Abstract
Objective
To evaluate the frequency, timing, and clinical features of relapses in giant cell arteritis (GCA).
Methods
Patients with GCA enrolled in a prospective, multicenter, longitudinal study were included in the analysis. Relapse was defined as either new disease activity after a period of remission or worsening disease activity.
Results
The study included 128 subjects: 102 women (80%) and 26 men (20%). Mean ± SD age at diagnosis of GCA was 69.9 ± 8.6 years. Mean followup for the cohort was 21.4 ± 13.9 months. Median (interquartile range) duration of disease at study enrollment was 4.6 months (1.2, 16.8). During followup, 59 relapses were observed in 44 patients (34%). Ten patients (8%) experienced 2 or more relapses. The most common symptoms at relapse were headache (42%) and polymyalgia rheumatica (51%), but ischemic (some transient) manifestations (visual symptoms, tongue or jaw claudication, and/or limb claudication) occurred in 29% of relapses (12% cohort). Forty-three relapses (73%) occurred while patients were taking glucocorticoid therapy at a median (range) prednisone dose of 7.5 (0–35) mg. In 21% of relapses, both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were normal. Among 69 patients enrolled in the cohort with newly diagnosed disease, 24% experienced a first relapse within 12 months after diagnosis.
Conclusion
Among patients with GCA, relapses are common, often occurring during treatment. ESR and CRP are frequently normal at times of clinical relapse, highlighting the need for better biomarkers to assess disease activity in GCA. There remains a need for effective therapeutic alternatives to glucocorticoids in GCA.
Key Indexing Terms: GIANT CELL ARTERITIS, RELAPSES, DISEASE ACTIVITY, COHORT STUDY
Giant cell arteritis (GCA) is a granulomatous vasculitis affecting large arteries, including the aorta and its primary branches. It is the most common vasculitis in individuals over the age of 50 years1. Clinically relevant extracranial manifestations, including large-artery stenoses, aneurysms, and/or aortic disease occur in at least one-third of patients with GCA1.
Glucocorticoids remain the mainstay of treatment for patients with GCA and should be initiated promptly if cranial manifestations occur. While symptoms respond to therapy, relapses are common2,3,4,5,6,7,8,9. Further, in a single autopsy series, nearly all patients with GCA were noted to have changes of active arteritis even though the disease was thought to be clinically quiescent10. Therapy with glucocorticoids is associated with significant morbidity with at least 1 glucocorticoid-associated adverse event occurring in 86% of patients2. The effectiveness of adjunctive treatment with other immunosuppressive medications has yielded mixed results11,12,13.
A better understanding of relapses in GCA would provide patients and physicians with prognostic information and help identify patients who may require longer duration of glucocorticoid therapy. The objectives of this study were to evaluate the frequency, timing, and clinical features of relapses in GCA in a prospective, longitudinal cohort.
MATERIALS AND METHODS
The study was approved by institutional review boards at each participating site. All participants provided informed consent.
Study population
The Vasculitis Clinical Research Consortium (VCRC) Longitudinal Study of GCA is a multicenter, longitudinal, observational cohort of patients with GCA. GCA was diagnosed based on modified 1990 American College of Rheumatology (ACR) classification criteria, which required age above 50 years with ≥ 2 of the following features: (1) new localized headache, (2) temporal artery abnormality on examination, (3) erythrocyte sedimentation rate (ESR) > 40 mm/h by Westergren method, (4) abnormal temporal artery biopsy, and (5) large vessel vasculitis by angiography or biopsy. Subjects with quarterly evaluations were included in this study. Subjects with newly diagnosed or established GCA were enrolled.
Clinical and laboratory assessments
All patients were followed prospectively with standardized quarterly clinical assessments that included any documentation of new disease-related symptom (since the previous visit, in the preceding 28 days, and on the day of evaluation), vascular physical examination findings, laboratory evaluations, results of vascular imaging studies, and medication usage. Additional visits occurred when clinically indicated, at which time additional data were obtained. Disease activity on the day of evaluation was classified by the treating physician as being in “remission” or “active” (categorized in their opinion as “low,” “moderate,” or “high” activity). Normal ESR was defined as a value of ≤ 20 mm/h by the Westergren method. Normal C-reactive protein (CRP) was defined as a value of ≤ 5 mg/l.
