Abstract
This review highlights a unique research area in polymer-based nanomedicine designs. Drug-free macromolecular therapeutics induce apoptosis of malignant cells by the crosslinking of surface non-internalizing receptors. The receptor crosslinking is mediated by the biorecognition of high-fidelity natural binding motifs (such as antiparallel coiled-coil peptides or complementary oligonucleotides) that are grafted to the side chains of polymers or attached to targeting moieties against cell receptors. This approach features the absence of low-molecular-weight cytotoxic compounds. Here, we summarize the rationales, different designs, and advantages of drug-free macromolecular therapeutics. Recent developments of novel therapeutic systems for B-cell lymphomas are discussed, as well as relevant approaches for other diseases. We conclude by pointing out various potential future directions in this exciting new field.
1. Introduction
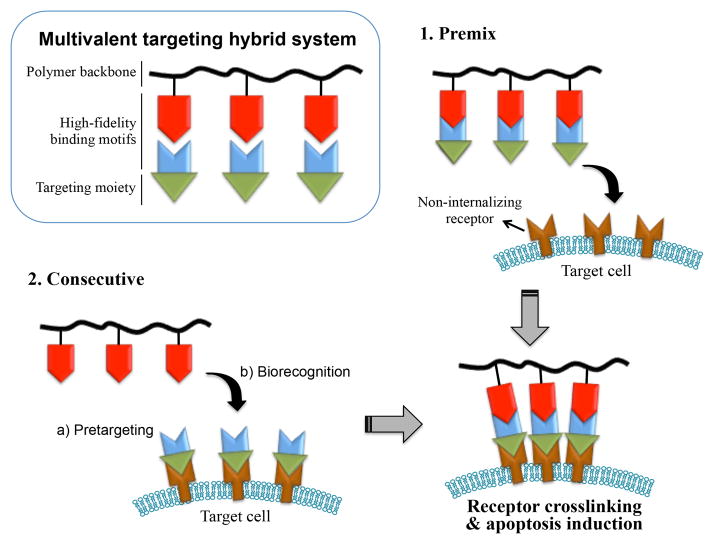
Macromolecular therapeutics, also referred to as polymeric nanomedicines, are a diverse group of drugs characterized by their large molecular weight (MW), including polymer-drug conjugates, polymeric micelles, polymer-modified liposomes, etc. The advantages of macromolecular therapeutics when compared to low-molecular-weight compounds are reviewed elsewhere.1–3 In particular, water-soluble polymeric drugs (MW > 40 kDa) attain prolonged plasma half-lives and achieve tumoritropic accumulation due to the enhanced permeability and retention (EPR) effect.4,5 Conventional polymeric nanomedicines utilize polymers as delivery vehicles to carry anticancer therapeutic agents. Many of these approaches are under clinical development.6–12 Increasingly the role of nanomedicine is not only to deliver a given drug to diseased tissues efficiently but also to trigger or improve therapeutic effects through innate biological responses.13,14 The design of macromolecular therapeutics has extended towards a unique paradigm where biomimetic strategies are employed to incite or control specific cellular activities.15–17 For instance, receptor coupling (or clustering) can be used to sensitize diseased tissues to a therapeutic agent.18–21 In this review, we highlight a novel paradigm in the nanomedicine research area – drug-free macromolecular therapeutics. This approach was firstly proposed by our laboratory in 2010.21 The basic idea is to induce apoptosis by crosslinking of cell-surface non-internalizing receptors mediated by the biorecognition of high-fidelity natural binding motifs, such as antiparallel coiled-coil peptides or complementary oligonucleotides. The general design concept of drug-free macromolecular therapeutics is shown in Fig. 1. An important feature of these designs is the absence of low-molecular-weight cytotoxic compounds (thus named “drug-free”). This paper discusses recent developments in this exciting new area, which mainly includes research performed in our laboratory using B-cell malignancies as a disease model and the CD20 receptor as a pharmacological target, as well as relevant approaches reported by other researchers.
Fig. 1.
Drug-free macromolecular therapeutics for apoptosis induction. Crosslinking of cell surface non-internalizing receptors is mediated by the biorecognition of natural binding motifs. Two hybrid conjugates can be administered consecutively as pretargeting and crosslinking doses, or premixed to form a multivalent construct and used as a single dose.
1.1. B-cell lymphoma and CD20
Non-Hodgkin’s lymphoma (NHL) is a prevalent cancer with over a half-million individuals having a history in the United States and an estimated 70,800 new cases diagnosed in 2014.22 Over the past 3 decades, the incidence of NHL has continuously increased (doubled since 1980). NHL has a high mortality rate; from 2006 to 2010, there were 18,990 deaths for every 100,000 patients in the U.S.22 The disease is comprised of a diverse and heterogeneous group of lymphatic malignancies, which makes the treatment challenging. About 85% of NHLs are cancers originating from B-cells; the remaining diseases are mostly of T-cell origin.23 This review focuses mainly on designs and developments of novel therapeutics against B-cell lymphomas (or B-NHLs), including Burkitt’s, diffuse large B-cell, follicular, immunoblastic large cell, precursor B-lymphoblastic, and mantle cell lymphomas. These malignancies are generally classified as either indolent or aggressive, which then dictates the type of therapy the patient may receive.23,24 Besides conventional chemotherapy and radiotherapy, which are usually accompanied by severe adverse reactions, monoclonal antibodies (mAbs) targeted to the B-cell surface antigen CD20 have become common treatments.25 Such “immunotherapies” have revolutionized the field. The current standard of B-NHL treatment is rituximab (the most commonly used anti-CD20 mAb) in combination with chemotherapy.26,27 However, large populations of patients exist who do not respond or develop resistance to these therapies. For example, the overall response rates for the treatment of relapsed/refractory low-grade or follicular NHL typically ranged from 40 to 50% (complete response 6, 3, 17, 3, and 14%; overall response 48, 46, 47, 39, and 43% in five different clinical trials).28 The nonresponsiveness and/or resistance have been attributed to the inability of immune effector cells (e.g., macrophages, natural killer cells) to hypercrosslink ligated mAbs,29,30 and Fc receptor (FcR)-mediated endocytosis31 or “trogocytosis”32 of CD20 antigens. These clinical obstacles create the need for new, improved therapeutic strategies.
CD20 is a 35–37 kDa integral membrane protein highly expressed on more than 95% of B-cell lymphomas.33,34 Free CD20 antigen is not present in serum, and there is no known natural ligand of CD20. When bound by antibodies, CD20 has a very low intracellular internalization rate;35,36 it is often considered a non-internalizing receptor. Studies suggest that CD20 functions as a store-operated calcium channel and a cell cycle regulator.37–39 It is one of the most reliable biomarkers of B-lymphocytes, thus providing an ideal target for treatment of B-NHL.23,24 CD20 is also expressed on normal B-cells; however, it is not expressed on stem cells or progenitor cells and mature or activated plasma cells.33 Therefore, the “B-cell depletion” therapeutic approach is considered safe; normal numbers of B-cells can be restored after treatment.25–27 The therapeutic efficacy of anti-CD20 mAbs is ascribed to three cellular events: antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and CD20-mediated apoptosis.40–42 All of these mechanisms require immune effector cells to function.41 In contrast, drug-free macromolecular therapeutics trigger direct and specific apoptosis of B-cell lymphomas without the help of effector cells. This is achieved by the design of synthetic effectors that reproduce the function of immune effector cells. The advantages of such an approach will be further discussed in this review.
1.2. Receptor crosslinking and apoptosis
Cell receptor clustering (crosslinking) is a natural process and driving force for numerous biological responses. For instance, the following cellular events have been reported to result from receptor clustering: hormone uptake,43 cell adhesion,44 cell activation45 and apoptosis.42,46 In particular, crosslinking of the surface antigen CD20 induces apoptosis of B-cells. Research have shown that when CD20-bound antibodies are hypercrosslinked by FcR-expressing immune effector cells (or polyclonal secondary antibodies), CD20 receptors tend to cluster as dimers or tetramers, redistribute and become localized into lipid rafts.47 Such events mediate the interaction between clustered CD20 and Src-family kinases (which are also located in lipid rafts), and trigger apoptotic signaling.48,49 Without the hypercrosslinking, apoptosis initiated by ligated mAbs is limited.50–52 These mechanistic studies warranted various earlier designs of multivalent mAb constructs. For example, Ghetie et al. synthesized a homodimer of rituximab by using a heterobifunctional crosslinker and showed that the mAb dimer potentiated apoptosis in human B-cell lymphomas, which synergized with a chemotherapeutic agent and an immunotoxin.51 Rossi et al. produced a hexavalent anti-CD20 antibody by covalently assembling 6 Fab′ to 1 Fc.53 Anti-lymphoma efficacy of this hexavalent construct in mouse xenografts was comparable to that of the monovalent mAb, but it was independent of effector mechanisms such as CDC. Stein et al. used a monomeric Ab that lacks effector cell functions hypercrosslinked by a secondary Ab to specifically facilitate apoptosis.54 These previous research showed that approaches aiming at direct apoptosis induction via cell surface receptor clustering are becoming attractive.
