Abstract
Purpose
To define rod and cone function further in terms of visual cycle mechanism, the retinal phenotype resulting from Rpe65 (retinoid isomerase I) deficiency in Nrl−/− mice having a single class of photoreceptors resembling wild-type cones was characterized and outcomes of retinoid supplementation evaluated.
Methods
Rpe65−/−/Nrl−/− mice were generated by breeding Rpe65−/− and Nrl−/− strains. Retinal histology, protein expression, retinoid content, and electroretinographic (ERG) responses were evaluated before and after treatment with 11-cis retinal by intraperitoneal injection.
Results
Retinas of young Rpe65−/−/Nrl−/− mice exhibited normal lamination, but lacked intact photoreceptor outer segments at all ages examined. Rpe65, Nrl, and rhodopsin were not detected, and S-opsin and M/L-opsin levels were reduced. Retinyl esters were the only retinoids present. In contrast, Nrl−/− mice exhibited decreased levels of retinaldehydes and retinyl esters, and elevated levels of retinols. ERG responses were elicited from Rpe65−/−/Nrl−/− mice only at the two highest intensities over a 4-log-unit range. Significant retinal thinning and outer nuclear layer loss occurred in Rpe65−/−/Nrl−/− mice with aging. Administration of exogenous 11-cis retinal did not rescue retinal morphology or markedly improve ERG responses.
Conclusions
The findings provide clarification of reported cone loss of function in Rpe65−/−/Nrl−/− mice, now showing that chromophore absence results in destabilized cone outer segments and rapid retinal degeneration. The data support the view that rod-dominant retinas do not have a cone-specific mechanism for 11-cis retinal synthesis and have potential significance for therapeutic strategies for rescue of cone-rich retinal regions affected by disease in the aging human population.
The basic mechanisms used by rod and cone photoreceptor cells to detect and respond to light are similar in many respects; however, the molecular bases underlying differences in kinetics and sensitivity, as well as disease involvement and effects of aging are not well understood. Such issues critically relate to understanding cone disease in macular and cone-rod dystrophies and age-related macular degeneration.1,2 Furthermore, successful therapeutic intervention relative to visual cycle defects is likely to depend on accommodating differential responses of rod and cone cells to various treatment paradigms.
RPE65 encodes an abundant protein present in the retinal pigment epithelium (RPE). Studies in mice have shown that Rpe65 is necessary for 11-cis retinal synthesis needed for generation of the rod photopigment rhodopsin, with Rpe65 deficiency resulting in massive accumulation of its substrate, all-trans retinyl ester, in the RPE.3 Recombinant RPE65 exhibits retinoid isomerase activity, generating 11-cis retinal from added all-trans retinol when coexpressed with lecithin retinol acyl transferase (LRAT).4-6 Mutations in human RPE65 cause severe early-onset retinal degeneration, including Leber congenital amaurosis.7,8 Constitutive opsin activity and cone pigment instability likely contribute to associated pathogenesis, with cone cell death preceding that of rods.9,10
A body of evidence indicates that there are fundamental differences in the mechanisms of photopigment regeneration in rod-dominant versus cone-dominant retinas (reviewed in Ref. 11). It has been further questioned whether 11-cis retinal supplied by the RPE is sufficient to sustain daylight vision of cones.12,13 An RPE65-independent visual cycle has been proposed to operate in the cone-dominant retinas of ground squirrels and chickens involving novel enzyme activities, including an all-trans retinol isomerase and a retinyl ester synthase located in cone and Müller cells.13,14 These enzyme activities have not yet been detected in the retinas of rod-dominant species, including humans and mice.
Mice lacking the transcription factor Nrl experience a block in the differentiation of rod precursor cells, resulting in retinas containing a single class of photoreceptors that are indistinguishable from authentic cones on the basis of number of criteria.15-18 Outer segments are short, contain a large proportion of discs not enclosed by plasma membrane, and are covered with a cone sheath. The nuclei contain condensed chromatin and mitochondria that are typical of mouse cones. The cells express several cone-specific proteins, synapse with rod and cone bipolar cells, and exhibit the spectral sensitivity and time constants of photoresponses typical of cones.17,18 Although the retinas of Nrl-knockout mice experience age-related changes after 3 months of age, studies in young animals provide a powerful approach to understanding cone cell function in a normally rod-dominant retina.
With an interest in further defining the role of RPE65 in visual cycle function and cone cell viability, we generated Nrl−/− mice deficient in Rpe65 by mating the two null strains and characterizing the resulting retinal phenotype. Our studies expanded on recent analysis of visual cycle function in Rpe65−/−/Nrl−/− mice,19 now showing that loss of 11-cis retinal synthesis is accompanied by defects in outer segment formation and retinal degeneration that cannot be readily reversed by retinoid replacement therapy.