Definition of disease relapse
Relapse was defined as new disease activity after a period of remission, or worsening disease activity that occurred during followup. Active disease at baseline evaluation was not considered a relapse. If a patient had new symptoms between followup visits but symptoms had resolved by the time of evaluation, the midpoint was chosen as the time of relapse.
Treatment
Treatment decisions were left to the individual physician. Use and timing of the following medications was collected systematically at each visit: prednisone or other glucocorticoids, cyclophosphamide, azathioprine, methotrexate (MTX), mycophenolate mofetil (MMF), etanercept, infliximab, adalimumab, other immunosuppressive drugs, aspirin, clopidogrel, and statins.
Data analysis
Descriptive statistics were used. The Kaplan-Meier method was used to evaluate the time to first relapse for the cohort of patients with newly diagnosed disease. JMP Version 9 (SAS Institute Inc.) was used for all analyses except the Kaplan-Meier analysis, which was performed using SAS Version 9.2 (SAS Institute Inc.).
RESULTS
Cohort
The study included 128 subjects — 102 women (80%) and 26 men (20%). Mean (± SD) age at diagnosis of GCA was 69.9 years (± 8.6). Temporal artery biopsy was not required because patients with extracranial large vessel disease were included in the study cohort. Temporal artery biopsy was performed in 103 subjects and was consistent with GCA in 80 (78%). Sixty patients (47%) of the cohort had large-vessel imaging (aorta and branches), of whom 25 subjects (42%) had abnormalities at the aorta and/or its branches. Among the subjects with abnormalities, large-artery stenosis was noted in 24 patients (96%) and 7 subjects (28%) had aortic abnormalities. Median duration of disease at baseline (study enrollment) was 4.6 months (interquartile range 1.2–16.8). Mean followup for the cohort was 21.4 months (± 13.9).
Forty-nine patients (39%) reported at least 1 prior relapse before study enrollment. At study entry, 118 patients (94%) were receiving glucocorticoids. Other immunosuppressive medications at baseline visit included MTX (19 patients, 15%), azathioprine (3 patients, 2.4%), cyclophosphamide (2 patients, 1.6%), and MMF (1 patient, 0.7%).
Relapses
During followup, 59 relapses were observed in 44 patients (34%). Of the 44 patients, temporal artery biopsy at diagnosis was positive in 34 (77%), negative in 6 (14%), and not performed in 4 (9%). Ten patients (8% of cohort) experienced > 1 relapse; including 6 patients with 2 relapses, 3 patients with 3 relapses, and 1 patient with 4 relapses. Disease was still active on the day of evaluation in 43 relapses (73%), with current disease activity rated as “low” in 28 relapses and “moderate” in 15 relapses. Evaluating the subset of 60 patients with imaging at baseline, 9 of 25 subjects (36%) with large-vessel manifestations experienced a relapse compared to 11 of 35 patients (31%) without large-vessel manifestations (p = 0.71). In 16 cases (27%), the patients had experienced a relapse between visits, but the symptoms had resolved by the time of evaluation. A greater proportion of patients with relapses had a history of relapse (23 of 44 patients, 52%) compared to 26 of 84 patients (31%) who did not experience a relapse (p = 0.02).
Symptoms
The symptoms attributed to GCA at the time of 59 relapses are summarized in Table 1. The most common symptom was headache followed by polymyalgia rheumatica (PMR). The median number of symptoms at relapse was 2 (range 1–6). In 16 relapses (25%), patients had isolated PMR symptoms. Three patients (7% of relapse group, 2% cohort) developed new visual symptoms while receiving treatment; 2 had ocular involvement at presentation, with new symptoms during relapse. One patient with no history of ocular involvement at diagnosis developed new transient partial vision loss. Findings included ischemic neuropathy in 1 patient, retinal vein occlusion in another patient and diplopia in the third patient. All 3 patients were receiving glucocorticoids (8 mg, 9 mg, and 10 mg daily) at the time of development of visual symptoms, and 2 were receiving low-dose aspirin therapy. Other ischemic symptoms at relapse included tongue or jaw claudication (14% relapses) and limb claudication (10% relapses), with 1 patient developing new limb claudication. At least 1 ischemic symptom was observed in 29% of relapses (15 patients).
