Abstract
A series of N-benzylated-5-methoxytryptamine analogues was prepared and investigated, with special emphasis on substituents in the meta position of the benzyl group. A parallel series of several N-benzylated analogues of 2,5-dimethoxy-4-iodophenethylamine (2C-I) also was included for comparison of the two major templates (i.e., tryptamine and phenethylamine). A broad affinity screen at serotonin receptors showed that most of the compounds had the highest affinity at the 5-HT2 family receptors. Substitution at the para position of the benzyl group resulted in reduced affinity, whereas substitution in either the ortho or the meta position enhanced affinity. In general, introduction of a large lipophilic group improved affinity, whereas functional activity often followed the opposite trend. Tests of the compounds for functional activity utilized intracellular Ca2+ mobilization. Function was measured at the human 5-HT2A, 5-HT2B, and 5-HT2C receptors, as well as at the rat 5-HT2A and 5-HT2C receptors. There was no general correlation between affinity and function. Several of the tryptamine congeners were very potent functionally (EC50 values from 7.6 to 63 nM), but most were partial agonists. Tests in the mouse head twitch assay revealed that many of the compounds induced the head twitch and that there was a significant correlation between this behavior and functional potency at the rat 5-HT2A receptor.
Keywords: Serotonin, 5-HT2 receptors, 5-HT2A, agonist, phenethylamine, 5-methoxytryptamine, mouse head twitch
Introduction
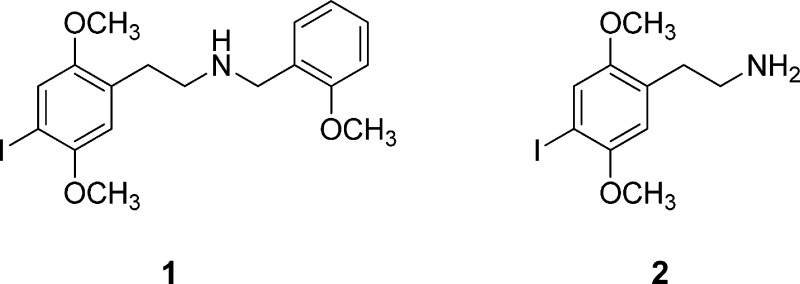
Recently, an extremely potent hallucinogenic phenethylamine, 25I-NBOMe (N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine; “smiles”) 1 has been available on the illicit drug market.1 For purposes of enforcement, it is presently considered by the Drug Enforcement Administration (DEA) to be an analogue of 2C-I (2), which is currently a Schedule I controlled substance. The procedure to classify 1 as a Schedule I substance has been initiated, and it has been placed temporarily into Schedule I.2 Unfortunately, several deaths have been associated with the use of 1,3−5 but it is not clear whether the deaths resulted from the ingestion of lethal amounts of pure solid drug, or whether the drug has some inherent toxicity that is not normally associated with other hallucinogens.
There has been increasing global interest in 1 and closely related analogues. For example, the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) has received a range of notifications from EU Member States about analytically confirmed nonfatal and fatal intoxications associated with 1. This was then followed by a risk assessment conducted by the Scientific Committee of the EMCDDA in order to assess health and social risks associated with this particular analogue.6 In addition, the World Health Organization’s Expert Committee on Drug Dependence reviewed the status of a range of new substances for its 36th meeting in June 2014, which included 1 and its 4-bromo and 4-chloro analogues.7 In September 2014, the Council of the European Union decided to subject 1 to control measures and criminal penalties throughout the European Union.8
Typically, simple N-alkylation dramatically attenuates or abolishes hallucinogenic activity in phenethylamines.9,10 The N-benzyl moiety, however, confers exceptionally high potency onto the molecule,11−15 and we have presented evidence that the N-benzyl may engage F339 in the human 5-HT2A receptor.14 We also examined various N-arylmethyl substituents and found that a variety of aryl groups were effective in enhancing potency.16,17 In addition, the presence of a polar substituent at the ortho position of the aryl ring (a possible hydrogen bond acceptor) further enhances activity.18 Silva et al.18 also have reported that in an in vitro cylindrical rat tail artery strip 1 had a pEC50 of 10.09 and an Emax of 30%.
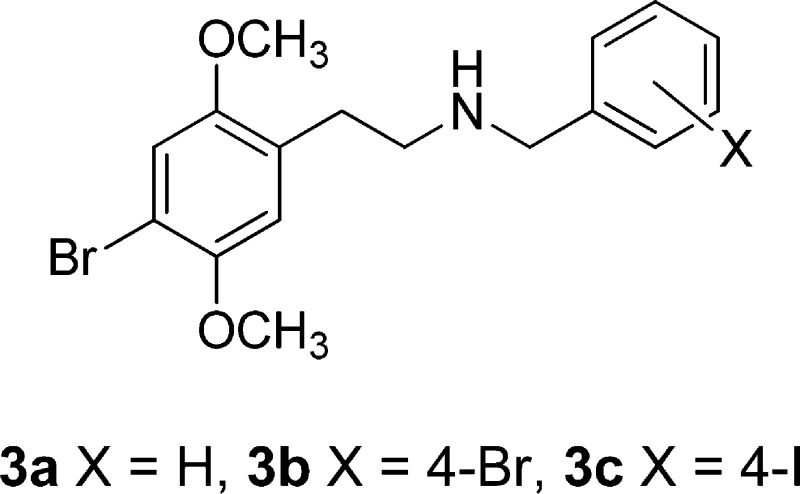
Two decades ago, Glennon et al.19 reported that the affinities of the N-benzyl compound 3a, as well as the 4-bromo- and 4-iodo-N-benzyl compounds, 3b and 3c, respectively, were 2–3 times higher than that of the parent primary amine. There have been no further reports on these compounds, and in our own work, we had never examined 3- or 4-substituted benzyl substituents in the phenethylamine series.
In addition to the phenethylamine type 5-HT2A agonists, certain simple tryptamines possess similar pharmacology, particularly 4- or 5-oxygenated molecules. In the report by Glennon et al., placing an N-benzyl moiety on the amine of 5-methoxytryptamine had essentially no effect on affinity. Interestingly, N-benzyl-5-methoxytryptamine previously had been reported to be an antagonist of serotonin-induced contraction in the rat stomach fundus, the isolated guinea pig uterus, and the isolated guinea pig taenia cecum.20 In addition, Leff et al.21 had shown that N-benzyl-5-methoxytryptamine had only weak partial agonist activity at 5-HT2 type receptors in rabbit aorta and rat jugular vein.
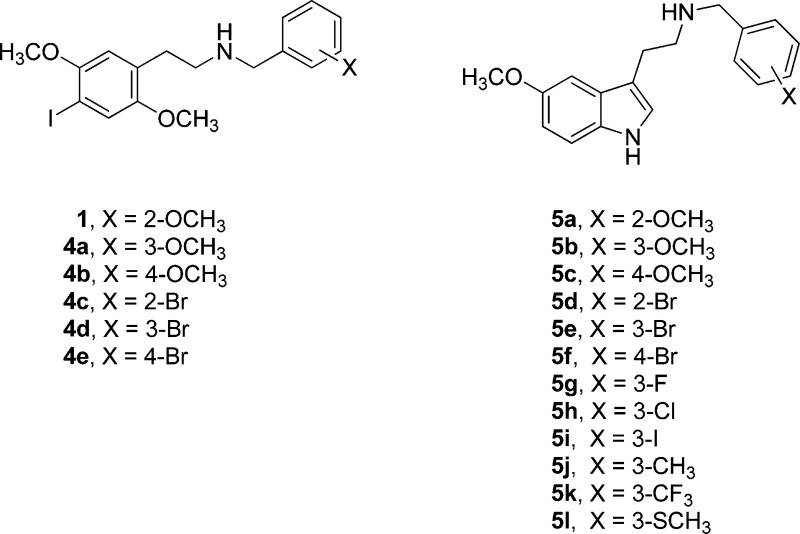
Surprisingly, however, in the Glennon report,19 a 5-HT2A receptor affinity of 0.1 nM was reported for the N-4-bromobenzyl compound (compound 33 in the Glennon report, numbered here as 5f), with 1000-fold selectivity for 5-HT2A over 5-HT2C receptors. We found these data particularly intriguing. This degree of selectivity was overestimated, however, because affinity at the 5-HT2A receptor was measured by displacement of an agonist ragioligand, whereas affinity at the 5-HT2C receptor was measured by displacement of an antagonist radioligand. Nonetheless, no specific 5-HT2A-selective agonist has been available, although such a compound would be very valuable for serotonin neuroscience research.
Although it was reported19 that 4-bromo compound 5f had 0.1 nM affinity at the human 5-HT2A receptor, the 4-fluoro-, 4-chloro-, and 4-iodo-substituted benzyl congeners had reported affinities of 40, 105, and 120 nM, respectively, in that same report. We found this discontinuity in the structure–activity relationship (SAR) puzzling, where the 4-bromo compound would be such an outlier in the family of halogen-substituted benzyls. Further investigation by Jensen, however, revealed that the authentic 4-bromo compound 5f actually had relatively low affinity for the 5-HT2A receptor, more consistent with the reported affinities of the other halogenated compounds.22 Although spectroscopic data were not reported by Glennon et al.19 that might explain the basis for this discrepancy, their publication indicated elemental analysis data to be consistent with the proposed structure. If the elemental analysis data were correct, the mostly likely explanation for the discordant biological data therefore seemed to be that 5f might have been an isomer other than the 4-substituted compound.
On the basis of the hypothesis that the original data were associated with an isomer other than the 4-bromo compound, we subsequently discovered that N-3-bromobenzyl compound 5e did have higher affinity for the 5-HT2A receptor (Ki 1.48 nM), compared to that of the 4-bromo congener 5f (Ki 11.2 nM). Further, the effect of an ortho-oxygenated N-benzyl appeared not to be significant for affinity in the tryptamine series, suggesting perhaps different binding orientations of the N-benzyltryptamines versus the N-benzylphenethylamines within the receptor. That is, compound 5a has been reported to have agonist potency (pEC50 7.08) in a rat tail artery assay not significantly different from the compound with an unsubstituted N-benzyl moiety (pEC50 7.00), although the Emax was slightly higher for the 2′-methoxy compound.18 These findings prompted us to synthesize a small series of structurally related congeners to determine whether other substitutions might have even greater affinity and/or selectivity for the 5-HT2A receptor.
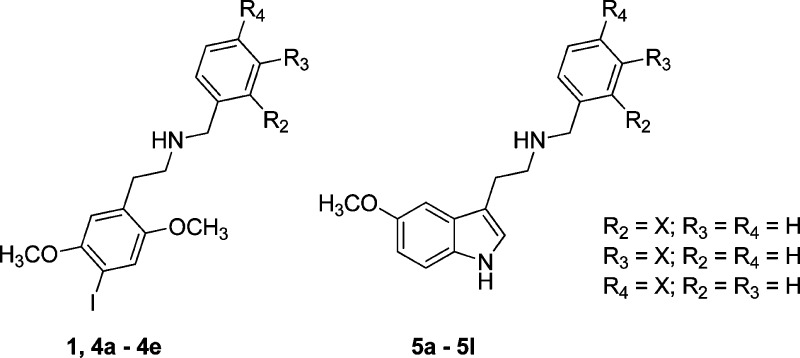
Thus, in this article we describe the facile synthesis of compounds 1, 4a–4e, and 5a–5l, preliminary screening at a variety of 5HT family receptors, and more detailed testing at human 5-HT2A, 5-HT2B, and 5-HT2C receptors, including affinity measurements using displacement of the agonist radioligand [125I]-DOI and functional effects in elevating intracellular calcium. We also present behavioral data for the mouse head twitch response (HTR) as a measure of in vivo 5-HT2A receptor activation.23
Compound 1 has been previously reported,24 and the NMR and electron ionization mass spectra of 4a and 4b have been reported but without any biological data.25 We thus decided to compare all of the series members at the same time to elucidate a consistent SAR.