2. Origin of drug-free macromolecular therapeutics
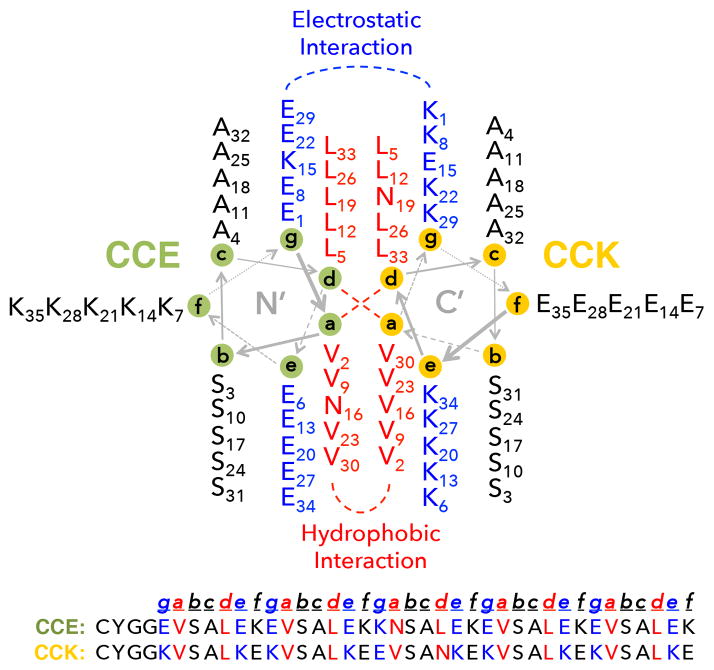
The initial design of drug-free macromolecular therapeutics was inspired by our previous work on hybrid hydrogels self-assembled from synthetic polymers and coiled-coil protein domains. We developed “smart” biomaterials composed of N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers grafted with biorecognition domains.55–58 The biorecognition of complementary grafts resulted in physical crosslinking of polymer chains and formation of 3D networks (hydrogels). In particular, a pair of oppositely charged pentaheptad peptides (CCE and CCK) that form antiparallel coiled-coil heterodimers were designed (Fig. 2). Multiple copies of CCE or CCK were grafted to the HPMA polymer (P) backbones to produce P-(CCE)x and P-(CCK)y, respectively. Equimolar mixtures of P-(CCE)x and P-(CCK)y solutions self-assembled into hydrogels where the coiled-coil peptides served as macromolecular physical crosslinkers.58,59 The excellent CCE/CCK biorecognition was also employed by Lv et al. for the development of tandem modular protein-based hydrogels.60 On the other hand, our laboratory pioneered the design of HPMA copolymers as anticancer drug carriers,61,62 which led to the development of PK1 (HPMA copolymer–doxorubicin conjugate), the first polymeric drug that entered clinical trials.63 HPMA copolymers are water-soluble, biocompatible, and long circulating in the bloodstream.3,64 They have flexible (random-coil) conformation in aqueous solutions; thus, targeting moieties or biorecognition motifs that are grafted to the side chains can be effectively presented.65 Based on these studies58,59 and the above-mentioned mechanism of receptor clustering mediated apoptosis, we hypothesized that the unique biorecognition of the CCE/CCK peptide motifs could be used to crosslink not only polymer chains but also cell surface receptors (e.g., CD20) to induce apoptosis of target cells (e.g., B-cell). Such an application of hybrid materials to biological systems to mediate specific cellular events (i.e., apoptosis) provides a bridge between the designs of biomaterials and novel nanomedicines.
Fig. 2.
Helical wheel diagram of the CCE/CCK coiled-coil antiparallel heterodimer.58 The heptad repeat of each peptide is labeled a–f. Both CCE and CCK were modified with a YGG peptide spacer (to prevent steric hindrance of binding after grafted to polymer chains) and functionalized with a cysteine (for conjugation).
3. Design of drug-free macromolecular therapeutics
3.1. Design based on formation of antiparallel coiled-coil peptides
Coiled-coils are common structural motifs in proteins where two or more right-handed α-helical peptides wind together to form a left-handed super-helix.66 The primary structure of the coiled-coil motif is characterized by a sequence of repeating seven-amino-acid residues (heptad) designated as [a, b, c, d, e, f, g]x; a and d are usually hydrophobic amino acids while the other residues are often polar.67,68 Each peptide first folds into an α-helix, and the hydrophobic residues present as a “stripe” that coils around the helix to form an amphipathic structure. The hydrophobic interface then occurs between two helices, making b, c, and f face outward. Interhelical ionic interactions (between e and g) further stabilize (or destabilize) the coiled-coil conformation. These specific intermolecular interactions offer a high degree of structural control based on primary sequences. Consequently, coiled-coil peptides have become attractive as a building block for nanomedicine design.58,69–71 We pioneered the development of drug-free macromolecular therapeutics, which employed a pair of 35-amino-acid coiled-coil forming peptides (CCE/CCK; see Fig. 2) as the biorecognition motif.21 Two macromolecular conjugates were synthesized: (1) CCE attached to a Fab′ fragment of anti-CD20 1F5 mAb (Fab′-CCE); (2) an HPMA copolymer grafted with multiple CCK peptides (P-(CCK)y). Exposure of a CD20+ human B-NHL cell line (Raji) to the Fab′-CCE conjugate first decorated the cell surfaces with CCE. Further treatment of the decorated cells with P-(CCK)y resulted in formation of antiparallel coiled-coils at cell surfaces, which crosslinked CD20 receptors and induced apoptosis.
3.1.1. In vitro and in vivo efficacies
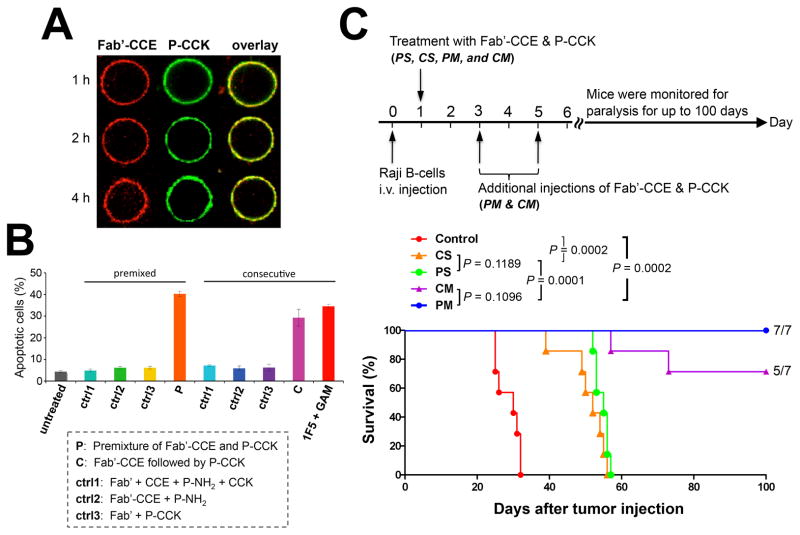
The concept of drug-free macromolecular therapeutics was firstly proven by Wu et al. with the above-mentioned Fab′-CCE/P-(CCK)y CD20-crosslinking system in vitro21 and in vivo.72 The coiled-coil formation was characterized by circular dichroism (CD) spectroscopy, and the biorecognition of the two conjugates occurred at the B-cell surface (Fig. 3A). Successful apoptosis induction of Raji cells was achieved after co-treatment with Fab′-CCE and P-(CCK)y, either consecutively or as a premixture (Fig. 3B). The apoptosis-inducing activity, under different conditions, was comparable to or better than a mouse anti-CD20 mAb (1F5)73 hypercrosslinked with a goat anti-mouse (GAM) secondary antibody.21 In vivo anticancer efficacy of this novel system was further evaluated in mice bearing systemically disseminated B-NHL.72 Both the consecutive (C) and the premixed (P) treatments were able to eradicate lymphoma cells in the blood and in the bone marrow, which produced long-term survivors (Fig. 3C).
Fig. 3.
Coiled-coil based drug-free macromolecular therapeutics. (A) Cell surface biorecognition of Fab′-CCE (labeled with rhodamine; red) and P-(CCK)9 (labeled with FITC; green). Raji B-cells were exposed to the mixture of the two conjugates and imaged by confocal fluorescence microscopy within 4 h. (B) In vitro apoptosis induction of Raji B-cells as analyzed by annexin V/propidium iodide assay. Cells were treated with Fab′-CCE and P-(CCK)9, or 1F5 mAb hypercrosslinked by goat anti-mouse (GAM) 2° Ab. Control (ctrl) groups are as indicated. P-NH2: polymer precursor of P-(CCK)9. Data are presented as mean ± SD (n = 3). (C) In vivo therapeutic efficacy against systemic B-NHL. Four million Raji cells were injected via the tail vein of SCID mice (n = 7 per group), which induced hind-limb paralysis. Control: untreated. CS: consecutive, single-dose. PS: premixed, single-dose. CM: consecutive, multiple-doses. PM: premixed, multiple-doses. Paralysis-free animal survival is presented in a Kaplan-Meier plot. Numbers of long-term survivors are indicated. Figures are adapted from Wu et al., 201021 and Wu et al., 2012.72
3.1.2. Imaging studies
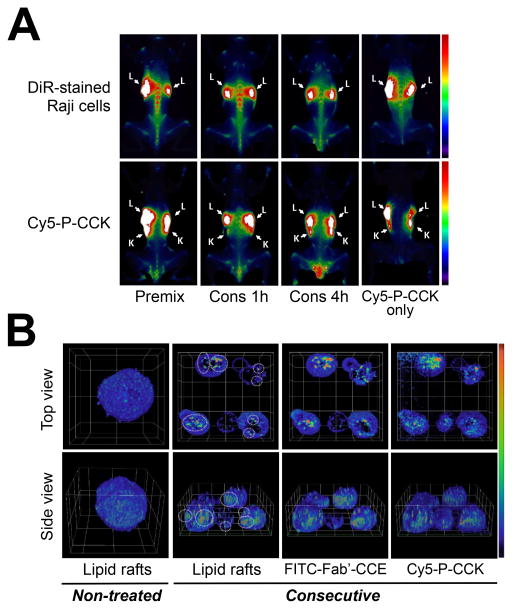
To study in vivo targeting of the Fab′-CCE/P-(CCK)y system, we recently performed multimodality imaging at the whole-body, tissue, and cellular levels.74 Excellent cell surface biorecognition was observed in the spine, femur, tibia, liver, and spleen of mice, which are common “hot spots” of B-NHL dissemination.75,76 After the first treatment with Fab′-CCE, high accumulation of P-(CCK)y was found within these lymphoma-enriched tissues (Fig. 4A). In contrast, mice injected with only P-(CCK)y (no Fab′-CCE) did not have such favorable tumor uptake. Whole body FMT (fluorescence molecular tomography) imaging confirmed the co-localization of signals indicating tumors, Fab′-CCE, and P-(CCK)y, respectively. To elucidate the mechanism(s) involved in the apoptosis induction, plasma membrane lipid rafts of Raji cells were counterstained with a marker (AF555-CTB).74 Under normal condition, lipid rafts spread throughout the plasma membrane, resulting in the AF555-CTB signal diffusion in a random punctate staining pattern on the cell surface (Fig. 4B, left panel). However, the consecutive treatment with Fab′-CCE and P-(CCK)y disrupted normal lipid distribution on the cell surface and caused the formation of several intense fluorescent spots (indicating lipid raft clusters), which co-localized with patches of the two conjugates (Fig. 4B, right panel). These studies suggested that the apoptosis was indeed mediated by CD20 clustering (in lipid rafts) as a result of the Fab′-CCE/P-(CCK)y biorecognition.
Fig. 4.