Materials and Methods
Breeding and Genotyping
Rpe65−/− mice3 and Nrl−/− mice15 were mated, Rpe65+/−/Nrl+/− offspring were identified by genotyping and then crossbred to generate animals homozygous null for both genes. Rpe65−/− mice were maintained on an Sv129;C57BL/6J mixed background. The Nrl−/− mice were on a C57BL/6J (Rpe65-Met450) background. Wild-type control animals were C57BL/6J. Mice were reared in a 12-/12-hour light-dark cycle (~800 lux room light), or in total darkness and were euthanatized by CO2 inhalation. All experimental procedures complied with the regulations of the University of Michigan Committee on Use and Care of Animals, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Histology
For phase-contrast microscopy, anesthetized mice were perfused with 4% paraformaldehyde in PBS, the eyes were embedded in JB-4 plastic, and sections (5 μm) were stained with Lee’s stain and imaged on a microscope (Eclipse E800; Nikon, Tokyo, Japan) with a digital camera. For electron microscopy, mice were perfused with 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4), the eyes were postfixed in 1% OsO4, dehydrated, and embedded in Epon 812. Ultrathin sections (70 nm) were stained with uranyl acetate and lead citrate for analysis by electron microscopy.20,21
Immunohistochemical and Western Analysis
Eyes from mice perfused with 4% paraformaldehyde were washed in PBS, transitioned to sucrose/OCT, and flash frozen. Sections (10 μm) were blocked with 20% sheep serum and 0.2% Triton X-100 and incubated with primary antibodies for 2 hours, then with fluoro-chrome-conjugated secondary antibody for 1 hour. Species and dilutions of primary antibodies were as follows: rabbit anti-S-opsin (1:500) and anti-M/L-opsin (1:500; Chemicon, Temecula, CA), mouse anti-rhodopsin (1D4; 1:500 of 1 mg/mL stock), mouse anti-RPE65 (8B11; 1:250 of 1 mg/mL stock), Alexa Fluor 555-conjugated anti-mouse IgG (H+L; 1:500) and Alexa Fluor 488-conjugated anti-rabbit IgG (H+L; 1:500; Invitrogen, Carlsbad CA). Images were obtained by fluorescence microscopy (Eclipse E800; Nikon). For Western analysis, total mouse eye proteins were separated by SDS-PAGE, transferred to nitrocellulose, blocked with 5% milk and 0.1% Tween 20, and incubated with primary antibody overnight at 4°C and then with alkaline phosphatase-conjugated secondary antibody for 1 hour at RT. The immunoblots were developed by using standard methods.22
Bleaching and Regeneration
The Nrl−/− mice were dark adapted overnight. The pupils were dilated with topical 1% phenylephrine and 1% atropine, and the mice were placed in a plastic box lined with aluminum foil and illuminated with UV light produced by four black-light UV tubes (cat no. 0014687; Bulbtronics, Farmingdale, NY) for 1 minute. The mice were returned to complete darkness for the times indicated in the figures, and the retinoid content was assayed.
Retinoid Analysis
All procedures were performed in dim red or infrared light using a modification of the lipid-extraction protocol of Bligh and Dyer.23 One to two frozen mouse eyes were placed in a centrifuge tube on dry ice, 150 to 200 μL dissection medium was added (vol/vol: 60% MeOH, 30% chloroform, 10% 1 M NH2OH; pH 7), the eyes were minced, and medium was added to 0.6 mL final volume. Samples were cooled on dry ice for 3 to 5 minutes, homogenized (Pellet Pestle; Kontes, Vineland, NJ), and sonicated with a microtip probe (30 pulses at 0.3 power and 0.1 duty cycle; Branson, Danbury, CT) with temperature maintained at 15 to 20°C (BAT-10 microthermocouple; Physitemp, Clifton, NJ). Chloroform (200 μL) was added, samples were vortexed for 5 seconds, 250 μL water was added, and samples were vortexed and then centrifuged at 14,000 rpm for 5 minutes. The chloroform phase was collected, dried under nitrogen, and dissolved in 200 μL HPLC mobile phase. HPLC was performed with a column (Hypersil column; 5.0 μm, 4.6 × 100 mm; Thermo Fisher Scientific, Waltham, MA) developed with n-hexane/ethanol 99.5:0.5 (A) or n-hexane/ethanol 99.75:0.25 (B) on an HPLC system (model 1100; Agilent, Palo Alto, CA) equipped with multidiode array detector and software (Chemstation; Thermo Fisher Scientific). Retinyl esters were collected and saponified (as described in Ref. 24), and head groups were evaluated by using the same run conditions. All-trans retinal, 9-cis retinal, retinyl palmitate, and all-trans retinol reference compounds were from Sigma-Aldrich (St. Louis, MO), and 11-cis retinal was the kind gift of Rosalie Crouch (Medical University of South Carolina, Charleston). Retinal oximes were obtained by reaction with NH2OH. 11-cis retinol was obtained by reduction of 11-cis retinal with sodium borohydride. Peak identification was by comparison to retention times and spectra for reference samples, with quantification calculated by scaling peak integrals to reference compounds. Yields of retinyl esters were 20% to 30% higher, and yields of retinal oximes and retinols were comparable to those obtained for extractions using NH2OH in methanol or ethanol.24,25 The limit of sensitivity of was ~2 pmol retinoid/eye.
11-cis Retinal Supplementation
Mice reared in complete darkness received intraperitoneal injections of 11-cis retinal in 10% ethanol, 10% BSA, and 0.9% NaCl (30 μL) or injections of vehicle alone.26 Single injections were given to the mice at 4 weeks of age (10 or 100 μg 11-cis retinal/dose), or multiple injections (three to six) were given from 1 to 4 weeks of age (1.25-50 μg 11-cis retinal/dose). ERG recordings were performed 24 hours after the final injection, with subsequent processing of the eyes for Western, retinoid, and TEM analyses.
