SUMMARY
Normal brain functioning relies critically on the ability to control appropriate behavioral responses to fearful stimuli. Overgeneralized fear is the major symptom of anxiety disorders including posttraumatic stress disorder. This review describes recent data demonstrating that the medial prefrontal cortex (mPFC) plays a critical role in the refining of cues that drive the acquisition of fear response. Recent studies on molecular mechanisms that underlie the role of mPFC in fear discrimination learning are discussed. These studies suggest that prefrontal N-methyl-D-aspartate receptors expressed in excitatory neurons govern fear discrimination learning via a mechanism involving cAMP response element-binding protein–dependent engagement of acetyltransferase.
Keywords: fear discrimination, prefrontal cortex, overgeneralization, NMDA Receptor, CBP
The concepts of fear memory accuracy and generalization reflect the ability of a subject to respond properly to a similar stimulus to that of the trained stimulus presented during fear conditioning procedure. Specifically, the ability to distinguish between these two stimuli indicates the level of fear memory accuracy (discrimination), whereas elevated level of fearful responses to harmless stimuli is an indicative of fear generalization. Fear memory accuracy is critical for survival, while fear generalization is effective for recalling, threat assessment, and avoiding dangerous situations. An extreme or excessive fear of stimuli that are not harmful is referred to as fear overgeneralization. Overgeneralized fear is the major symptom of anxiety disorders including phobia, panic disorders, generalized anxiety disorder,1,2 and posttraumatic stress disorder,3 triggered by secure environment cues resembling those of the traumatic experience. Fear behavior is controlled by adaptive processes including discrimination, generalization, and extinction. These concepts were initially developed by Pavlov4 and have been extensively studied for a century. Discriminatory fear learning involves fear conditioning, which is a form of classical Pavlovian conditioning4 and has become the best studied behavioral model for associative learning and its underlying synaptic and circuit-level plasticity.5–7 Multiple memory systems theory postulates that different types of memory are consolidated via hardwired pathways.8 In tone fear conditioning, tone [conditional stimulus (CS)]-foot shock [unconditional stimulus (US)] associations are directly encoded through synaptic plasticity in the amygdala, which receives direct auditory inputs. During the contextual fear conditioning, the contextual stimulus (CS) is encoded by the dorsal hippocampus (and later consolidated by the hippocampus–prefrontal circuitry), whose outputs are subsequently associated with the US through synaptic plasticity in the amygdala.5–7
The fact that expression of recent and remote long-term fear memories requires the dorsal hippocampus and medial prefrontal cortex (mPFC), respectively,9,10 suggests that the communication between these two brain regions controls transition from a recent state to remote state during system-level memory consolidation. Both regions appear to be engaged in context coding. In fact, context-specific neuronal ensembles were found in both regions.11,12 While the dorsal hippocampus and mPFC appear to track spatial information, the mPFC is likely to integrate contextual recognition of fear–context association with distinctive roles of infralimbic (IL) and prelimbic (PL) subregions of the mPFC.13,14 Fear behavior is differentially regulated by PL and IL of the mPFC15–18 via increase or decrease of fear expression, respectively,18,19 which may be due to differential connectivity with the amygdala (Fig. 1).20–22 This differential role of IL versus PL is of particular interest since, under the Hull-Spence theory of discrimination learning postulates, conditioned excitation (the result of reinforcement) and inhibition (the result of nonreinforcement) are postulated to have generalization gradients.23–25 The algebraic summation of excitatory and inhibitory strength determines the response rate to test stimuli.26 For example, differential conditioning increases unit and field responses to the conditioned stimulus (CS+), reinforced with an electric shock CS+, whereas responses to the second stimulus that was nonreinforced (CS−) decreased.27 Both excitatory and inhibitory neurons in the mPFC play important roles in the regulation of fear responses. For example, direct inhibition of fast-spiking interneurons in the dorsomedial prefrontal cortex disinhibits prefrontal excitatory neurons and promotes fear expression.28 The mPFC can compensate for absence of dorsal hippocampus in contextual fear learning, while IL mPFC lesions enhance generalization of contextual fear and interfere with discriminatory fear learning.29 In addition, the interaction between the thalamus and mPFC has been implicated in the contextual fear generalization30,31 and the retrieval of long-term fear memories.32
Figure 1.