Multimodality imaging of coiled-coil based drug-free macromolecular therapeutics. The Fab′-CCE conjugate was labeled with FITC, and P-(CCK)9 with Cy5. (A) In vivo biorecognition as characterized by fluorescence molecular tomography (FMT) imaging. Raji B-cells were stained with the DiR dye and intravenously injected to mice. One day later, mice were administered (i.v.) Fab′-CCE and P-(CCK)9, as a premixture (Premix) or consecutively (Cons) using different time lags (1 h or 4 h). Tumor-inoculated mice injected with P-(CCK)9 only served as controls. L: liver. K: kidney. (B) Three-dimensional z-stack confocal images showing distribution patterns of lipid rafts on the surfaces of Raji cells. Cells were treated consecutively with the two conjugates. Non-treated cells served as controls. Circular regions indicate lipid rafts clusters. Graticule size: 10 μm. Figures are adapted from Zhang et al., 2014.74
3.1.3. Immunogenicity
Despite excellent efficacy of the Fab′-CCE/P-(CCK)y system, potential immunogenicity of the peptide conjugates is a concern before clinical applications. Short α-helical peptides are usually weak immunogens, unless administered with adjuvants.77 Similarly, short peptides attached to HPMA copolymers have a minimal immunostimulatory response.78 However, longer peptides (up to 40 residues)79 can be immunogenic, especially when attached to macromolecular carriers to act as haptens.80 Immunogenicity may change upon self-assembly,81,82 which might result in the production of conformation-specific antibodies.83,84 We evaluated the potential of individual peptides (L- and D- CCE, CCK), coiled-coils (CCE + CCK) and polymer conjugates (P-(CCK)y) to activate RAW264.7 macrophages in vitro.85 RAW264.7 cells were cultivated together with the tested compounds, and cytokine production was determined by ELISA (TNF-α, IL-1β, IL-6 and IL-10), and viability or changes in the surface markers (M1 vs. M2 polarization, activation markers) by flow cytometry. Lipopolysaccharide (LPS) served as the positive control of activation (and M1 shift). Neither HPMA copolymer nor any peptide, either L- or D-, induced any response in murine macrophages. The component responsible for macrophage activation was the 1F5 mAb or its Fab′ fragment.
We further tested the in vivo immunogenicity of the conjugates in mice.85 In vivo the therapeutics based on L-peptides (MIX L = Fab′-L-CCE + P-(L-CCK)y) did not induce substantially different Ab response than those based on D-peptides (MIX D = Fab′-D-CCE + P-(D-CCK)y). The titer and avidity of Ab induced by i.v. treatment with MIX L or MIX D were generally low, slightly lower in the case of MIX D, except for anti-Fab′-CCE IgM Ab. In general, there were detectable Abs, but no cellular response to the therapeutics administered intravenously. Intravenous injection of Fab′-CCE, as well as the mixture of Fab′-CCE and P-(CCK)y, triggered humoral immune responses, which led to the production of Abs directed against the Fab′ part of the therapeutics. Nevertheless, P-(CCK)y, when administered alone, was immunocompatible in mice. The major component responsible for the immunogenicity was again identified as the 1F5 mAb or its Fab′ fragment.85
The biocompatibility and immunocompatibility of HPMA copolymers have been widely proven.78,86–88 HPMA copolymers with oligopeptide side chains behaved as thymus-independent antigens with very limited immunogenicity and no mitogenic activity.78 In clinical trials, patients were administered up to 30 g (in 6 infusions; 3 weeks apart) of HPMA copolymer-doxorubicin conjugates that contained GFLG tetrapeptide sequences, and no immunogenicity-associated side effects were observed.63 In addition, it has been shown that conjugation of immunogens such as peptides or antibodies to HPMA copolymers could reduce the immunogenicity.87,88 Therefore, we anticipate a favorable safety profile of the P-(CCK)y conjugate in the clinical setting. Since the immunological response was predominantly directed against Fab′, which was from a mouse mAb (1F5), it is likely that Fab′-CCE will be immunostimulatory in humans. Interestingly, Press et al. treated 4 patients with the 1F5 mAb and observed minimal treatment toxicities.73,89 Thus, immunogenicity may be acceptable for translation; however, to be on the safe side, humanization of the Fab′ fragment is recommended before clinical applications. Alternatively, a system composed of human anti-CD20 mAbs (ofatumumab, veltuzumab, etc.) can be used.90
3.2. Design based on hybridization of morpholino oligonucleotides
The biorecognition of the coiled-coil forming oligopeptides, CCE and CCK, in the “drug-free” system worked well both in vitro21,74 and in vivo.72 However, to achieve a strong anticancer effect (produce tumor-free long-term survivors), we used a 1:25 molar ratio of CCE equivalent (in Fab′-CCE) to CCK equivalent (in P-(CCK)9).72 This is because the individual peptide sequences (CCE and CCK) do not have a pronounced secondary structure at pH 7 and are in a random coil conformation.58 Binding of oligopeptides to macromolecules increases their secondary structure only slightly.58,91 Consequently, Fab′-CCE and P-(CCK)y interact first via hydrophobic and electrostatic interactions, and then the oligopeptides fold into a strong antiparallel coiled-coil heterodimer. Such relatively complex binding pattern likely results in inadequate interaction of polymer conjugates with Fab′ conjugates when administered at the 1:1 molar ratio condition. Therefore, we tried to identify a biorecognition pair that would bind efficiently at the 1:1 molar ratio. Morpholino oligonucleotides have been selected due to their fast hybridization, excellent binding affinity and stability in plasma as well as water-solubility.
Nucleic acid hybridization is a crucial biorecognition event in life. A DNA double helix is composed of Watson-Crick base paring, i.e., hydrogen bonding of A/T and C/G, between two single-stranded polynucleotides with complementary sequences. The conformation is further stabilized by base stacking, i.e., π-π interaction of neighboring bases on the same strand. Such a self-recognition property plays the central role for coding, storing and transferring of genetic information; it possesses high fidelity feature. Since early 1980s, DNA has been used as building blocks for biomaterials design,92,93 and more recently, functional nanostructures for drug delivery.94,95 In particular, hybrid materials comprising oligonucleotides and synthetic polymers can be utilized to fabricate nanoconstructs with precise geometry and versatile functionality.96–98
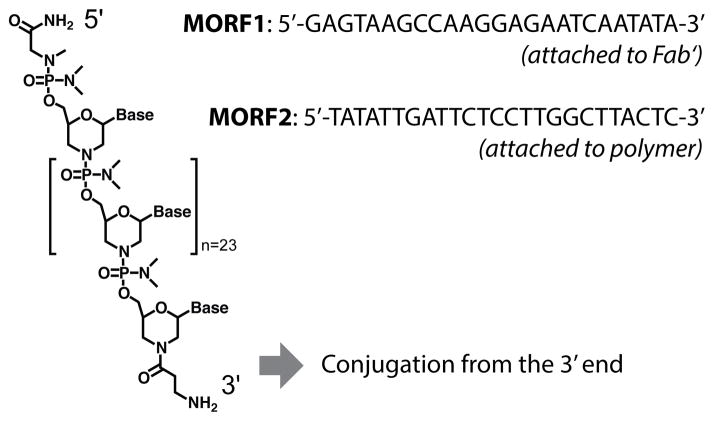
Over the years, a variety of artificial oligonucleotides with chemically modified backbones have been synthesized.99 These nonphosphodiester backbones are nuclease resistant and stable in the body; thus, they are suitable for biopharmaceutical applications. We designed a pair of phosphorodiamidate morpholino (MORF) oligomers, MORF1 and MORF2 (Fig. 5), as the biorecognition motifs for the second-generation “drug-free” therapeutic system.100 The MORF oligos are charge neutral, resulting in significantly stronger binding than natural DNA and RNA.101 Hybridization of the MORF pair has well-defined binding specificity, which prevents potential off-target effects.102,103 In addition, MORF oligos have good aqueous solubility and favorable pharmacokinetics.104,105 The sequences of MORF1 and MORF2 were designed to achieve optimal binding efficiency and minimal off-targets with human and murine mRNA, and to prevent self-complementarity.100 This new therapeutic system was composed of two hybrid conjugates: (1) anti-CD20 Fab′ linked to MORF1 (Fab′-MORF1), and (2) HPMA copolymers grafted with multiple MORF2 (P-(MORF2)x). The two conjugates self-assembled via MORF1-MORF2 hybridization at the surface of CD20+ B-cells, which crosslinked CD20 and initiated apoptosis.100
Fig. 5.
Structure and base sequences of the morpholino oligonucleotide pair, MORF1 (8630.5 Da) and MORF2 (8438.5 Da).100
3.2.1. In vitro improvement
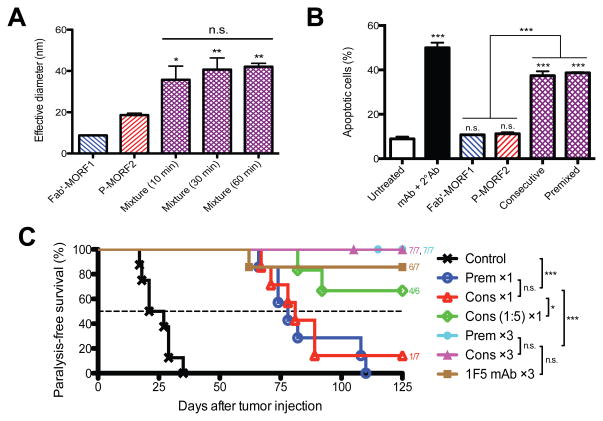
The efficacies of this new binary system (Fab′-MORF1/P-(MORF2)x) was shown by Chu et al.100 In vitro characterization by dynamic light scattering demonstrated that the two nanoconjugates, when mixed together at physiological conditions, rapidly reached a maximal binding within 10 min (Fig. 6A). In contrast, the CCE/CCK coiled-coil formation required a much longer time (~60 min).21 Further analysis with UV-visible and CD spectroscopy indicated that such binding was indeed mediated by MORF1/MORF2 hybridization and that the melting temperature was about 59 °C, well above body temperature. These results suggested a fast and stable self-assembly of the two conjugates, which are favorable for the design of drug-free macromolecular therapeutics. Importantly, when cell surface biorecognition and apoptosis were evaluated in Raji cells, we found that the treatment with equimolar MORF1/MORF2 was sufficient to achieve substantial efficacies in this hybridization system (Fig. 6B).100 However, for the coiled-coil design, a 25-time excess of the second peptide (CCE:CCK=1:25) was used.21 Table 1 shows the side-by-side comparison of apoptosis induction between the two designs. Apoptotic index (%) of Raji cells was assessed under identical cell number and concentration; MW of the polymer backbones was both ~100 kDa. These data indicate that the oligonucleotide system induced higher levels of apoptosis when compared to the peptide system. Such phenomenon was observed in both the consecutive and the premixed treatment regimens. Comparison between the two systems in these designs suggests superior binding and accessibility of the MORF oligos on the HPMA polymer chains as compared to the coiled-coil forming peptides.
