitization and is dependent upon activity from the central terminals of sensitized DRG neurons. Elevated neurotransmission from the nociceptor terminals to dorsal horn neurons alters synaptic density, kinetics and threshold of activation, resulting in increase transmission of pain signals [97]. The focus of this review is on signaling mechanisms that mediate peripheral sensitization in DRG neurons.
1.1 Peripheral sensitization
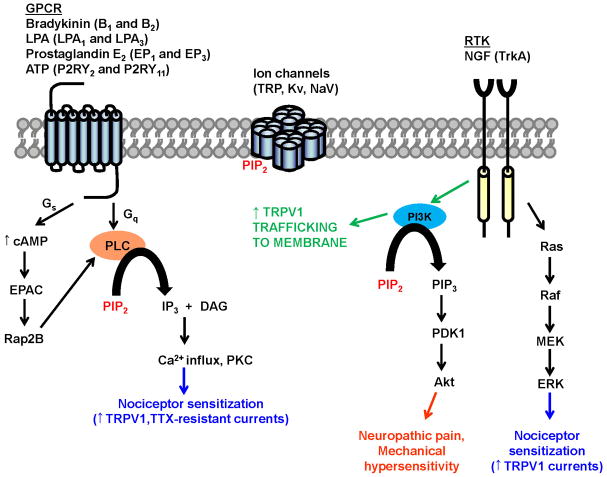
Nociceptive, neuropathic, and inflammatory pain are mediated by several different molecular mechanisms; some of these mechanisms are unique to one type of pain while others are involved in multiple pain modalities [74]. Nerve injury and inflammation result in the release of multiple pronociceptive molecules, including bradykinin (BK), lysophosphatidic acid (LPA), adenosine triphosphate (ATP), prostaglandins (PGE2) and nerve growth factor (NGF) [28]. These ligands signal through a diverse set of Gq- and Gs-coupled G-protein coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) to sensitize nociceptors [28]. Activation of Gq-coupled GPCRs via canonical Gq-coupling results in phospholipase C (PLC)-catalyzed hydrolysis of phosphatidylinositol 4,5 bisphosphate (PIP2) to produce diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 binds to IP3 receptors on the endoplasmic reticulum (ER) to release calcium from intracellular stores, causing an increase in cytoplasmic calcium. DAG activates protein kinase Cs (PKCs) which can also activate the mitogen-activated protein kinase (MAPK) cascade. PKC and MAPK signaling cascades have been implicated in nociceptor sensitization associated with inflammatory and neuropathic pain [32]. Gs-coupled GPCRs can contribute to the PKC- pathway by exchange protein activated by cAMP (EPAC) activation of PLC [33]. In addition, RTKs recruit phosphoinositide 3-kinase (PI3K), a lipid kinase that phosphorylates PIP2 to generate phosphatidylinositol 3,4,5 triphosphate (PIP3) which activates Rac-alpha serine/threonine kinase (Akt or also known as protein kinase B, PKB) [101]. Importantly, downstream effector activation by GPCRs and RTKs can potentiate the activity and expression of a variety of ion channels and modulate the hyperexcitability of nociceptors following nerve injury and inflammation (Figure 1). Nociceptor sensitization goes beyond acute modification of ion channels and includes the generation of a “primed state”, a state where nociceptors are primed for activation but are inactive without overt stimulation [32]. This “primed state” is primarily mediated by PKC epsilon (ε) where rearrangement of cellular cytoskeleton, modulation of subcellular compartments and extracelluar matrices is observed [32]. Due to text constraints, we will focus on the signaling-mediated modulatory effects on ion channel activity only.
Figure 1. GPCR- and RTK- mediated signaling that leads to sensitization of nociceptors.
Nociceptive sensitization is dependent on PIP2-sensitive GPCRs, RTKs and ion channels that mediate hyperexcitability following nerve injury and inflammation. Activation of GPCRs leads to PKC-mediated enhancement of TRPV1 and Tetrodotoxin (TTX)-resistant Voltage-gated Sodium Channel (NaV) activity. Stimulation of RTKs leads to activation of the PI3K/PDK1/Akt signaling cascade.
Of particular interest to this review is the non-selective cation channel, transient receptor vanilloid 1 (TRPV1), which is selectively expressed in the small and medium diameter unmyelinated sensory neurons. Capsaicin, noxious heat (>43°C), protons, ethanol, and many endogenous lipid metabolites can activate TRPV1 channels to allow cation influx, leading to membrane depolarization and subsequently result in action potential firing [40]. TRPV1 activity is also regulated by PIP2 [68; 49]. Although the TRPV1 channel is activated by noxious temperatures (>43°C), during tissue injury and inflammatory conditions, the thermal activation threshold drops well below normal physiological temperatures, which serves as the cellular basis for inflammatory thermal hyperalgesia [41]. The reduction in activation threshold is due to post-translational modulation of TRPV1 by various kinases such as PKA, PKC and Proto-oncogene tyrosine-kinase Src [8; 63; 7; 39; 104]. Activation of p38 MAPK through NGF signaling, as well as prolonged activation of PKC has been reported to enhance the expression of TRPV1 channel protein, thereby playing a role in nociceptor sensitization [38; 15; 102]. Indeed, mice lacking the TRPV1 gene do not develop inflammatory thermal hyperalgesia, and show modest impairment of noxious acute heat sensitivity [12; 17].
In addition to TRP channels, a variety of ion channels are responsible for regulating neuronal excitability—and more importantly hyperexcitability following nerve injury and inflammation—via mechanisms that are both independent of and dependent upon modulation by GPCRs and RTKs [23; 94]. Furthermore, an increase in excitability is crucial for prolonged nociceptive sensitization and persistent pain [6; 23; 28]. Many different classes of ion channels regulate neuronal excitability including sodium, potassium, calcium and hyperpolarization-activated (non-specific cation) channels [25; 26; 80; 6; 28]. Although a review of the functions of each of these ion channels is beyond the scope of this review, it is important to note that many of these ion channels depend on PIP2 for activity [25; 80; 23; 81] (Figure 1).
One important commonality between GPCR-, RTK- and ion-channel mediated nociceptive signaling and sensitization is their dependence upon the lipid second messenger, PIP2 (Figure 1). PIP2 regulates TRPV1 as well as other ion channels responsible for the regulation of neuronal excitability and is a critical component of the Gq-coupled GPCR and RTK signaling pathways, which mediate nociceptive sensitization following nerve injury and inflammation. Thus, PIP2 sits at a critical convergence point for many pain promoting pathways.
