Abstract
Objective
Glomerulonephritis (GN) is common in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), but tools for early detection of renal involvement are imperfect. We investigated 4 urinary proteins as markers of active renal AAV: alpha-1 acid glycoprotein (AGP), kidney injury molecule-1 (KIM-1), monocyte chemoattractant protein-1 (MCP-1), and neutrophil gelatinase-associated lipocalin (NGAL).
Methods
Patients with active renal AAV (n = 20), active nonrenal AAV (n = 16), and AAV in longterm remission (n = 14) were identified within a longitudinal cohort. Urinary biomarker concentrations (by ELISA) were normalized for urine creatinine. Marker levels during active AAV were compared to baseline remission levels (from 1–4 visits) for each patient. Areas under receiver-operating characteristic curves (AUC), sensitivities, specificities, and likelihood ratios (LR) comparing disease states were calculated.
Results
Baseline biomarker levels varied among patients. All 4 markers increased during renal flares (p < 0.05). MCP-1 discriminated best between active renal disease and remission: a 1.3-fold increase in MCP-1 had 94% sensitivity and 89% specificity for active renal disease (AUC = 0.93, positive LR 8.5, negative LR 0.07). Increased MCP-1 also characterized 50% of apparently nonrenal flares. Change in AGP, KIM-1, or NGAL showed more modest ability to distinguish active renal disease from remission (AUC 0.71–0.75). Hematuria was noted in 83% of active renal episodes, but also 43% of nonrenal flares and 25% of remission samples.
Conclusion
Either urinary MCP-1 is not specific for GN in AAV, or it identifies early GN not detected by standard assessment and thus has potential to improve care. A followup study with kidney biopsy as the gold standard is needed.
Key Indexing Terms: Vasculitis, Biomarkers, Glomerulonephritis, Wegener Granulomatosis, Monocyte Chemoattractant Protein-1
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) refers to a group of closely related systemic diseases [granulomatosis with polyangiitis (GPA; Wegener's), microscopic polyangiitis (MPA), and Churg-Strauss syndrome (CSS)] that typically involve multiple organ systems. Renal involvement is manifested as necrotizing glomerulonephritis (GN), which can lead to rapid and irreversible renal damage. More than 70% of patients with GPA or MPA1,2,3,4 and 15%–25% of patients with CSS5,6,7,8 will have GN during the course of their illness. Patients with GPA are at particular risk for relapse of renal disease or a first presentation of GN after initial diagnosis and treatment of GPA1. The high rates of GN at presentation and at relapse call for vigilance in monitoring patients for evidence of GN.
Renal biopsy is the gold standard for diagnosis of active GN, but the procedure is not without risk, and repeated renal biopsies are not practical. Noninvasive indicators of GN such as a rise in serum creatinine and/or the presence of new hematuria, worsening proteinuria, and urinary red blood cell casts in particular can be effective indicators of active renal disease, but there are problems with each of these methods. A rise in serum creatinine provides no information about the cause of renal injury and may not occur until there has been substantial, and sometimes permanent, loss of function9. While urine sediment analysis is helpful in identifying a relapse of GN, detection of red blood cell casts is insensitive and is operator-dependent. Objective and more sensitive components of urinalysis are problematic for the opposite reason: hematuria and proteinuria can persist long after remission is achieved10, and nonglomerular hematuria can be caused by previous exposure to cyclophosphamide, which is commonly used to treat AAV.
Thus, additional biomarkers of renal inflammation and injury are needed for detecting relapsing disease and would ideally provide sensitive and disease-specific evidence of activity prior to decline of the glomerular filtration rate (GFR)9. Urinary biomarkers of renal disease have several potential advantages. Collection of urine is noninvasive, inexpensive, and reflects disease activity throughout the kidney rather than in the limited sample of glomeruli obtained in a biopsy. Proteomic techniques, such as 2-dimensional electrophoresis and mass spectrometry, have led to identification of urinary biomarkers of renal disease11, particularly in patients with systemic lupus erythematosus12,13. In AAV, the few studies of urinary biomarkers14,15,16,17 have suggested that these are potential markers of active renal disease: urinary monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor (TNF), interleukin 6 (IL-6), IL-8, vascular cell adhesion molecule-1 (VCAM-1), and tissue inhibitor of matrix metalloproteinases-1 (TIMP-1).
We evaluated potential urinary biomarkers of active kidney injury in AAV using a candidate protein approach. Markers were selected based on confirmation as markers of renal injury in AAV or in other kidney diseases, and relative ease of measurement using commercially available ELISA kits. We selected 4 candidate proteins: 3 that have not been reported previously in AAV [kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and alpha-1 acid glycoprotein (AGP)], and 1 protein for which data in AAV seemed particularly impressive (MCP-1)14.
KIM-1 (also known as HAVCR1 and TIM-1) was first identified as a protein upregulated during ischemic kidney injury in rats18. KIM-1 was then found in the urine of human patients with ischemic acute tubular necrosis19. Urinary KIM-1 is elevated in rats exposed to nephrotoxic and ischemic insults20,21 and in a variety of human kidney diseases22,23. The urinary concentration of KIM-1 correlates directly with staining for KIM-1 in renal biopsies, and varies inversely with renal function23. Although KIM-1 staining occurs in the tubules rather than the glomeruli, elevation of urinary KIM-1 has also been observed in diseases that are primarily glomerular23.