Chemistry
All of the compounds were most easily prepared using a modification of the facile method first reported by Abdel-Magid et al.26 The free base of 2 was stirred in 3 mL of MeOH for 30 min with the appropriate aldehyde, followed by reduction of the intermediate enamine with NaBH4. Following appropriate workup, the bases were converted to their HCl or maleate salts and crystallized in good to excellent yields.
Pharmacology
Affinities at a panel of 5-HT receptors were determined by the NIMH-sponsored PDSP program (http://pdsp.med.unc.edu/kidb.php). Affinities at both the human and rat 5-HT2A and 5-HT2C receptors also were determined, using both agonist and antagonist radioligands. As a measure of functional potency and efficacy, changes in intracellular Ca2+ levels were measured using a fluorometric imaging plate reader (FLIPRTETRA, Molecular Devices), at the human 5-HT2A, 5-HT2B, and 5-HT2C receptors, and at the rat 5-HT2A and 5-HT2C receptors. Finally, as a measure of in vivo 5-HT2A receptor activation, we assessed the ability of all compounds to induce the mouse HTR.23 We hypothesized that functional potency at the rat 5-HT2A receptor might correlate best with the mouse head twitch behavioral data because ligand affinities at the rat 5-HT2A receptor correlate with the mouse 5-HT2A receptor but not with the human 5-HT2A receptor.27
Results
Further exploration of a small library of 3-substituted N-benzyl tryptamines allowed us to develop a tentative SAR for this series, and it is clear that substituents on the N-benzyl 3-position do modulate affinity in the tryptamine series. In the broad screening of 5-HT receptor types, all of the compounds had the highest affinity at the 5-HT2 family of receptors (Tables 1 and 2).
Table 1. Affinities of New Compounds for the Human 5-HT2A and 5-HT2C Receptors Using Both Agonist and Antagonist Radioligandsa.
| h5-HT2A pKi ±
SEM (Ki nM) |
h5-HT2C pKi ± SEM (Ki nM) |
|||
|---|---|---|---|---|
| cmpd | [3H]ketanserin | [125I]DOI | [3H]mesulergine | [125I]DOI |
| 1 | 9.28 ± 0.11 (0.52) | 9.80 ± 0.15 (0.16) | 9.16 ± 0.09 (0.69) | 9.30 ± 0.16 (0.50) |
| 4a | 8.81 ± 0.17 (1.5) | 9.57 ± 0.09 (0.27) | 8.38 ± 0.01 (4.17) | 9.90 ± 0.07 (0.13) |
| 4b | 7.93 ± 0.13 (11.7) | 9.15 ± 0.16 (0.70) | 7.85 ± 0.02 (14.1) | 8.44 ± 0.14 (3.63) |
| 4c | 8.63 ± 0.18 (2.34) | 9.42 ± 0.09 (0.38) | 8.06 ± 0.07 (8.71) | 8.99 ± 0.18 (1.02) |
| 4d | 8.40 ± 0.04 (3.98) | 9.24 ± 0.12 (0.57) | 8.12 ± 0.02 (7.59) | 8.79 ± 0.08 (1.62) |
| 4e | 7.28 ± 0.14 (52.5) | 8.49 ± 0.09 (3.24) | 7.34 ± 0.02 (45.7) | 8.48 ± 0.25 (3.31) |
| 5a | 7.78 ± 0.05 (16.6) | 8.82 ± 0.19 (1.51) | 7.49 ± 0.14 (32.4) | 8.47 ± 0.10 (3.39) |
| 5b | 8.11 ± 0.10 (7.76) | 8.98 ± 0.14 (1.05) | 7.42 ± 0.12 (38.0) | 8.23 ± 0.09 (5.89) |
| 5c | 7.16 ± 0.16 (69.2) | 7.98 ± 0.04 (10.5) | 6.90 ± 0.03 (126) | 7.85 ± 0.13 (14.1) |
| 5d | 7.60 ± 0.12 (25.1) | 8.63 ± 0.19 (2.34) | 7.00 ± 0.01 (100) | 7.85 ± 0.10 (14.1) |
| 5e | 8.17 ± 0.11 (6.76) | 8.83 ± 0.10 (1.48) | 7.58 ± 0.05 (26.3) | 8.25 ± 0.11 (5.62) |
| 5f | 6.37 ± 0.12 (427) | 7.95 ± 0.22 (11.2) | 6.60 ± 0.15 (251) | 7.54 ± 0.19 (28.8) |
| 5g | 7.67 ± 0.04 (21.4) | 8.58 ± 0.17 (2.63) | 7.32 ± 0.09 (47.9) | 8.06 ± 0.14 (8.71) |
| 5h | 8.28 ± 0.08 (5.25) | 8.98 ± 0.10 (1.05) | 7.55 ± 0.06 (28.2) | 8.37 ± 0.05 (4.27) |
| 5i | 8.46 ± 0.09 (3.47) | 9.21 ± 0.16 (0.62) | 8.19 ± 0.09 (6.46) | 8.98 ± 0.08 (1.05) |
| 5j | 8.32 ± 0.17 (4.79) | 8.93 ± 0.11 (1.17) | 7.65 ± 0.03 (22.4) | 8.47 ± 0.08 (3.39) |
| 5k | 7.55 ± 0.05 (28.2) | 8.53 ± 0.19 (2.95) | 6.99 ± 0.06 (102) | 7.83 ± 0.26 (14.8) |
| 5l | 8.05 ± 0.15 (8.91) | 8.51 ± 0.17 (3.09) | 7.88 ± 0.23 (13.2) | 8.68 ± 0.30 (2.09) |
pKi ± SEM (affinities in nM); n = 3–5 separate displacement curves.
Table 2. PDSP Screening Affinities for All Compounds at Other Human Serotonin Receptor Typesa.
| cmpd | 5-HT2B | 5-HT1A | 5-HT1B | 5-HT1D | 5-ht1e | 5-HT3 | 5-ht5a | 5-HT6 | 5-HT7 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.86 ± 0.03 (1.4) | 5.99 ± 0.05 (1033) | 5.23 ± 0.06 (5886) | 6.27 ± 0.05 (533) | >10,000 | >10,000 | 5.55 ± 0.07 (2795) | 7.5 ± 0.06 (32) | 5.81 ± 0.06 (1542) |
| 4a | 8.34 ± 0.03 (4.6) | 6.03 ± 0.05 (925) | 5.49 ± 0.05 (3232) | 6.36 ± 0.05 (439) | 5.77 ± 0.05 (1707) | >10,000 | 7.24 ± 0.06 (57) | 7.17 ± 0.06 (67) | 6.23 ± 0.06 (583) |
| 4b | 7.78 ± 0.03 (17) | 5.97 ± 0.05 (1064) | 5.8 ± 0.05 (1592) | 6.49 ± 0.05 (325) | 5.89 ± 0.05 (1285) | >10,000 | 5.99 ± 0.06 (1020) | 7.12 ± 0.03 (75) | 5.8 ± 0.06 (1575) |
| 4c | 7.7 ± 0.04 (20) | 5.94 ± 0.06 (1155) | >10,000 | 6.37 ± 0.05 (423) | >10,000 | >10,000 | 5.64 ± 0.09 (2290) | 6.59 ± 0.06 (257) | 5.59 ± 0.05 (2547) |
| 4d | 7.89 ± 0.04 (13) | 6.17 ± 0.06 (670) | 5.80 ± 0.05 (1568) | 6.79 ± 0.05 (162) | 6.10 ± 0.04 (792) | >10,000 | 6 ± 0.08 (1009) | 6.76 ± 0.06 (175) | 6.45 ± 0.05 (355) |
| 4e | 7.17 ± 0.04 (68) | 6.19 ± 0.06 (649) | 5.22 ± 0.05 (5093) | 6.51 ± 0.05 (311) | >10,000 | 5.61 ± 0.05 (2460) | 5.73 ± 0.06 (1848) | 6.46 ± 0.05 (350) | 6.19 ± 0.05 (641) |
| 5a | 8.04 ± 0.03 (9) | 6.64 ± 0.05 (231) | >10,000 | 5.89 ± 0.05 (1292) | >10,000 | >10,000 | >10,000 | 7.06 ± 0.03 (87) | 5.75 ± 0.06 (1770) |
| 5b | 8.6 ± 0.03 (2.5) | 6.48 ± 0.05 (335) | >10,000 | 6.48 ± 0.06 (334) | >10,000 | >10,000 | 5.9 ± 0.06 (1261) | 7.6 ± 0.03 (25) | 6.39 ± 0.05 (406) |
| 5c | 7.49 ± 0.03 (33) | 7.12 ± 0.06 (76) | 5.97 ± 0.04 (1060) | 6.79 ± 0.06 (161) | >10,000 | >10,000 | 5.62 ± 0.09 (2388) | 6.45 ± 0.03 (353) | 7.44 ± 0.05 (37) |
| 5d | 7.62 ± 0.03 (24) | 6.54 ± 0.05 (286) | >10,000 | 6.11 ± 0.05 (782) | >10,000 | 5.21 ± 0.07 (6169) | >10,000 | 6.69 ± 0.05 (203) | 5.96 ± 0.05 (1086) |
| 5e | 8.45 ± 0.03 (3.6) | 6.81 ± 0.05 (155) | 5.19 ± 0.06 (6433) | 6.42 ± 0.05 (381) | >10,000 | >10,000 | 6.21 ± 0.06 (612) | 7.34 ± 0.03 (45) | 6.93 ± 0.06 (116) |
| 5f | 6.83 ± 0.03 (150) | 7.11 ± 0.05 (78) | 5.35 ± 0.05 (4374) | 6.57 ± 0.05 (271) | >10,000 | >10,000 | 5.99 ± 0.08 (1034) | 6.25 ± 0.03 (566) | 6.45 ± 0.06 (358) |
| 5g | 7.66 ± 0.03 (22) | 6.53 ± 0.04 (295) | 5.57 ± 0.06 (2674) | 6.50 ± 0.06 (319) | >10,000 | >10,000 | 5.61 ± 0.05 (2450) | 7.23 ± 0.03 (59) | 6.62 ± 0.05 (242) |
| 5h | 8.16 ± 0.02 (6.6) | 6.71 ± 0.05 (195) | 5.36 ± 0.06 (4392) | 6.55 ± 0.06 (282) | >10,000 | >10,000 | 5.64 ± 0.06 (2310) | 7.30 ± 0.03 (50) | 6.55 ± 0.05 (281) |
| 5i | 9.12 ± 0.03 (0.76) | 6.91 ± 0.05 (122) | 5.53 ± 0.05 2963) | 6.70 ± 0.06 (199) | >10,000 | >10,000 | 5.81 ± 0.05 (1536) | 7.58 ± 0.03 (27) | 7.66 ± 0.05 (22) |
| 5j | 8.71 ± 0.03 (1.9) | 6.57 ± 0.04 (271) | 5.37 ± 0.07 (4241) | 6.55 ± 0.06 (283) | 5.41 ± 0.05 (3876) | >10,000 | 5.41 ± 0.06 (3852) | 7.21 ± 0.03 (62) | 6.67 ± 0.05 (212) |
| 5k | 7.56 ± 0.02 (28) | 6.62 ± 0.05 (240) | >10,000 | 6.56 ± 0.06 (278) | >10,000 | >10,000 | 5.51 ± 0.06 (3091) | 7.06 ± 0.03 (87) | 6.58 ± 0.05 (262) |
| 5l | 8.39 ± 0.04 (4.1) | 6.90 ± 0.05 (127) | >10,000 | 6.18 ± 0.05 (659) | >10,000 | >10,000 | 6.08 ± 0.08 (841) | 8.01 ± 0.06 (9.7) | 6.87 ± 0.05 (136) |
pKi ± SEM, (affinity in nM).