Fig. 6.
Oligonucleotide hybridization mediated drug-free macromolecular therapeutics. (A) Effective hydrodynamic diameters of the two conjugates and their mixture (equimolar MORF1/MORF2; different times after mixing) as characterized by dynamic light scattering. Statistics, unless otherwise indicated, was performed by comparing the mixture with P-(MORF2)3. (B) Apoptosis of Raji B-cells as analyzed by annexin V assay. Consecutive, Fab′-MORF1 followed by equimolar P-(MORF2)3; Premixed, equimolar mixture of Fab′-MORF1 and P-(MORF2)3; mAb + 2° Ab, 1F5 mAb followed by goat anti-mouse secondary Ab. Statistics, unless otherwise indicated, was performed by comparing each group with the untreated cells. (C) Therapeutic efficacy against systemic B-lymphoma in SCID mice (n = 6–8 per group). Four million Raji cells were injected via tail vein on day 0. Cons ×1, consecutive treatment of equimolar Fab′-MORF1 and P-(MORF2)10, 1-dose; Prem ×1, equimolar mixture of Fab′-MORF1 and P-(MORF2)10, 1-dose; Cons (1:5) ×1, consecutive treatment, MORF1:MORF2 = 1:5, 1-dose; Cons ×3, 3 doses of consecutive treatment; Prem ×3, 3 doses of premixture; 1F5 mAb ×3, 3 doses of 1F5 mAb. One-dose treatment on day 1; three doses on days 1, 3 and 5. Statistics was performed with the log-rank test. *p < 0.05, **p < 0.005, ***p < 0.0001, n.s.: no significant difference. Figures are adapted from Chu et al., 2014.100
Table 1.
Comparison of in vitro and in vivo anti-B-NHL efficacies between the coiled-coil based and the oligonucleotide hybridization mediated drug-free macromolecular therapeutics.
| In vitro – Apoptotic Index* | ||||
|---|---|---|---|---|
| CCE/CCK | MORF1/MORF2 | |||
| Consecutive | (1 μM, valence=9) | (0.5 μM, valence=3) | (1 μM, valence=3) | (0.5 μM, valence=9) |
| 12% | 23% | 37% | 50% | |
| Premixed | (1 μM, valence=9) | (0.5 μM, valence=3) | (1 μM, valence=3) | (0.5 μM, valence=9) |
| 16% | 17% | 39% | 43% | |
| In vivo – Median Survival Time† | ||
|---|---|---|
| CCE/CCK | MORF1/MORF2 | |
| Consecutive | (1 nmol, 1:25) | (1 nmol, 1:1) |
| 50 days | 81 days | |
| Premixed | (1 nmol, 1:25) | (1 nmol, 1:1) |
| 55 days | 78 days | |
3.2.2. In vivo improvement
To evaluate in vivo anticancer efficacy of the hybridization system and to compare it with the previous design, we performed animal experiments using the same mouse model of systemic human B-NHL (Fig. 6C).100 Mice were intravenously injected with Raji cells, followed by administration (i.v.) of the two conjugates. Results showed that, at equivalent doses, a single treatment of Fab′-MORF1 and P-(MORF2)x (MORF1:MORF2=1:1) was significantly more effective than a single treatment of Fab′-CCE and P-(CCK)y (CCE:CCK=1:25) on preventing lymphoma dissemination and on extending the animal survival (Table 1). The efficacy can be further improved by using a 5-time excess of P-(MORF2)x (MORF1:MORF2=1:5).100 Moreover, the time lag in the consecutive treatment can be optimized based on biodistribution and pharmacokinetics of the Fab′-MORF1 conjugate.106 The comparison between the coiled-coil and the oligonucleotide designs clearly indicates that the hybridization system is advantageous for the drug-free approach. This is likely due to a more direct and specific binding pattern of the oligonucleotide base pairing at physiological conditions, when compared to the binding of the peptides, CCE and CCK. In addition, the charge neutral property of MORFs may help to prevent potential off-targets. These results indicate that, to improve therapeutic outcomes of drug-free macromolecular therapeutics, it is important to select a biorecognition pair with high binding efficiency.
3.2.3. Evaluation in patient samples
We evaluated the drug-free approach in chronic lymphocytic leukemia (CLL) cells isolated from 10 patients.107 Primary cells were treated with Fab′-MORF1 and P-(MORF2)x, and apoptosis and cytotoxicity were observed in 8 samples, including 2 samples with the 17p13 deletion. Chromosome 17p deletions are associated with the loss of one allele of the p53 gene, which portend an ultrahigh-risk prognostic factor.108 The data suggest a p53-independent mechanism of apoptosis induction. This constitutes potential treatment for chemoresistant malignancies109 and may synergize with other therapies.110 Similarly, the approach also worked in cells from patients with mantle cell lymphoma, an aggressive subset of B-NHL that is particularly difficult to treat.106 When compared to anti-CD20 mAbs 1F5 and rituximab, drug-free macromolecular therapeutics showed significantly more potent apoptosis-inducing activity and cytotoxicity. These results highlight the promising potential of the drug-free approach for clinical translation, as novel treatments against NHL, CLL, and other B-cell associated malignancies.
3.3. Other approaches
Another strategy is to use multivalent polymer-mAb or polymer-Fab′ conjugates for direct CD20 crosslinking and apoptosis induction. For instance, multimeric rituximab bound to activated dextran111 or lipid nanoparticles112 have been produced. Our laboratory synthesized multivalent anti-CD20 Fab′ attached to HPMA copolymer backbones, which successfully induced apoptosis of malignant B-cells.113–115 The advantage of these approaches is the more straightforward onestep treatment, likely resulting to better patient compliance. However, due to the large size of the antibodies or their fragments, it is difficult to synthesize such constructs with high valency (due to steric hindrance). This undesirable feature may drastically limit the therapeutic efficiency. Previously we attempted to increase the valence by using branched polymer backbones113,114 or linear, high-MW copolymers synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization.115 Other researchers used biological polymers such as DNA or polypeptides as scaffolds to attach antibodies or the smaller size single-chain variable fragments (scFv).17,116 These polyvalent constructs indeed achieved better efficacies than their monovalent counterparts. In addition, other targeting moieties such as aptamers have been employed for the crosslinking of different receptors, e.g., CD30 (for Hodgkin’s lymphoma)117 and HER2 (for breast and gastric cancers).118 Another approach is to use HPMA copolymers grafted with coiled-coil forming peptides and attach a complementary peptide terminated either in an anticancer drug or a single chain fragment as a targeting moiety.70,71,119–121 Nevertheless, one fundamental difference between these single-treatment designs and the aforementioned binary systems is that the binary systems have the opportunity of performing pretargeting. This significant advantage will be further discussed in the next section.
4. Advantages of drug-free macromolecular therapeutics
The most important feature of drug-free macromolecular therapeutics is the lack of low-MW cytotoxic compounds and, thus, the absence of non-specific toxicities. The apoptosis induction is highly specific against the targeted cells, which will likely result in a better adverse effects profile when compared to conventional chemo- and radiotherapies. The mechanism of receptor crosslinking is unique. For instance, the CD20-clustering-mediated B-cell death has been identified as a distinct pathway that can bypass mitochondria and caspase activation, which offers the opportunity to treat chemoresistant malignancies.109 Besides the drug-free feature, other favorable aspects are: (1) the proposed “two-step” treatment is suitable for pretargeting; (2) multivalency of the polymer conjugates has potential to improve therapeutic performance; (3) the immune-independent feature addresses the concern of mAbs nonresponiveness or resistance. The following subsections will discuss these advantages in details.
4.1. Pretargeting
The proposed two-step approach, i.e., consecutive administration of Fab′-MORF1 (or Fab′-CCE) followed by P-(MORF2)x (or P-(CCK)y), offers the opportunity of pretargeting. The pretargeting strategy is commonly used in cancer radioimmunotherapy.122,123 The purpose is to achieve desirable pharmacokinetic goals by separating therapeutic modalities (e.g., radionuclides) from targeting functionality (e.g., antibodies). For instance, tumors have been pretargeted with an antibody conjugated to a MORF oligo; after a time lag to clear out nonspecific binding, radiolabeled complementary MORF oligos (therapeutic effectors) were administered for radiotherapy.124,125 Over the years, the concept of pretargeting has been expanded and applied for such strategies as amplified therapeutic delivery126 and universal targeting of different tumor ligands.127 These approaches aim to improve therapeutic efficacies and reduce adverse side reactions. Similarly, for drug-free macromolecular therapeutics, the Fab′ conjugates can be used as a pretargeting agent, and then the multivalent polymer conjugates are delivered as the therapeutically active dose. The time lag between the two doses can be adjusted based on pharmacokinetics and biodistribution of the Fab′ conjugates, in order to optimize pretargeting efficiency and achieve maximum tumor-to-tissue accumulation in individual patients. We have recently proven this concept in mice.106 This was achieved by, first, finding a time lag when the pretargeting agent (Fab′-MORF1) was mostly cleared from the blood and reached a steady plasma concentration, and, second, by determining the tumor targeting efficiency when using this time interval. Results indicated a suitable timing for P-(MORF2)x administration at 5 h (in female SCID mice); at this time, Fab′-MORF1 was efficiently distributed to the tumors. Based on this result, we further performed therapy experiments in a disseminated B-NHL mouse model. When the optimized pretargeting time lag (5 h) was used, the therapeutic efficacy was significantly better than that of identical experimental conditions but with a 1 h interval. A low dose (58 μg × 3) of Fab′-MORF1 with a 5× excess P-(MORF2)x resulted in significantly delayed tumor growth and substantially improved animal survival.106 The optimized therapeutic system surpassed rituximab in anticancer efficacy and completely eradicated lymphoma B-cells in 83% of the animals. This pretargeting approach may constitute a novel personalized nanotherapy to enable more efficient treatment and limit potential side effects associated with off-target binding.