Electroretinogram Recording and Analysis
Mice were dark adapted for 12 hours and prepared in dim red illumination. Animals were anesthetized with a loading dose of ketamine (93 mg/kg, IP) and xylazine (8 mg/kg, IP). Pupils were dilated with topical 1% atropine and 0.5% tropicamide. Body temperature was maintained at 37°C with a heating pad. Corneal ERGs were recorded from both eyes using gold wire loops with 0.5% tetracaine topical anesthesia and a drop of 2% methylcellulose for corneal hydration. A gold wire loop placed in the mouth served as the reference, and a ground electrode was placed on the tail. ERGs were recorded in a Ganzfeld configuration with brief xenon white flashes, with flash intensity varied using combinations of neutral density filters and photostimulator intensity settings (−0.5-3.5 log cd · s/m2 per flash measured with a IL1700 Research Radiometer; International Light, Newburyport, MA). Responses were amplified (model CP5111 AC amplifier; Grass-Telefactor, West Warwick, RI) at 10,000 gain at 0.1 to 1000 Hz, and digitized (PCI-1200 I/O board; National Instruments, Austin, TX). A notch filter was used to remove 60-Hz line noise. Dark-adapted ERGs were recorded at 3- to 60-second intervals depending on the stimulus intensity. Animals were then light adapted for 10 minutes by exposure to a white 32 cd/m2 rod-saturating background, and photopic ERGs were recorded for single flash white stimuli over a 4-log-unit range. Responses were computer-averaged at all intensities with at least 25 records averaged for the weakest signals. Flash stimuli had a duration of 10 μs. ANOVA was performed to determine statistical significance at P ≤ 0.05.
Results
Alterations in Retinoid Metabolism in Eyes of Nrl−/− Mice
In baseline studies, we evaluated retinoid content and processing in eyes from Nrl−/− mice using HPLC. In dark-adapted animals, we found that total retinoid levels in the Nrl−/− mice were three to four times less than in the wild-type mice, because of decreases in 11-cis retinal, all-trans retinal, and (total) retinyl esters (Figs. 1A, 1B). The decrease in 11-cis retinal content (~106 pmol/eye in Nrl−/− versus ~528 pmol/eye in wild type) is consistent with earlier studies showing that opsin levels are also decreased in Nrl−/− mice (~90 pmol cone opsin/eye versus ~520 pmol rhodopsin/eye in wild type17), due to the smaller outer segment size of cones than rods. Thus, Nrl−/− mice retain a chromophore-to-opsin ratio that is close to unity, suggesting that nearly all the 11-cis retinal present in the retina is bound in visual pigment, as in wild-type mice.27 After exposure to high-intensity illumination that isomerizes more than 90% of the 11-cis retinal, and return to the dark, the Nrl−/− mice rapidly converted all-trans retinal to all-trans retinol, followed by a recovery of 11-cis retinal (Fig. 1C). Although absolute rates of 11-cis retinal synthesis were slower in the Nrl−/− mice than in the wild type (1.77 vs. 3.06 pmol · min−1 · eye−1), relative rates (calculated as the percentage from dark value) were faster in the Nrl−/− mice (1.77% min−1 versus 0.58% min−1; Fig. 1D). The Nrl−/− mice also exhibited four- and sixfold increases in percentage of total 11-cis retinol and all-trans retinol, retinoids normally present in the retina in minor amounts.
Figure 1.

Retinoid content and processing in Nrl−/− and wild-type mice at 5 weeks of age. (A, B) Quantitative analysis of retinoid compounds present in whole-eye extracts. Nrl−/− (◻); wild-type (∎). (C) Time course of retinoid intercon-version during recovery from >90% bleaching, shown as a percentage of the total. 11-cis retinal (◯); all-trans retinal (●); all-trans retinol (◻); retinyl esters (∎). (D) Time course of 11-cis retinal recovery after an exposure to intense light, shown as a percentage of dark value.
Photoreceptor Layer Changes in Rpe65−/−/Nrl−/− Mice
The mice homozygous null for both Rpe65 and Nrl were viable and could not be distinguished from wild-type littermates by simple observation. Their eyes were unremarkable in overall appearance, with sections of retina/RPE/choroid showing normal cellular lamination in light micrographs. However, the layer normally containing photoreceptor inner and outer segments was extremely thin in the double-knockout mice, even when compared with that in the Nrl−/− mice, which had shorter outer segments typical of cones (Fig. 2).
Figure 2.
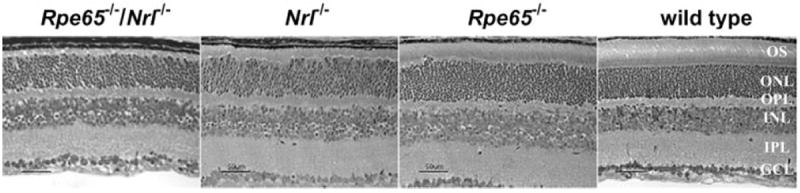
Retinal histology of Rpe65−/−/Nrl−/−, single-knockout, and wild-type mice. Phase-contrast micrographs of toluidine blue-stained sections from animals at 4 weeks of age. OS, photoreceptor outer segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Ultrastructural analysis using transmission electron microscopy showed a widespread lack of outer segment disc membranes in retinas of Rpe65−/−/Nrl−/− mice at all ages examined (1-5 weeks old). In mice at 2 weeks of age, there were some cells with disc membranes, but nothing like a complete outer segment (Fig. 3). The short stacks of membranes were evident among the apical processes of the RPE (Fig. 3A). Many cilia that did not bear an outer segment at all were observed (Figs. 3B, 3C). At 5 weeks of age, disc membranes were not seen, but some cilia were still evident (not shown). Thus, it appears that many photoreceptor cells form at least part of an outer segment by 2 weeks of age, but most of these cells are lost due to cell death by 5 weeks of age. The remaining photoreceptor cells have only a few or no discs—although they all appear to have a cilium. Oil droplets similar to those present in Rpe65−/− mice3 were occasionally observed in the RPE of the Rpe65−/−/Nrl−/− mice (data not shown), with the RPE otherwise normal in appearance, with its apical processes seen as long parallel membranes adjacent to the inner segments.
Figure 3.