1.2 Phosphatidylinositol (4,5)-bisphosphate (PIP2)
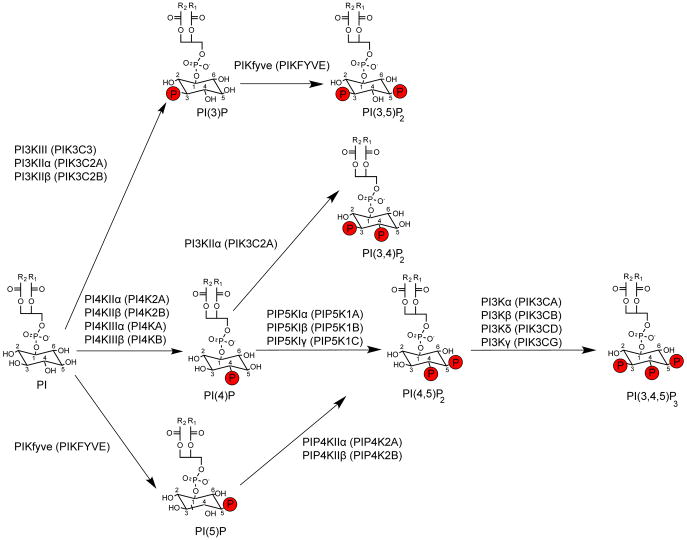
Although PIP2 is only a minor constituent (<1%) of the plasma membrane, it is very important to a multitude of cellular processes and serves as prerequisite to other regulatory lipids in the phosphatidylinositol (PI) synthetic cascade [54; 103] (Figure 2). Many of the mentioned processes in section 1.1 are dependent upon adequate PIP2 synthesis via phosphatidylinositol kinases. Type 1 phosphatidylinositol 4-phosphate 5-kinases (PIP5KIs) and type 2 phosphatidylinositol 5-phosphate 4-kinases (PIP4KIIs) synthesize PI(4,5)P2 by phosphorylating phosphatidylinositol 4-phosphate, [PI(4)P] and phosphatidylinositol 5-phosphate [PI(5)P], respectively (Figure 2). PI(4)P is the most abundant monophosphoinositide and is present at 10-fold greater concentrations than PI(5)P in erythrocytes, suggesting that PIP5KIs are the predominant PIP2 synthesizing enzymes [46]. It must be noted that PIKfyve can also phosphorylate phosphatidylinositol (PI) to generate PI(5)P that is subsequently phosphorylated by PIP4Ks to generate PI(4,5)P2, and phosphatidylinositol 3-phosphate, PI(3)P to form PI(3,5)P2. However, it has been shown that PIP5KI generation of PI(4,5)P2 is the major regulatory mechanism for GPCRs and ion channels [92; 80; 103] (Figure 2).
Figure 2. Phosphatidylinositol (PI) synthetic pathways.
Phosphatidylinositol (PI) can be phosphorylated at the D3, D4 and D5 position of the inositol ring by PI3K, PI4K and PIKfyve respectively. The majority of all phosphatidylinositol 4,5-bisphosphate (PIP2) is synthesized via phosphorylation of PI(4)P by PIP5KIs. PIP2 can undergo further phosphorylation by class I PI3Ks to generate PIP3. R2 and R1 are the fatty acid chains that make up diacylglycerol (DAG). Phosphate groups are red. Figure based on reactions catalyzed in vivo [70]. Image adapted from [75]. Gene names for respective kinases are shown in parentheses.
Rapid synthesis of PIP2 by activated lipid kinases has been suggested to feed into PIP2-mediated pathways to amplify signaling downstream of stimulated receptors in non-neuronal cells [91; 61]. A similar mechanism could be at play in nociceptive neurons but further studies will be required for confirmation. As lipid kinases gain recognition for their ability to alter pain sensitivity, we review the roles of various lipid kinases in regulating pain signaling and sensitization, with a primary focus on TRPV1 activity.
2. Lipid kinases that regulate nociceptive sensitization
2.1 Phosphoinositide 3-kinases (PI3Ks)
PI3Ks are the most studied group of lipid kinases. There are 3 classes of mammalian PI3Ks. Class I kinases (4 genes that give rise to α,β,δ and γ isoforms) are receptor-regulated PI(4,5)P2 kinases that produce PI(3,4,5)P3. Class II kinases (3 genes that give rise to α,β and γ isoforms) are larger monomeric enzymes known as PI3K-C2 kinases that phosphorylate PI to generate PI(3)P, and phosphorylate PI(4)P to generate PI(3,4)P2 (Figure 2; table 1). Class III kinase (only one isoform) is the “housekeeping” PI-specific enzyme responsible for generating PI(3)P. This review focuses on class 1 PI3Ks as their involvement in regulating receptor-activated signaling is well-established.
Table 1.