4.2. Multivalency
A significant advantage of drug-free macromolecular therapeutics is the multivalency of the polymer conjugates, i.e., multiple peptides or oligonucleotides per polymer chain. The multivalent effect describes the simultaneous interaction of repeated binding moieties in one molecular entity. Such interaction is superior to the monovalent binding kinetically and thermodynamically.128,129 It has been reported that the multivalency of anti-CD20 constructs can magnify binding affinity and apoptosis induction by several folds, when compared to their monovalent or divalent counterparts.17,113,114,116 In our drug-free approach, P-(CCK)y or P-(MORF2)x with valences up to 9 or 10 have been synthesized (using ~100 kDa polymer backbones). These conjugates would have multimeric interactions with targets, which possibly accounted for their significantly better therapeutic performance than the divalent mAbs as observed by Chu et al.106,107. Previously we have shown that, in addition to the valence, the MW of the polymer backbone also had a positive influence on the efficiency of CD20 crosslinking and apoptosis in vitro.115 In the body, HPMA copolymers with larger MW tend to circulate longer in the blood.64,65 This characteristic is favorable for targeting blood cancers such as lymphomas. Based on these promising aspects, the efficacy of the drug-free design may be further improved by using polymer conjugates with higher valences and/or larger polymer backbones. One method to approach this is to synthesize multiblock backbone-degradable HPMA copolymers by RAFT polymerization.130–133 The MW (and valence) of the polymer conjugates can be substantially increased, while the biocompatibility is maintained.
4.3. Immune-independency
Distinct from mAb-based immunotherapy, drug-free macromolecular therapeutics directly induce apoptosis in diseased cells without the need for immune activation. Successful treatment with mAbs requires FcR-expressing immune effector cells (macrophages, neutrophils, natural killer cells, etc.) to recognize the Fc region of ligated antibodies and trigger immune responses such as ADCC or CDC.40,41 However, a common clinical failure of immunotherapy is the inactivation of these effector mechanisms.29,30 For instance, many rituximab nonresponders harbor polymorphism in the IgG FcR gene, which leads to the inability of effector cells to hypercrosslink mAbs that are bound to the surfaces of B-cells.29 In the drug-free design, we used synthetic effectors to reproduce and enhance the function of immune effector cells. High-fidelity biorecognition pairs are introduced externally to replace the Fc–FcR binding. This approach may benefit patients who do not respond to immunotherapies, which constitute about half of all B-NHLs.28 In addition, the Fab′ conjugates (without Fc) are used for pretargeting. This is advantageous because various reported mechanisms attributed to the mAb resistance are directly or partly mediated by the Fc–FcR recognition, for example, CD20 downregulation,134,135 internalization,31,36 and “trogocytosis” (shaving of receptors from cell surfaces by macrophages).32 The designed Fc-independent apoptosis induction may circumvent these mechanisms, resulting in a potential to target mAb-resistant diseases. Michel and Mattes have shown that the 1F5 mAb/CD20 complex becomes non-internalizing when the Fc region of the mAb is removed.36 This observation further strengthens our point of view. Moreover, mAb therapies may “over-activate” the immune responses, which results in adverse side reactions, e.g., hypersensitivity due to complement activation that requires discontinuation of treatment and administration of corticosteroids.136 These side effects are sometimes fatal (e.g., cytokine storm137 and rituximab-associated lung injury138,139). In contrast, our direct apoptosis induction strategy does not rely on immune functions; this may ease such concerns. We have demonstrated that, at equivalent doses, the 2nd-generation (hybridization-mediated) drug-free design possesses superior or comparable anti-lymphoma efficacies to type I anti-CD20 mAbs.106 These data suggest significant advantages of the drug-free therapeutics paradigm over conventional immunotherapies. Mechanistic studies to compare drug-free macromolecular therapeutics with Type II mAbs (e.g., obinutuzumab) which may also induce direct apoptosis140,141 will provide further evaluation of the clinical potential.
5. Conclusions and beyond
In summary, drug-free macromolecular therapeutics constitute a new paradigm of polymer-based nanomedicines that are free of toxins and immune activation. Cell surface biorecognition of hybrid nanomaterials translates into innate biological responses, i.e., apoptosis. The apoptosis induction is direct (without the help of effector cells) and specific (targeted to certain receptors) and suitable for the design of precisely pretargeted nanotherapies. This novel approach has significant advantages over conventional chemo-, radio-, and immunotherapies. These promising perspectives warrant further developments within the same pipeline and may stimulate other designs. Here, we suggest potential future directions and provide supporting literature for each direction:
Targeting moieties: peptide ligands identified by combinatorial methods,142,143 oligosaccharides,144,145 and oligonucleotide aptamers.146,147 An aptamer for the B-cell receptor has been identified.146 Bifunctional nucleic acids can be produced that contain aptamers (targeting moieties) and crosslinkers (binding motifs) on each end of one molecule.147
Binding motifs: different sequences and lengths (e.g., longer motifs with spacers may result in less steric hindrance of binding148), other types of binders such as peptide nucleic acids (PNA),149,150 locked nucleic acids (LNA),146,151 and 2′-O-methyloligoribonucleotides (2′-OMe-RNA).151
Polymer backbones (or other carriers): liposomes,152 carbon nanotubes,126 or genetically engineered biopolymers (e.g., polypeptides,116 poly-DNA17). Mobility and biodistribution of carriers should be characterized. Flexible backbones are generally preferred for receptor crosslinking.
Different diseases: CD20 crosslinking and B-cell depletion can be used for autoimmune disorders such as rheumatoid arthritis,153 multiple sclerosis,154 and systemic lupus erythematosus.155 The same approach can potentially be used for anti-rejection treatment of organ transplants, e.g., rituximab is used off-label for kidney transplant recipients.156
Cell receptors: potentially any non- or slowly internalizing cell surface antigen can be a target, such as CD45 (T-cell, B-cell, macrophage),157 death receptor 4 (breast and colon cancers, etc.),158 prostate stem cell antigen (prostate cancer),159 and carcinoembryonic antigen (many tumor types, but not on normal cells).160,161 The crosslinking of these antigens can induce cell apoptosis.
Other directions: crosslinking two different receptors simultaneously to achieve synergistic effects (e.g., CD20/CD40,162 CD20/FGFR3,163 CD37/CD20 or CD37/CD19152), and designed as a switch for ON-OFF regulation of cellular events.158,164
For further translation into the clinic, the drug-free therapeutic approach will ultimately require validation and confirmation in properly conducted clinical trials, as well as carefully designed in vivo biocompatibility/toxicity studies. For applications in cancer, the tumor penetration capability of each of the therapy components shall be evaluated. It will be interesting to compare the mobility of the nano-sized therapeutic conjugates with that of the immune effector cells, which have limited penetration into solid tumors. In conclusion, we anticipate more designs and research in this exciting new field of polymeric nanomedicines.
Supplementary Material
Acknowledgments
The research on drug-free macromolecular therapeutics was supported in part by NIH grant GM95606 (to J.K.) from the National Institute of General Medical Sciences and by the University of Utah Research Foundation. The authors thank Dr. Jiyuan Yang, Dr. Rui Zhang, and Jonathan M. Hartley for helpful discussions, and Yu-Chan Chen for assisting with figure preparation.
Footnotes
Conflict of interest
J.K. and T.-W.C. are inventors on a pending US patent application (PCT/US2014/023784; assigned to the University of Utah) related to drug-free macromolecular therapeutics. J.K. is Chief Scientific Advisor for Bastion Biologics. Otherwise, the authors declare no competing financial interests.