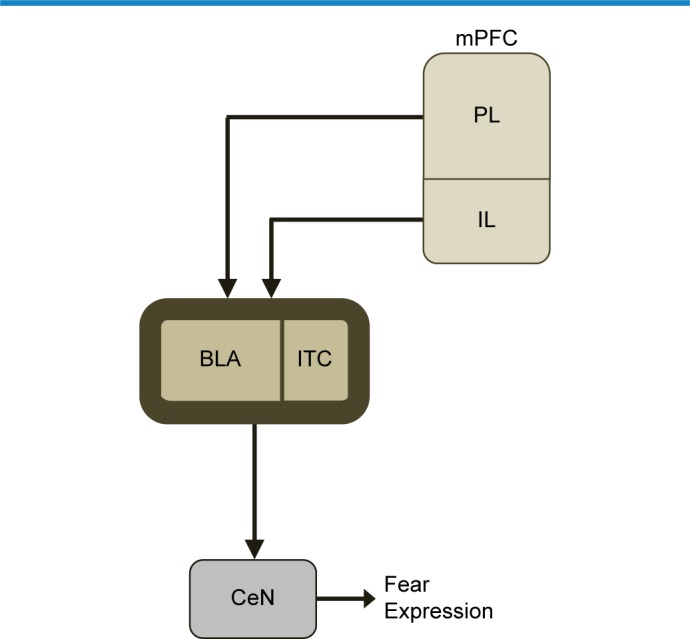
Fear behavior is differentially regulated by IL and PL subregions of the mPFC (see text).
Notes: Complex interactions and circuitry for fear discrimination learning also comprise the basolateral nucleus of the amygdala (BLA), the central nucleus of the amygdala (CeN), and the amygdala intercalated neurons (ITC).
That the mPFC is a locus for gating fear discrimination and danger assessment is supported by additional evidence that includes animal studies in which an mPFC lesion impairs the ability to guide behavior, specifically when memory retrieval resolves conflicting dangerous and harmless contextual cues.33–36 A fear decline is associated with elevated activity in the mPFC as determined by activation of immediate-early genes,37,38 blood oxygenation levels,39 cell firing,40 and local field potentials.41 The mPFC has dense reciprocal anatomical and functional connections with sensory cortices, thalamic sensory relays, and memory systems including the hippocampus (context, multisensory processing) and the amygdala, the critical locus for fear processing. Considerable evidence indicates that neurons in mPFC, basolateral amygdala (BLA), and hip are functionally coupled at the theta range (4–12 Hz oscillations) during fear conditioning,42,43 conditioned extinction,41 and discriminative fear learning.44 Moreover, memory retrieval elicits mPFC neuronal activity patterns reminiscent of neural representations of behavioral contexts that govern successful recollection. Different behavioral contexts evoke distinct firing of neuronal ensembles,12 which can reset during uncertainty following environmental change45 or induce sudden transitions between neural ensemble states accompanied by behavioral transitions.46
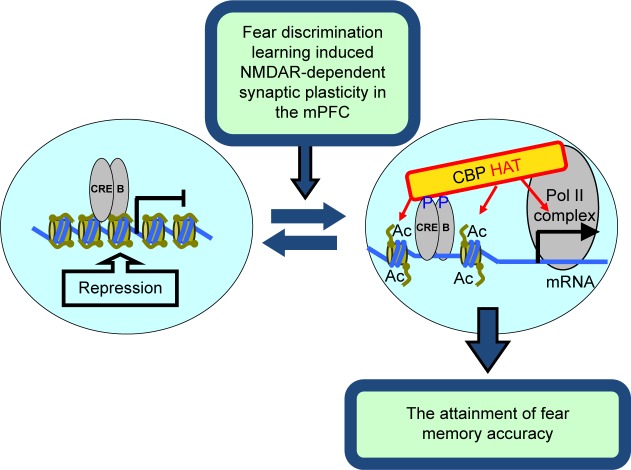
Multiple memory systems theory postulates that different types of memory are consolidated via hardwired pathways;8 however, how memories are integrated47,48 to specific neurons and synapses in a circuit remains unclear. While the circuit-, cellular-, and molecular-level mechanisms of fear extinction and contextual conditioning have been studied in the mPFC, much less is known about how the neural circuitry of the mPFC contributes to fear discriminatory learning. Glutamate, the main excitatory neurotransmitter is critically involved in fear memory.49 In addition, it has been demonstrated that specific glutamate postsynaptic receptors such as N-methyl-D-aspartate receptors (NMDARs) are directly involved in various learning mechanisms including modulation of fear memory.50–53 Furthermore, NMDAR, Ca2+ signaling, transcription factor cAMP response element-binding protein (CREB), and CREB-binding protein’s (CBP’s) intrinsic histone acetyltransferase activity (HAT) have been implicated as putative mechanisms for long-term memory encoding into cortical circuits.54–56 Using fear discrimination learning assays, it was recently demonstrated that NMDAR-, CREB-, and CBP’s HAT-dependent signaling in the mPFC is required for successful fear discrimination learning (Fig. 2).57,58 Thus, fear discrimination is attained via the mPFC-dependent reduction of generalized fear responses to harmless stimuli.57,58 Both selective inhibition of CBP HAT or CREB function in the mPFC circuitry show strong deficit in fear discrimination learning.58 A similar effect was observed in mice with selective deletion of NMDAR from excitatory neurons in the mPFC.57 These data suggest that successful fear discrimination involves prefrontal NMDAR-dependent mechanism governing decline of generalized fear responses to harmless nonreinforced stimuli.