Representative lipid kinase inhibitors
| Drug | Kinases inhibited | References |
|---|---|---|
| Wortmannin | PI3K and class III PI4Ks | Powis et al. (1994) [67] Nakanishi et al. (1995) [58] |
| GDC-0941 | PI3K | Folkes et al. (2008) [24] |
| LY29002 | PI3K | Vlahos et al. (1994) [88] |
| Compound 15e | PI3Kα | Hayakawa et al. (2006) [30] |
| TGX221 | PI3Kβ | Jackson et al. (2005) [35] |
| CAL-101 | PI3Kδ | Lanutti et al. (2011) [44; 66] |
| AS252424 | PI3Kγ | Pomel et al. (2006) [66] |
| Phenylarsine Oxide (PAO) | PI4K | Wiedeman et al. (1996) [96] |
| PIK-93 | PI4KIIIβ | Burke et al. (2014) [11] |
| 4-anilinoquinazolines | PI4KIIIα | Bianco et al. (2012) [9] |
| Adenosine | Class II PI4Ks (low dose), Class III PI4Ks (high dose) | Guo et al. (2003) [29] |
| SAR088 | PIP4KIIβ | Voss et al. (2014) [90] |
| UNC3230 | PIP5K1γ | Wright et al. (2014) [99] |
| YM201636 | PIKfyve | Jefferies et al. (2008) [37] |
| R59022 | strongly inhibits DGKα, moderately inhibits DGKε and θ | Sato et al. (2013) [72] |
| R59949 | strongly inhibits DGKα, moderately inhibits DGKδ and κ | Sato et al. (2013) [72] |
PI3Ks are comprised of 2 subunits, a catalytic subunit which binds to PIP2 and phosphorylates at the 3′ position and a regulatory subunit, which recognizes phosphorylated tyrosine residues and binds to SRC homology 2 (SH2) domains [85]. While all PI3K isoforms have a p110 (protein with molecular weight of 110 kilodaltons, kDa) catalytic subunit, PI3Kα, β and δ binds to a p85 (protein with molecular weight of 85 kDa) regulatory subunit whereas PI3Kγ binds to a p101 (protein with molecular weight of 101 kDa) regulatory subunit [85]. SH2 domains on the p85 regulatory subunit allow for interaction with phosphorylated tyrosine in membrane-associated proteins such as RTKs [108], recruiting p110 to the membrane to phosphorylate PIP2 to generate PIP3 [85]. A well-studied example is the nerve growth factor (NGF)-TrkA receptor-PI3K signaling cascade. NGF is released in the vicinity of peripheral nerve endings during inflammation and sensitizes TRPV1 responses via activation of its receptor tyrosine kinase, TrkA, which subsequently recruits PI3K [76; 10]. PI3K binds to TRPV1 directly via its p85α subunit, which presumably recognizes the phosphorylated Y200 of TRPV1, to enhance TRPV1 surface trafficking upon NGF stimulation of TrkA in DRG neurons [78]. Furthermore, PI3K sensitizes TRPV1 via activation of extracellular signal-regulated kinase (ERK) in sensory neurons and mediates NGF-induced inflammatory heat hyperalgesia and mechanical hyperalgesia [108; 51; 107]. Besides NGF-TrkA induced TRPV1 sensitization, PI3K recruitment of Akt/PKB also contributes to neuropathic pain induced by spinal nerve ligation and mechanical hypersensitivity induced by capsaicin in rats [20; 82; 101]. PI3K is also a major factor in central sensitization after noxious inflammatory stimuli [65]. Hence, inhibiting class I PI3Ks could provide a way to attenuate nociceptive sensitization.
However, pan-PI3K inhibitors, such as wortmannin and LY29002 (Table 1), may produce unwanted side effects due to the expression of class I PI3K in various cell types. Therefore, it is important to study the expression patterns of these isoforms and fully dissect the signaling pathways that each is involved in. PI3Kα, β and γ but not δ are expressed in DRGs [45; 5]. PI3Kα is ubiquitously expressed in sensory neurons. PI3Kβ is expressed in spinal cord dorsal horn neurons and enhances AMPA receptor trafficking upon inflammation, resulting in increase excitatory synaptic transmission [45]. PI3Kδ is reportedly not expressed in the DRG but is found in astrocytes in the spinal cord dorsal horn [45]. Even though this isozyme does not seem to regulate nociceptive sensitization, it has important roles during development and in nerve regeneration after injury [21]. The only GPCR-coupled PI3K, PI3Kγ, is expressed in nociceptive neurons and has been implicated in morphine-induced peripheral analgesia and tolerance [16; 42]. Surprisingly, PI3Kγ knockout (Pik3cg−/−) mice exhibit enhanced responses to heat and capsaicin, suggesting that PI3Kγ acts as a negative regulator of thermal and TRPV1 responses [60]. Interestingly, antagonism of this isozyme with a specific inhibitor in the periphery (via intraplantar injections) was shown to be anti-allodynic in a carrageenan-induced allodynia model [45]. The earlier finding of PI3Kγ negatively regulating TRPV1 activity only focused on acute thermal nociception and TRPV1 channel activity but did not look at the role of PI3Kγ in NGF-induced TRPV1 sensitization or in neuropathic and inflammatory pain models. The latter finding from a different group indicated that inhibition of PI3Kγ inhibited allodynia in a carrageenan-induced inflammatory pain model. The difference in observations could be due to the mode of nociception being investigated. The prominent role of PI3Kγ in positively regulating GPCR-signaling may overrule its negative effects on TRPV1 activity.
2.2 Phosphotidylinositol-4 kinases (PI4Ks)
PI(4)Ks phosphorylate PI to generate PI(4)P, the immediate precursor for PI(4,5)P2 (Figure 2). Furthermore, PI(4)P itself is essential for TRPV1 activity as its depletion reduces the channel’s response to capsaicin [48]. There are 2 classes of mammalian PI4Ks, wortmannin-sensitive class III enzymes, PI4KIIIα and PI4KIIIβ, and wortmannin-insensitive class II enzymes, PI4KIIα and PI4KIIβ. Class III enzymes exhibit a higher degree of similarity to PI3Ks, thus likely contributing to their sensitivity to wortmannin [55]. The subcellular location of these isozymes has been extensively characterized in various cell types. Their subcellular location governs the intracellular trafficking processes in which they are involved [13; 55; 56]. However, the functions of these kinases in regulating nociceptive sensitization in peripheral sensory neurons are unknown. Moreover, it is unknown which PI(4)Ks are expressed in DRG neurons. Interestingly, PI4K-mediated PIP2 production is crucial for the adaptation (response magnitude diminishes with sustained presence of stimulus, also known as desensitization) of ion channels such as inward-rectifier potassium channels (Kir) and voltage-gated potassium channels (Kv) in rat taste receptor cells, suggesting a plausible role for PI4Ks in regulating adaptation of Kv channels in pain-sensing neurons as well [106]. Prevention of desensitization of Kv channels via inhibition of PI4K could lead to decrease in neuronal excitability.