References
- 1.Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda PH. Macromolecular therapeutics. Clin Pharmacokinet. 2003;42:1089–1105. doi: 10.2165/00003088-200342130-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kopeček J. The potential of water-soluble polymeric carriers in targeted and site-specific drug delivery. J Control Release. 1990;11:279–290. [Google Scholar]
- 3.Yang J, Kopeček J. Macromolecular therapeutics. J Control Release. 2014;190:288–303. doi: 10.1016/j.jconrel.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 5.Kopeček J. Polymer-drug conjugates: origins, progress to date and future directions. Adv Drug Deliv Rev. 2013;65:49–59. doi: 10.1016/j.addr.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour LW, et al. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int J Oncol. 2009;34:1629–1636. doi: 10.3892/ijo_00000293. [DOI] [PubMed] [Google Scholar]
- 7.Pasut G, Veronese FM. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv Drug Deliv Rev. 2009;61:1177–1188. doi: 10.1016/j.addr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Nowotnik DP, Cvitkovic E. ProLindac (AP5346): a review of the development of an HPMA DACH platinum polymer therapeutic. Adv Drug Deliv Rev. 2009;61:1214–1219. doi: 10.1016/j.addr.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Chipman SD, Oldham FB, Pezzoni G, Singer JW. Biological and clinical characterization of paclitaxel poliglumex (PPX, CT-2103), a macromolecular polymer-drug conjugate. Int J Nanomedicine. 2006;1:375–383. doi: 10.2147/nano.2006.1.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss GJ, et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Invest New Drugs. 2013;31:986–1000. doi: 10.1007/s10637-012-9921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yurkovetskiy AV, Fram RJ. XMT-1001, a novel polymeric camptothecin pro-drug in clinical development for patients with advanced cancer. Adv Drug Deliv Rev. 2009;61:1193–1202. doi: 10.1016/j.addr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Zuckerman JE, et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci U S A. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 14.Vicent MJ, Ringsdorf H, Duncan R. Polymer therapeutics: clinical applications and challenges for development. Adv Drug Deliv Rev. 2009;61:1117–1120. doi: 10.1016/j.addr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Kellner C, et al. Mimicking an induced self phenotype by coating lymphomas with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J Immunol. 2012;189:5037–5046. doi: 10.4049/jimmunol.1201321. [DOI] [PubMed] [Google Scholar]
- 16.Destouches D, et al. A simple approach to cancer therapy afforded by multivalent pseudopeptides that target cell-surface nucleoproteins. Cancer Res. 2011;71:3296–3305. doi: 10.1158/0008-5472.CAN-10-3459. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, et al. DNA-scaffolded multivalent ligands to modulate cell function. Chembiochem. 2014;15:1268–1273. doi: 10.1002/cbic.201402100. [DOI] [PubMed] [Google Scholar]
- 18.Kiessling LL, Gestwicki JE, Strong LE. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr Opin Chem Biol. 2000;4:696–703. doi: 10.1016/s1367-5931(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 19.Bollinger CR, Teichgräber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Stephens B, Handel TM. Chemokine receptor oligomerization and allostery. Prog Mol Biol Transl Sci. 2013;115:375–420. doi: 10.1016/B978-0-12-394587-7.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu K, Liu J, Johnson RN, Yang J, Kopeček J. Drug-free macromolecular therapeutics: induction of apoptosis by coiled-coil-mediated cross-linking of antigens on the cell surface. Angew Chem Int Ed. 2010;49:1451–1455. doi: 10.1002/anie.200906232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 23.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 24.Zelenetz AD, et al. Non-Hodgkin’s lymphomas, version 4.2014. J Natl Compr Cancer Netw. 2014;12:1282–1303. doi: 10.6004/jnccn.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 26.Maloney DG, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 27.Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366:2008–2016. doi: 10.1056/NEJMct1114348. [DOI] [PubMed] [Google Scholar]
- 28.Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin’s lymphoma. Annu Rev Med. 2008;59:237–250. doi: 10.1146/annurev.med.59.060906.220345. [DOI] [PubMed] [Google Scholar]
- 29.Cartron G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 30.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 31.Dransfield I. Inhibitory FcγRIIb and CD20 internalization. Blood. 2014;123:606–607. doi: 10.1182/blood-2013-12-539874. [DOI] [PubMed] [Google Scholar]
- 32.Pham T, Mero P, Booth JW. Dynamics of macrophage trogocytosis of rituximab-coated B cells. PloS One. 2011;6:e14498. doi: 10.1371/journal.pone.0014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678–1685. [PubMed] [Google Scholar]
- 34.Anderson KC, et al. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63:1424–1433. [PubMed] [Google Scholar]
- 35.Press OW, Farr AG, Borroz KI, Anderson SK, Martin PJ. Endocytosis and degradation of monoclonal antibodies targeting human B-cell malignancies. Cancer Res. 1989;49:4906–4912. [PubMed] [Google Scholar]
- 36.Michel RB, Mattes MJ. Intracellular accumulation of the anti-CD20 antibody 1F5 in B-lymphoma cells. Clin Cancer Res. 2002;8:2701–2713. [PubMed] [Google Scholar]
- 37.Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol. 1993;121:1121–1132. doi: 10.1083/jcb.121.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 39.Janas E, Priest R, Malhotra R. Functional role of lipid rafts in CD20 activity? Biochem Soc Symp. 2005:165–175. doi: 10.1042/bss0720165. [DOI] [PubMed] [Google Scholar]
- 40.Boross P, Leusen JHW. Mechanisms of action of CD20 antibodies. Am J Cancer Res. 2012;2:676–690. [PMC free article] [PubMed] [Google Scholar]
- 41.Okroj M, Österborg A, Blom AM. Effector mechanisms of anti-CD20 monoclonal antibodies in B cell malignancies. Cancer Treat Rev. 2013;39:632–639. doi: 10.1016/j.ctrv.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood. 1998;91:1644–1652. [PubMed] [Google Scholar]
- 43.Kahn CR, Baird KL, Jarrett DB, Flier JS. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978;75:4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu Y, et al. Crosslinking of the T cell-specific accessory molecules CD7 and CD28 modulates T cell adhesion. J Exp Med. 1992;175:577–582. doi: 10.1084/jem.175.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fourcin M, et al. gp130 transducing receptor cross-linking is sufficient to induce interleukin-6 type responses. J Biol Chem. 1996;271:11756–11760. doi: 10.1074/jbc.271.20.11756. [DOI] [PubMed] [Google Scholar]
- 46.Vallat LD, Park Y, Li C, Gribben JG. Temporal genetic program following B-cell receptor cross-linking: altered balance between proliferation and death in healthy and malignant B cells. Blood. 2007;109:3989–3997. doi: 10.1182/blood-2006-09-045377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: signalling through lipid rafts. Immunology. 2002;107:176–182. doi: 10.1046/j.1365-2567.2002.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmeister JK, Cooney D, Coggeshall KM. Clustered CD20 induced apoptosis: src-family kinase, the proximal regulator of tyrosine phosphorylation, calcium influx, and caspase 3-dependent apoptosis. Blood Cells Mol Dis. 2000;26:133–143. doi: 10.1006/bcmd.2000.0287. [DOI] [PubMed] [Google Scholar]
- 49.Unruh TL, et al. Cholesterol depletion inhibits src family kinase-dependent calcium mobilization and apoptosis induced by rituximab crosslinking. Immunology. 2005;116:223–232. doi: 10.1111/j.1365-2567.2005.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghetie M-A, et al. Homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells. Proc Natl Acad Sci U S A. 1997;94:7509–7514. doi: 10.1073/pnas.94.14.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghetie M-A, Bright H, Vitetta ES. Homodimers but not monomers of Rituxan (chimeric anti-CD20) induce apoptosis in human B-lymphoma cells and synergize with a chemotherapeutic agent and an immunotoxin. Blood. 2001;97:1392–1398. doi: 10.1182/blood.v97.5.1392. [DOI] [PubMed] [Google Scholar]
- 52.Polyak MJ, Deans JP. Alanine-170 and proline-172 are critical determinants for extracellular CD20 epitopes; heterogeneity in the fine specificity of CD20 monoclonal antibodies is defined by additional requirements imposed by both amino acid sequence and quaternary structure. Blood. 2002;99:3256–3262. doi: 10.1182/blood.v99.9.3256. [DOI] [PubMed] [Google Scholar]
- 53.Rossi EA, et al. Novel designs of multivalent anti-CD20 humanized antibodies as improved lymphoma therapeutics. Cancer Res. 2008;68:8384–8392. doi: 10.1158/0008-5472.CAN-08-2033. [DOI] [PubMed] [Google Scholar]
- 54.Stein R, et al. Characterization of a humanized IgG4 anti-HLA-DR monoclonal antibody that lacks effector cell functions but retains direct antilymphoma activity and increases the potency of rituximab. Blood. 2006;108:2736–2744. doi: 10.1182/blood-2006-04-017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, Stewart RJ, Kopeček J. Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature. 1999;397:417–420. doi: 10.1038/17092. [DOI] [PubMed] [Google Scholar]
- 56.Kopeček J. Hydrogel biomaterials: a smart future? Biomaterials. 2007;28:5185–5192. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopeček J, Yang J. Smart self-assembled hybrid hydrogel biomaterials. Angew Chem Int Ed. 2012;51:7396–7417. doi: 10.1002/anie.201201040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J, Xu C, Wang C, Kopeček J. Refolding hydrogels self-assembled from N-(2-hydroxypropyl)methacrylamide graft copolymers by antiparallel coiled-coil formation. Biomacromolecules. 2006;7:1187–1195. doi: 10.1021/bm051002k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J, Wu K, Koňák Č, Kopeček J. Dynamic light scattering study of self-assembly of HPMA hybrid graft copolymers. Biomacromolecules. 2008;9:510–517. doi: 10.1021/bm701001f. [DOI] [PubMed] [Google Scholar]
- 60.Lv S, Cao Y, Li H. Tandem modular protein-based hydrogels constructed using a novel two-component approach. Langmuir. 2012;28:2269–2274. doi: 10.1021/la2038526. [DOI] [PubMed] [Google Scholar]
- 61.Kopeček J. Soluble biomedical polymers. Polim Med. 1997;7:191–221. [PubMed] [Google Scholar]
- 62.Kopeček J. Controlled biodegradability of polymers – a key to drug delivery systems. Biomaterials. 1984;5:19–25. doi: 10.1016/0142-9612(84)90062-0. [DOI] [PubMed] [Google Scholar]