NGAL (lipocalin 2) was originally purified from human neutrophils24. NGAL mRNA was found to be dramatically upregulated following ischemic renal injury in mice25, and subsequently in acute tubular necrosis and other forms of kidney injury in humans26. Increased NGAL can be detected in biopsy specimens, serum, and urine of patients with acute tubular necrosis26. As with KIM-1, expression is predominantly in tubules, but urinary NGAL can be elevated in disease that is primarily glomerular; for example, urinary NGAL levels correlate with disease activity and are predictive of flares of lupus nephritis27,28.
MCP-1 (CCL2) is a chemokine that participates in recruitment of leukocytes to areas of inflammation by binding its receptor, CCR229. MCP-1 is upregulated in a rodent model of GN as well as in human inflammatory glomerulopathies30. MCP-1 may play a direct role in promoting renal fibrosis in diabetic nephropathy31. Urinary MCP-1 correlates well with disease activity in lupus nephritis32 and has been shown to be a marker of both active renal disease and poor prognosis in AAV14,17.
AGP (orosomucoid) is an acute-phase protein released in response to systemic inflammation. Proteomic analysis of urine from patients with lupus nephritis identified AGP as a potential biomarker. Subsequent work showed that urinary AGP can be used to predict renal flares in lupus nephritis13,33.
We compared the urinary concentrations of these proteins before, during, and after renal flares of AAV, and also measured concentrations in patients with active vasculitis but no current evidence of renal involvement and in patients in longterm remission.
Materials and Methods
Patients and clinical data collection
Patients were enrolled in the Vasculitis Clinical Research Consortium (VCRC) longitudinal studies of patients with granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), or Churg-Strauss syndrome (CSS), from 8 referral centers in the United States and Canada. Patients had to meet American College of Rheumatology (ACR) criteria for GPA modified to include ANCA, or the Chapel Hill Consensus Conference definition of MPA, or ACR criteria for CSS adapted so that biopsy proof of small-vessel vasculitis was not required. Clinical data, including measures of disease activity (see below), were collected on a quarterly or annual basis and at times of increased activity of vasculitis. Treatment with immune-suppressive medications (yes/no) was recorded at each visit. All patients were enrolled using protocols approved by the institutional review boards or ethics committees of all participating sites and written informed consent documents in keeping with The Declaration of Helsinki.
Measures of vasculitis disease activity
Information on specific manifestations of vasculitis was recorded using the Birmingham Vasculitis Activity Score34 as well as a more detailed form designed for the VCRC studies. Data elements that defined active renal disease included hematuria, urinary red blood cell casts, and/or rise in serum creatinine > 30% that were interpreted by the clinician as being due to active vasculitis. Only 2 patients had renal biopsies done at the time of this clinical assessment. Physician global assessment (PGA) of current overall vasculitis activity was assessed with 2 measures: a 0–10 Likert scale and a categorical assessment (remission or low, moderate, or high disease activity).
Study design
The goals of the study were (1) to assay 4 urinary biomarkers in samples from every subject in the VCRC cohort in whom an episode of active renal disease had been confirmed; (2) to compare those results to results obtained during remission in the same patients; (3) to compare to results obtained from patients in whom nonrenal disease flares were identified; (4) to compare to results from patients in remission for at least 2 years; (5) to assay multiple samples during remission; and (6) to limit the study size to about 180 assays per marker because of cost.
Twenty patients with an episode of active renal disease and at least 1 remission visit were identified (Group 1). These episodes all occurred among patients who were experiencing relapses rather than first-ever episodes of vasculitis, and 16 of these patients had had prior episodes of renal disease. Since overall vasculitis disease activity was characterized as being “moderate” or “high” in the categorical assessment (and/or PGA ≥ 3) for all but 2 episodes characterized as having active renal involvement, the comparison group of 16 patients with active vasculitis but without active renal disease (Group 2) was limited to subjects with episodes of active vasculitis of similar severity using the same categorical and PGA scales. For both groups, the 2 visits in remission preceding and the 2 visits in remission following the visit during active disease were selected if available (4 per patient). Fourteen patients in longterm remission, based on absence of active disease during any assessment for at least 2 consecutive years in the VCRC study, were also selected (Group 3), with 3 consecutive visits from the middle of the observational period selected for each such patient. Patients in Groups 2 and 3 were selected to include patients with a history of renal involvement from vasculitis prior to enrollment in the VCRC (7/16 in Group 2, 7/14 in Group 3), based on information collected on the baseline VCRC medical history form.
Collection and storage of urine samples
Urine was collected by the patients in sterile cups (the same ones subsequently sent to the clinical laboratory for urinalyses), with processing time typical of outpatient sample collection. Urine was aliquoted without further manipulation, frozen at −80°C at each participating clinical site, shipped on dry ice to the VCRC specimen repository, and stored at −80°C until use for this study.
Quantification of urine protein, urine creatinine, and biomarker concentrations
Urine samples were thawed at room temperature, then gently centrifuged to remove solid debris. There was no adjustment of pH. Urine protein concentrations were determined by microscale Bradford assay (Sigma; catalog no. TP0100) using 5 μl of urine. Albumin was used for the standard curve, and concentrations > 0.35 mg/ml were regarded as reliable after inspection of those data. Urine creatinine was quantified using a microplate assay (Arbor Assays, DetectX; catalog no. K002). AGP, KIM-1, MCP-1, and lipocalin-2/NGAL were quantified by ELISA (R&D; catalog numbers DAGP00, DKM100, DCP100, DLCN20). Urine was diluted 10-fold for the AGP assay, 2-fold for MCP-1, and undiluted for KIM-1 and NGAL assays. Absorbances were measured using a plate reader. Polynomial trendlines were fitted to standard curve data using Microsoft Excel, and the trendline equations were then used to calculate concentrations for each sample. Marker concentrations were then divided by urine creatinine concentrations prior to analysis.