PI4KIIIα and PI4KIIα are the primary producers of plasma membrane PI(4)P [59; 58; 29; 4]. Both kinases are widely expressed in mammalian tissues, with enrichment in the brain. PI4KIIIα is primarily localized to the ER and Golgi membranes whereas PI4KIIα is expressed on golgi networks and endosomes [29; 2]. Although they are primarily localized within intracellular membranes, they replenish the PI(4)P pools at the plasma membrane with PI4KIIIα shown to be essential for the maintenance of GPCR-responsive pool of PI(4)P [86; 3]. Minor axon loss was observed in DRG neurons of PI4KIIα knockout (Pi4k2a−/−) mice, suggesting that another PI4K regulates the majority of PI(4)P production in peripheral neurons [77]. PI4KIIIα conditional knockout in primary cultures of mouse embryonic fibroblasts (MEFs) led to significant reduction in PI(4)P levels and plasma membrane PI(4,5)P2 levels even though global PI(4,5)P2 levels were only modestly reduced due to compensatory upregulation of PIP5KI expression [59]. Studies are still needed to evaluate inhibition of PI4KIIIα as an approach to reduce TRPV1 activity via reduction in PI(4)P levels. The dosing and route of administration of PI4KIIIα-specific inhibitors may be limited as conditional PI4KIIIα knockout mice develop lethal gastrointestinal disorders [84]. That said, the deletion of a gene in an entire organism after development could still be highly detrimental whereas an inhibitor administered at a specific site (such as intrathecal or topical) limits the exposure of the drug and may reduce unwanted side effects.
PI4KIIIβ is localized to the ER and Golgi membrane where it mediates endosomal/vesicular trafficking and perhaps plays a role in synaptic development and plasticity [27; 31; 79]. PI4KIIβ is mainly cytosolic and its translocation to the plasma membrane is promoted by platelet-derived growth factor [93]. PI4KIIβ activity is enhanced upon membrane insertion. It would be interesting to investigate if other growth factors such as NGF could induce a similar increase in PI4KIIβ membrane translocation and enhancement in activity in DRG neurons. The resultant upregulation of PI(4)P production and subsequent increase in PIP2 pools could serve as a mechanism to amplify pronociceptive signaling by NGF.
2.3 Type 1 Phosphatidylinositol 4-phosphate 5-kinases (PIP5KIs)
The subsequent step of producing PIP2 from PI(4)P is mediated by PIP5KIs (Figure 2). There are three mammalian PIP5KI isozymes: PIP5KIα, PIP5KIβ, and PIP5KIγ (Figure 2). The three isozymes are >80% identical at the amino acid level within the kinase catalytic domain. However, they have very little sequence homology within their N and C termini; these isozyme-specific regions allow differentiated functions of each isoform within cells [34; 100]. Each isoform has differential expression within cells and across murine tissues. It must be noted that human PIP5KIα is homologous to murine PIP5KIβ and human PIP5KIβ is homologous to murine PIP5KIα. PIP5KIα is ubiquitously expressed in murine tissue, is primarily expressed in the nucleus, and translocates to the membrane following receptor activation [34; 19; 100]. PIP5KIβ is also ubiquitously expressed in murine tissue but is located in the perinuclear region [19]. Unlike ubiquitously expressed PIP5KIα and PIP5KIβ, PIP5KIγ is expressed predominantly in neuronal tissue, with some expression detected in the lung and kidney. PIP5KIγ localizes to the cytoplasm, plasma membrane and intracellular membranes [95; 18; 92].
Characterization of PIP5KIα and PIP5KIβ has been carried out utilizing a variety of cell types and roles in membrane ruffling, endocytosis, and actin dynamics have all been elucidated [53; 52]. It is common for PIP5KIα and PIP5KIβ to have overlapping functions; however, like the specialized expression profile of PIP5KIγ, it is rare that PIP5KIγ shares common functionality with PIP5KIα and PIP5KIβ [53; 52]. Endocytosis is the one function in which all three isozymes play a role; however, it is suggested that PIP5KIγ has a specialized role in interacting with adaptor protein 2 (AP-2) in this process [1; 53]. Furthermore, in bone marrow macrophages, PIP5KIα and PIP5KIγ have very distinct functions that mediate different steps in phagocytosis [53; 52]. The role of PIP5KIγ has primarily been studied in cortical synaptic transmission [18], GPCR-mediated signaling [92], regulation of focal adhesions [47], and AP-2 mediated endocytosis [1]. Moreover, the functions of PIP5KIγ can be further differentiated by the involvement of the two different splice isoforms, PIP5KIγ635 and PIP5KIγ661. PIP5KIγ635 is the primary splice variant responsible for the regulation of GPCR-mediated signaling whereas PIP5KIγ661 is the primary splice variant responsible for the interactions with talin and AP-2 which mediate endocytosis and focal adhesions [47; 92; 1; 53].
Our recent study indicates that PIP5KIγ is the predominant PIP2-producing PIP5K1 in DRG neurons and is an important regulator of nociceptive signaling and sensitization [99]. Thermal and mechanical hypersensitivity in models of neuropathic and inflammatory pain as well as TRPV1 sensitization were significantly reduced in PIP5KIγ heterozygous (Pip5k1c+/−) mice. Constitutive PIP5KIγ homozygous knockouts (Pip5k1c−/−) are embryonically lethal [99], and hence should not be studied. We independently validated our genetic observations with a small molecule inhibitor of PIP5KIγ, UNC3230 (Table 1). Intrathecal delivery of UNC3230 had induced antinociceptive effects in our rodent pain models, recapitulating the antinociceptive phenotypes observed in Pip5k1c+/− mice, suggesting that localized inhibition of PIP5KIγ in adults is sufficient to reduce nociceptive sensitization [99].
A recent functional genomics study identified phospholipid signaling and lipid kinases as key regulators of heat nociception in flies [60]. It was also found that PIP5KIα knockout (Pip5k1a−/−) mice displayed hypersensitivity to noxious heat and capsaicin but the precise underlying mechanism is unknown. PIP5KIα is expressed at much lower levels in DRG and does not contribute to PIP2 levels in the nervous system [89]. Given the sometimes differing or opposing roles of PIP5KIα and PIP5KIγ in the same processes [53; 52; 87; 62; 61], it is reasonable to speculate that PIP5KIα and PIP5KIγ may have opposing functions in DRG neurons. Furthermore, given the complexity of nociceptive signaling and the low level of expression of PIP5KIα in DRG neurons, PIP5KIα could be modulating nociceptive processes at the level of the spinal cord or brain.