Figure 2.
Model of acetylation-dependent fear discrimination learning.
Notes: In the absence of appropriate stimulation, the target genes are repressed. Recent studies reveal that NMDARs expressed in prefrontal excitatory neurons control the ability to distinguish between dangerous and harmless stimuli via a mechanism that involves CREB-dependent engagement of acetyltransferase. This model suggests that NMDAR-induced plasticity, NMDAR-dependent CREB phosphorylation at Serine-131, and NMDAR-dependent CREB-dependent engagement of CBP acetyltransferase control the consolidation of specific memories required for successful fear discrimination learning.
Abbreviations: Ac, acetylated histone; Pol II complex, Polymerase II complex.
While both CBP and CREB are partners and both are implicated in long-term plasticity and memory consolidation in Aplysia, Drosophila, and mice, CREB has been strongly implicated in adaptive alteration of neuronal excitability and memory allocation48 and it is possible that CBP HAT may mediate CREB-dependent changes in neuronal excitability. Four independent manipulations to downregulate CBP acetyltransferase activity specifically in an adult living brain to avoid development confound have been reported.55,59,60 Histones are believed to be the primary targets for CBP’s HAT activity; however, a number of nonhistone targets for CBP’s HAT activity, which are involved in chromatin remodeling and gene expression regulation, have been discovered.61–67 While the impact of histone and nonhistone protein acetylation by CBP is not fully understood, CBP’s HAT appears to be a critical component of a putative epigenetic mechanism that controls long-term memory.55,60
Recent data indicate that the mPFC plays a critical role in the refining of cues that drive the acquisition of fear response, including some molecular mechanisms that underlie this role. These data are in line with previous work showing that the mPFC supports fear extinction, because fear discrimination involves selective reduction of the response to nonreinforced stimuli, perhaps through interaction between the amygdala, hippocampal system, and mPFC during a consolidation of selective memories. The findings indicating that three components of the molecular mechanism underlying long-term plasticity in the mPFC (NMDAR, CREB and CBP HAT) are directly implicated in appropriate disambiguation of fear signals provide direct evidence that fear discrimination involves long-term memory coding into the prefrontal excitatory circuitry. Modern neuroscience now has the tools to begin to characterize the mechanisms and neural circuits responsible for fear memories remaining distinct and resistant to confusion, and further experiments are needed to reveal what type of information is consolidated in the mPFC that is required for the attainment of fear memory accuracy after initial fear generalization.