Table 1.
Symptoms of active giant cell arteritis during all 59 relapses, and by prednisone dose at time of relapse. Values expressed as no. relapses with the symptom recorded divided by total no. relapses at that dose of prednisone (%). Steroid data were unavailable in 2 relapses.
| Symptoms | All, n = 59 | Prednisone, 0 mg, n =11 | Prednisone, 1–10 mg, n = 32 | Prednisone, 11–20 mg, n = 11 | Prednisone, > 20 mg, n = 3 |
|---|---|---|---|---|---|
| Cranial | |||||
| Headache | 25 (42) | 1 (9) | 12 (38) | 8 (73) | 3 (100) |
| Scalp tenderness | 19 (32) | 0 (0) | 7 (22) | 9 (82) | 2 (67) |
| New temporal artery pain | 6 (10) | 0 (0) | 2 (6) | 2 (18) | 2 (67) |
| Carotidynia | 2 (3) | 0 (0) | 2 (6) | 0 (0) | 0 (0) |
| Jaw/tongue claudication | 8 (14) | 1 (9) | 4 (13) | 3 (27) | 0 (0) |
| Visual manifestations | 3 (5) | 0 (0) | 3 (9) | 0 (0) | 0 (0) |
| PMR/arthralgia | 30 (51) | 8 (73) | 16 (50) | 5 (45) | 1 (33) |
| Upper extremity claudication | 6 (10) | 2 (18) | 3 (9) | 0 (0) | 0 (0) |
| Lower extremity claudication | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) |
| Constitutional (weight loss, fatigue) | 8 (14) | 2 (18) | 4 (13) | 1 (9) | 1 (33) |
PMR: polymyalgia rheumatica.
Medications
Forty-three relapses (73%) occurred while patients were taking glucocorticoids. Median (range) prednisone dose at time of relapse was 7.5 mg (0–35). Eleven relapses (17%) occurred after discontinuation of prednisone, 32 (54%) at a daily dose of prednisone 1–10 mg, 11 (17%) at prednisone doses between 11–20 mg, and 3 (5%) at prednisone doses > 20 mg. In the subset of patients with newly diagnosed GCA, median (range) prednisone at first relapse was 10 mg (0–35) with 15 relapses at daily dose of 0–10 mg, 4 at doses of 11–20 mg, and 2 at > 20 mg. Other medication use at relapse included MTX (13 relapses, 22%), anti-tumor necrosis factor therapy (2 relapses, 3%), and MMF (2 relapses, 3%).
A distribution of the clinical manifestations at relapse by the dose of prednisone at relapse is in Table 1. Cranial manifestations at relapse were noted at all doses of prednisone. However, symptoms of PMR were most frequently observed only at doses ≤ 10 mg prednisone.
Laboratory findings
In 43 flares (73% relapses) with active disease at the time of evaluation, mean hemoglobin was 12.7 g/dl (± 0.98), mean white blood cell count was 10.9 (± 13.0) × 109/l, and mean platelet count was 313 (± 70.7) × 109/l.
Data for ESR and CRP were available in 39 relapses. Mean ESR (± SD) was 31 mm/h (± 15.3) with a mean CRP (± SD) of 16.3 mg/l (± 14.3). In 30 relapses (77%), ESR was elevated at > 20 mm/h while CRP was elevated (> 5 mg/l) in 29 relapses (74%). Both ESR and CRP were normal (ESR < 20 mm/h, CRP < 5 mg/l) in 8 relapses (21%).