3. Additional lipid kinases that might regulate nociceptive signaling and sensitization
3.1 PIKfyve generates PI(3,5)P2
Besides producing PI(5)P from PI, PIKfyve also phosphorylates PI(3)P to generate PI(3,5)P2. PIKfyve negatively regulates exocytosis in the neurosecretory cells [64] while levels of PI(3,5)P2 are important in maintaining the health of peripheral neurons [105]. Although their role in regulating peripheral nociceptor sensitization is unknown, PIKfyve has been shown to downregulate the expression of Cav1.2 in cortical neurons. Interestingly this voltage-gated calcium channel is upregulated in spinal dorsal horn in chronic neuropathic pain [22; 83].
3.2 Phosphatidylinositol 4-phosphate kinase (PIP4K)
As pools of PI(5)P are relatively low compared to PI(4)P, as studied in erythrocytes [46], PIP4Ks are assumed to not have a major role in regulating the levels of PI(4,5)P2 in cells. Studies are required to identify their expression and role in regulating signaling in nociceptive neurons.
3.3 Diacylglycerol Kinase (DGKs)
PIP2 hydrolysis by activated PLC produces DAG and IP3. DAG, which activates PKC, can be phosphorylated by DGKs to generate phosphatidic acid (PA). A study has shown that DGKí and ζ are expressed in the DRG but their roles in regulating GPCR signaling in DRG neurons remain uninvestigated [71]. Recently, we found that overexpression of DGKη leads to sustained GPCR signaling in HEK cells [69], suggesting that inhibition of DGKη, and possibly other DGK isoforms, may blunt GPCR signaling. Furthermore, DGKζ-produced PA can activate PIP5KIα, suggesting that DGK contributes to a forward feedback mechanism that can further enhance PIP2 production [50]. Hence, inhibition of DGKs could serve as an approach to desensitize and reduce signaling in DRG neurons, provided future studies confirm a regulatory role in nociceptive signaling similar to those observed in HEK cells.
4. Future Directions
Bypassing nociceptor and receptor diversity by targeting convergence points downstream of multiple pronociceptive receptors and ion channels provides a promising approach to inhibit nociceptive sensitization. Lipid second messengers such as PIP2 are attractive candidates due to their involvement in regulating the activity of various ion channels and serving as precursors for downstream effectors of GPCR- and RTK- signaling pathways. Targeting lipid kinases that produce these regulatory lipid second messengers could provide novel approaches to attenuate pain signaling (Figure 3). Many of the proposed mechanisms that involve lipid kinases in this review are speculative due to a lack of understanding of their expression and function in DRG neurons. This area is thus ripe for further research and therapeutic intervention, particularly given that kinases are highly druggable targets. However, caution is warranted when targeting these lipid kinases as they are widely expressed and are involved in regulating many physiological processes.
Figure 3. Lipid kinases (in blue) that regulate levels of PIP2 (PI(4,5)P2) could affect nociceptive sensitization when inhibited or genetically deleted.
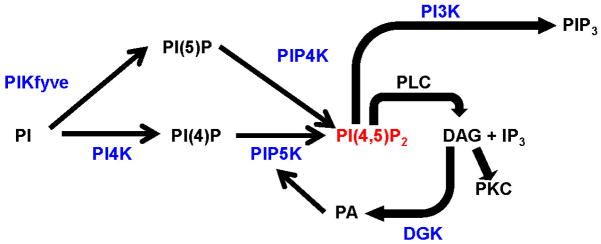
PIP2 levels decrease significantly via inhibition of PIP5K and modestly when PI4K is inhibited[59]. Whether PIKfyve and PIP4K contribute to PIP2 levels in DRG neurons is unknown. PI3K inhibition leads to significant attenuation of NGF-induced TRPV1 sensitization. DGK phosphorylation of DAG produces PA, which has been implicated in PIP5K activation, suggesting a feedforward mechanism for PIP2 signaling.
Acknowledgments
Research in the lab of M.J.Z. is supported by grants from NINDS (R01NS081127, R01NS067688).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Bairstow SF, Ling K, Su X, Firestone AJ, Carbonara C, Anderson RA. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J Biol Chem. 2006;281(29):20632–20642. doi: 10.1074/jbc.M601465200. [DOI] [PubMed] [Google Scholar]
- 2.Balla A, Balla T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 2006;16(7):351–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Balla A, Kim YJ, Varnai P, Szentpetery Z, Knight Z, Shokat KM, Balla T. Maintenance of hormone-sensitive phosphoinositide pools in the plasma membrane requires phosphatidylinositol 4-kinase IIIalpha. Mol Biol Cell. 2008;19(2):711–721. doi: 10.1091/mbc.E07-07-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balla A, Tuymetova G, Tsiomenko A, Varnai P, Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol Biol Cell. 2005;16(3):1282–1295. doi: 10.1091/mbc.E04-07-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett SE, Reynolds AJ, Tan T, Heydon K, Hendry IA. Differential mRNA expression and subcellular locations of PI3-kinase isoforms in sympathetic and sensory neurons. J Neurosci Res. 1999;56(1):44–53. doi: 10.1002/(SICI)1097-4547(19990401)56:1<44::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2003;100(21):12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35(4):721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 9.Bianco A, Reghellin V, Donnici L, Fenu S, Alvarez R, Baruffa C, Peri F, Pagani M, Abrignani S, Neddermann P, De Francesco R. Metabolism of phosphatidylinositol 4-kinase IIIalpha-dependent PI4P Is subverted by HCV and is targeted by a 4-anilino quinazoline with antiviral activity. PLoS Pathog. 8(3):e1002576. doi: 10.1371/journal.ppat.1002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551(Pt 2):433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke JE, Inglis AJ, Perisic O, Masson GR, McLaughlin SH, Rutaganira F, Shokat KM, Williams RL. Structures of PI4KIIIbeta complexes show simultaneous recruitment of Rab11 and its effectors. Science. 344(6187):1035–1038. doi: 10.1126/science.1253397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 13.Chu KM, Minogue S, Hsuan JJ, Waugh MG. Differential effects of the phosphatidylinositol 4-kinases, PI4KIIalpha and PI4KIIIbeta, on Akt activation and apoptosis. Cell Death Dis. 1:e106. doi: 10.1038/cddis.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Committee on Advancing Pain Research C, and Education; Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. 2011. p. 1. [Google Scholar]