Transmission electron micrographs of the RPE/photoreceptor layer of Rpe65−/−/Nrl−/− mice at 2 weeks of age. (A-C) Images at different levels of magnification. Arrows: basal bodies of photoreceptor cilia. N, RPE nuclei; M, RPE melanosomes; V, RPE apical villi; OS, outer segment membranes. Scale bars, 1 μm.
Decreased Expression of Outer Segment Proteins in Rpe65−/−/Nrl−/− Mice
In animals at 4 weeks of age, Western analysis of whole-eye lysates showed the expected absence of Nrl and rhodopsin in the Nrl−/− and Rpe65−/−/Nrl−/− mice and the absence of Rpe65 in the Rpe65−/− and Rpe65−/−/Nrl−/− mice (Fig. 4A). In addition, rhodopsin levels were markedly decreased in the Rpe65−/− mice, whereas slight reductions in Nrl and Rpe65 were present in the Rpe65−/− and Nrl−/− mice, respectively. The expression of Rpe65 in Nrl−/− mice was relatively stable, showing little reduction from 1 to 7.5 months of age (Fig. 4B). Expression of Rpe65 was not observed in the rosette formations that were present in the ONL of Nrl−/− retinas (Fig. 4C). Expression of Cralbp, arrestin, Rdh5, and Gapdh was observed in all four genotypes.
Figure 4.

Analysis of protein expression in Rpe65−/−/Nrl−/−, Rpe65−/−, Nrl−/−, and wild-type mice. (A) Immunoreactivity in whole-eye extracts from mice at 4 weeks of age probed with antibodies specific for the proteins indicated. (B) Rpe65 immunoreactivity in whole-eye extracts from Nrl−/− mice at the ages indicated. (C) Rpe65 immunoreactivity in RPE/retina cryosections from Nrl−/− mice. P, phase contrast; D, DAPI (4′,6-diamidino-2-phenylindole)-stained nuclei; R, immunoreactivity with mAb 8B11 specific for Rpe65.
Immunohistochemical analysis showed S-opsin and M/Lopsin were decreased in the Rpe65−/− and Rpe65−/−/Nrl−/− mice relative to wild type, and relative to Nrl−/− mice that have elevated levels of both opsins (Fig. 5). In the double knockout animals, diffuse, low-level S-opsin reactivity was apparent in most photoreceptor cells, with distinct M/L-opsin labeling present in a subset of the cells. In both cases, opsin reactivity was mislocalized and detected throughout the photoreceptor inner segment, as previously reported for Rpe65−/− mice.26 The downregulation and mislocalization of opsins and failed outer segment formation in the Rpe65−/−/Nrl−/− mice suggests that little or no light-responsive signal transduction machinery remained intact in the double-knockout animals.
Figure 5.

Immunohistochemistry of S-opsin and M/L opsin in Rpe65−/−/Nrl−/−, Nrl−/−, Rpe65−/−, and wild-type mice in RPE/retina cryosections. Green: opsin immunoreactivity (AlexaFluor-conjugated secondary antibody). Blue: DAPI (4′,6-diamidino-2-phenylindole)-stained nuclei.
Absence of 11-cis Retinoids in Eyes of Rpe65−/−/Nrl−/−
HPLC analysis of retinoid content showed that only retinyl esters were present in significant amounts in the eyes of Rpe65−/−/Nrl−/− mice, at levels approximately one-third those in Rpe65−/− mice (Fig. 6). In both Rpe65-deficient genotypes, more than 98% of the total retinoid content was in the form of retinyl esters, with 11-cis retinal and all-trans retinal below the level of detection, and levels of all-trans retinol intermediate between those in wild-type and Nrl−/− mice. Analysis of the ester fraction from Rpe65−/−/Nrl−/− mice by saponification and head group identification showed that only all-trans retinyl esters were present, with complete absence of 11-cis isomers in the double-knockout mice.
Figure 6.

HPLC analysis of the retinoid content of eyes of Rpe65−/−/Nrl−/− mice. (A) HPLC traces of total retinoid extracts from two eyes of a Rpe65−/−/Nrl−/− mouse. According to retention times and absorbance spectra, the peaks were assigned as follows (arrows): 1, retinyl esters; 2, syn 11-cis retinal oxime; 3, syn all-trans retinal oxime; 4, anti-11-cis retinal oxime; 5, 11-cis retinol; 6, all-trans retinol. (B) HPLC traces of the retinyl ester fraction collected (as denoted with a bar over the peak on trace a), and after saponification and rechromatography (trace b). Inset: Peak absorbance spectra for the saponification product on trace b and the standard compounds 11-cis retinol (trace c, peak 1) and all-trans retinol (trace c, peak 2). (C) Retinoid content of eyes from Rpe65−/−/Nrl−/−, Nrl−/−, Rpe65−/−, and wild-type mice.