- 63.Vasey PA, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 64.Kopeček J, Kopečková P. HPMA copolymers: origins, early developments, present, and future. Adv Drug Deliv Rev. 2010;62:122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ulbrich K, Šubr V. Structural and chemical aspects of HPMA copolymers as drug carriers. Adv Drug Deliv Rev. 2010;62:150–166. doi: 10.1016/j.addr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Parry DAD, Fraser RDB, Squire JM. Fifty years of coiled-coils and alpha-helical bundles: a close relationship between sequence and structure. J Struct Biol. 2008;163:258–269. doi: 10.1016/j.jsb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Woolfson DN. The design of coiled-coil structures and assemblies. Adv Protein Chem. 2005;70:79–112. doi: 10.1016/S0065-3233(05)70004-8. [DOI] [PubMed] [Google Scholar]
- 68.Oakley MG, Hollenbeck JJ. The design of antiparallel coiled coils. Curr Opin Struct Biol. 2001;11:450–457. doi: 10.1016/s0959-440x(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 69.Yu YB. Coiled-coils: stability, specificity, and drug delivery potential. Adv Drug Deliv Rev. 2002;54:1113–1129. doi: 10.1016/s0169-409x(02)00058-3. [DOI] [PubMed] [Google Scholar]
- 70.Pechar M, et al. Coiled coil peptides as universal linkers for the attachment of recombinant proteins to polymer therapeutics. Biomacromolecules. 2011;12:3645–3655. doi: 10.1021/bm200897b. [DOI] [PubMed] [Google Scholar]
- 71.Pechar M, et al. Coiled coil peptides and polymer-peptide conjugates: synthesis, self-assembly, characterization and potential in drug delivery systems. Biomacromolecules. 2014;15:2590–2599. doi: 10.1021/bm500436p. [DOI] [PubMed] [Google Scholar]
- 72.Wu K, Yang J, Liu J, Kopeček J. Coiled-coil based drug-free macromolecular therapeutics: in vivo efficacy. J Control Release. 2012;157:126–131. doi: 10.1016/j.jconrel.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Press OW, et al. Monoclonal antibody 1F5 (anti-CD20) serotherapy of human B cell lymphomas. Blood. 1987;69:584–591. [PubMed] [Google Scholar]
- 74.Zhang R, Yang J, Chu T-W, Hartley JM, Kopeček J. Multimodality imaging of coiled-coil mediated self-assembly in a “drug free” therapeutic system. Adv Healthcare Mat. 2015 doi: 10.1002/adhm.201400679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghetie M-A, et al. Disseminated or localized growth of a human B-cell tumor (Daudi) in SCID mice. Int J Cancer. 1990;45:481–485. doi: 10.1002/ijc.2910450318. [DOI] [PubMed] [Google Scholar]
- 76.Griffiths GL, et al. Cure of SCID mice bearing human B-lymphoma xenografts by an anti-CD74 antibody-anthracycline drug conjugate. Clin Cancer Res. 2003;9:6567–6571. [PubMed] [Google Scholar]
- 77.Goodman-Snitkoff G, et al. Defining minimal requirements for antibody production to peptide antigens. Vaccine. 1990;8:257–262. doi: 10.1016/0264-410x(90)90055-q. [DOI] [PubMed] [Google Scholar]
- 78.Říhová B, Kopeček J, Ulbrich K, Chytrý V. Immunogenicity of N-(2-hydroxypropyl)methacrylamide copolymers. Makromol Chem. 1985;(Suppl 9):13–24. [Google Scholar]
- 79.O’Hern PA. Immunogenicity of peptides having pre-determined alpha-helical and alpha-alpha fold topologies. Mol Immunol. 1991;28:1047–1053. doi: 10.1016/0161-5890(91)90019-g. [DOI] [PubMed] [Google Scholar]
- 80.Lövgren K, Larsson M. Conjugation of synthetic peptides to carrier iscoms: factors affecting the immunogenicity of the conjugate. J Immunol Methods. 1994;173:237–243. doi: 10.1016/0022-1759(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 81.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 82.Rudra JS, Tripathi PK, Hildeman DA, Jung JP, Collier JH. Immune responses to coiled coil supramolecular biomaterials. Biomaterials. 2010;31:8475–8483. doi: 10.1016/j.biomaterials.2010.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gras-Masse H, et al. Influence of helical organization on immunogenicity and antigenicity of synthetic peptides. Mol Immunol. 1988;25:673–678. doi: 10.1016/0161-5890(88)90102-2. [DOI] [PubMed] [Google Scholar]
- 84.Lu SM, Hodges RS. A de novo designed template for generating conformation-specific antibodies that recognize alpha-helices in proteins. J Biol Chem. 2002;277:23515–23524. doi: 10.1074/jbc.M201981200. [DOI] [PubMed] [Google Scholar]
- 85.Kverka M, et al. Immunogenicity of coiled-coil based drug-free macromolecular therapeutics. Biomaterials. 2014;35:5886–5896. doi: 10.1016/j.biomaterials.2014.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Říhová B, Větvička V, Strohalm J, Ulbrich K, Kopeček J. Action of polymeric prodrugs based on N-(2-hydroxypropyl)methacrylamide copolymers. I. Suppression of the antibody response and proliferation of mouse splenocytes in vitro. J Control Release. 1989;9:21–32. [Google Scholar]
- 87.Říhová B, Kovář M. Immunogenicity and immunomodulatory properties of HPMA-based polymers. Adv Drug Deliv Rev. 2010;62:184–191. doi: 10.1016/j.addr.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Říhová B. Biocompatibility of biomaterials: hemocompatibility, immunocompatiblity and biocompatibility of solid polymeric materials and soluble targetable polymeric carriers. Adv Drug Deliv Rev. 1996;21:157–176. [Google Scholar]
- 89.Johnson TA, Press OW. Therapy of B-cell lymphomas with monoclonal antibodies and radioimmunoconjugates: the Seattle experience. Ann Hematol. 2000;79:175–182. doi: 10.1007/s002770050576. [DOI] [PubMed] [Google Scholar]
- 90.Cang S, Mukhi N, Wang K, Liu D. Novel CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol. 2012;5:64. doi: 10.1186/1756-8722-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pechar M, Kopečková P, Joss L, Kopeček J. Associative diblock copolymers of poly(ethylene glycol) and coiled-coil peptides. Macromol Biosci. 2002;2:199–206. [Google Scholar]
- 92.Seeman NC. Nucleic acid junctions and lattices. J Theor Biol. 1982;99:237–247. doi: 10.1016/0022-5193(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 93.Seeman NC. DNA in a material world. Nature. 2003;421:427–431. doi: 10.1038/nature01406. [DOI] [PubMed] [Google Scholar]
- 94.Wang Z-G, Ding B. DNA-based self-assembly for functional nanomaterials. Adv Mater. 2013;25:3905–3914. doi: 10.1002/adma.201301450. [DOI] [PubMed] [Google Scholar]
- 95.Li J, Fan C, Pei H, Shi J, Huang Q. Smart drug delivery nanocarriers with self-assembled DNA nanostructures. Adv Mater. 2013;25:4386–4396. doi: 10.1002/adma.201300875. [DOI] [PubMed] [Google Scholar]
- 96.Nagahara S, Matsuda T. Hydrogel formation via hybridization of oligonucleotides derivatized in water-soluble vinyl polymers. Polym Gels Netw. 1996;4:111–127. [Google Scholar]
- 97.Ding K, Alemdaroglu FE, Börsch M, Berger R, Herrmann A. Engineering the structural properties of DNA block copolymer micelles by molecular recognition. Angew Chem Int Ed. 2007;46:1172–1175. doi: 10.1002/anie.200603064. [DOI] [PubMed] [Google Scholar]
- 98.Pan P, et al. Thermoresponsive micellization and micellar stability of poly(N-isopropylacrylamide)-b-DNA diblock and miktoarm star polymers. Langmuir. 2012;28:14347–14356. doi: 10.1021/la303128y. [DOI] [PubMed] [Google Scholar]
- 99.Nielsen PE. DNA analogues with nonphosphodiester backbones. Annu Rev Biophys Biomol Struct. 1995;24:167–183. doi: 10.1146/annurev.bb.24.060195.001123. [DOI] [PubMed] [Google Scholar]
- 100.Chu TW, Yang J, Zhang R, Sima M, Kopeček J. Cell surface self-assembly of hybrid nanoconjugates via oligonucleotide hybridization induces apoptosis. ACS Nano. 2014;8:719–730. doi: 10.1021/nn4053827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 102.Summerton JE. Morpholino, siRNA, and S-DNA compared: impact of structure and mechanism of action on off-target effects and sequence specificity. Curr Top Med Chem. 2007;7:651–660. doi: 10.2174/156802607780487740. [DOI] [PubMed] [Google Scholar]
- 103.Stein D, Foster E, Huang SB, Weller D, Summerton J. A specificity comparison of four antisense types: morpholino, 2′-O-methyl RNA, DNA, and phosphorothioate DNA. Antisense Nucleic Acid Drug Dev. 1997;7:151–157. doi: 10.1089/oli.1.1997.7.151. [DOI] [PubMed] [Google Scholar]
- 104.Iversen PL. Phosphorodiamidate morpholino oligomers: favorable properties for sequence-specific gene inactivation. Curr Opin Mol Ther. 2001;3:235–238. [PubMed] [Google Scholar]
- 105.Amantana A, Iversen PL. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Curr Opin Pharmacol. 2005;5:550–555. doi: 10.1016/j.coph.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 106.Chu T-W, et al. A two-step pretargeted nanotherapy for CD20 crosslinking may achieve superior anti-lymphoma efficacy to rituximab. Cancer Res. 2015 doi: 10.7150/thno.12040. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chu T-W, Kosak KM, Shami PJ, Kopeček J. Drug-free macromolecular therapeutics induce apoptosis of patient chronic lymphocytic leukemia cells. Drug Deliv Transl Res. 2014;4:389–394. doi: 10.1007/s13346-014-0209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Méhes G. Chromosome abnormalities with prognostic impact in B-cell chronic lymphocytic leukemia. Pathol Oncol Res. 2005;11:205–210. doi: 10.1007/BF02893852. [DOI] [PubMed] [Google Scholar]
- 109.van der Kolk LE, et al. CD20-induced B cell death can bypass mitochondria and caspase activation. Leukemia. 2002;16:1735–1744. doi: 10.1038/sj.leu.2402559. [DOI] [PubMed] [Google Scholar]
- 110.Byrd JC, Jones JJ, Woyach JA, Johnson AJ, Flynn JM. Entering the era of targeted therapy for chronic lymphocytic leukemia: impact on the practicing clinician. J Clin Oncol. 2014;32:3039–3047. doi: 10.1200/JCO.2014.55.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang N, Khawli LA, Hu P, Epstein AL. Generation of rituximab polymer may cause hyper-cross-linking–induced apoptosis in non-Hodgkin’s lymphomas. Clin Cancer Res. 2005;11:5971–5980. doi: 10.1158/1078-0432.CCR-05-0554. [DOI] [PubMed] [Google Scholar]
- 112.Popov J, et al. Multivalent rituximab lipid nanoparticles as improved lymphoma therapies: indirect mechanisms of action and in vivo activity. Nanomed. 2011;6:1575–1591. doi: 10.2217/nnm.11.50. [DOI] [PubMed] [Google Scholar]
- 113.Johnson RN, Kopečková P, Kopeček J. Synthesis and evaluation of multivalent branched HPMA copolymer Fab′ conjugates targeted to the B-cell antigen CD20. Bioconjug Chem. 2009;20:129–137. doi: 10.1021/bc800351m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson RN, Kopečková P, Kopeček J. Biological activity of anti-CD20 multivalent HPMA copolymer Fab′ conjugates. Biomacromolecules. 2012;13:727–735. doi: 10.1021/bm201656k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chu T-W, Yang J, Kopeček J. Anti-CD20 multivalent HPMA copolymer Fab′ conjugates for the direct induction of apoptosis. Biomaterials. 2012;33:7174–7181. doi: 10.1016/j.biomaterials.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aluri SR, et al. A hybrid protein-polymer nanoworm potentiates apoptosis better than a monoclonal antibody. ACS Nano. 2014;8:2064–2076. doi: 10.1021/nn403973g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parekh P, et al. Immunotherapy of CD30-expressing lymphoma using a highly stable ssDNA aptamer. Biomaterials. 2013;34:8909–8917. doi: 10.1016/j.biomaterials.2013.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mahlknecht G, et al. Aptamer to ErbB-2/HER2 enhances degradation of the target and inhibits tumorigenic growth. Proc Natl Acad Sci U S A. 2013;110:8170–8175. doi: 10.1073/pnas.1302594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pola R, et al. Polymer therapeutics with a coiled coil motif targeted against murine bcl1 leukemia. Biomacromolecules. 2013;14:881–889. doi: 10.1021/bm3019592. [DOI] [PubMed] [Google Scholar]