- 15.Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28(19):5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunha TM, Roman-Campos D, Lotufo CM, Duarte HL, Souza GR, Verri WA, Jr, Funez MI, Dias QM, Schivo IR, Domingues AC, Sachs D, Chiavegatto S, Teixeira MM, Hothersall JS, Cruz JS, Cunha FQ, Ferreira SH. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci U S A. 107(9):4442–4447. doi: 10.1073/pnas.0914733107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 18.Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431(7007):415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 19.Doughman RL, Firestone AJ, Wojtasiak ML, Bunce MW, Anderson RA. Membrane ruffling requires coordination between type Ialpha phosphatidylinositol phosphate kinase and Rac signaling. J Biol Chem. 2003;278(25):23036–23045. doi: 10.1074/jbc.M211397200. [DOI] [PubMed] [Google Scholar]
- 20.Duan B, Liu DS, Huang Y, Zeng WZ, Wang X, Yu H, Zhu MX, Chen ZY, Xu TL. PI3-kinase/Akt pathway-regulated membrane insertion of acid-sensing ion channel 1a underlies BDNF-induced pain hypersensitivity. J Neurosci. 32(18):6351–6363. doi: 10.1523/JNEUROSCI.4479-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eickholt BJ, Ahmed AI, Davies M, Papakonstanti EA, Pearce W, Starkey ML, Bilancio A, Need AC, Smith AJ, Hall SM, Hamers FP, Giese KP, Bradbury EJ, Vanhaesebroeck B. Control of axonal growth and regeneration of sensory neurons by the p110delta PI 3-kinase. PLoS One. 2007;2(9):e869. doi: 10.1371/journal.pone.0000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favereaux A, Thoumine O, Bouali-Benazzouz R, Roques V, Papon MA, Salam SA, Drutel G, Leger C, Calas A, Nagy F, Landry M. Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: role in pain. EMBO J. 30(18):3830–3841. doi: 10.1038/emboj.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer MJM, Mak SWK, McNaughton PA. Sensitisation of Nociceptors--What are Ion Channels Doing? The Open Pain Journal. 2010;3:82–96. [Google Scholar]
- 24.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, Friedman LS, Hayes A, Hancox TC, Kugendradas A, Lensun L, Moore P, Olivero AG, Pang J, Patel S, Pergl-Wilson GH, Raynaud FI, Robson A, Saghir N, Salphati L, Sohal S, Ultsch MH, Valenti M, Wallweber HJ, Wan NC, Wiesmann C, Workman P, Zhyvoloup A, Zvelebil MJ, Shuttleworth SJ. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51(18):5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 25.Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Phosphatidylinositol [correction] 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J Neurosci. 2004;24(48):10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamper N, Shapiro MS. Target-specific PIP(2) signalling: how might it work? J Physiol. 2007;582(Pt 3):967–975. doi: 10.1113/jphysiol.2007.132787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1(5):280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 28.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nature medicine. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo J, Wenk MR, Pellegrini L, Onofri F, Benfenati F, De Camilli P. Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100(7):3995–4000. doi: 10.1073/pnas.0230488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayakawa M, Kaizawa H, Moritomo H, Koizumi T, Ohishi T, Okada M, Ohta M, Tsukamoto S, Parker P, Workman P, Waterfield M. Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg Med Chem. 2006;14(20):6847–6858. doi: 10.1016/j.bmc.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Hilfiker S. Neuronal calcium sensor-1: a multifunctional regulator of secretion. Biochem Soc Trans. 2003;31(Pt 4):828–832. doi: 10.1042/bst0310828. [DOI] [PubMed] [Google Scholar]
- 32.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55(3):365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25(26):6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishihara H, Shibasaki Y, Kizuki N, Katagiri H, Yazaki Y, Asano T, Oka Y. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem. 1996;271(39):23611–23614. doi: 10.1074/jbc.271.39.23611. [DOI] [PubMed] [Google Scholar]
- 35.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, Sturgeon SA, Prabaharan H, Thompson PE, Smith GD, Shepherd PR, Daniele N, Kulkarni S, Abbott B, Saylik D, Jones C, Lu L, Giuliano S, Hughan SC, Angus JA, Robertson AD, Salem HH. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11(5):507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 36.Jansen PH, Lecluse RG, Verbeek AL. Past and current understanding of the pathophysiology of muscle cramps: why treatment of varicose veins does not relieve leg cramps. J Eur Acad Dermatol Venereol. 1999;12(3):222–229. [PubMed] [Google Scholar]
- 37.Jefferies HB, Cooke FT, Jat P, Boucheron C, Koizumi T, Hayakawa M, Kaizawa H, Ohishi T, Workman P, Waterfield MD, Parker PJ. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9(2):164–170. doi: 10.1038/sj.embor.7401155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 39.Jin X, Morsy N, Winston J, Pasricha PJ, Garrett K, Akbarali HI. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am J Physiol Cell Physiol. 2004;287(2):C558–563. doi: 10.1152/ajpcell.00113.2004. [DOI] [PubMed] [Google Scholar]
- 40.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 41.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 42.Konig C, Gavrilova-Ruch O, von Banchet GS, Bauer R, Grun M, Hirsch E, Rubio I, Schulz S, Heinemann SH, Schaible HG, Wetzker R. Modulation of mu opioid receptor desensitization in peripheral sensory neurons by phosphoinositide 3-kinase gamma. Neuroscience. 169(1):449–454. doi: 10.1016/j.neuroscience.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 43.Kuner R. Central mechanisms of pathological pain. Nat Med. 16(11):1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 44.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, Druker BJ, Puri KD, Ulrich RG, Giese NA. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 117(2):591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leinders M, Koehrn FJ, Bartok B, Boyle DL, Shubayev V, Kalcheva I, Yu NK, Park J, Kaang BK, Hefferan MP, Firestein GS, Sorkin LS. Differential distribution of PI3K isoforms in spinal cord and dorsal root ganglia: potential roles in acute inflammatory pain. Pain. 155(6):1150–1160. doi: 10.1016/j.pain.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9(2):99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 47.Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420(6911):89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 48.