Absence of ERG Responses in Rpe65−/−/Nrl−/− Mice
Previous studies of Nrl−/− mice showed ERG responses typical of cones, with markedly reduced responses under scotopic conditions, and supranormal b-wave amplitudes under photopic conditions.15,17,18 In contrast, Rpe65−/−/Nrl−/− mice exhibited no recordable ERG responses when subjected to a series of flashes in dark-adapted conditions (−5.81-1.09 log cd · s/m2 per flash) or light-adapted conditions (−0.91-1.09 log cd · s/m2 per flash) that elicited robust responses from Nrl−/− and wild-type mice and reduced responses from Rpe65−/− mice (Figs. 7A, 7B). However, when ERGs were recorded from the Rpe65−/−/Nrl−/− mice over an extended intensity range of 4.0 log units (−0.5-3.5 log cd · s/m2 xenon flashes delivered from a photographic flash unit), significant b-wave responses were evoked in two of five animals, in both dark-adapted (data not shown) and light-adapted conditions (Fig. 7C). There were no recordable responses for flashes less than 2.5 cd · s/m2 in intensity under dark- or light-adapted conditions. The a-wave was absent except for a very small signal seen with the highest intensity flash (3.5 log cd · s/m2), with previous studies showing recordable a-waves in Nrl−/− mice.15,17,18 As the a-wave arises primarily from the suppression of the photoreceptors’ light-sensitive current, the near complete absence of a-waves in the double-knockout mice indicates that the photoreceptor current has declined to undetectable levels, or that its sensitivity to light has become so low that it can be only minimally activated. In contrast, the b-wave arises from inner retinal neurons (primarily, ON-bipolar cells), and its presence indicates that some photoreceptor function remains, whereas its low amplitude reflects insensitivity and/or decline in inner retinal integrity.
Figure 7.
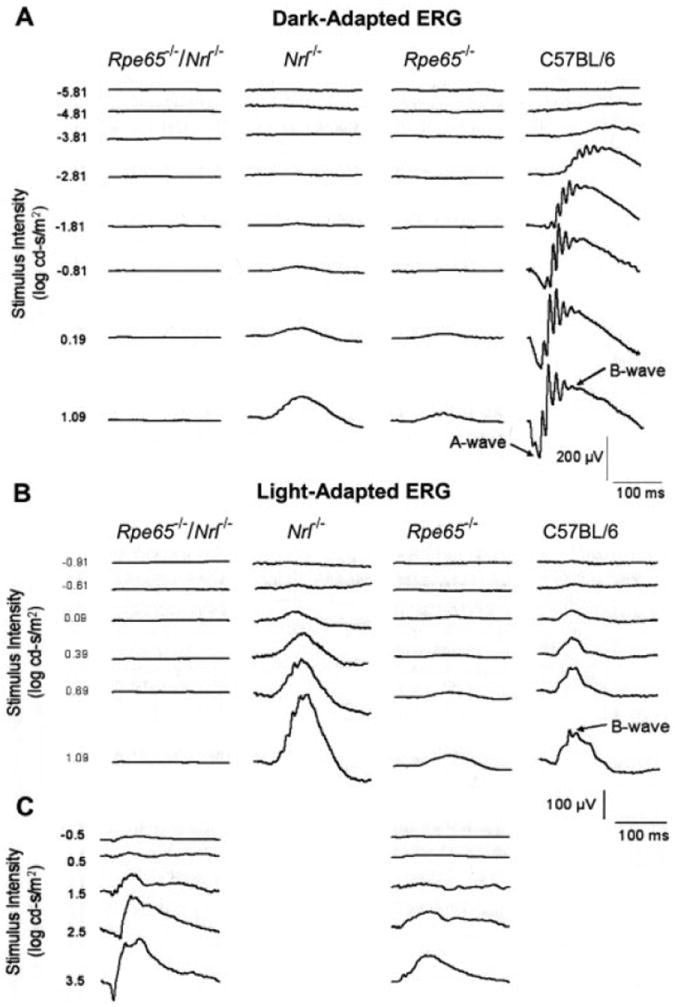
ERG waveform series of Rpe65−/−/Nrl−/−, single-knockout, and wild-type mice recorded over a range of stimulus intensities. (A) Dark-adapted flash ERG responses (scotopic). (B) Light-adapted flash ERG responses (photopic). (C) Light-adapted flash ERG responses (photopic) for an extended range of stimulus intensity, maximum 3.5 log cd · s/m2. Representative responses are shown. The number of traces averaged differed for different genotypes, with approximately 25 traces averaged for the Rpe65−/−/Nrl−/− mice.
Retinal Pathology in Eyes of Rpe65−/−/Nrl−/− Mice
At ages older than 4 weeks, the retinas of the Rpe65−/−/Nrl−/− mice exhibited striking pathology that increased over time. In sections of central retina inferior to the optic nerve head, ONL thickness was approximately equivalent in mice of all four genotypes, consisting of 9 to 10 rows of nuclei at 4 weeks of age (Figs. 2, 8A). At 7 weeks of age, the Rpe65−/−/Nrl−/− mice had lost at least two rows of photoreceptor nuclei, and overall thinning of the retina was evident. At 12 weeks of age, the total retinal thickness of the Rpe65−/−/Nrl−/− mice was approximately half that of Nrl−/− mice, with further losses in the ONL that were more severe in the superior than in the inferior central retina. Rosette formations present in most sections from the Nrl−/− mice at 12 weeks of age were seen only occasionally in the Rpe65−/−/Nrl−/− mice, perhaps because the conditions needed for rosette formation are not maintained as the number of cells in the ONL of the double-knockout mouse decreases with aging. Morphometric analysis of ONL thickness in the Rpe65−/−/Nrl−/− and wild-type mice at 4, 7, and 12 weeks of age clearly demonstrated the progressive cell loss in the double-knockout animals, with the superior and temporal regions affected most severely (Fig. 8B).
Figure 8.

Retinal histology and thickness measurements in Rpe65−/−/Nrl−/−, single-knockout, and wild-type mice at 4, 7, and 12 weeks of age. (A) Phase-contrast micrographs of toluidine-stained RPE/retina sections. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. CI, central retina inferior to optic nerve head; CS, central retina superior to optic nerve head. (B, C) Plots of ONL thickness in Rpe65−/−/Nrl−/− and wild-type mice, with measurements made on sections parallel (superior, inferior) and orthogonal (nasal, temporal) to the vertical meridian of the eye with standard deviations shown: 4 weeks (▲); 7 weeks (∎); 12 weeks (∎).