Discordance between ESR and CRP was only noted in 3 of 39 relapses (8%; only subset with active symptoms on the day of evaluation included). In those with discordant ESR/CRP, 2 had elevated ESR and normal CRP.
Time to first relapse
To evaluate the time to first relapse following diagnosis, relapses in the 69 patients enrolled within 4 months of diagnosis were examined. Twenty-four percent of subjects experienced a first relapse by 12 months and 50% experienced a first relapse by 24 months (Figure 1).
Figure 1.
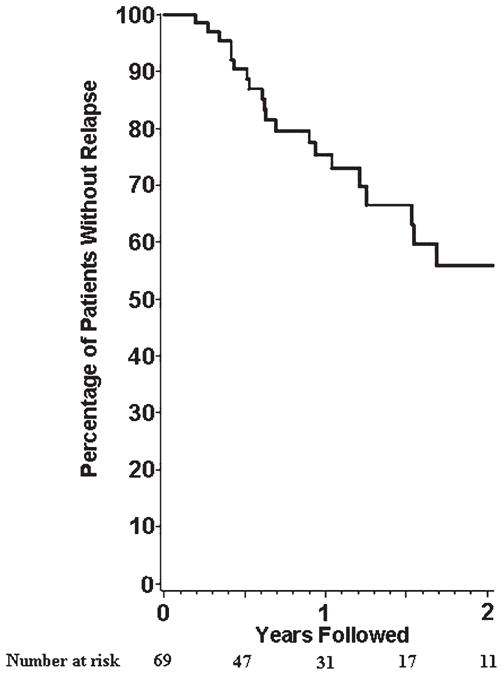
Relapses in 69 patients with newly diagnosed giant cell arteritis.
DISCUSSION
Relapses are common in GCA. In this prospective study, 34% of patients with GCA experienced at least 1 relapse during a median followup of 21 months. Ischemic symptoms (some transient) were observed in 29% of relapses. Nearly three-quarters of all relapses occurred during treatment with glucocorticoids and one-third of relapses occurred despite other immunosuppressive therapy.
The frequency of relapses reported in observational cohorts of patients with GCA varies widely based on the study type and definition of relapse. In retrospective studies, at least 1 disease relapse was reported in 28% to 62% of patients2,3,5,7,8. Of the 3 prospective observational studies to date evaluating relapses in GCA, relapses were reported in 36% (21 of 58), 60% (15 of 25), and 68% (68 of 106) of patients4,6,9. The current study includes a large prospective cohort of patients with GCA with standardized assessments at regular intervals including comprehensive information on symptoms at relapse. In contrast to a recent prospective study where only patients with biopsy-positive GCA were included9, our cohort also included patients with imaging findings of large-artery stenosis from GCA.
While headache and PMR were the most commonly reported symptoms during relapse, ischemic manifestations such as limb claudication, jaw/tongue claudication, and vision loss were noted in 29% of relapses in our study. This is at the higher range of what has been reported. In clinical trials, 12–22% subjects developed ischemic symptoms9,13 while in a retrospective study, 13% (7 of 54 patients) experienced symptomatic vascular complications7. In a prospective cohort of 106 patients with biopsy-positive GCA, only 1 patient developed new visual changes while none developed manifestations of large-vessel involvement during relapses9. The observation in our study may have to do with the inclusion of patients with large-artery manifestations from GCA.
Incident vision loss after initiation of treatment was estimated to be 1% at 5 years in a retrospective study14. A higher incidence was reported in a prospective clinical trial, where the cumulative incidence of vision loss at 1 year was 14%15. In an observational study of patients with GCA and ischemic optic neuropathy at diagnosis, recurrent visual symptoms were noted in 10% of patients16. In our study, new visual symptoms were present during relapse in 3 patients (5%), all of whom were receiving glucocorticoid therapy. Fortunately, they did not experience new permanent vision loss.