At the 5-HT2A and 5-HT2C receptors, the highest affinity was observed in the competition displacements with [125I]-DOI. Except for 5c and 5f, all of the tryptamine compounds had low nanomolar or subnanomolar affinity for the human 5-HT2A receptor. The known phenethylamine 1 had by far the highest affinity at 5-HT2A/2C receptors, with subnanomolar affinity at both subtypes. We have previously reported an affinity for 1 at the human 5-HT2A receptor of 0.04 nM.14 Of the tryptamines, only the 3-iodobenzyl compound 5i, had subnanomolar affinity at the 5-HT2A receptor, although all of the tryptamines had high affinity at this receptor. It should be noted that N-methylation of 5e completely abolished affinity at the 5-HT2A receptor (Ki > 10 μM; data not shown), indicating that tertiary amines are not tolerated in the N-benzyltryptamines.
The rank order of affinity of all compounds at the [125I]-DOI-labeled h5-HT2C receptor generally paralleled that measured at the 5-HT2A receptor, although the affinities tended to be somewhat lower. Again, among the tryptamines studied 5i had the highest affinity at this receptor, as well as at the 5-HT2B receptor. Affinities measured at the [125I]-DOI site tended to be on the order of 5–10 times higher than that at the antagonist labeled sites at both receptors.
Functional potencies at the rat and human 5-HT2A and 5-HT2C receptors and the human 5-HT2B receptor are shown in Table 3. Compound 1 was a nearly full agonist at both receptor types, with a 4.2 nM EC50 at the human 5-HT2A receptor and 11 nM EC50 at the rat 5-HT2A receptor. The most potent compound was 5a, with an EC50 of 1.9 nM and 85% efficacy at the h5-HT2A. Notably, this compound has the N-2-methoxybenzyl substituent, the same as the most potent phenethylamine 1, suggesting that it may be optimal for activation of the 5-HT2A receptor when placed at the 2-position of the N-benzyl moiety. Efficacies of the tryptamines at the rat and human 5-HT2A receptors and human 5-HT2C receptor varied from about 40% to 80%, with a few compounds that were full agonists (e.g., 5a and 5c), whereas at the rat 5-HT2C receptor all of the compounds were full agonists.
Table 3. Functional Data for New Compounds in Rat and Human 5-HT2A and 5-HT2C and Human 5-HT2B Receptorsa.
| r5-HT2A |
h5-HT2A |
h5-HT2B |
r5-HT2C |
h5HT2C |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cmpd | pEC50 (EC50 nM) | Emax % | pEC50 (EC50 nM) | Emax % | pEC50 (EC50 nM) | Emax % | pEC50 (EC50 nM) | Emax % | pEC50 (EC50 nM) | Emax % |
| 5-HT | 8.3 ± 0.04 (5.4) | 100 ± 1.5 | 8.7 ± 0.05 (2.0) | 100 ± 1.6 | 9.31 ± 0.04 (0.49) | 99.9 ± 1.1 | 9.70 ± 0.03 (0.20) | 99.6 ± 0.73 | 9.52 ± 0.08 (0.30) | 98.5 ± 2.4 |
| 1 | 8.0 ± 0.04 (11) | 79.4 ± 1.1 | 8.4 ± 0.05 (4.2) | 86.4 ± 1.4 | 7.81 ± 0.09 (15) | 65 ± 2 | 7.02 ± 0.05 (95) | 104 ± 2 | 7.38 ± 0.12 (41.7) | 92 ± 0 |
| 4a | 7.6 ± 0.04 (27) | 51.7 ± 0.9 | 7.6 ± 0.03 (28) | 71.6 ± 0.9 | 7.4 ± 0.3 (38) | NAb | 6.88 ± 0.06 (133) | 91 ± 3 | 7.47 ± 0.36 (33.8) | 41 ± 6 |
| 4b | 7.3 ± 0.04 (50) | 53.8 ± 0.9 | 7.2 ± 0.03 (60) | 74.1 ± 0.8 | 7.1 ± 0.1 (87) | 38 ± 2 | 6.88 ± 0.05 (132) | 97 ± 2 | 7.36 ± 0.31 (43.2) | 50 ± 7 |
| 4c | 7.4 ± 0.06 (36) | 65.6 ± 1.6 | 7.4 ± 0.04 (42) | 88.0 ± 1.5 | 6.82 ± 0.07 (134) | 83 ± 3 | 6.98 ± 0.02 (105) | 104 ± 1 | 7.24 ± 0.13 (57.6) | 87 ± 5 |
| 4d | 7.8 ± 0.03 (14) | 68.5 ± 0.9 | 7.8 ± 0.04 (17) | 87.5 ± 1.3 | 7.05 ± 0.05 (85) | 90 ± 2 | 7.44 ± 0.05 (36) | 101 ± 2 | 7.28 ± 0.17 (57.6) | 74 ± 5 |
| 4e | 6.8 ± 0.03 (150) | 67.3 ± 0.9 | 6.8 ± 0.03 (170) | 88.0 ± 1.4 | 6.21 ± 0.04 (610) | 90 ± 2 | 6.54 ± 0.04 (290) | 105 ± 2 | 6.66 ± 0.14 (200) | 77 ± 5 |
| 5a | 7.7 ± 0.03 (21) | 80.9 ± 1.1 | 8.7 ± 0.05 (1.9) | 85.2 ± 1.4 | 8.2 ± 0.1 (6.7) | 52 ± 2 | 7.79 ± 0.04 (16) | 102 ± 2 | 7.24 ± 0.12 (57.1) | 119 ± 6 |
| 5b | 7.5 ± 0.04 (34) | 52.2 ± 0.9 | 8.2 ± 0.04 (6.2) | 70.0 ± 1.0 | 6.0 ± 0.4 (949) | NAb | 6.78 ± 0.05 (168) | 102 ± 2 | 6.75 ± 0.15 (178) | 65 ± 5 |
| 5c | 6.7 ± 0.03 (190) | 75.0 ± 1.3 | 7.4 ± 0.04 (42) | 84.1 ± 1.3 | 7.64 ± 0.04 (23) | 81 ± 1 | 7.73 ± 0.04 (19) | 102 ± 2 | 7.12 ± 0.11 (75.1) | 112 ± 5 |
| 5d | 6.3 ± 0.04 (450) | 49.7 ± 1.2 | 7.5 ± 0.05 (30) | 74.7 ± 1.5 | 6.8 ± 0.3 (168) | NAb | 6.05 ± 0.05 (898) | 104 ± 3 | 6.36 ± 0.09 (439) | 94 ± 5 |
| 5e | 6.9 ± 0.03 (130) | 65.5 ± 0.8 | 7.9 ± 0.04 (13) | 73.8 ± 1.1 | 7.5 ± 0.2 (29) | 20 ± 2 | 6.38 ± 0.04 (422) | 112 ± 3 | 6.49 ± 0.23 (321) | 64 ± 8 |
| 5f | 5.8 ± 0.04 (1500) | 77.6 ± 2.4 | 6.4 ± 0.02 (430) | 90.3 ± 1.2 | 6.54 ± 0.05 (290) | 90 ± 2 | 6.69 ± 0.03 (204) | 108 ± 2 | 6.28 ± 0.14 (529) | 83 ± 7 |
| 5g | 7.1 ± 0.04 (80) | 69.1 ± 1.3 | 8.0 ± 0.1 (10) | 89.3 ± 1.1 | 7.42 ± 0.08 (38) | 37 ± 1 | 7.34 ± 0.07 (46) | 100 ± 3 | 6.72 ± 0.13 (192) | 83 ± 5 |
| 5h | 7.1 ± 0.03 (83) | 70.1 ± 1.0 | 7.9 ± 0.04 (14) | 81.2 ± 1.3 | 7.3 ± 0.2 (50) | NAb | 6.54 ± 0.04 (286) | 105 ± 2 | 6.50 ± 0.13 (316) | 85 ± 6 |
| 5i | 6.9 ± 0.04 (120) | 73.4 ± 1.4 | 7.8 ± 0.04 (16) | 79.0 ± 1.1 | 7.4 ± 0.2 (43) | 31 ± 2 | 6.51 ± 0.05 (313) | 110 ± 3 | 6.35 ± 0.09 (445) | 94 ± 5 |
| 5j | 7.6 ± 0.04 (26) | 56.2 ± 0.9 | 8.2 ± 0.04 (6.5) | 73.3 ± 1.0 | NAb | 6.72 ± 0.04 (192) | 104 ± 2 | 6.54 ± 0.10 (289) | 75 ± 4 | |
| 5k | 6.1 ± 0.03 (770) | 69.6 ± 1.4 | 7.1 ± 0.04 (87) | 75.5 ± 1.2 | 6.97 ± 0.07 (107) | 51 ± 2 | 6.79 ± 0.03 (162) | 104 ± 2 | 6.29 ± 0.11 (512) | 75 ± 5 |
| 5l | 6.9 ± 0.05 (120) | 32.0 ± 0.7 | 7.5 ± 0.04 (32) | 46.9 ± 0.8 | NAb | 6.69 ± 0.05 (205) | 101 ± 2 | 6.55 ± 0.11 (283) | 60 ± 4 | |
Values are pEC50 ± SEM, with (EC50) values in nM and Emax given in percentage of the maximum response to 5-HT.
NA, not active; Emax ≤ 15%.
It is noteworthy that the functional potencies in the rat and human 5-HT2A receptors are essentially identical for phenethylamine compounds 1, and 4a–4e, yet the potencies for tryptamine compounds 5a–5l are 4–10-fold higher at the human 5-HT2A receptor than at the rat 5-HT2A receptor. This finding may reflect the single amino acid difference in the orthosteric binding site of these two receptors at position 5.46. In the rat or mouse 5-HT2A receptor, residue 5.46 is an alanine, whereas in the human receptor it is a serine. We have previously shown that mutation of this residue in the human receptor from serine to alanine has little effect on affinity or function for phenethylamine 5-HT2A agonists but does have a significant effect for tryptamines.28 One might infer, therefore, from these potency differences that the indole NH in the present series also engages this serine in the human receptor but not the alanine in the rat receptor, consistent with mutagenesis studies reported by others.29,30
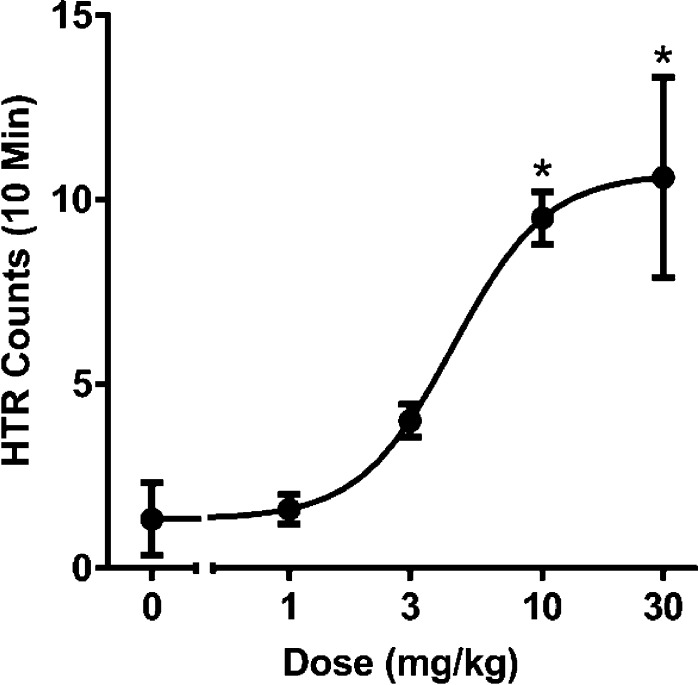
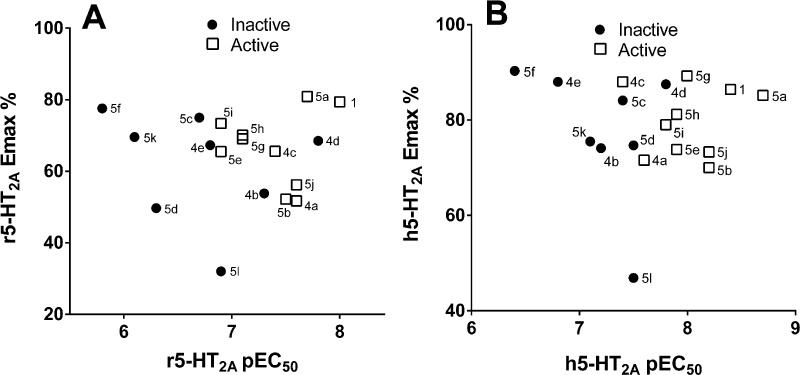
Figure 1 shows an illustrative dose–response curve for compound 5h in the mouse HTR. HTR data for all compounds are given in Table 4. Although some of the compounds failed to induce the HTR at doses up to 30 mg/kg, most of the “inactive” compounds displayed relatively low potency at 5-HT2A (see Figure 2), so it is possible that they would induce the HTR if tested at higher doses. Importantly, for the subset of compounds that induced the HTR, behavioral potency was significantly correlated with functional potency at the r5-HT2A receptor (r = 0.69, p < 0.03; Figure 2), but there was no correlation with functional EC50 values at the r5-HT2C receptor (r = 0.17, p > 0.1). Despite the overall correlation between mouse HTR and r5-HT2A potency, the relationship was not always orderly for individual compounds. Compound 1 was by far the most potent compound in that assay, with an ED50 of 0.078 mg/kg (data taken from Halberstadt and Geyer31). It is not clear why 1 should be so much more potent than any other compound because, for example, 4d is inactive but appears nearly comparable functionally, with an EC50 of 14 nM and efficacy of 69%, compared with an EC50 of 11 nM for 1 with an efficacy of 79%. The next most potent compounds in the mouse HTR are 4c and 5j, with identical ED50s of 2.31 mg/kg, about 300-fold less potent than 1. Although they have similar functional EC50 values (36 and 26 nM), nothing in the functional or binding data can explain their lower potency compared to that of 1. Further, compounds 5a, 5b, and 5g have virtually identical ED50 values in the mouse HTR, yet their functional EC50s at the rat 5-HT2A receptor are 21, 34, and 80 nM, respectively.