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25(19):4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27(26):7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo B, Prescott SM, Topham MK. Diacylglycerol kinase zeta regulates phosphatidylinositol 4-phosphate 5-kinase Ialpha by a novel mechanism. Cell Signal. 2004;16(8):891–897. doi: 10.1016/j.cellsig.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21(12):3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- 52.Mao YS, Yamaga M, Zhu X, Wei Y, Sun HQ, Wang J, Yun M, Wang Y, Di Paolo G, Bennett M, Mellman I, Abrams CS, De Camilli P, Lu CY, Yin HL. Essential and unique roles of PIP5K-gamma and -alpha in Fcgamma receptor-mediated phagocytosis. J Cell Biol. 2009;184(2):281–296. doi: 10.1083/jcb.200806121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao YS, Yin HL. Regulation of the actin cytoskeleton by phosphatidylinositol 4-phosphate 5 kinases. Pflugers Arch. 2007;455(1):5–18. doi: 10.1007/s00424-007-0286-3. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 55.Minogue S, Waugh MG. The phosphatidylinositol 4-kinases: don’t call it a comeback. Subcell Biochem. 58:1–24. doi: 10.1007/978-94-007-3012-0_1. [DOI] [PubMed] [Google Scholar]
- 56.Minogue S, Waugh MG, De Matteis MA, Stephens DJ, Berditchevski F, Hsuan JJ. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J Cell Sci. 2006;119(Pt 3):571–581. doi: 10.1242/jcs.02752. [DOI] [PubMed] [Google Scholar]
- 57.Moller AR. Pain: Its Anatomy, Physiology and Treatment. 2012 [Google Scholar]
- 58.Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci U S A. 1995;92(12):5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakatsu F, Baskin JM, Chung J, Tanner LB, Shui G, Lee SY, Pirruccello M, Hao M, Ingolia NT, Wenk MR, De Camilli P. PtdIns4P synthesis by PI4KIIIalpha at the plasma membrane and its impact on plasma membrane identity. J Cell Biol. 199(6):1003–1016. doi: 10.1083/jcb.201206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neely GG, Rao S, Costigan M, Mair N, Racz I, Milinkeviciute G, Meixner A, Nayanala S, Griffin RS, Belfer I, Dai F, Smith S, Diatchenko L, Marengo S, Haubner BJ, Novatchkova M, Gibson D, Maixner W, Pospisilik JA, Hirsch E, Whishaw IQ, Zimmer A, Gupta V, Sasaki J, Kanaho Y, Sasaki T, Kress M, Woolf CJ, Penninger JM. Construction of a global pain systems network highlights phospholipid signaling as a regulator of heat nociception. PLoS genetics. 2012;8(12):e1003071. doi: 10.1371/journal.pgen.1003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen TT, Kim YM, Kim TD, Le OT, Kim JJ, Kang HC, Hasegawa H, Kanaho Y, Jou I, Lee SY. Phosphatidylinositol 4-phosphate 5-kinase alpha facilitates Toll-like receptor 4-mediated microglial inflammation through regulation of TIRAP location. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M112.410126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noda Y, Niwa S, Homma N, Fukuda H, Imajo-Ohmi S, Hirokawa N. Phosphatidylinositol 4-phosphate 5-kinase alpha (PIPKalpha) regulates neuronal microtubule depolymerase kinesin, KIF2A and suppresses elongation of axon branches. Proc Natl Acad Sci U S A. 2012;109(5):1725–1730. doi: 10.1073/pnas.1107808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277(16):13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 64.Osborne SL, Wen PJ, Boucheron C, Nguyen HN, Hayakawa M, Kaizawa H, Parker PJ, Vitale N, Meunier FA. PIKfyve negatively regulates exocytosis in neurosecretory cells. J Biol Chem. 2008;283(5):2804–2813. doi: 10.1074/jbc.M704856200. [DOI] [PubMed] [Google Scholar]
- 65.Pezet S, Marchand F, D’Mello R, Grist J, Clark AK, Malcangio M, Dickenson AH, Williams RJ, McMahon SB. Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J Neurosci. 2008;28(16):4261–4270. doi: 10.1523/JNEUROSCI.5392-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pomel V, Klicic J, Covini D, Church DD, Shaw JP, Roulin K, Burgat-Charvillon F, Valognes D, Camps M, Chabert C, Gillieron C, Francon B, Perrin D, Leroy D, Gretener D, Nichols A, Vitte PA, Carboni S, Rommel C, Schwarz MK, Ruckle T. Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase gamma. J Med Chem. 2006;49(13):3857–3871. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- 67.Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54(9):2419–2423. [PubMed] [Google Scholar]
- 68.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300(5623):1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 69.Rittiner JE, Brings VE, Zylka MJ. Overexpression of diacylglycerol kinase eta enhances Galphaq-coupled G protein-coupled receptor signaling. Mol Pharmacol. 85(5):800–810. doi: 10.1124/mol.113.091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudge SA, Wakelam MJ. SnapShot: lipid kinases and phosphatases. Cell. 155(7):1654–1654. e1651. doi: 10.1016/j.cell.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Sasaki H, Hozumi Y, Hasegawa H, Ito T, Takagi M, Ogino T, Watanabe M, Goto K. Gene expression and localization of diacylglycerol kinase isozymes in the rat spinal cord and dorsal root ganglia. Cell Tissue Res. 2006;326(1):35–42. doi: 10.1007/s00441-006-0219-z. [DOI] [PubMed] [Google Scholar]
- 72.Sato M, Liu K, Sasaki S, Kunii N, Sakai H, Mizuno H, Saga H, Sakane F. Evaluations of the selectivities of the diacylglycerol kinase inhibitors R59022 and R59949 among diacylglycerol kinase isozymes using a new non-radioactive assay method. Pharmacology. 92(1–2):99–107. doi: 10.1159/000351849. [DOI] [PubMed] [Google Scholar]
- 73.Schaible HG. Peripheral and central mechanisms of pain generation. Handb Exp Pharmacol. 2007;(177):3–28. doi: 10.1007/978-3-540-33823-9_1. [DOI] [PubMed] [Google Scholar]
- 74.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5 (Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 75.Shisheva A. Phosphoinositides in insulin action on GLUT4 dynamics: not just PtdIns(3,4,5)P3. Am J Physiol Endocrinol Metab. 2008;295(3):E536–544. doi: 10.1152/ajpendo.90353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274(3):159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 77.Simons JP, Al-Shawi R, Minogue S, Waugh MG, Wiedemann C, Evangelou S, Loesch A, Sihra TS, King R, Warner TT, Hsuan JJ. Loss of phosphatidylinositol 4-kinase 2alpha activity causes late onset degeneration of spinal cord axons. Proc Natl Acad Sci U S A. 2009;106(28):11535–11539. doi: 10.1073/pnas.0903011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128(5):509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strahl T, Grafelmann B, Dannenberg J, Thorner J, Pongs O. Conservation of regulatory function in calcium-binding proteins: human frequenin (neuronal calcium sensor-1) associates productively with yeast phosphatidylinositol 4-kinase isoform, Pik1. J Biol Chem. 2003;278(49):49589–49599. doi: 10.1074/jbc.M309017200. [DOI] [PubMed] [Google Scholar]