The majority of relapses in the current study occurred while patients were taking glucocorticoids. Few relapses were observed at prednisone doses ≥ 20 mg. In general, in the 0–20 mg/day range of prednisone dosing, relapses occurred irrespective of glucocorticoid dose at relapse including in the subset of patients with newly diagnosed disease. This finding was also reported in another prospective study of patients with GCA, using a standardized protocol of glucocorticoid taper6. In contrast, in a randomized clinical trial also with a standardized glucocorticoid taper, most relapses occurred between the fourth and sixth months after randomization17. In the present cohort, cranial symptoms at relapse were observed at all doses of prednisone while symptoms of PMR were more frequently observed at relapse at prednisone doses of ≤ 10 mg.
Acute-phase reactants (APR) are commonly measured serially in the clinical care of patients with GCA. In previous studies, APR have been found to be neither sensitive nor specific markers of disease activity6,18,19. While the definition of relapse used in our study did not require an increase in ESR or CRP, physicians did have access to APR data during the clinical evaluation, which may have influenced their decision about whether a patient had a relapse. In our study, 21% of relapses had normal ESR and normal CRP even in the presence of symptoms of active disease. Clinicians should be cautious about relying upon ESR or CRP to monitor disease activity in patients with GCA.
A better understanding of the timing of relapses may have clinical implications in the followup of patients with GCA. In 1 prospective observational study of patients with newly diagnosed GCA, relapses appeared to be evenly distributed over the entire duration of followup (median 550 days)6. In another prospective incident cohort of patients with GCA, median time to first relapse was 51 weeks, and while relapses were observed throughout the followup, majority occurred within the first 2 years after diagnosis9. In our study, when we evaluated the subset with newly diagnosed GCA, relapse was observed throughout the duration of followup.
The strengths of this study are the large cohort size, the multicenter, prospective design with comprehensive standardized evaluations at regular time intervals at expert centers, and a uniform definition of relapse, which did not rely on markers of inflammation. The cohort also includes patients with GCA with large-vessel manifestations in addition to those with cranial manifestations. Collection of data on symptoms attributable to disease activity occurring between study visits ensured more complete ascertainment of any relapses between visits.
There are limitations in our study to consider. First, the ACR classification criteria were used as inclusion criteria for the cohort. They are classification, not diagnostic, criteria. However, a substantial proportion of patients in this cohort had other diagnostic modalities such as positive biopsy or radiographic findings consistent with large-vessel vasculitis, making the possibility of misclassification small. The patients were evaluated at tertiary care referral centers, possibly enriching the cohort with patients with more relapsing disease and those with large-artery stenosis. However, the relapse rate was within the ranges that have been reported. Glucocorticoid doses and taper schedule were not standardized, but that reflects clinical practice. Similarly, adjunctive immunosuppressive therapy use was also not standardized. While this analysis was restricted regarding measurement of APR only to individuals who had active symptoms the day of evaluation, it is possible that the prednisone had been increased prior to assessment at the study center, thereby influencing the results.
Our study demonstrates that relapses are common in GCA. Despite treatment, ischemic symptoms were reported in 29% of relapses; some fortunately transient. Relapses often occurred in the context of glucocorticoid therapy, and in some cases, adjunctive immunosuppressive therapy. There remains a need for therapeutic agents that are better able to induce and sustain longterm remission in patients with GCA and for the discovery of reliable biomarkers of disease activity.
Acknowledgments
Dr. Kermani was supported by the Vasculitis Clinical Research Consortium (VCRC), which has received support from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319), the US National Center for Research Resources (U54 RR019497), the US Office of Rare Diseases Research, and the US National Center for Advancing Translational Science. The VCRC is part of the Rare Diseases Clinical Research Network.