Figure 1.
Representative dose–response plot in the mouse head twitch assay for compound 5h. *p < 0.05 versus vehicle (Tukey’s test).
Table 4. Activity of New Compounds in Producing the Mouse Head Twitch.
| ED50 mg/kg (95% CI) | test duration (min) | N | dose range | active doses (mg/kg) | max counts | maximally effective dose(mg/kg) | magnitude of peak effect × vehicle | |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.078 (0.055–0.111) | 30 | 5 | 0.03–1.0 | 0.1, 0.3, 1 | 102.6 | 1 | 16.0 |
| 4a | 4.34 (1.41–13.32) | 10 | 10 | 0.3–30 | 3, 10, 30 | 11.4 | 30 | 5.7 |
| 4b | inactive | 5 | 0.3–30 | |||||
| 4c | 2.31 (1.41–3.77) | 20 | 5 | 0.3–30 | 3, 10, 30 | 23.2 | 10 | 3.0 |
| 4d | inactive | 5–7 | 0.3–10 | |||||
| 4e | inactive | 6 | 1–30 | |||||
| 5a | 3.15 (1.94–5.12) | 20 | 10 | 0.3–30 | 10, 30 | 25.4 | 10 | 3.9 |
| 5b | 3.28 (1.53–7.04) | 10 | 5–6 | 1–30 | 10 | 9.2 | 10 | 3.7 |
| 5c | inactive | 5 | 30 | |||||
| 5d | inactive | 5 | 0.3–30 | |||||
| 5e | 5.18 (2.35–11.38) | 10 | 5–6 | 1–30 | 10, 30 | 14.2 | 30 | 4.4 |
| 5f | inactive | 5 | 0.3–30 | |||||
| 5g | 3.33 (2.25–4.93) | 10 | 6 | 1–30 | 10, 30 | 14.5 | 10 | 7.3 |
| 5h | 4.43 (2.03–9.69) | 10 | 5–6 | 1–30 | 10, 30 | 10.6 | 30 | 8.0 |
| 5i | 7.77 (3.40–17.53) | 10 | 6 | 1–30 | 10, 30 | 20.2 | 30 | 3.4 |
| 5j | 2.31 (0.82–6.51) | 10 | 5 | 0.3–30 | 10 | 14.6 | 10 | 3.5 |
| 5k | inactive | 5 | 30 | |||||
| 5l | inactive | 4–5 | 30 |
Figure 2.
Plots of active and inactive compounds as a function of potency and efficacy at the rat 5-HT2A receptor (panel A) and the human 5-HT2A receptor (panel B).
With the exception of 5k and 5l, which had relatively low functional potencies at the r5-HT2A (EC50 values of 770 and 120 nM, respectively), all of the meta-substituted N-benzyl derivatives of 5-methoxytryptamine induced the HTR. That included the 3-methyl (5j; ED50 = 2.31 mg/kg), 3-methoxy (5b; ED50 = 3.28 mg/kg), 3-fluoro (5g; ED50 = 3.33 mg/kg), 3-chloro (5h; ED50 = 4.43 mg/kg), 3-bromo (5e; ED50 = 5.18 mg/kg), and 3-iodo (5i; ED50 = 7.77 mg/kg) compounds.
The HTR produced by compounds 5b and 5j showed a biphasic bell-shaped dose–response function (the response peaked at 10 mg/kg and 30 mg/kg was inactive). Other 5-HT2A agonists, including DOI, DOM, 2C-T-7, and 5-MeO–DIPT, have been shown to produce similar nonmonotonic responses.32−ref34 Fantegrossi et al.ref34 have argued that the descending arm of the biphasic HTR dose–response is a consequence of 5-HT2C activation, which attenuates the response to 5-HT2A activation. Recently, however, it was reported that N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenethylamine (25CN-NBOH), a 5-HT2A agonist with 100-fold selectivity over 5-HT2C, also induces the HTR with a biphasic dose–response.ref35 The fact that the descending arm of the response to 25CN-NBOH was not affected by a 5-HT2C antagonistref35 demonstrates that the inhibition of the HTR at high doses does not necessarily result from competing activity at 5-HT2C. One potential alternative explanation for the biphasic HTR is that high levels of 5-HT2A activation may produce competing behaviors that interfere with expression of head shaking. Along those lines, it has been reported that high doses of quipazine, 5-MeO-DMT, and (+)-LSD produce stereotypic behaviors that preclude head shakes and wet dog shakes in rats.ref36,ref37
Discussion
Unfortunately, despite the report by Glennon et al.,19 compound 5e was not selective for the h5-HT2A receptor versus the h5-HT2C receptor. Using affinity at the [125I]-DOI-labeled receptors, the selectivity of 5e was slightly less than 4-fold. Even using affinity at the [125I]-DOI-labeled h5-HT2A receptor and the [3H]-mesulergine-labeled h5-HT2C receptor, “selectivity” was only about 18-fold. The most selective compound in the entire series, with respect to affinity, was 5d, but with only 6-fold selectivity.
With respect to selectivity in function at the h5-HT2A vs h5-HT2C, the most selective tryptamine was 5j, with 44-fold selectivity and less than a 3-fold difference in affinity at the agonist-labeled receptors. Indeed, we were disappointed that none of the compounds had high selectivity for the h5-HT2A receptor.
Overall, with the exception of compound 1, none of the compounds was particularly potent in producing the HTR. This low potency is somewhat surprising, given that many known hallucinogens with high affinity for the 5-HT2A receptor, such as 2,5-dimethoxy-4-iodoamphetamine (DOI), R-(−)-2,5-dimethoxy-4-methylamphetamine (R-DOM), R-(−)-2,5-dimethoxy-4-bromoamphetamine (R-DOB), 2,5-dimethoxy-4-propylthiophenethylamine (2C-T-7), psilocin, and 5-MeO-N,N-diisopropyltryptamine (5-MeO–DIPT) produce the head twitch in mice at doses of ≤1 mg/kg.32,33,34−36 However, certain tryptamine hallucinogens, including 5-MeO-N,N-dimethyltryptamine (5-MeO-DMT) and α-methyltryptamine, are active within the same dose range (3–30 mg/kg) as the N-benzyltryptamines tested herein.36−38 It is unlikely that the low in vivo potencies of the compounds studied here are related to the use of an automated HTR detection system because we have confirmed that the results obtained using this system are consistent with published data based on visual scoring.23 For example, the potency of LSD measured using the automated system (ED50 = 0.13 μmol/kg)23 is almost exactly the same as the potency assessed using direct observation (ED50 = 0.14 μmol/kg).37 One possible explanation for the low potencies might be rapid first pass metabolism of N-benzyl-analogues in general39 combined with a slow release from subcutaneous tissue due to the highly hydrophobic nature of the compounds.
Substitution on the N-benzyl ring has different effects, depending on whether the phenethylamines or the tryptamines are being studied. For example, ortho-bromo-substituted tryptamine congener 5d failed to induce the HTR when tested at doses up to 30 mg/kg (∼60 μmol/kg), yet N-3-bromobenzyl 5e is active. By contrast, N-2-bromobenzyl phenethylamine 4c is active, whereas N-3-bromobenzyl 4d is inactive in the HTR assay.
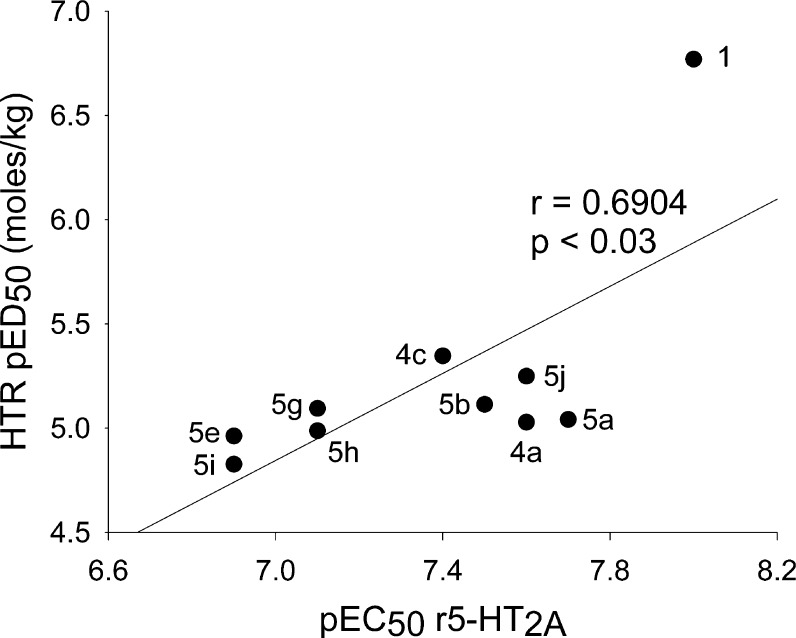
None of the phenethylamines or tryptamines with 4-substituted N-benzyl groups, 4b, 4e, 5c, or 5f, was active in the HTR. All of these compounds were partial agonists with relatively low potency in the r5-HT2A functional assay. Although 5e, with a 3-substituted N-benzyl, has an EC50 and Emax virtually identical to 4e, it is active in the HTR assay. It is possible that differences in pharmacokinetics or metabolic lability could explain these data. Nevertheless, if only the compounds active in the mouse HTR assay are compared, one finds a significant correlation between potency in the rat 5-HT2A receptor and potency in the HTR assay, as shown in Figure 3.
Figure 3.