Clinical laboratory tests
Serum creatinine and urinalyses (dipstick and microscopy) were performed in CLIA-approved laboratories at the clinical sites according to standard practice for collection and processing of out-patient specimens. Results of dipstick and microscopic urinalyses were recorded in the VCRC database as positive or negative (without further quantification) for blood, protein, red blood cells (RBC), and RBC casts, based on the official urinalysis results. RBC and RBC casts could also be noted as positive based on examination by the investigator or a nephrologist colleague at the time of the patient visit, but such an examination was not required nor was the method standardized, nor was it recorded whether assessment for RBC or casts was made by the clinical laboratory or the investigator. The presence or absence of dysmorphic RBC was not recorded. Glomerular filtration rate (GFR, ml/min per 1.73 m2 body surface area) was calculated from serum creatinine using the MDRD formula35.
Statistical analysis: Analysis of marker levels in remission
To determine whether levels in remission differed pre- or post-flare, pre-flare and post-flare values were compared by paired T test. In addition, the difference between pre- and post-flare values was determined, and the distribution analyzed by signed-rank test.
To determine whether remission values varied significantly with time before or after flare, the difference between the flare level and each remission level was used as the dependent variable in a generalized linear model (GLM) in which time pre/post flare and a unique patient identifier served as the independent variables.
Data from patients with multiple remission visits and the manufacturer's reported coefficient of variation (CV) for analytical imprecision (6%–7% for each assay) were used to calculate within-subject normal biological variation, between-subject variation, and the index of individuality36,37.
Comparison of marker levels in active vasculitis and remission
For each patient, marker level during active vasculitis was divided by the mean of marker levels during remission for that patient to calculate a fold-change. Groups were defined by the presence or absence of active renal disease. Statistical significance within each group was determined by signed-rank test.
Analysis of change in marker level
Fold-change relative to the patient's baseline was calculated for each individual sample (active or remission), from each patient who had at least 2 samples during remission. Fold-changes during remission were pooled across the 3 clinical groups (n = 125 samples), and fold-changes associated with active vasculitis were pooled within each clinical group (n = 17 renal, n = 14 nonrenal). Logistic regression, with fold-change in marker level as the independent variable and disease state as the dichotomous dependent variable, was used to generate receiver-operating characteristic (ROC) curves for comparisons of 3 pairs of disease states: active renal disease versus remission, active nonrenal disease versus remission, and active renal versus active nonrenal disease. The optimal cutpoint for distinguishing 2 disease states was determined using the Youden index, which is the maximum of the sum of sensitivity and specificity38. Sensitivity and specificity at this cutpoint were used to calculate the positive likelihood ratio [sensitivity/(1 – specificity)] and negative likelihood ratio [(1 – sensitivity)/specificity]. Ninety-five percent CI of sensitivity and specificity were determined by the asymptotic method.
Analysis of the effect of proteinuria on marker level
Association of marker level with proteinuria was measured using Pearson correlation coefficients and linear regression. The primary analysis was limited to the 12 samples (from 8 patients) with protein > 0.35 mg/ml during remission, but larger subsets of remission samples were also analyzed to ensure consistency as noted in the text. Multiple linear regression was also performed using all samples (active and remission), with MCP-1/creatinine as the dependent variable and protein/creatinine, age, sex, active disease (vs remission), and active renal disease (vs not) as independent variables. Marker levels during remission in groups defined by presence or absence of urinary RBC (by microscopic examination) and/or protein (by dipstick urinalysis) were compared by Kruskal-Wallis test.
Effects of demographic and disease-related factors
The mean marker levels during remission were determined for each patient. Marker levels between groups (male vs female, GPA vs MPA/CSS/unknown) were compared using Wilcoxon rank-sum tests. Marker levels were compared to age and GFR using Spearman correlation coefficients.
Since treatment could change longitudinally, GLM was performed using data from all samples, with marker level as the dependent variable and treatment (yes/no) as one of the independent variables, along with active vasculitis versus remission, active renal disease versus not, protein/creatinine (continuous variable), and either (1) patient ID (as a composite of all variables that change minimally over time); or (2) age, sex, and AAV subtype (GPA, MPA, CSS, or unknown).
All analyses were performed using SAS 9.1 (SAS Institute) or InStat (GraphPad Software).
Results
Patient characteristics
As shown in Table 1, most patients had GPA with anti-PR3 antibodies, had longstanding disease (median duration 6.5 yrs at study enrollment), and were being treated with immune-suppressive drugs at times of both active disease and remission. Each patient was classified into 1 of 3 groups (described above): active renal disease, active nonrenal disease, and longterm remission.
Table 1.