Failure of Exogenous 11-cis Retinal to Rescue the Phenotype of the Rpe65−/−/Nrl−/− Mice
Dark-reared Rpe65−/−/Nrl−/− and Rpe65−/− mice were dosed with 11-cis retinal by intraperitoneal injection, an approach previously shown to rescue the retinal phenotype of Rpe65−/− mice.26,28,29 Protocols involving single and multiple doses were used, starting at 1 week to 4 weeks of age. Approximately 24 hours after the final injection, ERGs were recorded under dark-adapted and light-adapted conditions, over a range of flash intensities from −0.5 to 3.5 log cd · s/m2. In control experiments, Rpe65−/− mice that received either single or multiple doses of 11-cis retinal exhibited improved ERG responses in both dark-adapted and light-adapted states. Figure 9A summarizes data for Rpe65−/− mice given six injections of 11-cis retinal (1.25-5.0 μg) at postnatal day (PN)10 to PN30. In the treated animals, maximum responses significantly increased for the dark-adapted b-wave (P = 0.042) and appeared to be increased for the light-adapted b-wave (although statistical significance was not reached due to the small sample size). In contrast, fewer than half of the Rpe65−/−/Nrl−/− mice that received exogenous 11-cis retinal showed any improvement in ERG recordings, and only when multiple injections were given. Figure 9B summarizes the data for the Rpe65−/−/Nrl−/− mice injected with 11-cis retinal. No significant difference in dark-adapted b-wave responses recorded at the brightest flash intensity was seen between the treated and untreated animals. Under light-adapted conditions, an apparent doubling of the b-wave amplitude was evident in treated Rpe65−/−/Nrl−/− mice (P = 0.036). However, the maximum response obtained in the treated double-knockout animals (~75 μV) was less than half that of untreated Rpe65−/− mice (~165 μV), and a fraction of that elicited from untreated Nrl−/− mice using stimulus intensities 2 log units lower. Intraperitoneal injection of 11-cis retinal did not increase expression of S-opsin and M/L-opsin in Rpe65−/−/Nrl−/− mice or increase chromophore accumulation in the retina (data not shown). Neither was there improvement in photoreceptor morphology, with continued absence of intact outer segment structures in mice given either single or multiple injections of 11-cis retinal beginning as early as 1 week of age. A representative image is shown in Figure 10.
Figure 9.

ERG responses to maximum flash intensity of 3.5 log cd · s/m2 for mice treated with 11-cis retinal by intraperitoneal injection. (A) Rpe65−/− and (B) Rpe65−/−/Nrl−/− mice housed in complete darkness received injections of 11-cis retinal as follows: postnatal day (PN)10 and PN14, 1.25 μg; PN17, 2.5 μg; PN21, 3.75 μg; PN27 and PN33, 5.0 μg. ERG analysis was performed 24 hours after the final injection. (∎) 11-cis retinal injected (n = 4 Rpe65−/−, n = 2 Rpe65−/−/Nrl−/−); (▨) vehicle injected (n = 3 Rpe65−/−, n = 3 Rpe65−/−/Nrl−/−). Error bars, mean ± SEM.
Figure 10.

Transmission electron micrographs of the RPE/photoreceptor layer of a 37-day-old Rpe65−/−/Nrl−/− mouse, treated by intraperitoneal injection of 11-cis retinal, twice weekly, beginning at 7 days of age. Arrows: basal bodies of photoreceptor cilia. N, RPE nucleus; M, RPE melanosome; V, RPE apical microvilli. Scale bar, 1 μm.
Discussion
Our characterization of the phenotype resulting from Rpe65 deficiency in the all-cone retina of Nrl−/− mice represents an important extension of findings in a previous study,19 with our studies now showing that the absence of chromophore results in decreased opsin content, failed outer segment morphogenesis, and severe retinal degeneration in Rpe65−/−/Nrl−/− mice. As the cones of Nrl−/− mice appear to resemble those of the wild type in both structure and function,15-18 these combined observations suggest that normally rod-dominant mice do not have an independent mechanism for cone photopigment regeneration. Our studies also suggest that cone-rich regions of the retina affected by visual cycle defects may be difficult to rescue with retinoid therapy.
In mice deficient for only Nrl, we found that retinoid processing in the retina remained intact, consistent with previous measures of the ERG responses in these animals.15,17 During recovery after bleaching, reduction of all-trans retinal occurred rapidly, with relatively, but not absolutely, higher rates of 11-cis retinal synthesis compared with wild type. Dark-adapted levels of 11-cis retinal in Nrl−/− mice were decreased; however, cone opsin levels were also decreased relative to rhodopsin levels in wild-type mice17 (reflecting the smaller outer segments of cones), suggesting that virtually all the 11-cis retinal present in the retina is bound to opsin protein, as in wild type.27 A distinct feature of Nrl−/− mice was relatively increased levels of all-trans and 11-cis retinol, present in minor amounts in wild-type mice. Thus, it may be interesting to consider whether cone-specific stores of retinoids exist in the retina. We did not observe increased retinyl ester content, increased levels of Rpe65 protein, or localization of Rpe65 in ONL rosettes, as reported by Wenzel et al.19 Similarly, no increase in Rpe65 transcript levels in Nrl−/− mice was detected using microarray analysis (Mears A, personal communication, September 2005). As the Nrl−/− mice in all three studies were derived from the same source,15 and carry the same Rpe65-Met450 polymorphism,30 other genetic background effects may underlie the observed differences, as the breeders used were mixed with respect to Sv129 and C57BL/6 backgrounds. Previous studies have shown that photoreceptor survival in Rho−/− mice is increased on the C57BL/6 genetic background relative to that on Sv129.31
In mice deficient for both Rpe65 and Nrl, we found that retinoid processing was blocked after uptake and storage of vitamin A in the RPE, in agreement with the findings of Wenzel et al.19 Only all-trans retinyl esters were detected in significant amounts, with levels of 11-cis retinal below the limit of detection, and no ERG responses were elicited at flash intensities that produce supranormal responses in Nrl−/− mice.15,17 However, our studies also showed that Rpe65−/−/Nrl−/− mice had dramatically reduced levels of S-opsin and M/L opsin, as well as profound loss of stable photoreceptor outer segment structures. Although intact ciliary structures were visible by transmission electron microscopy, stacks of disc membranes were found to be associated with only a fraction of photoreceptor cells and only in mice at very young ages (2 weeks). It is not yet clear whether the absence of disc membranes in older animals simply results from wide-spread photoreceptor cell death, or whether the outer segment structures that initially form are unstable and degenerate over time.