Regression analysis of pED50 for the mouse head twitch response on the pEC50 for function for active compounds at the rat 5-HT2A receptor; n = 10.
Taken together, these data show that for N-benzylphenethylamines the highest in vivo potency in mice is associated with an ortho-substituent on the benzyl group, whereas the N-benzyltryptamines are more active in vivo when a meta-substituent is present. Hence, there are SAR differences between the N-benzyltryptamines and the N-benzylphenethylamines for the induction of the HTR, which likely reflect different binding orientations in the 5-HT2A receptor. Obviously, the indole system is larger than a simple phenyl ring, something that would clearly affect the binding modes for the two different series at the orthosteric site. For example, the distance from the indole C(3) atom to the 5-oxygen atom is 4.94 Å, whereas the corresponding distance from the 5-methoxy oxygen to C(1) of the aryl ring is only 3.70 Å. Even the distance of 4.85 Å from C(1) of the aryl ring to the 4-iodo atom of the phenethylamines is less than the 4.94 Å distance measured from C(3) of the indole to the 5-methoxy.
One exception is that for both the N-benzyltryptamines and N-benzylphenethylamines, oxygenated substituents are tolerated at the ortho- and meta-positions of the benzyl moiety. For example, 1, 4a, 5a, and 5b are all active in the HTR assay, whereas 4d and 5d are inactive over a range of doses. This observation again would be consistent with some structural feature in the 5-HT2A receptor that could engage a polar oxygen atom at the ortho-position of the N-benzyl moiety. There has been speculation, based on virtual docking studies with phenethylamines and tryptamines, that an oxygen atom in the ortho-position of the N-benzyl moiety may interact with a hydrogen bond donor (possibly the OH of Tyr 370(7.43) in the h5-HT2A receptor.14,18 It is conceivable that an oxygen atom at the meta-position in N-benzyltryptamines also could form a hydrogen bond with Tyr 370, possibly involving a water molecule.
Unfortunately, a 5-HT2A selective agonist did not emerge from this small library of compounds. There are now only two selective 5-HT2A agonists reported,40,41 but they have not been available for extensive study. Thus, research on 5-HT2A receptor function has been forced to employ either a mixed 5-HT2A/2C agonist such as DOI in combination with a specific 5-HT2C antagonist, or to administer antagonists alone, the latter paradigm really being appropriate to study receptor function only when there are high levels of endogenous receptor activation or constitutive activity of the receptors. Genetic knockout mice have not revealed particular behavioral phenotypes and have served primarily to demonstrate that a particular drug depends on the presence of 5-HT2A or 5-HT2C receptors for its effect. Hence, the psychopharmacology of a “pure” 5-HT2A agonist remains completely unknown. Furthermore, the tremendous present interest in the role of the 5-HT2A receptor in normal brain function makes it imperative that scientists in the field gain access to a 5-HT2A specific agonist so that research into the roles of the 5-HT2A receptor can be more fully elucidated.
Methods
Chemistry
General Methods
Reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO) or Alfa Aesar (Ward Hill, MA) and used as delivered, unless otherwise specified. Thin layer chromatography was carried out using J. T. Baker flexible sheets (silica gel IB2-F) with fluorescent indicator, visualizing with UV light at 254 nm or iodine stain. Melting points were determined using a Mel-Temp apparatus and are uncorrected. NMR experiments were carried out using a Bruker Advance 300 MHz instrument, and the chemical shift (δ) values are in parts per million (ppm) relative to tetramethylsilane at 0.00 ppm. The solvent was CD3OD. NMR samples were dissolved in MeOD. Ph = aromatic protons/carbons of benzyl group; In = aromatic protons/carbons of the indole nucleus; Ar = either phenyl or indole resonances, or phenyl in the case of compounds 1–4f. Coupling constants (J) are presented in Hertz. Abbreviations used in the reporting of NMR spectra include: br = broad, s = singlet, d = doublet, t = triplet, q = quartet, and quint = quintuplet.
Mass spectra were performed by high resolution LC-QTOF-MS on protonated molecules [M + H]+. UHPLC-Q-TOF-MS conditions for UHPLC separation employed a mobile phase consisting of 100% MeCN that included 1% formic acid (organic phase) and an aqueous solution of 1% formic acid (aqueous phase). The column was maintained at 40 °C with a 0.6 mL/min flow rate and 5.5 min acquisition time. The elution was a 5–70% MeCN gradient ramp over 3.5 min, then up to 95% MeCN in 1 min and held for 0.5 min before returning to 5% MeCN in 0.5 min. Q-TOF-MS data were acquired in positive mode scanning from 100 to 1000 m/z with and without auto MS/MS fragmentation. Ionization was achieved with an Agilent JetStream electrospray source and infused internal reference masses. Agilent 6540 Q-TOF-MS parameters: gas temperature, 325 °C; drying gas, 10 L/min; and sheath gas temperature, 400 °C. Internal reference masses of 121.05087 and 922.00979 m/z were used.
For compounds 1 and 4a–4e, 0.5 mmol of the free base of 4-iodo-2,5-dimethoxyphenethylamine10,42 was stirred for 30 min at room temperature with 0.55 mmol of the appropriate aldehyde in 3 mL of methanol. The reaction was then placed on an ice bath, and 48 mg (1.25 mmol) of NaBH4 was added in three portions over 15 min. The ice bath was removed and the reaction allowed to stir for an additional 15 min. The reaction was then transferred to a separatory funnel with 50 mL of EtOAc. The organic phase was washed three times with saturated NaCl, then dried overnight over Na2SO4. The drying agent was removed by suction filtration, and the filtrate was concentrated under reduced pressure. EtOH (1 mL) was added to the amber residue, and the HCl salt was prepared by acidification with 0.5 mL of 1 N HCl/EtOH. Dilution with EtOAc or diethyl ether then led to crystallization of the HCl salts, generally in good yields. In most cases, the supernatant was simply decanted from the crystalline product, followed by resuspension of the crystals in Et2O and decantation, then air drying to afford the products as white to off-white fine needles. No attempt was made to optimize the yields, but in one case the supernatant was reduced to dryness and the residue crystallized from EtOH/Et2O to afford an additional 6% of product. This small additional recovery was not deemed sufficient to warrant the extra effort. Thus, all reported yields are those obtained after the first crystallization.
The synthesis of tryptamines 5a–5l followed essentially the same procedure, except that maleate salts were prepared. As an example, 1.0 mmol of 5-methoxytryptamine free base (Aldrich) was stirred for 30 min with 1.10 mmol of the appropriate aldehyde in 5 mL of methanol. The reaction was then placed on an ice bath, and 96 mg (2.5 mmol) of NaBH4 was added in three portions over 15 min. The ice bath was removed and the reaction allowed to stir for an additional 15 min. The reaction was then transferred to a separatory funnel with 50 mL of EtOAc and was washed three times with saturated NaCl. The organic phase was dried overnight over Na2SO4, then filtered and concentrated under reduced pressure. Maleic acid (116 mg, 1 mmol) and 1.0 mL of acetone were then added to the residual amber oil, and the solution swirled until all of the maleic acid had dissolved. The reaction was then diluted with 10 mL of EtOAc, and Et2O was added nearly to the cloud point. In most cases, crystallization occurred rapidly and spontaneously, and the product solution was stored overnight in a cold room. Crystalline products were collected by suction filtration, washed on the filter with EtOAc, and then air-dried to afford white to off-white fine needles.
N-(2-Methoxybenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethan-1-amine Hydrochloride (1)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 86%; mp 168–170 °C, Lit24 mp 162–166 °C, 166.131H NMR (300 MHz, CD3OD) δ ppm 7.46 (1H, td, J = 8.2, 1.7 Hz, Ar–H), 7.37 (1H, dd, J = 7.6, 1.6 Hz, Ar–H), 7.35 (1H, s, Ar–H), 7.09 (1H, d, J = 8.3 Hz, Ar–H), 7.02 (1H, td, J = 7.5, 1.0 Hz, Ar–H), 6.86 (1H, s, Ar–H), 4.24 (2H, s, NB-CH2), 3.88 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.78 (3H, s, OCH3), 3.20–3.25 (2H, m, α-CH2), 3.03–2.98 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 159.37 (Ar–Cq), 154.44 (Ar–Cq), 153.60 (Ar–Cq), 132.81 (Ar–CH), 132.73 (Ar–CH), 126.99 (Ar–Cq), 123.19 (Ar–CH), 122.13 (Ar–CH), 120.29 (Ar–Cq), 114.98 (Ar–CH), 112.16 (Ar–CH), 85.04 (Ar–Cq-iodine), 57.59 (OCH3), 56.71 (OCH3), 56.24 (OCH3), 48.1 (NB-CH2), 48.0 (α-CH2), 28.49 (β-CH2). HRMS calculated for C18H23INO3 [M + H]+, 428.07171; observed [M + H]+, 428.07239. The EI mass spectrum also has been reported by Casale and Hays.25
N-(3-Methoxybenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethan-1-amine Hydrochloride (4a)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 85%; mp 171–2 °C. 1H NMR (300 MHz, CD3OD) δ ppm 7.38 (1H, t, J = 7.7 Hz, Ar–H), 7.34 (1H, s, Ar–H), 6.98–7.10 (3H, m, Ar–H), 6.86 (1H, s, Ar–H), 4.19 (2H, s, NB-CH2), 3.83 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.79 (3H, s, OCH3), 3.22–3.27 (2H, m, α-CH2), 2.99–3.04 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 161.77 (Ar–Cq), 154.43 (Ar–Cq), 153.63 (Ar–Cq), 133.82 (Ar–Cq), 131.48 (Ar–CH), 127.01 (Ar–Cq), 123.14 (Ar–CH), 122.92 (Ar–CH), 116.53 (Ar–CH), 116.13 (Ar–CH), 114.95 (Ar–CH), 85.00 (Ar–Cq-iodine), 57.59 (OCH3), 56.68 (OCH3), 55.93 (OCH3), 52.23 (NB-CH2), 48.1 (α-CH2), 28.65 (β-CH2). HRMS calculated for C18H23INO3 [M + H]+, 428.07171; observed [M + H]+, 428.07319. The EI mass spectrum has also been reported by Casale and Hays.25
N-(4-Methoxybenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethan-1-amine Hydrochloride (4b)
This particular compound was extremely difficult to crystallize, providing unfilterable gels upon attempts to crystallize it from EtOH, EtOH/Et2O, or MeOH/Et2O. It was finally obtained by dissolving in a minimum amount of boiling acetonitrile and allowing the solution to cool. Upon cooling, the solution also took on a gel-like appearance, but unlike other attempts, this material could be collected by vacuum filtration through a sintered glass filter funnel. The voluminous white solid was washed on the filter with a small amount of cold acetonitrile, then left on the funnel with suction until dry; yield 72%; mp 180–182 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.41 (2H, d, J = 8.7 Hz, 2 x Ar–H), 7.34 (1H, s, Ar–H), 7.00 (2H, d, J = 8.5 Hz, 2 x Ar–H), 6.85 (1H, s, Ar–H), 4.15 (2H, s, NB-CH2), 3.82 (3H, s, OCH3), 3.81 (3H, s OCH3), 3.79 (3H, s OCH3), 3.18–3.23 (2H, m, α-CH2), 2.96–3.01 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 162.27 (Ar–Cq), 154.42 (Ar–Cq), 153.64 (Ar–Cq), 132.59 (2 x Ar–CH), 127.05 (Ar–Cq), 124.22 (Ar–Cq), 123.16 (Ar–CH), 115.64 (2 x Ar–CH), 114.94 (Ar–CH), 84.98 (Ar–Cq-iodine), 57.59 (OCH3), 56.68 (OCH3), 55.90 (OCH3), 51.90 (NB-CH2), 47.8 (α-CH2), 28.67 (β-CH2). HRMS calculated for C18H23INO3 [M + H]+, 428.07171; observed [M + H]+, 428.07320. The EI mass spectrum has also been reported by Casale and Hays.25
N-(2-Bromobenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethan-1-amine Hydrochloride (4c)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 79%; mp 170–1 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.74 (1H, dd, J = 7.9, 1.3 Hz, Ar–H), 7.61 (1H, dd, J = 7.7, 1.7 Hz, Ar–H), 7.49 (1H, td, J = 7.5, 1.3 Hz), 7.39 (1H, td, J = 7.9, 1.9 Hz), 7.35 (1H, s, Ar–H), 6.89 (1H, s, Ar–H), 4.42 (2H, s, NB-CH2), 3.82 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.31–3.36 (2H, m, α-CH2), 3.03–3.08 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 154.46 (Ar–Cq), 153.62 (Ar–Cq), 134.74 (Ar–CH), 133.03 (Ar–CH), 132.81(Ar–CH), 132.32 (Ar–Cq), 129.70 (Ar–CH), 126.87 (Ar–Cq), 125.94 (Ar–Cq), 123.19 (Ar–CH), 114.98 (Ar–CH), 85.08 (Ar–Cq-iodine), 57.60 (OCH3), 56.73 (OCH3), 51.99 (NB-CH2), 48.7 (α-CH2), 28.62 (β-CH2). HRMS calculated for C17H20BrINO2 [M + H]+, 475.97166; observed [M + H]+, 475.97212.