Characteristics of patients in this study.
| Characteristic | Active Renal, n = 20 |
Active Nonrenal, n = 16 |
Longterm Remission n = 14 |
Total, n = 50 |
|---|---|---|---|---|
| Sex | ||||
| Female | 8 | 8 | 4 | 20 |
| Male | 12 | 8 | 10 | 30 |
| Age, yrs* | 60 (48–67; 26–80) | 50 (34–57; 17–71) | 48 (40–56; 19–63) | 52 (44–61; 17–80) |
| Disease | ||||
| GPA | 17 | 13 | 10 | 40 |
| MPA | 1 | 0 | 1 | 2 |
| CSS | 0 | 3 | 3 | 6 |
| Unknown | 2 | 0 | 0 | 2 |
| ANCA specificity | ||||
| PR3 | 17 | 11 | 7 | 35 |
| MPO | 1 | 1 | 2 | 4 |
| Disease duration, yrs* | 6.9 (3.6–9.1; 1.2–18) | 4.9 (3.0–8.8; 0.6–21) | 5.2 (2.9–11; 1.9–28) | 6.5 (3.2–9.8; 0.6–28) |
| GFR* | 59 (48–71; 24–78) | 87 (74–99; 55–175) | 71 (51–86; 25–115) | 70 (52–86; 24–175) |
| Treatment: yes/no** | ||||
| Active | 15/4 | 14/2 | NA | 29/6 |
| Remission | 33/14 | 34/4 | 41/1 | 108/19 |
| Proteinuria: yes/no** | ||||
| Active | 14/5 | 2/12 | NA | 16/17 |
| Remission | 14/31 | 4/29 | 9/31 | 27/91 |
| Hematuria: yes/no** | ||||
| Active | 15/3 | 6/8 | NA | 21/11 |
| Remission | 13/31 | 7/25 | 8/30 | 28/86 |
| RBC: yes/no** | ||||
| Active | 15/3 | 4/8 | NA | 19/11 |
| Remission | 10/28 | 8/20 | 9/31 | 27/78 |
| RBC casts: yes/no** | ||||
| Active | 8/8 | 1/10 | NA | 9/18 |
| Remission | 3/28 | 0/26 | 1/35 | 4/89 |
For age, disease duration, and GFR, numbers indicate median (25%–75%; range).
Data on treatment and urinalyses are reported per sample, stratified by the presence or absence of active vasculitis. Because of missing data, numbers do not always sum to the numbers of patients in the group. GPA: granulomatosis with polyangiitis; MPA: microscopic polyangiitis; CSS: Churg-Strauss syndrome; PR3: proteinase-3; MPO: myeloperoxidase; GFR: glomerular filtration rate, ml/min per 1.73 m2. NA: not applicable; ANCA: antineutrophil cytoplasmic antibody; RBC: red blood cells.
Results of urinalyses were available for most visits (Table 1). Hematuria (dipstick), proteinuria, and RBC were each present during 74%–83% of episodes of active renal disease, and there was always at least 1 such abnormality. Hematuria was also seen during episodes of active nonrenal disease (43% by dipstick, 33% by microscopy) and during remission in 25% of visits across all 3 clinical groups. RBC casts were rarely seen during remission or in active nonrenal disease. RBC casts were reported in only 50% of cases of active renal disease, although we could not determine in how many cases a microscopic examination for casts was performed by the investigator or a colleague. These results highlight the challenge of assessing for active GN in this cohort. Although “grumbling” renal disease cannot be ruled out as a cause of hematuria in any individual sample, the frequent finding of hematuria or proteinuria in patients without progression of renal dysfunction during a prolonged period of continuous remission, without escalation in treatment, argues that in most cases these patients truly were in remission.
Variation of marker concentrations during remission
Trajectories of marker concentrations in individual patients are shown in Figure 1. Baseline (remission) levels of all markers varied among patients (p = 0.004 for NGAL, p < 0.001 for the other 3 markers). Within-subject normal biological variation (expressed as a CV) was estimated at 79% for AGP, 40% for KIM-1, 45% for MCP-1, and 56% for NGAL. Between-subject variation was 56% for AGP, 85% for KIM-1, 104% for MCP-1, and 99% for NGAL. Therefore, KIM-1, MCP-1, and NGAL all had indices of individuality < 0.6, indicating that change in marker level for a given person was more appropriate for analysis than use of population reference intervals (“normal ranges”)39,40.
Figure 1.
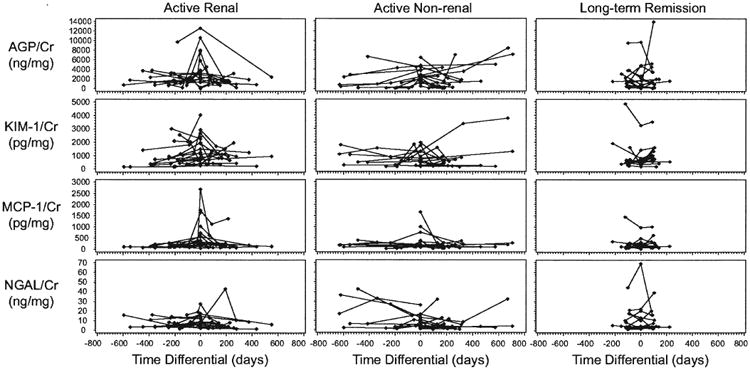
Trajectories of urinary biomarker concentrations in individual patients, divided into groups based on the presence of a flare of vasculitis with renal involvement (left panels), flare without evidence of renal involvement (middle panels), or remission for at least 2 years (right panels). Flare was defined as Day 0, and time before or after that visit was calculated for every other sample. For patients in longterm remission, the middle of the 3 visits was defined as Day 0. Concentrations of all markers were divided by the urine creatinine concentration. AGP: alpha-1 acid glycoprotein; KIM-1: kidney injury molecule-1; MCP-1: monocyte chemoattractant protein-1; NGAL: neutrophil gelatinase-associated lipocalin; Cr: urine creatinine.
There was no significant association of any marker level with time pre- or post-flare, and no significant differences between levels at pre-flare and post-flare remission visits (data not shown). Therefore, it was appropriate to average all the remission values for each patient.