- 120.Apostolovic B, Deacon SP, Duncan R, Klok HA. Hybrid polymer therapeutics incorporating bioresponsive, coiled coil peptide linkers. Biomacromolecules. 2010;11:1187–1195. doi: 10.1021/bm901313c. [DOI] [PubMed] [Google Scholar]
- 121.Apostolovic B, Deacon SP, Duncan R, Klok HA. Cell uptake and trafficking behavior of non-covalent, coiled-coil based polymer-drug conjugates. Macromol Rapid Commun. 2011;32:11–18. doi: 10.1002/marc.201000434. [DOI] [PubMed] [Google Scholar]
- 122.Goodwin DA, Meares CF. Advances in pretargeting biotechnology. Biotechnol Adv. 2001;19:435–450. doi: 10.1016/s0734-9750(01)00065-9. [DOI] [PubMed] [Google Scholar]
- 123.Sharkey RM, et al. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin Cancer Res. 2005;11:7109s–7121s. doi: 10.1158/1078-0432.CCR-1004-0009. [DOI] [PubMed] [Google Scholar]
- 124.Liu G, et al. Tumor pretargeting in mice using (99m)Tc-labeled morpholino, a DNA analog. J Nucl Med. 2002;43:384–391. [PubMed] [Google Scholar]
- 125.Liu G, et al. Successful radiotherapy of tumor in pretargeted mice by 188Re-radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analogue. Clin Cancer Res. 2006;12:4958–4964. doi: 10.1158/1078-0432.CCR-06-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mulvey JJ, et al. Self-assembly of carbon nanotubes and antibodies on tumours for targeted, amplified delivery. Nat Nanotechnol. 2013;8:763–771. doi: 10.1038/nnano.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gunn J, Park SI, Veiseh O, Press OW, Zhang M. A pretargeted nanoparticle system for tumor cell labeling. Mol Biosyst. 2011;7:742–748. doi: 10.1039/c005154c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chittasupho C. Multivalent ligand: design principle for targeted therapeutic delivery approach. Ther Deliv. 2012;3:1171–1187. doi: 10.4155/tde.12.99. [DOI] [PubMed] [Google Scholar]
- 129.Kitov PI, Bundle DR. On the nature of the multivalency effect:_ a thermodynamic model. J Am Chem Soc. 2003;125:16271–16284. doi: 10.1021/ja038223n. [DOI] [PubMed] [Google Scholar]
- 130.Pan H, Yang J, Kopečková P, Kopeček J. Backbone degradable multiblock N-(2-hydroxypropyl)methacrylamide copolymer conjugates via reversible addition-fragmentation chain transfer polymerization and thiol-ene coupling reaction. Biomacromolecules. 2011;12:247–252. doi: 10.1021/bm101254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang J, Luo K, Pan H, Kopečková P, Kopeček J. Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelicPolyHPMA conjugates. React Funct Polym. 2011;71:294–302. doi: 10.1016/j.reactfunctpolym.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luo K, Yang J, Kopečková P, Kopeček J. Biodegradable multiblock Poly[N-(2-hydroxypropyl)methacrylamide] via reversible addition fragmentation chain transfer polymerization and click chemistry. Macromolecules. 2011;44:2481–2488. doi: 10.1021/ma102574e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang R, Yang J, Sima M, Zhou Y, Kopeček J. Sequential combination therapy of ovarian cancer with degradable N-(2-hydroxypropyl)methacrylamide copolymer paclitaxel and gemcitabine conjugates. Proc Natl Acad Sci U S A. 2014;111:12181–12186. doi: 10.1073/pnas.1406233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davis TA, Czerwinski DK, Levy R. Therapy of B-cell lymphoma with anti-CD20 antibodies can result in the loss of CD20 antigen expression. Clin Cancer Res. 1999;5:611–615. [PubMed] [Google Scholar]
- 135.Tsai P-C, Hernandez-Ilizaliturri FJ, Bangia N, Olejniczak SH, Czuczman MS. Regulation of CD20 in rituximab-resistant cell lines and B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18:1039–1050. doi: 10.1158/1078-0432.CCR-11-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van der Kolk LE, Grillo-López AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115:807–811. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 137.Ponce R. Adverse consequences of immunostimulation. J Immunotoxicol. 2008;5:33–41. doi: 10.1080/15476910801897920. [DOI] [PubMed] [Google Scholar]
- 138.Lands LC. New therapies, new concerns: rituximab-associated lung injury. Pediatr Nephrol. 2010;25:1001–1003. doi: 10.1007/s00467-010-1476-3. [DOI] [PubMed] [Google Scholar]
- 139.Chaumais M-C, et al. Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol. 2009;24:1753–1755. doi: 10.1007/s00467-009-1195-9. [DOI] [PubMed] [Google Scholar]
- 140.Mössner E, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell–mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Herter S, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12:2031–2042. doi: 10.1158/1535-7163.MCT-12-1182. [DOI] [PubMed] [Google Scholar]
- 142.Ding H, Prodinger WM, Kopeček J. Identification of CD21-binding peptides with phage display and investigation of binding properties of HPMA copolymer-peptide conjugates. Bioconjug Chem. 2006;17:514–523. doi: 10.1021/bc0503162. [DOI] [PubMed] [Google Scholar]
- 143.Ding H, Prodinger WM, Kopeček J. Two-step fluorescence screening of CD21-binding peptides with one-bead one-compound library and investigation of binding properties of N-(2-hydroxypropyl)methacrylamide copolymer-peptide conjugates. Biomacromolecules. 2006;7:3037–3046. doi: 10.1021/bm060508f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.David A, Kopečková P, Rubinstein A, Kopeček J. Enhanced biorecognition and internalization of HPMA copolymers containing multiple or multivalent carbohydrate side-chains by human hepatocarcinoma cells. Bioconjug Chem. 2001;12:890–899. doi: 10.1021/bc010026v. [DOI] [PubMed] [Google Scholar]
- 145.David A, Kopečková P, Minko T, Rubinstein A, Kopeček J. Design of a multivalent galactoside ligand for selective targeting of HPMA copolymer-doxorubicin conjugates to human colon cancer cells. Eur J Cancer. 2004;40:148–157. doi: 10.1016/j.ejca.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 146.Mallikaratchy PR, et al. A multivalent DNA aptamer specific for the B-cell receptor on human lymphoma and leukemia. Nucleic Acids Res. 2011;39:2458–2469. doi: 10.1093/nar/gkq996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou J, Soontornworajit B, Snipes MP, Wang Y. Development of a novel pretargeting system with bifunctional nucleic acid molecules. Biochem Biophys Res Commun. 2009;386:521–525. doi: 10.1016/j.bbrc.2009.06.090. [DOI] [PubMed] [Google Scholar]
- 148.Peng Z, Sima M, Salama ME, Kopečková P, Kopeček J. Spacer length impacts the efficacy of targeted docetaxel conjugates in prostate-specific membrane antigen expressing prostate cancer. J Drug Target. 2013;21:968–980. doi: 10.3109/1061186X.2013.833207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rusckowski M, Qu T, Chang F, Hnatowich DJ. Pretargeting using peptide nucleic acid. Cancer. 1997;80:2699–2705. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2699::aid-cncr48>3.3.co;2-a. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, et al. Pretargeting with amplification using polymeric peptide nucleic acid. Bioconjug Chem. 2001;12:807–816. doi: 10.1021/bc0100307. [DOI] [PubMed] [Google Scholar]
- 151.Mallikaratchy P, et al. A self-assembling short oligonucleotide duplex suitable for pretargeting. Nucleic Acid Ther. 2013;23:289–299. doi: 10.1089/nat.2013.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu B, et al. Targeted drug delivery and cross-linking induced apoptosis with anti-CD37 based dual-ligand immunoliposomes in B chronic lymphocytic leukemia cells. Biomaterials. 2013;34:6185–6193. doi: 10.1016/j.biomaterials.2013.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yazici Y. Rheumatoid arthritis: When should we use rituximab to treat RA? Nat Rev Rheumatol. 2011;7:379–380. doi: 10.1038/nrrheum.2011.79. [DOI] [PubMed] [Google Scholar]
- 154.Hauser SL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 155.Chan VS-F, Tsang HH-L, Tam RC-Y, Lu L, Lau C-S. B-cell-targeted therapies in systemic lupus erythematosus. Cell Mol Immunol. 2013;10:133–142. doi: 10.1038/cmi.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ramanath V, Nistala R, Chaudhary K. Update on the role of rituximab in kidney diseases and transplant. Expert Opin Biol Ther. 2012;12:223–233. doi: 10.1517/14712598.2012.646984. [DOI] [PubMed] [Google Scholar]
- 157.Huang Y-J, et al. Multivalent structure of galectin-1-nanogold complex serves as potential therapeutics for rheumatoid arthritis by enhancing receptor clustering. Eur Cell Mater. 2012;23:170–181. doi: 10.22203/ecm.v023a13. [DOI] [PubMed] [Google Scholar]
- 158.Cho MH, et al. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat Mater. 2012;11:1038–1043. doi: 10.1038/nmat3430. [DOI] [PubMed] [Google Scholar]
- 159.Gu Z, Yamashiro J, Kono E, Reiter RE. Anti-prostate stem cell antigen monoclonal antibody 1G8 induces cell death in vitro and inhibits tumor growth in vivo via a Fc-independent mechanism. Cancer Res. 2005;65:9495–9500. doi: 10.1158/0008-5472.CAN-05-2086. [DOI] [PubMed] [Google Scholar]
- 160.Wirth T, Soeth E, Czubayko F, Juhl H. Inhibition of endogenous carcinoembryonic antigen (CEA) increases the apoptotic rate of colon cancer cells and inhibits metastatic tumor growth. Clin Exp Metastasis. 2002;19:155–160. doi: 10.1023/a:1014566127493. [DOI] [PubMed] [Google Scholar]
- 161.Santoro L, et al. Noninternalizing monoclonal antibodies are suitable candidates for 125I radioimmunotherapy of small-volume peritoneal carcinomatosis. J Nucl Med. 2009;50:2033–2041. doi: 10.2967/jnumed.109.066993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Al-Zoobi L, et al. Enhancement of Rituximab-induced cell death by the physical association of CD20 with CD40 molecules on the cell surface. Int Immunol. 2014;26:451–465. doi: 10.1093/intimm/dxu046. [DOI] [PubMed] [Google Scholar]
- 163.Kotani N, Ishiura Y, Yamashita R, Ohnishi T, Honke K. Fibroblast growth factor receptor 3 (FGFR3) associated with the CD20 antigen regulates the rituximab-induced proliferation inhibition in B-cell lymphoma cells. J Biol Chem. 2012;287:37109–37118. doi: 10.1074/jbc.M112.404178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Karlsson M, et al. Pharmacologically controlled protein switch for ON-OFF regulation of growth factor activity. Sci Rep. 2013;3:2716. doi: 10.1038/srep02716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.