N-(3-Bromobenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethan-1-amine Hydrochloride (4d)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 89%; mp 199–201 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.69–7.74 (1H, m, Ar–H), 7.60–7.66 (1H, m, Ar–H), 7.45–7.51 (1H, m, Ar–H), 7.41 (1H, d, J = 7.7 Hz, Ar–H), 7.35 (1H, s, Ar–H), 6.86 (1H, s, Ar–H), 4.22 (2H, s, NB-CH2), 3.81 (3H, s, OCH3), 3.79 (3H, s, OCH3), 3.22–3.27 (2H, m, α-CH2), 2.98–3.03 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 154.44 (Ar–Cq), 153.63 (Ar–Cq), 134.97 (Ar–Cq), 134.03 (Ar–CH), 133.87, (Ar–CH), 132.12 (Ar–CH), 129.89 (Ar–CH), 126.93 (Ar–Cq), 124.00 (Ar–Cq), 123.18 (Ar–CH), 114.95 (Ar–CH), 85.06 (Ar–Cq-iodine), 57.59 (OCH3), 56.70 (OCH3), 51.51 (NB-CH2), 48.3 (α-CH2), 28.68 (β-CH2). HRMS calculated for C17H20BrINO2 [M + H]+, 475.97166; observed [M + H]+, 475.97281.
N-(4-Bromobenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethan-1-amine Hydrochloride (4e)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 81%; mp 196–7 °C. 1H NMR (300 MHz, CD3OD) δ ppm 7.64 (2H, d, J = 8.7 Hz, 2 x Ar–H), 7.42 (2H, d, J = 8.5 Hz, 2 x Ar–H), 7.34 (1H, s, Ar–H), 6.86 (1H, s, Ar–H), 4.21 (2H, s, NB-CH2), 3.81 (3H, s, OCH3), 3.79 (3 H, s, OCH3), 3.22–3.27 (2H, m, α-CH2), 2.98–3.03 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 154.43 (Ar–Cq), 153.62 (Ar–Cq), 133.50 (2 x Ar–CH), 133.00 (2 x Ar–CH), 131.71 (Ar–Cq), 126.92 (Ar–Cq), 124.92 (Ar–Cq), 123.16 (Ar–CH), 114.94 (Ar–CH), 85.02 (Ar–Cq-iodine), 57.60 (OCH3), 56.68 (OCH3), 51.58 (NB-CH2), 48.2 (α-CH2), 28.66 (β-CH2). HRMS calculated for C17H20BrINO2 Calculated [M + H]+, 475.97166; observed [M + H]+, 475.97268.
N-(2-Methoxybenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Hydrochloride (5a)
Obtained as needles following crystallization from EtOH/EtOAc; yield 91%; mp 232–4 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.42 (1H, td, J = 7.9, 1.7 Hz, Ph-H), 7.33 (1H, dd, J = 7.4, 1.6 Hz, Ph-H), 7.28 (1H, dd, J = 8.9, 0.6 Hz, In–H), 7.16 (1H, s, In–H), 6.97–7.03 (2H, m, Ph-H), 6.95 (1H, d, J = 2.4 Hz, In–H), 6.81 (1H, dd, J = 8.8, 2.4 Hz, In–H), 4.23 (2H, s, NB-CH2), 3.78 (3H, s, OCH3), 3.67 (3H, s, OCH3), 3.28–3.33 (2H, m, α-CH2, overlapping with solvent), 3.12–3.17 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 159.25 (Ph–Cq), 155.42 (In-Cq), 133.67 (Ar–Cq), 132.77 (Ph–CH), 132.68 (Ph–CH), 128.34 (Ar–Cq), 125.35 (In-CH), 122.12 (In-CH), 120.13 (Ar–Cq), 113.41 (Ph–CH), 113.21 (In-CH), 112.06 (Ph–CH), 109.51 (Ar–Cq), 101.00 (In-CH), 56.35 (OCH3), 55.93 (OCH3), 48.90 (α-CH2), 48.3 (NB-CH2), 23.21 (β-CH2). HRMS calculated for C19H23N2O2 [M + H]+, 311.17540; observed [M + H]+, 311.17548.
N-(3-Methoxybenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5b)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 84%; mp 124–5 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.33–7.39 (1H, m, Ph-H), 7.26 (1H, dd, J = 8.9, 0.6 Hz, In–H), 7.13 (1H, s, In–H), 6.99–7.01 (4H, m, overlapping 3 x Ph-H, 1 x In–H), 6.80 (1H, dd, J = 8.8, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.18 (2H, s, NB-CH2), 3.81 (3H, s, OCH3), 3.80 (3H, s, OCH3), 3.28–3.35 (2H, α-CH2, overlapping with solvent), 3.11–3.16 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 170.89 (maleate), 161.79 (Ph–Cq), 155.39 (In-Cq), 136.79 (maleate), 133.89 (Ar–Cq), 133.60 (Ar–Cq), 131.50 (Ph–CH), 128.44 (Ar–Cq), 125.00 (In-CH), 122.87 (Ph–CH), 116.40 (Ph–CH), 116.15 (Ph–CH), 113.35 (In-CH), 113.07 (In-CH), 109.84 (Ar–Cq), 101.04 (In-CH), 56.39 (OCH3), 55.88 (OCH3), 52.16 (NB-CH2), 49.0 (α-CH2), 23.36 (β-CH2). HRMS calculated for C19H23N2O2 [M + H]+, 311.17540; observed [M + H]+, 311.17572
N-(4-Methoxybenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5c)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 82%; mp 172–3 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.36 (2H, d, J = 8.0 Hz, 2 x Ph-H), 7.26 (1 H, dd, J = 8.8, 0.5 Hz, In–H), 7.12 (1H, s, In–H), 6.99 (1H, d, J = 2.5 Hz, In–H), 6.97 (2H, d, J = 6.6 Hz, 2 x Ph-H), 6.80 (1H, dd, J = 8.9, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.15 (2H, s, NB-CH2), 3.81 (3H, s, OCH3), 3.80 (3H, s, OCH3), 3.27–3.32 (2H, m, α-CH2, overlapping with solvent), 3.09–3.14 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 170.90 (maleate), 162.24 (Ph–Cq), 155.37 (In-Cq), 136.78 (maleate), 133.60 (Ar–Cq), 132.52 (2 x Ph–CH), 128.45 (Ar–Cq), 124.97 (In-CH), 124.27 (Ar–Cq), 115.64 (2 x Ph–CH), 113.34 (In-CH), 113.06 (In-CH), 109.89 (Ar–Cq), 101.06 (In-CH), 56.39 (OCH3), 55.89 (OCH3), 51.78 (NB-CH2), 48.5 (α-CH2), 23.38 (β-CH2). HRMS calculated for C19H23N2O2 [M + H]+, 311.17540; observed [M + H]+, 311.17632
N-(2-Bromobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5d)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 72%; mp 93–5 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.70 (1H, dd, J = 7.9, 1.3 Hz, Ph-H), 7.54 (1H, dd, J = 7.7, 1.9 Hz, Ph-H), 7.45 (1H, td, J = 7.5, 1.4 Hz, Ph-H), 7.36 (1H, td, J = 7.8, 1.8 Hz, Ph-H), 7.26 (1H, dd, J = 8.9, 0.6 Hz, In–H), 7.16 (1H, s, In–H), 7.02 (1H, d, J = 2.3 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.41 (2H, s, NB-CH2), 3.82 (3H, s, OCH3), 3.40–3.45 (2H, m, α-CH2), 3.16–3.21 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 170.89 (maleate), 155.41 (In-Cq), 136.75 (maleate), 134.71 (Ph–CH), 133.64 (Ar–Cq), 133.04 (Ph–CH), 132.76 (Ph–CH), 132.40 (Ar–Cq), 129.65 (Ph–CH),128.44 (Ar–Cq), 125.94 (Ar–Cq), 125.12 (In-CH), 113.38 (In-CH), 113.10 (In-CH), 109.67 (Ar–Cq), 101.06 (In-CH), 56.40 (OCH3), 51.90 (NB-CH2), 49.3 (α-CH2), 23.32 (β-CH2). HRMS calculated for C18H20BrN2O [M + H]+, 359.07535; observed [M + H]+, 359.07581.
N-(3-Bromobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5e)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 86%; mp 137–8 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.67–7.68 (1H, m, Ph-H), 7.61 (1H, dt, J = 7.7, 1.6 Hz, Ph-H), 7.34–7.45 (2H, m, Ph-H), 7.27 (1H, d, J = 8.7 Hz, In–H), 7.14 (1H, s, In–H), 7.01 (1H, d, J = 2.3 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.21 (2H, s, NB-CH2), 3.82 (3H, s, OCH3), 3.31–3.36 (2H, m, α-CH2, overlapping with solvent), 3.11–3.16 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 170.92 (maleate), 155.40 (In-Cq), 136.78 (maleate), 135.10 (Ar–Cq), 134.00 (Ph–CH), 133.82 (Ph–CH), 133.61 (Ar–Cq), 132.10 (Ph–CH), 129.81 (Ph–CH), 128.45 (Ar–Cq), 125.01 (In-CH), 124.03 (Ar–Cq), 113.37 (In-CH), 113.08 (In-CH), 109.82 (Ar–Cq), 101.06 (In-CH), 56.42 (OCH3), 51.52 (NB-CH2), 49.1 (α-CH2), 23.40 (β-CH2). HRMS calculated for C18H20BrN2O [M + H]+, 359.07535; observed [M + H]+, 359.07547
N-(4-Bromobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5f)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 75%; mp 181–3 °C. 1H NMR (CD3OD): δ ppm 7.60 (2H, d, J = 8.5 Hz, 2 x Ph-H), 7.37 (2H, d, J = 8.5 Hz, 2 x Ph-H), 7.26 (1H, d, J = 8.9 Hz, In–H), 7.13 (1H, s, In–H), 6.99 (1H, d, J = 2.3 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.20 (2H, s, NB-CH2), 3.81 (3H, s, OCH3), 3.31–3.36 (2H, m, α-CH2, overlapping with solvent), 3.11–3.16 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 170.89 (maleate), 155.38 (In-Cq), 136.7 5 (maleate), 133.61 (Ar–Cq), 133.51 (2 x Ph–CH), 132.92 (2 x Ph–CH), 131.77 (Ar–Cq), 128.43 (Ar–Cq), 125.02 (In-CH), 124.90 (Ar–Cq), 113.36 (In-CH), 113.06 (In-CH), 109.76 (Ar–Cq), 101.06 (In-CH), 56.41 (OCH3), 51.50 (NB-CH2), 48.90 (α-CH2), 23.40 (β-CH2). HRMS calculated for C18H20BrN2O [M + H]+, 359.07535; observed [M + H]+, 359.07597.