Sensitivity, specificity, likelihood ratio, and ROC curve for change in marker level
All markers were significantly (p < 0.05) higher during active renal disease than remission in the same patient (data not shown). We then analyzed how well the fold-change from baseline distinguished disease states in pairwise comparisons: active renal disease to remission, active nonrenal disease to remission, and renal to nonrenal active disease (Figure 2). Change in MCP-1 showed the best ability to distinguish active renal disease from remission, with area under the ROC curve (AUC) = 0.93; a 1.3-fold increase in MCP-1 showed 94% sensitivity (95% CI 74%–100%) and 89% specificity (95% CI 81%–93%), yielding positive likelihood ratio (LR) = 8.5 and negative LR = 0.07. Change in AGP, KIM-1, or NGAL showed more modest ability to distinguish active renal disease from remission (AUC 0.71–0.75).
Figure 2.
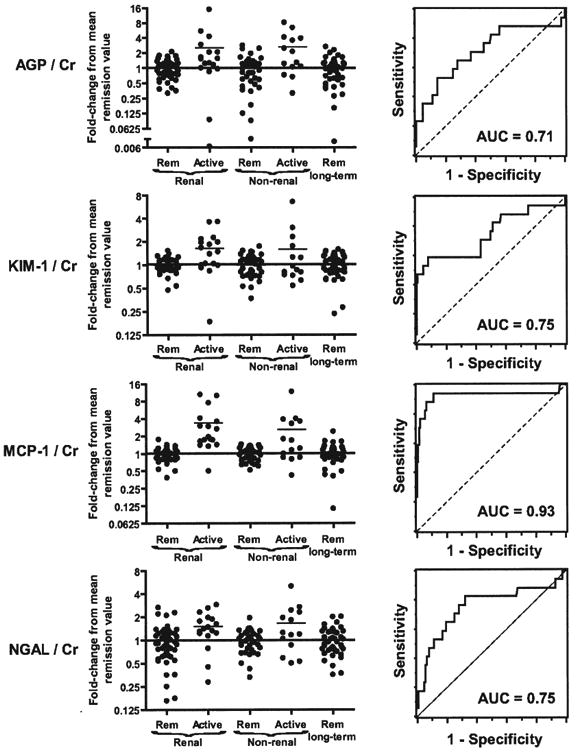
Ability of fold-change in biomarker concentration to distinguish active renal disease, active nonrenal disease, and remission. Concentrations of all markers were divided by the urine creatinine (Cr) concentration. The marker level for each sample was divided by the mean level during remission for each patient. Such fold-changes are shown in the left panels, with patients divided into groups as in Figure 1. Receiver-operating characteristic curves (ROC; right panels) were calculated using fold-changes in marker levels as the independent variables and disease state (active renal disease vs remission) as the dichotomous dependent variable; areas under the ROC curves (AUC) are shown. AGP: alpha-1 acid glycoprotein; KIM-1: kidney injury molecule-1; MCP-1: monocyte chemoattractant protein-1; NGAL: neutrophil gelatinase-associated lipocalin.
All 4 markers were also modestly but significantly higher in nonrenal disease than in remission (AUC 0.52–0.73). No marker showed a strong ability to separate active renal disease from active nonrenal vasculitis (AUC 0.48–0.63), but power for this analysis was low. Seven of the 14 samples from patients with nonrenal flares showed increases in MCP of at least 1.3-fold. It was impossible to determine whether elevated marker levels during (apparently) nonrenal disease reflected poor specificity or improved sensitivity, i.e., renal involvement that was not apparent to the clinician.
Analyses limited to the 40 patients with GPA gave similar results: AUC distinguishing active renal disease from remission were almost identical to what was obtained using the full dataset, and AUC continued to indicate modest distinction of nonrenal vasculitis from remission (range 0.60–0.79) and poor distinction of renal from nonrenal active vasculitis (range 0.42–0.61), with caveats regarding limited power as above.
Effects of proteinuria, hematuria, and demographic and disease-related factors on marker levels
The effect of nonspecific proteinuria on marker concentrations was measured using samples from patients clinically in remission but with accurately measurable urine protein (> 0.35 mg/ml). As shown in Figure 3A, there was a moderate correlation between proteinuria and urinary marker level for each of the biomarkers studied. Although this analysis was limited by the small numbers of samples, inclusion of data from samples with lower levels of proteinuria did not change this interpretation: associations showed strong statistical significance, but correlation coefficients were lower, consistent with increased noise at low levels of proteinuria (Figure 4A).
Figure 3.