A previous study of mice deficient in only Rpe65 showed that cone opsin stability is decreased in the absence of chromophore, resulting in subcellular mislocalization of cone opsin and early cone cell death in the central retina.32 However, at young ages, cone structures in the peripheral retina remain intact in Rpe65−/− mice, and administration of exogenous 9-cis or 11-cis retinal effectively corrects opsin expression and revives cone ERG responses.26,28,29,33,34 In addition, gene-replacement therapy has been shown to rescue the function of peripheral cone photoreceptors remaining in the rodless retinas of Rho−/− mice that are also deficient in Rpe65.26 In contrast, in Rpe65−/−/Nrl−/− mice, we found that administration of 11-cis retinal (beginning as young as 1 week of age) did not significantly increase opsin expression, correct opsin mislocalization, improve outer segment morphogenesis, or increase retinoid accumulation in the retina. In addition, the ERG responses obtained in treated double-knockout mice were much lower than those obtained in treated Rpe65−/− mice. Thus, our findings add to the body of evidence suggesting that the efficacy of therapeutic intervention in the visual cycle is likely to differ in rod- versus cone-rich regions of the retina.
In some respects, the retinal phenotype of Rpe65−/−/Nrl−/− mice appears more similar to that resulting from rhodopsin deficiency35 than that due to Rpe65 deficiency alone, both in terms of defective photoreceptor outer segment formation and early-onset retinal degeneration. In Rho−/− mice, stable rod outer segments fail to form due to loss of a major structural component of the disc membrane that may also influence the trafficking of additional proteins from the inner to outer segment.36,37 In contrast, in Rpe65−/− mice, chromophore loss has minor effects on rod structure, retinal degeneration is slow, and rescue by administration of exogenous 11-cis retinal is effective until advanced ages.29 Differences in the stability of rod versus cone outer segments and photopigments are likely to reside in several factors, including affinity for the chromophore and signals involved in outer segment morphogenesis. Additional factors, including rod-derived cone viability factor (RdCVF)38 may also be important. In this regard, it is interesting to note that many patients with mutations in visual cycle genes show signs of macular degeneration and scarring consistent with increased damage occurring in cone-rich regions of the retina.39-41
In summary, our data support the view that the RPE-based visual cycle is the only significant source of chromophore in the rod-dominant retina, as our data indicate that Rpe65 expression is restricted to the RPE of both rod- and cone-dominant retinas,42 and others who report Rpe65 expression in cones find only minute levels.43 A role for Rpe65 in facilitating entry into a cone-centric pathway of 11-cis retinal synthesis cannot be excluded; however, there is no experimental evidence of such a mechanism. Although both rods and cones in the mouse retina appear dependent on Rpe65 for 11-cis retinal synthesis, for cone cells, loss of chromophore results in devastating photoreceptor instability and rapid degeneration. Recent studies of individuals with RPE65-mutations also suggest that RPE65-based mechanisms are critical for 11-cis retinal production necessary for cone function and maintenance of normal numbers of cone cells.44 Thus, special considerations may be needed to achieve rescue of the cone-rich central retina in patients with mutations in visual cycle genes resulting in decreased chromophore synthesis, or in other conditions resulting in decreased cone stability. We further conclude that alternative mechanisms of 11-cis retinal synthesis associated with cone photoreceptors described in chicken and ground squirrel13,14 are likely to represent specializations of cone-dominant retinas.
Acknowledgments
The authors thank T. Michael Redmond (National Eye Institute) for the Rpe65−/− mice; Robert Molday (University of British Columbia, Van-couver) for the 1D4 antibody; Bret Hughes (University of Michigan, Ann Arbor) for many helpful discussions; and Elena Filippova, Austra Liepa, and Mitchell Gillett for expert technical assistance.
Supported by National Eye Institute Grants EY12298 (DAT), EY02660 (ENP), EY07042 (DSW), EY11115 (AS), and P30-EY07003 (Vision Research Core Grant); National Institutes of Health Research Grant M01-RR00042 (UM-General Clinical Research Center); The Foundation Fighting Blindness (DAT, AS); and Research to Prevent Blindness (DAT, AS, ENP).
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May, 2006.