N-(3-Fluorobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5g)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 78%; mp 150–2 °C. 1H NMR (300 MHz CD3OD): δ ppm 7.44–7.51 (1H, m, Ph-H), 7.16–7.29 (4H, m, overlapping 3 x Ph-H, 1 x In–H), 7.14 (1H, s, In–H), 7.01 (1H, d, J = 2.4 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.24 (2H, s, NB-CH2), 3.81 (3H, s, OCH3), 3.31–3.37 (2H, m, α-CH2, overlapping with solvent), 3.12–3.17 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 170.89 (maleate), 164.38 (Ph–Cq-3′, d, J = 246.2 Hz), 155.40 (In-Cq), 136.74 (maleate), 135.08 (Ph–Cq-1′, d, J = 7.5 Hz), 133.61 (In-Cq), 132.31 (Ph–C-5′, d, J = 8.3 Hz), 128.45 (In-Cq), 126.88 (Ph–C-6′, d, J = 3.0 Hz), 125.00 (In-CH), 117.79 (Ph–C-2′, d, J = 22.5 Hz), 117.60 (Ph–C-4′, d, J = 21.8 Hz), 113.37 (In-CH), 113.08 (In-CH), 109.79 (In-Cq), 101.06, (In-CH), 56.41 (OCH3), 51.58 (NB-CH2, J = 1.5 Hz), 49.1 (α-CH2), 23.38 (β-CH2). HRMS calculated for C18H20FN2O [M + H]+, 299.15542; observed [M + H]+, 299.15602.
N-(3-Chlorobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5h)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 79%; mp 116–8 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.52 (1H, br, s, Ph-H), 7.34–7.49 (3H, m, Ph-H), 7.26 (1H, d, J = 8.9 Hz, In–H), 7.14 (1H, s, In–H), 7.01 (1H, d, J = 2.4 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.22 (2H, s, NB-CH2), 3.82 (3H, s, OCH3), 3.31–3.37 (2H, m, α-CH2, overlapping with solvent), 3.12–3.17 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 155.41 (In-Cq), 136.76 (maleate), 136.10 (Ar–Cq), 134.82 (Ar–Cq), 133.60 (Ar–Cq), 131.89 (Ph–CH), 131.04 (Ph–CH), 130.84 (Ph–CH), 129.38 (Ph–CH), 128.45 (Ar–Cq), 125.01 (In-CH), 113.36 (In-CH), 113.08 (In-CH), 109.78 (In-Cq), 101.04 (In-CH), 56.40 (OCH3), 51.54 (NB-CH2), 49.1 (α-CH2), 23.39 (β-CH2). HRMS calculated for C18H20ClN2O [M + H]+, 315.12587; observed [M + H]+, 315.12666
N-(3-Iodobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5i)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 84%; mp 131–2 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.87 (1H, brs, Ph-H), 7.81 (1H, d, J = 7.9 Hz, Ph-H), 7.45 (1H, d, J = 7.7 Hz, Ph-H), 7.27 (1H, d, J = 8.3 Hz, In–H), 7.21 (1H, t, J = 7.8 Hz, Ph-H), 7.13 (1H, s, In–H), 7.01 (1H, d, J = 2.3 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.3 Hz, In–H), 6.24 (2H, s, maleate), 4.18 (2H, s, NB-CH2), 3.82 (3H, s, OCH3), 3.31–3.36 (2H, m, α-CH2, overlapping with solvent), 3.11–3.16 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 155.39 (In-Cq), 139.98 (Ph–CH), 139.88 (Ph–CH), 136.76 (maleate), 134.98 (Ar–Cq), 133.58 (Ar–Cq), 132.03 (Ph–CH), 130.31 (Ph–CH), 128.46 (Ar–Cq), 124.99 (In-CH), 113.36 (In-CH), 113.08 (In-CH), 109.80 (Ar–Cq), 101.03 (In-CH), 95.42 (Ar–Cq-iodine), 56.42 (OCH3), 51.41 (NB-CH2), 49.1 (α-CH2), 23.37 (β-CH2). HRMS calculated for C18H20IN2O [M + H]+, 407.06148; observed [M + H]+, 407.06188.
N-(3-Methylbenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5j)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 78%; mp 125–7 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.22–7.35 (5H, m, overlapping 4 x Ph-H and 1 x In–H), 7.13 (1H, s, In–H), 6.99 (1H, d, J = 2.3 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.17 (2H, s, NB-CH2), 3.82 (3H, s, OCH3), 3.29–3.35 (2H, m, α-CH2, overlapping with solvent), 3.10–3.15 (2H, m, β-CH2), 2.36 (3H, s, CH3). 13C NMR (CD3OD): δ ppm 170.90 (maleate), 155.38 (In-Cq), 140.47 (Ar–Cq), 136.80 (maleate), 133.61 (Ar–Cq), 132.46 (Ar–Cq), 131.49 (Ph–CH), 131.40 (Ph–CH), 130.27 (Ph–CH), 128.46 (Ar–Cq), 127.93 (Ph–CH), 125.00 (In-CH), 113.35 (In-CH), 113.06 (In-CH), 109.87 (Ar–Cq), 101.07 (In-CH), 56.40 (OCH3), 52.24 (NB-CH2), 48.9 (α-CH2), 23.37 (β-CH2), 21.36 (CH3). HRMS calculated for C19H23N2O [M + H]+, 295.18049; observed [M + H]+, 295.18090.
N-(3-Methylthiobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5j)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 80%; mp 151–2 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.31–7.39 (3H, m, Ph-H), 7.26 (1H, d, J = 8.9 Hz, In–H), 7.19 (1H, dt, J = 7.0, 1.9 Hz, Ph-H), 7.13 (1H, s, In–H), 7.00 (1H, d, J = 2.4 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.4 Hz, In–H), 6.24 (2H, s, maleate), 4.19 (2H, s, NB-CH2), 3.81 (3H, s, OCH3), 3.30–3.35 (2H, m, α-CH2, overlapping with solvent), 3.11–3.16 (2H, m, β-CH2), 2.48 (3H, s, CH3). 13C NMR (CD3OD): δ ppm 170.91 (maleate), 155.40 (Ar–Cq), 141.95 (Ar–Cq), 136.78 (maleate), 133.60 (Ar–Cq), 133.34 (Ar–Cq), 130.74 (Ph–CH), 128.46 (Ar–Cq), 128.40 (Ph–CH), 128.30 (Ph–CH), 127.20 (Ph–CH), 125.00 (In-CH), 113.36 (In-CH), 113.08 (In-CH), 109.84 (Ar–Cq), 101.04 (In-CH), 56.41 (OCH3), 52.05 (NB-CH2), 49.1 (α-CH2), 23.38 (β-CH2), 15.37 (CH3). HRMS calculated for C19H23N2OS [M + H]+, 327.15256; observed [M + H]+, 327.15362.
N-(3-Trifluoromethylbenzyl)-2-(5-methoxy-1H-indol-3-yl)ethan-1-amine Maleate (5k)
Obtained as needles following crystallization from acetone/EtOAc/Et2O; yield 62%; mp 161–2 °C. 1H NMR (300 MHz, CD3OD): δ ppm 7.84 (1H, brs, Ph-H), 7.62–7.78 (3H, m, Ph-H), 7.26 (1H, d, J = 8.8 Hz, In–H), 7.14 (1H, s, In–H), 7.02 (1H, d, J = 2.1 Hz, In–H), 6.80 (1H, dd, J = 8.9, 2.3 Hz, In–H), 6.24 (2H, s, maleate), 4.32 (2H, s, NB-CH2), 3.81 (3H, s, OCH3), 3.35–3.40 (2H, m, α-CH2), 3.13–3.18 (2H, m, β-CH2). 13C NMR (CD3OD): δ ppm 170.91 (maleate), 155.41 (In-Cq), 136.74 (maleate), 134.85 (Ph–CH), 134.04 (Ph–Cq), 133.60 (In-Cq), 132.56 (Ph-Cq, d, J = 32.3 Hz), 131.24 (Ph–CH), 128.46 (In-Cq), 127.84 (Ph–CH, q, J = 4.0 Hz), 127.46 (Ph–CH, q, J = 3.8 Hz), 125.4 (CF3, q, J = 272 Hz), 125.01 (In-CH), 113.36 (In-CH), 113.06 (In-CH), 109.79 (In-Cq), 101.06 (In-CH), 56.39 (OCH3), 51.63 (NB-CH2), 49.20 (α-CH2), 23.41 (β-CH2). HRMS calculated for C19H20F3N2O [M + H]+, 349.15222; observed [M + H]+, 349.15259
Pharmacology
Receptor Affinity
Receptor affinity values for a panel of human serotonin receptors were obtained for all compounds through the NIMH-sponsored PDSP program (www.pdsp.med.unc.edu). Affinity data from screening are reported in Table 1. Following the initial screen, more detailed values were obtained for affinity at the human 5-HT2A and 5-HT2C receptors using both an antagonist radioligand ([3H]ketanserin for 5-HT2A) and ([3H]mesulergine for 5-HT2C) and an agonist radioligand ([3H]-DOI) for both receptors. Those data are reported in Table 2.
Receptor Efficacy and Potency in the Ca2+ Mobilization Assay
Changes in intracellular Ca2+ levels were measured using a Fluorometric Imaging plate reader (FLIPRTETRA, Molecular Devices), essentially as described in the PDSP (NIMH Psychoactive Drug Screening Program) Assay Protocol Book (www.pdsp.med.unc.edu). PO1C cells stably transfected with r5-HT2C or r5-HT2A receptors, and HEK 293 cells stably transfected with h5-HT2A, h5-HT2B, or h5-HT2C receptors were plated (20,000 cells/well) into poly-l-lysine coated 394-well clear-bottom black-walled microplates (Greiner Bio-one) with 50 μL of media (DMEM media supplemented with 500 μg/mL Geneticin sulfate (G-418), 10% dialyzed fetal bovine serum, and 50 U of penicillin/50 μg of streptomycin) and incubated overnight (37 °C, 5% CO2). The following day, media were replaced with 20 μL of FLIPR Calcium 4 Assay Kit (Molecular Devices) diluted in assay buffer (HBSS, 2.5 mM probenecid, and 20 mM HEPES, pH 7.4–7.8) and incubated for 45 min at 37 °C and 15 min at room temperature. Compounds were initially dissolved in DMSO. The 16-point curves were prepared as 3× serial dilutions for each compound with final concentrations ranging from 10 μM to 0.003 nM. Basal fluorescence was measured for 10 s, then 10 μL of test or control compounds was added followed by continued fluorescence measurement for an additional 120 s. Raw data were normalized to baseline fluorescence (0%) and 5HT at 10 μM (100%), expressed as percent activation, and plotted as a function of molar concentration of test compound using Prism 5.0 (GraphPad Software). These data are reported in Table 3.
Mouse Head Twitch Response
Animals
Male C57BL/6J mice (6–8 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and housed in a vivarium at the University of California, San Diego, an AAALAC-approved animal facility that meets Federal and State requirements for the care and treatment of laboratory animals. Mice were housed up to four per cage in a climate-controlled room with a reversed light-cycle (lights on at 1900 h, off at 0700 h). Food and water were provided ad libitum, except during behavioral testing. Testing was performed between 1000 and 1830 h. Experiments were conducted in accord with NIH guidelines and were approved by the UCSD animal care committee.