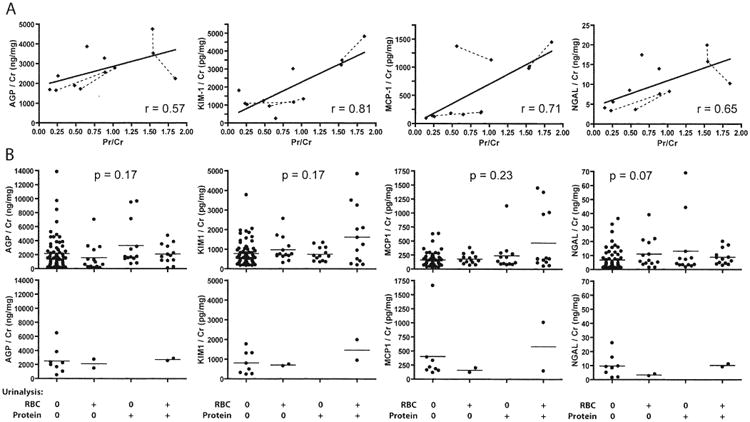
Association of urinary biomarker concentration with total urine protein concentration (A) and urinalysis results (B). A. Data points from the same patients are connected by broken lines. Solid lines show results of linear regression, and correlation coefficients (r) are shown. Both marker and protein concentrations were divided by urinary creatinine (Cr) concentrations, which is equivalent to simply plotting marker versus total protein concentration without such normalization. B. All samples from patients in remission (top panels) or with active nonrenal vasculitis (bottom panels) that also had urinalysis data were classified into 4 groups based on the presence of urinary red blood cells (RBC; by microscopy) and/or protein (on dipstick). P values from Kruskal-Wallis tests are shown in the top panels; p values for comparisons in the bottom panels were all > 0.3, but power to detect differences was low. AGP: alpha-1 acid glycoprotein; KIM-1: kidney injury molecule-1; MCP-1: monocyte chemoattractant protein-1; NGAL: neutrophil gelatinase-associated lipocalin.
Figure 4.
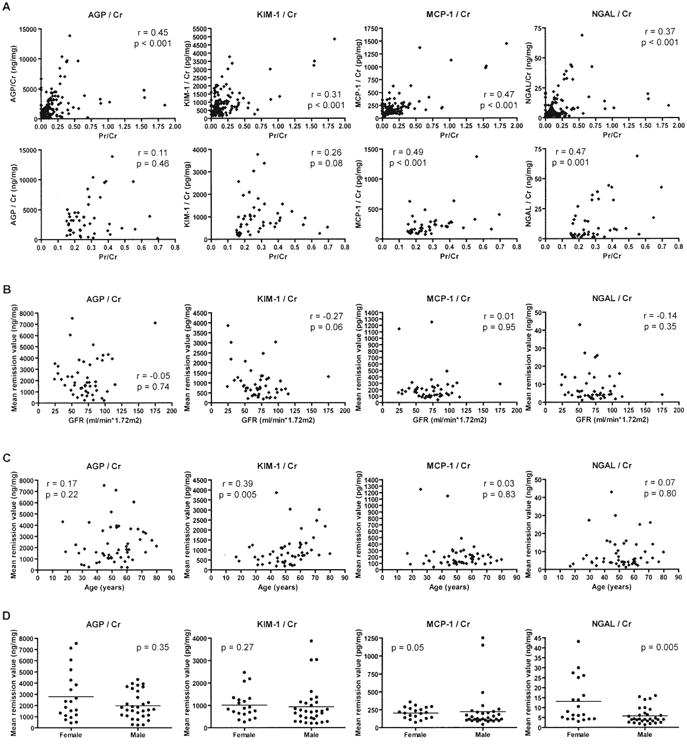
Relationship between urinary biomarker concentrations and urinary protein concentration (A), glomerular filtration rate (B), age (C), and sex (D). Concentrations of all markers were divided by the urine creatinine concentration. Results of all samples are shown in the top set of panels in A; only data from samples with urine protein/creatinine (Pr/Cr) between 0.15 and 0.7 are shown in the bottom set of panels in A. In B to D, the mean remission value for each patient is plotted, and in B, glomerular filtration rate (GFR) was calculated at the last remission visit. Spearman correlation coefficients (r) and associated p values are shown; in D, p values were determined by Wilcoxon rank-sum tests. AGP: alpha-1 acid glycoprotein; KIM-1: kidney injury molecule-1; MCP-1: monocyte chemoattractant protein-1; NGAL: neutrophil gelatinase-associated lipocalin.
MCP-1 was estimated to rise by 52–69 pg/mg creatinine for each increase in the urine protein/creatinine ratio of 0.1. However, active renal disease was still a significant predictor of MCP-1 level after accounting for proteinuria, using either multivariable models or adjusted MCP-1 levels (data not shown).
Marker levels during remission did not differ significantly in groups defined by the presence or absence of RBC and/or protein on urinalysis (Figure 3B). The small number of samples with abnormal urinalyses that also had high levels of KIM-1 and MCP-1 had the highest protein/creatinine ratios in the study (Figure 3A), suggesting that kidney damage or proteinuria per se was the driver of marker elevation, rather than unrecognized inflammation. Marker levels also did not appear to differ among patients with active nonrenal disease stratified by hematuria and/or proteinuria, although power to make such an assessment was low (Figure 3B).
In contrast to proteinuria, GFR did not correlate closely with marker level during remission: Spearman correlation coefficients ranged from 0.12 to 0.35 (Figure 4B). Remission levels of KIM-1 were significantly correlated with age (r = 0.39, p = 0.005), and remission levels of NGAL were higher in females than males (p = 0.005; Figure 4C, 4D). Multivariable models confirmed that associations of KIM-1 with age, NGAL with sex, and all 4 markers with proteinuria persisted after adjustment for the other variables. Such models also showed that association of MCP-1 with active renal disease persisted after adjustment for other variables, and that current treatment status was not associated with marker levels (data not shown).
Marker levels in remission did not differ significantly among patients with GPA, MPA, or CSS, although power to detect such differences was low. Marker levels during active renal disease could not be compared among the different subtypes of AAV, because only 1 patient with MPA (and none with CSS) had active renal disease.
Discussion
Urinary levels of MCP-1, AGP, KIM-1, and NGAL each showed statistically significant increases during renal flares among patients with AAV. MCP-1 showed the best discrimination between active renal disease and remission. KIM-1, NGAL, and AGP, despite being among the most promising markers in other kidney diseases, have lower prospects for clinical use in AAV.