References
- 1.Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372:234–45. doi: 10.1016/S0140-6736(08)61077-6. [DOI] [PubMed] [Google Scholar]
- 2.Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–8. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Lado L, Calvino-Diaz C, Pineiro A, Dierssen T, Vazquez-Rodriguez TR, Miranda-Filloy JA, et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine. 2011;90:186–93. doi: 10.1097/MD.0b013e31821c4fad. [DOI] [PubMed] [Google Scholar]
- 4.Liozon E, Roblot P, Paire D, Loustaud V, Liozon F, Vidal E, et al. Anticardiolipin antibody levels predict flares and relapses in patients with giant-cell (temporal) arteritis. A longitudinal study of 58 biopsy-proven cases. Rheumatology. 2000;39:1089–94. doi: 10.1093/rheumatology/39.10.1089. [DOI] [PubMed] [Google Scholar]
- 5.Hachulla E, Boivin V, Pasturel-Michon U, Fauchais AL, Bouroz-Joly J, Perez-Cousin M, et al. Prognostic factors and long-term evolution in a cohort of 133 patients with giant cell arteritis. Clin Exp Rheumatol. 2001;19:171–6. [PubMed] [Google Scholar]
- 6.Weyand CM, Fulbright JW, Hunder GG, Evans JM, Goronzy JJ. Treatment of giant cell arteritis: interleukin-6 as a biologic marker of disease activity. Arthritis Rheum. 2000;43:1041–8. doi: 10.1002/1529-0131(200005)43:5<1041::AID-ANR12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Martinez A, Hernandez-Rodriguez J, Espigol-Frigole G, Prieto-Gonzalez S, Butjosa M, Segarra M, et al. Clinical relevance of persistently elevated circulating cytokines (tumor necrosis factor alpha and interleukin-6) in the long-term followup of patients with giant cell arteritis. Arthritis Care Res. 2010;62:835–41. doi: 10.1002/acr.20043. [DOI] [PubMed] [Google Scholar]
- 8.Nesher G, Nesher R, Mates M, Sonnenblick M, Breuer GS. Giant cell arteritis: intensity of the initial systemic inflammatory response and the course of the disease. Clin Exp Rheumatol. 2008;26:S30–4. [PubMed] [Google Scholar]
- 9.Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, Tavera-Bahillo I, Corbera-Bellalta M, Planas-Rigol E, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine. 2014;93:194–201. doi: 10.1097/MD.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostberg G. Morphological changes in the large arteries in polymyalgia arteritica. Acta Med Scand (Suppl) 1972;533:135–59. [PubMed] [Google Scholar]
- 11.Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–30. doi: 10.7326/0003-4819-146-9-200705010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Taboada VM, Rodriguez-Valverde V, Carreno L, Lopez-Longo J, Figueroa M, Belzunegui J, et al. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Ann Rheum Dis. 2008;67:625–30. doi: 10.1136/ard.2007.082115. [DOI] [PubMed] [Google Scholar]
- 13.Mahr AD, Jover JA, Spiera RF, Hernandez-Garcia C, Fernandez-Gutierrez B, Lavalley MP, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–97. doi: 10.1002/art.22754. [DOI] [PubMed] [Google Scholar]
- 14.Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology. 1993;100:550–5. doi: 10.1016/s0161-6420(93)31608-8. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002;46:1309–18. doi: 10.1002/art.10262. [DOI] [PubMed] [Google Scholar]
- 16.Chan CC, Paine M, O’Day J. Predictors of recurrent ischemic optic neuropathy in giant cell arteritis. J Neuroophthalmol. 2005;25:14–7. doi: 10.1097/00041327-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Jover JA, Hernandez-Garcia C, Morado IC, Vargas E, Banares A, Fernandez-Gutierrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:106–14. doi: 10.7326/0003-4819-134-2-200101160-00010. [DOI] [PubMed] [Google Scholar]
- 18.Kyle V, Cawston TE, Hazleman BL. Erythrocyte sedimentation rate and C reactive protein in the assessment of polymyalgia rheumatica/giant cell arteritis on presentation and during follow up. Ann Rheum Dis. 1989;48:667–71. doi: 10.1136/ard.48.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pountain GD, Calvin J, Hazleman BL. Alpha 1-antichymotrypsin, C-reactive protein and erythrocyte sedimentation rate in polymyalgia rheumatica and giant cell arteritis. Br J Rheumatol. 1994;33:550–4. doi: 10.1093/rheumatology/33.6.550. [DOI] [PubMed] [Google Scholar]