- 80.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh BC, Leal K, Hille B. Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 2010;67(2):224–238. doi: 10.1016/j.neuron.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun RQ, Tu YJ, Yan JY, Willis WD. Activation of protein kinase B/Akt signaling pathway contributes to mechanical hypersensitivity induced by capsaicin. Pain. 2006;120(1–2):86–96. doi: 10.1016/j.pain.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 83.Tsuruta F, Green EM, Rousset M, Dolmetsch RE. PIKfyve regulates CaV1. 2 degradation and prevents excitotoxic cell death. J Cell Biol. 2009;187(2):279–294. doi: 10.1083/jcb.200903028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaillancourt FH, Brault M, Pilote L, Uyttersprot N, Gaillard ET, Stoltz JH, Knight BL, Pantages L, McFarland M, Breitfelder S, Chiu TT, Mahrouche L, Faucher AM, Cartier M, Cordingley MG, Bethell RC, Jiang H, White PW, Kukolj G. Evaluation of phosphatidylinositol-4-kinase IIIalpha as a hepatitis C virus drug target. J Virol. 86(21):11595–11607. doi: 10.1128/JVI.01320-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signaling: the path to discovery and understanding. Nature Reviews: Molecular Cell Biology. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 86.Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143(2):501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasudevan L, Jeromin A, Volpicelli-Daley L, De Camilli P, Holowka D, Baird B. The beta- and gamma-isoforms of type I PIP5K regulate distinct stages of Ca2+ signaling in mast cells. J Cell Sci. 2009;122(Pt 14):2567–2574. doi: 10.1242/jcs.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269(7):5241–5248. [PubMed] [Google Scholar]
- 89.Volpicelli-Daley LA, Lucast L, Gong LW, Liu L, Sasaki J, Sasaki T, Abrams CS, Kanaho Y, De Camilli P. Phosphatidylinositol-4-phosphate 5-kinases and phosphatidylinositol 4,5-bisphosphate synthesis in the brain. J Biol Chem. 2010;285(37):28708–28714. doi: 10.1074/jbc.M110.132191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Voss MD, Czechtizky W, Li Z, Rudolph C, Petry S, Brummerhop H, Langer T, Schiffer A, Schaefer HL. Discovery and pharmacological characterization of a novel small molecule inhibitor of phosphatidylinositol-5-phosphate 4-kinase, type II, beta. Biochem Biophys Res Commun. 449(3):327–331. doi: 10.1016/j.bbrc.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Chen X, Lian L, Tang T, Stalker TJ, Sasaki T, Kanaho Y, Brass LF, Choi JK, Hartwig JH, Abrams CS. Loss of PIP5KIbeta demonstrates that PIP5KI isoform-specific PIP2 synthesis is required for IP3 formation. Proc Natl Acad Sci U S A. 2008;105(37):14064–14069. doi: 10.1073/pnas.0804139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang YJ, Li WH, Wang J, Xu K, Dong P, Luo X, Yin HL. Critical role of PIP5KI{gamma}87 in InsP3-mediated Ca(2+) signaling. J Cell Biol. 2004;167(6):1005–1010. doi: 10.1083/jcb.200408008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei YJ, Sun HQ, Yamamoto M, Wlodarski P, Kunii K, Martinez M, Barylko B, Albanesi JP, Yin HL. Type II phosphatidylinositol 4-kinase beta is a cytosolic and peripheral membrane protein that is recruited to the plasma membrane and activated by Rac-GTP. J Biol Chem. 2002;277(48):46586–46593. doi: 10.1074/jbc.M206860200. [DOI] [PubMed] [Google Scholar]
- 94.Weng X, Smith T, Sathish J, Djouhri L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Adelta-nociceptors. Pain. 2012;153(4):900–914. doi: 10.1016/j.pain.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 95.Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32(1):79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 96.Wiedemann C, Schafer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 1996;15(9):2094–2101. [PMC free article] [PubMed] [Google Scholar]
- 97.Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 98.Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55(3):353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 99.Wright BD, Loo L, Street SE, Ma A, Taylor-Blake B, Stashko MA, Jin J, Janzen WP, Frye SV, Zylka MJ. The lipid kinase PIP5K1C regulates pain signaling and sensitization. Neuron. 82(4):836–847. doi: 10.1016/j.neuron.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD. Phosphatidylinositol-4-phosphate 5-kinase 1alpha mediates extracellular calcium-induced keratinocyte differentiation. Mol Biol Cell. 2009;20(6):1695–1704. doi: 10.1091/mbc.E08-07-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu JT, Tu HY, Xin WJ, Liu XG, Zhang GH, Zhai CH. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol. 2007;206(2):269–279. doi: 10.1016/j.expneurol.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 102.Xu X, Wang P, Zou X, Li D, Fang L, Lin Q. Increases in transient receptor potential vanilloid-1 mRNA and protein in primary afferent neurons stimulated by protein kinase C and their possible role in neurogenic inflammation. J Neurosci Res. 2009;87(2):482–494. doi: 10.1002/jnr.21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L, Mao YS, Janmey PA, Yin HL. Phosphatidylinositol 4, 5 bisphosphate and the actin cytoskeleton. Subcell Biochem. 2012;59:177–215. doi: 10.1007/978-94-007-3015-1_6. [DOI] [PubMed] [Google Scholar]
- 104.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, Piper RC, Yang B, Nau JJ, Westrick RJ, Morrison SJ, Meisler MH, Weisman LS. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci U S A. 2007;104(44):17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao FL, Herness S. Resynthesis of phosphatidylinositol 4,5-bisphosphate mediates adaptation of the caffeine response in rat taste receptor cells. J Physiol. 2009;587(Pt 2):363–377. doi: 10.1113/jphysiol.2008.165167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34(4):689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24(38):8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]