There is a growing body of evidence that urinary MCP-1 is elevated in a variety of renal diseases41. Our results largely confirm the findings of Tam, et al14 regarding increased urinary MCP-1 coinciding with renal flares of AAV, but with 3 important differences: (1) we observed multiple cases of active renal disease in which MCP-1 concentrations fell within the range of values seen during remission, likely because all the patients in our study were experiencing relapses rather than being studied at diagnosis; (2) we observed an association of urinary MCP-1 with proteinuria among patients in remission, whereas Tam, et al did not, although sample sizes in both studies were small; and (3) we found elevated concentrations of MCP-1 in multiple patients who had active AAV but no evidence of active renal involvement.
There are 2 potential interpretations for elevations of MCP-1 in active “nonrenal” disease: either urinary MCP-1 is not specific for GN in AAV, or it is a marker of early GN not detected by standard clinical assessment methods as interpreted by clinicians with expertise in vasculitis, and thus has potential to improve care. Results of urinalyses suggested that the latter explanation is plausible. A followup study in which kidney biopsy is used as the gold standard would be ideal.
Multiple studies have investigated circulating levels of MCP-1. Tam, et al reported that serum MCP-1 was similar in all study groups, which included patients with active GN, active AAV without GN, remission, and healthy controls14. Ohlsson, et al found higher levels of plasma MCP-1 in patients with AAV compared to healthy controls, but the difference was not significant after correction for renal function17. Tomasson, et al found that plasma MCP-1 was lower in patients with active GPA (regardless of status of renal disease) than in the same patients in remission42. These findings indicate that urinary MCP-1 is increased by local inflammation in the kidney rather than increased filtration of circulating MCP-1. Indeed, urinary MCP-1 levels correlate well with histologic changes and recruitment of CD68-positive cells in experimental GN43. Expression of MCP-1 was increased both in glomeruli and the tubulointerstitium and in parenchymal and infiltrating cells in biopsies from patients with GN due to AAV14.
Elevation of urinary MCP-1 in the absence of elevation in the circulation is an advantage in considering potential clinical use. Markers that are also elevated in serum or plasma, which have included TNF, IL-6, IL-8, VCAM-1, and TIMP-1 in previous studies15,16, should ideally be measured in urine and serum/plasma simultaneously, with calculation of fractional excretion15.
The main limitation of our study is its size, which reduced the precision of estimation of the tests' abilities to distinguish disease states. However, the fact that this small sample size was sufficient to find significant differences indicates that additional research is worth pursuing. We also cannot guarantee that the assessment of the urine sediment was of maximal accuracy (as might be achieved through a standardized review of slides or photographs by multiple skilled nephrologists). However, all the investigators are experienced in this technique and confer with nephrologist colleagues in cases of uncertainty, and our definition of active renal disease (an expert clinician's interpretation of standard clinical data) is more relevant to clinical practice than a more rigorous protocol would be. Assessment for differences between subtypes of AAV and for direct effects of treatment independent of disease activity was also limited. In addition, before urinary MCP-1 could be used clinically, effects of preanalytical factors such as time of collection, sample processing and storage, and urine pH would need to be determined, as would levels in renal and urologic conditions other than GN.
The strengths of the study include testing of multiple specimens from each patient, linkage of those data to detailed clinical data that had been collected prospectively in a standardized manner, and study of patients in different clinical states. This study design allowed us to estimate within-subject normal biological variation and between-subject variation, and thereby determine that establishing a baseline for each patient may be essential for any future use of urinary MCP-1 clinically. The importance of these factors is well known to professionals in laboratory medicine36,44 but, we suspect, underappreciated by translational researchers interested in developing new laboratory tests.
Acknowledgments
Supported by grants from the US National Institutes of Health (RC1 AR0508303-01 to Dr. Merkel and Dr. Monach, U54 AR057319, U01 AR51874-04, and U54 RR019497).