Disclosure: K.L. Feathers, None; A.L. Lyubarsky, None; N.W. Khan, None; K. Teofilo, None; A. Swaroop, None; D.S. Williams, None; E.N. Pugh, Jr, None; D.A. Thompson, None
References
- 1.Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler R, Curcio C, Hicks D, Price D, Wong F. Cell death in age-related macular degeneration. Mol Vis. 1999;5:31. [PubMed] [Google Scholar]
- 3.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 4.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122(3):449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci USA. 2005;102(35):12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA. 2005;102(38):13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17(2):194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 8.Marlhens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17(2):139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 9.Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35(2):158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 10.Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX. Down-regulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46(4):1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]
- 11.Muniz A, Villazana-Espinoza ET, Hatch AL, Trevino SG, Allen DM, Tsin AT. A novel cone visual cycle in the cone-dominated retina. Exp Eye Res. 2007;85:175–184. doi: 10.1016/j.exer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci USA. 1989;86(23):9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36(1):69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mata NL, Ruiz A, Radu RA, Bui TV, Travis GH. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44(35):11715–11721. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29(4):447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, Brown B, Li A, Mears AJ, Swaroop A, Craft CM. GRK1-dependent phosphorylation of S and M opsins and their binding to cone arrestin during cone phototransduction in the mouse retina. J Neurosci. 2003;23(14):6152–6160. doi: 10.1523/JNEUROSCI.23-14-06152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniele LL, Lillo C, Lyubarsky AL, et al. Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol Vis Sci. 2005;46(6):2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikonov SS, Daniele LL, Zhu X, Craft CM, Swaroop A, Pugh EN., Jr Photoreceptors of Nrl−/− mice coexpress functional S- and M-cone opsins having distinct inactivation mechanisms. J Gen Physiol. 2005;125(3):287–304. doi: 10.1085/jgp.200409208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzel A, von Lintig J, Oberhauser V, Tanimoto N, Grimm C, Seeliger MW. RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest Ophthalmol Vis Sci. 2007;48(2):534–542. doi: 10.1167/iovs.06-0652. [DOI] [PubMed] [Google Scholar]
- 20.Arikawa K, Molday LL, Molday RS, Williams DS. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol. 1992;116(3):659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs D, Azarian SM, Lillo C, et al. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J Cell Sci. 2004;117:6473–6483. doi: 10.1242/jcs.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birk HW, Koepsell H. Reaction of monoclonal antibodies with plasma membrane proteins after binding on nitrocellulose: renaturation of antigenic sites and reduction of nonspecific antibody binding. Anal Biochem. 1987;164(1):12–22. doi: 10.1016/0003-2697(87)90360-5. [DOI] [PubMed] [Google Scholar]
- 23.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Garwin GG, Saari JC. High-performance liquid chromatography analysis of visual cycle retinoids. Methods Enzymol. 2000;316:313–324. doi: 10.1016/s0076-6879(00)16731-x. [DOI] [PubMed] [Google Scholar]
- 25.von Lintig J, Vogt K. Filling the gap in vitamin A research: molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000;275(16):11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 26.Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46(10):3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel A, Oberhauser V, Pugh EN, Jr, et al. The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem. 2005;280(33):29874–29884. doi: 10.1074/jbc.M503603200. [DOI] [PubMed] [Google Scholar]
- 28.Ablonczy Z, Crouch RK, Goletz PW, et al. 11-cis-retinal reduces constitutive opsin phosphorylation and improves quantum catch in retinoid-deficient mouse rod photoreceptors. J Biol Chem. 2002;277(43):40491–40498. doi: 10.1074/jbc.M205507200. [DOI] [PubMed] [Google Scholar]
- 29.Rohrer B, Goletz P, Znoiko S, et al. Correlation of regenerable opsin with rod ERG signal in Rpe65−/− mice during development and aging. Invest Ophthalmol Vis Sci. 2003;44(1):310–315. doi: 10.1167/iovs.02-0567. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001;21(1):53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphries MM, Kiang S, McNally N, et al. Comparative structural and functional analysis of photoreceptor neurons of Rho−/− mice reveal increased survival on C57BL/6J in comparison to 129Sv genetic background. Vis Neurosci. 2001;18(3):437–443. doi: 10.1017/s0952523801183100. [DOI] [PubMed] [Google Scholar]
- 32.Fan J, Woodruff ML, Cilluffo MC, Crouch RK, Fain GL. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J Physiol. 2005;568:83–95. doi: 10.1113/jphysiol.2005.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Hooser JP, Aleman TS, He YG, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci USA. 2000;97(15):8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Hooser JP, Liang Y, Maeda T, et al. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem. 2002;277(21):19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphries MM, Rancourt D, Farrar GJ, et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15(2):216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 36.Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc Natl Acad Sci USA. 2005;102(9):3301–3306. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision Res. 2006;46(27):4427–4433. doi: 10.1016/j.visres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 38.Leveillard T, Mohand-Said S, Lorentz O, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36(7):755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 39.Thompson DA, Gyurus P, Fleischer LL, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2000;41(13):4293–4299. [PubMed] [Google Scholar]
- 40.Felius J, Thompson DA, Khan NW, et al. Clinical course and visual function in a family with mutations in the RPE65 gene. Arch Ophthalmol. 2002;120(1):55–61. doi: 10.1001/archopht.120.1.55. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson SG, Aleman TS, Cideciyan AV, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci USA. 2005;102(17):6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemati N, Feathers KL, Chrispell JD, Reed DM, Carlson TJ, Thompson DA. RPE65 surface epitopes, protein interactions, and expression in rod- and cone-dominant species. Mol Vis. 2005;11:1151–1165. [PubMed] [Google Scholar]
- 43.Znoiko SL, Crouch RK, Moiseyev G, Ma JX. Identification of the RPE65 protein in mammalian cone photoreceptors. Invest Ophthalmol Vis Sci. 2002;43(5):1604–1609. [PubMed] [Google Scholar]
- 44.Jacobson SG, Aleman TS, Cideciyan AV, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci USA. 2007;104(38):15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]