Procedures
The HTR was assessed using a head-mounted magnet and a magnetometer detection coil. Mice were anesthetized (100 mg/kg ketamine, 3 mg/kg acepromazine, and 20 mg/kg xylazine, IP), and a neodymium magnet (4.57 × 4.57 × 2.03 mm, 375 mg) was attached to the skull using dental cement. The magnet was positioned so that the N–S axis was parallel to the dorsoventral plane of the head. Mice were allowed to recover for 2 weeks after surgery. HTR experiments were conducted in a well-lit room. Test compounds were dissolved in water containing 5% Tween-80 and administered SC (5 or 10 mL/kg). Mice were injected with drug or vehicle and placed in a glass cylinder surrounded by a magnetometer coil. Head movements were recorded and analyzed for HTR as described previously.23,31 Coil voltage was low-pass filtered (5–10 kHz), amplified, and digitized (40 kHz sampling rate) using a Powerlab/8SP with LabChart v 7.3.2 (ADInstruments, Colorado Springs, CO, USA). The data were filtered off-line (40–200 Hz band-pass), and HTRs were identified by manually searching for sinusoidal wavelets possessing at least two bipolar peaks, spectrum in the 40–160 Hz range, amplitude exceeding the background noise level, and duration <0.15 s, with stable coil voltage during the period immediately before and after each response.
Analysis
HTR counts were analyzed using one-way analyses of variance (ANOVAs). Post-hoc comparisons were made using Tukey’s studentized range method. Significance was demonstrated by surpassing an α-level of 0.05. ED50 values and 95% confidence limits were calculated using nonlinear regression. These data are reported in Table 4.
Author Contributions
D.N. directed the project, synthesized all of the compounds, supervised the integration of the various studies, and was responsible for the writing and final editing of the manuscript, F.S. carried out the calcium mobilization functional assays, A.H. supervised the mouse head twitch assays, L.M.K. assisted with the mouse assays, S.D.B. and S.P.E. carried out the analytical chemistry assays, and W.F. made key suggestions for the project and edited the manuscript.
This work was supported by the NIMH Psychoactive Drug Screening Program, NIMH K01 MH100644, NIDA R01 DA002925, and the Brain and Behavior Research Foundation.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Lawn W.; Barratt M.; Williams M.; Horne A.; Winstock A. (2014) The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample. J. Psychopharmacol. 28, 780–788. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, D. o. J. Schedules of Controlled Substances: Temporary Placement of Three Synthetic Phenethylamines into Schedule I, 21 CFR Part 1308, 2013, [Docket NO. DEA-382].
- Poklis J. L.; Devers K. G.; Arbefeville E. F.; Pearson J. M.; Houston E.; Poklis A. (2014) Postmortem detection of 25I-NBOMe [2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine] in fluids and tissues determined by high performance liquid chromatography with tandem mass spectrometry from a traumatic death. Forensic Sci. Int. 234, e14–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterscheid J. P.; Phillips G. T.; Lopez A. E.; Gonsoulin M. L.; Chen H. H.; Sanchez L. A. (2014) Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am. J. Forensic Med. Pathol. 35, 20–25. [DOI] [PubMed] [Google Scholar]
- Nikolaou P.; Papoutsis I.; Stefanidou M.; Spiliopoulou C.; Athanaselis S. (2015) 2C-I-NBOMe, an “N-bomb” that kills with “Smiles”. Toxicological and legislative aspects. Drug Chem.Toxicol. 38, 113–119. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (2014) Risk Assessment Report of a New Psychoactive Substance: 2-(4-Iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25I-NBOMe), EMCDDA, Lisbon, Portugal. [Google Scholar]
- World Health Organization (2014) Thirty-Sixth Meeting of the Expert Committee on Drug Dependence, World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- (2014) 2014/688/EU: Council implementing decision of 25 December 2014 on subjecting −4-iodo-2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (25I-NBOMe), 3,4-dichloro-N-[[1-(dimethylamino)cyclohexyl]methyl]benzamide (AH-7921), 3,4-methylenedioxypyrovalerone (MDPV) and 2-(3-methoxyphenyl)-2-(ethylamino)cyclohexanone (methoxetamine) to control measures. Off. J. Eur. Union L287, 22. [Google Scholar]
- Shulgin A. T. (1978) Psychotomimetic Drugs: Structure-Activity Relationships, in Handbook of Psychopharmacology, Chapter 6, pp 243–333, Plenum Press, New York. [Google Scholar]
- Shulgin A., and Shulgin A. (1991) PIHKAL: A Chemical Love Story, Transform Press, Berkeley, CA. [Google Scholar]
- Heim R.; Pertz H. H.; Elz S. (1999) Preparation and in vitro pharmacology of novel secondary amine-type 5-HT2A receptor agonists: from submillimolar to subnanomolar activity. Arch. Pharm. Pharm. Med. Chem. 332, 34. [Google Scholar]
- Elz S.; Klass T. H. R.; Wamke U.; Pertz H. H. (2002) Development of highly potent partial agonists and chiral antagonists as tools fo the study of 5-HT2A-receptor mediated functions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 365(Suppl 1), R29. [Google Scholar]
- Heim R. (2003) Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur Entwicklung eines neuen Struktur-Wirkungskonzepts, Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany. [Google Scholar]
- Braden M. R.; Parrish J. C.; Naylor J. C.; Nichols D. E. (2006) Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol. Pharmacol. 70, 1956–1964. [DOI] [PubMed] [Google Scholar]
- Heim R.; Elz S. (2000) Novel extremely potent partial 5-HT2A receptor agonists: successful application of a new structure-activity concept. Arch. Pharm. Pharm. Med. Chem. 333, 39. [Google Scholar]
- Nichols D. E. (2012) Structure-activity relationships of serotonin 5-HT2A agonists. WIREs Membr. Transp. Signaling 1, 559–579. [Google Scholar]
- Braden M. R. (2007) Towards a Biophysical Understanding of Hallucinogen Action, Ph.D. Thesis, Purdue University, West Lafayette, IN. [Google Scholar]
- Silva M. E.; Heim R.; Strasser A.; Elz S.; Dove S. (2011) Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor. J. Comput.-Aided Mol. Des. 25, 51–66. [DOI] [PubMed] [Google Scholar]
- Glennon R. A.; Dukat M.; el Bermawy M.; Law H.; De Los Angeles J.; Teitler M.; King A.; Herrick-Davis K. (1994) Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines. J. Med. Chem. 37, 1929–1935. [DOI] [PubMed] [Google Scholar]
- Takagi K.; Takayanagi I.; Irikura T.; Nishino K.; Ito M. (1969) A potent competitive inhibitor of 5-hydroxytryptamine: 3-(2′-benzylaminoethyl)-5-methoxyindol hydrochloride. Jpn. J. Pharmacol. 19, 234–239. [DOI] [PubMed] [Google Scholar]
- Leff P.; Martin G. R.; Morse J. M. (1986) The classification of peripheral 5-HT2-like receptors using tryptamine agonist and antagonist analogues. Br. J. Pharmacol. 89, 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen N. (2004) Tryptamines as Ligands and Modulators of the Serotonin 5-HT2A Receptor and the Isolation of Aeruginascin from the Hallucinogenic Mushroom Inocybe aeruginascens, Ph.D. Thesis, Georg-August-Universität zu Göttingen, Göttingen, Germany. [Google Scholar]
- Halberstadt A. L.; Geyer M. A. (2013) Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology 227, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. (2010) Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain, Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark. [Google Scholar]
- Casale J. F.; Hays P. A. (2012) Characterization of eleven 2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (NBOMe) derivatives and differentiation from their 3- and 4-methoxybenzyl analogues - Part I. Microgram J. 9, 84–109. [Google Scholar]
- Abdel-Magid A. F.; Carson K. G.; Harrris B. D.; Maryanoff C. A.; Shah R. D. (1996) Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures. J. Org. Chem. 61, 3849–3862. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P.; Aloyo V. J. (2011) Pharmacological and behavioral characterization of the 5-HT2A receptor in C57BL/6N mice. Psychopharmacology 215, 581–593. [DOI] [PubMed] [Google Scholar]
- Braden M. R.; Nichols D. E. (2007) Assessment of the roles of serines 5.43(239) and 5.46(242) for binding and potency of agonist ligands at the human serotonin 5-HT2A receptor. Mol. Pharmacol. 72, 1200–1209. [DOI] [PubMed] [Google Scholar]
- Johnson M. P.; Loncharich R. J.; Baez M.; Nelson D. L. (1994) Species variations in transmembrane region V of the 5-hydroxytryptamine type 2A receptor alter the structure-activity relationship of certain ergolines and tryptamines. Mol. Pharmacol. 45, 277–286. [PubMed] [Google Scholar]
- Johnson M. P.; Audia J. E.; Nissen J. S.; Nelson D. L. (1993) N(1)-substituted ergolines and tryptamines show species differences for the agonist-labeled 5-HT2 receptor. Eur. J. Pharmacol. 239, 111–118. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. (2014) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Harrington A. W.; Eckler J. R.; Arshad S.; Rabin R. A.; Winter J. C.; Coop A.; Rice K. C.; Woods J. H. (2005) Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology 181, 496–503. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Harrington A. W.; Kiessel C. L.; Eckler J. R.; Rabin R. A.; Winter J. C.; Coop A.; Rice K. C.; Woods J. H. (2006) Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol., Biochem. Behav. 83, 122–129. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Simoneau J.; Cohen M. S.; Zimmerman S. M.; Henson C. M.; Rice K. C.; Woods J. H. (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J. Pharmacol. Exp. Ther. 335, 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Gray B. W.; Bailey J. M.; Smith D. A.; Hansen M.; Kristensen J. L.; Woods J. H.. Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylamino]methyl)phenol (25CN-NBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT receptors, in mice. Psychopharmacology (Berl). 2014, not provided [DOI] [PMC free article] [PubMed]
- Bedard P.; Pycock C. J. (1977) "Wet-dog" shake behaviour in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology 16, 663–670. [DOI] [PubMed] [Google Scholar]
- Matthews W. D.; Smith C. D. (1980) Pharmacological profile of a model for central serotonin receptor activation. Life Sci. 26, 1397–1403. [DOI] [PubMed] [Google Scholar]
- Benneyworth M. A.; Xiang Z.; Smith R. L.; Garcia E. E.; Conn P. J.; Sanders-Bush E. (2007) A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 72, 477–484. [DOI] [PubMed] [Google Scholar]
- Canal C. E.; Olaghere da Silva U. B.; Gresch P. J.; Watt E. E.; Sanders-Bush E.; Airey D. C. (2010) The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology 209, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Koedood L.; Powell S. B.; Geyer M. A. (2011) Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 25, 1548–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne S. J.; Pickering R. W. (1967) A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 11, 65–78. [DOI] [PubMed] [Google Scholar]
- Darmani N. A.; Martin B. R.; Glennon R. A. (1990) Withdrawal from chronic treatment with (±)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur. J. Pharmacol. 186, 115–118. [DOI] [PubMed] [Google Scholar]
- Leth-Petersen S.; Bundgaard C.; Hansen M.; Carnerup M. A.; Kehler J.; Kristensen J. L. (2014) Correlating the metabolic stability of psychedelic 5-HT agonists with anecdotal reports of human oral bioavailability. Neurochem. Res. 39, 2018–2023. [DOI] [PubMed] [Google Scholar]
- Juncosa J. I. Jr.; Hansen M.; Bonner L. A.; Cueva J. P.; Maglathlin R.; McCorvy J. D.; Marona-Lewicka D.; Lill M. A.; Nichols D. E. (2013) Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands. ACS Chem. Neurosci. 4, 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M.; Phonekeo K.; Paine J. S.; Leth-Petersen S.; Begtrup M.; Brauner-Osborne H.; Kristensen J. L. (2014) Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem. Neurosci. 5, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U.; Shulgin A. T.; Braun G.; Sargent T. III. (1977) Synthesis and body distribution of several iodine-131 labeled centrally acting drugs. J. Med. Chem. 20, 1543–1546. [DOI] [PubMed] [Google Scholar]