References
- 1.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 2.Reinhold-Keller E, Beuge N, Latza U, de Groot K, Rudert H, Nolle B, et al. An interdisciplinary approach to the care of patients with Wegener's granulomatosis: long-term outcome in 155 patients. Arthritis Rheum. 2000;43:1021–32. doi: 10.1002/1529-0131(200005)43:5<1021::AID-ANR10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum. 2008;58:2908–18. doi: 10.1002/art.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41:776–84. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 5.Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine. 1999;78:26–37. doi: 10.1097/00005792-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Solans R, Bosch JA, Perez-Bocanegra C, Selva A, Huguet P, Alijotas J, et al. Churg-Strauss syndrome: outcome and long-term follow-up of 32 patients. Rheumatology. 2001;40:763–71. doi: 10.1093/rheumatology/40.7.763. [DOI] [PubMed] [Google Scholar]
- 7.Della Rossa A, Baldini C, Tavoni A, Tognetti A, Neglia D, Sambuceti G, et al. Churg-Strauss syndrome: clinical and serological features of 19 patients from a single Italian centre. Rheumatology. 2002;41:1286–94. doi: 10.1093/rheumatology/41.11.1286. [DOI] [PubMed] [Google Scholar]
- 8.Dunogue B, Pagnoux C, Guillevin L. Churg-strauss syndrome: clinical symptoms, complementary investigations, prognosis and outcome, and treatment. Semin Respir Crit Care Med. 2011;32:298–309. doi: 10.1055/s-0031-1279826. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2008;23:2151–7. doi: 10.1007/s00467-007-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magrey MN, Villa-Forte A, Koening CL, Myles JL, Hoffman GS. Persistent hematuria after induction of remission in Wegener granulomatosis: a therapeutic dilemma. Medicine. 2009;88:315–21. doi: 10.1097/MD.0b013e3181c101cc. [DOI] [PubMed] [Google Scholar]
- 11.Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteomics. 2006;5:1760–71. doi: 10.1074/mcp.R600004-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Mosley K, Tam FW, Edwards RJ, Crozier J, Pusey CD, Lightstone L. Urinary proteomic profiles distinguish between active and inactive lupus nephritis. Rheumatology. 2006;45:1497–504. doi: 10.1093/rheumatology/kel351. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M, Wiers K, Brooks EB, Greis KD, Haines K, Klein-Gitelman MS, et al. Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr Res. 2009;65:530–6. doi: 10.1203/PDR.0b013e31819e4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam FW, Sanders JS, George A, Hammad T, Miller C, Dougan T, et al. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant. 2004;19:2761–8. doi: 10.1093/ndt/gfh487. [DOI] [PubMed] [Google Scholar]
- 15.Tesar V, Masek Z, Rychlik I, Merta M, Bartunkova J, Stejskalova A, et al. Cytokines and adhesion molecules in renal vasculitis and lupus nephritis. Nephrol Dial Transplant. 1998;13:1662–7. doi: 10.1093/ndt/13.7.1662. [DOI] [PubMed] [Google Scholar]
- 16.Sanders JS, Huitema MG, Hanemaaijer R, van Goor H, Kallenberg CG, Stegeman CA. Urinary matrix metalloproteinases reflect renal damage in anti-neutrophil cytoplasm autoantibody-associated vasculitis. Am J Physiol Renal Physiol. 2007;293:F1927–34. doi: 10.1152/ajprenal.00310.2007. [DOI] [PubMed] [Google Scholar]
- 17.Ohlsson S, Bakoush O, Tencer J, Torffvit O, Segelmark M. Monocyte chemoattractant protein 1 is a prognostic marker in ANCA-associated small vessel vasculitis. Mediators Inflamm. 2009;2009:584916. doi: 10.1155/2009/584916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–42. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 19.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 20.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 21.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–29. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 22.Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H. Kidney injury molecule-1 in renal disease. J Pathol. 2010;220:7–16. doi: 10.1002/path.2642. [DOI] [PubMed] [Google Scholar]
- 23.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–17. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 24.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 25.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 26.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–70. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 27.Pitashny M, Schwartz N, Qing X, Hojaili B, Aranow C, Mackay M, et al. Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum. 2007;56:1894–903. doi: 10.1002/art.22594. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein T, Pitashny M, Levine B, Schwartz N, Schwartzman J, Weinstein E, et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology. 2010;49:960–71. doi: 10.1093/rheumatology/kep468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–61. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rovin BH, Rumancik M, Tan L, Dickerson J. Glomerular expression of monocyte chemoattractant protein-1 in experimental and human glomerulonephritis. Lab Invest. 1994;71:536–42. [PubMed] [Google Scholar]
- 31.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 32.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol. 2005;16:467–73. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Ross GF, Wiers K, Nelson S, Bennett M, Passo MH, et al. Identification of a urinary proteomic signature for lupus nephritis in children. Pediatr Nephrol. 2007;22:2047–57. doi: 10.1007/s00467-007-0608-x. [DOI] [PubMed] [Google Scholar]
- 34.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener's granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Fraser CG. Biological variation: from principles to practice. Washington, DC: AACC Press; 2001. [Google Scholar]
- 37.Harris EK. Effects of intra- and interindividual variation on the appropriate use of normal ranges. Clin Chem. 1974;20:1535–42. [PubMed] [Google Scholar]
- 38.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Petersen PH, Fraser CG, Sandberg S, Goldschmidt H. The index of individuality is often a misinterpreted quantity characteristic. Clin Chem Lab Med. 1999;37:655–61. doi: 10.1515/CCLM.1999.102. [DOI] [PubMed] [Google Scholar]
- 40.Petersen PH, Sandberg S, Fraser CG, Goldschmidt H. Influence of index of individuality on false positives in repeated sampling from healthy individuals. Clin Chem Lab Med. 2001;39:160–5. doi: 10.1515/CCLM.2001.027. [DOI] [PubMed] [Google Scholar]
- 41.Kim MJ, Tam FW. Urinary monocyte chemoattractant protein-1 in renal disease. Clin Chim Acta. 2011;412:2022–30. doi: 10.1016/j.cca.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 42.Tomasson G, Lavalley M, Tanriverdi K, Finkielman JD, Davis JC, Jr, Hoffman GS, et al. Relationship between markers of platelet activation and inflammation with disease activity in Wegener's granulomatosis. J Rheumatol. 2011;38:1048–54. doi: 10.3899/jrheum.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada T, Furuichi K, Segawa-Takaeda C, Shimizu M, Sakai N, Takeda SI, et al. MIP-1-alpha and MCP-1 contribute to crescents and interstitial lesions in human crescentic glomerulonephritis. Kidney Int. 1999;56:995–1003. doi: 10.1046/j.1523-1755.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 44.Petersen PH, Fraser CG, Kallner A, Kenny D, editors. Strategies to set global quality specifications in laboratory medicine. Scand J Clin Lab Invest. 1999;59:475–585. [PubMed] [Google Scholar]