Footnotes
ACADEMIC EDITOR: Lora Tally Watts, Editor in Chief
PEER REVIEW: 5 peer reviewers contributed to the peer review report. Reviewers’ reports totaled 854 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by University of California Riverside Collaborative Research Seed Grant and National Institutes of Health grants. The author confirms that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the author was invited to submit this paper
Author Contributions
Conceived and designed the experiments: EK. Analyzed the data: EK. Wrote the first draft of the manuscript: EK. Contributed to the writing of the manuscript: EK. Agree with manuscript results and conclusions: EK. Jointly developed the structure and arguments for the paper: EK. Made critical revisions and approved final version: EK. Author reviewed and approved of the final manuscript.
REFERENCES
- 1.Reinecke A, Becker ES, Hoyer J, Rinck M. Generalized implicit fear associations in generalized anxiety disorder. Depress Anxiety. 2010;27:252–259. doi: 10.1002/da.20662. [DOI] [PubMed] [Google Scholar]
- 2.Gazendam FJ, Kamphuis JH, Kindt M. Deficient safety learning characterizes high trait anxious individuals. Biol Psychol. 2013;92:342–352. doi: 10.1016/j.biopsycho.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlov IP. In: Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Eds ed. Anrep GV, editor. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 6.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 7.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 8.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 9.Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- 10.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 11.Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2012;109:5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 14.Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci. 2014;34:8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtin J, Bienvenu TC, Einarsson EO, Herry C. Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience. 2013;240:219–242. doi: 10.1016/j.neuroscience.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2010;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 21.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 22.Strobel C, Marek R, Gooch HM, Sullivan RK, Sah P. Prefrontal and auditory input to intercalated neurons of the amygdala. Cell Rep. 2015 Mar 5; doi: 10.1016/j.celrep.2015.02.008. Published online. [DOI] [PubMed] [Google Scholar]
- 23.Klein SB. Learning: Principles and Applications. 7 ed. SAGE Publications Ltd; Thousands Oaks, CA: 2014. [Google Scholar]
- 24.Spence KW. The differential response in animals to stimuli varying within a single dimension. Psychol Rev. 1937;44:430. [Google Scholar]
- 25.Hull CL. A Behavior System. New Haven: Yale University Press; 1952. [Google Scholar]
- 26.Hearst E. Discrimination learning as the summation of excitation and inhibition. Science. 1968;162:1303–1306. doi: 10.1126/science.162.3859.1303. [DOI] [PubMed] [Google Scholar]
- 27.Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtin J, Chaudun F, Rozeske RR, et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- 29.Zelikowsky M, Bissiere S, Hast TA, et al. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci U S A. 2013;110:9938–9943. doi: 10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Sudhof TC. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73:990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Do-Monte FH, Quinones-Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519:460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 34.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. J Neurosci. 2007;27:4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- 37.Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- 38.Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 40.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- 43.Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010;107:6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlsson MP, Tervo DG, Karpova AY. Network resets in medial prefrontal cortex mark the onset of behavioral uncertainty. Science. 2012;338:135–139. doi: 10.1126/science.1226518. [DOI] [PubMed] [Google Scholar]
- 46.Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66:438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogerson T, Cai DJ, Frank A, et al. Synaptic tagging during memory allocation. Nat Rev Neurosci. 2014;15:157–169. doi: 10.1038/nrn3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin HC, Mao SC, Su CL, Gean PW. Alterations of excitatory transmission in the lateral amygdala during expression and extinction of fear memory. Int J Neuropsychopharmacol. 2010;13:335–345. doi: 10.1017/S1461145709990678. [DOI] [PubMed] [Google Scholar]
- 50.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 51.Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 52.Davis M. NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci. 2011;13:463–474. doi: 10.31887/DCNS.2011.13.4/mdavis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learn Mem. 2013;20:290–294. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bengtson CP, Bading H. Nuclear calcium signaling. Adv Exp Med Biol. 2012;970:377–405. doi: 10.1007/978-3-7091-0932-8_17. [DOI] [PubMed] [Google Scholar]
- 55.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Limback-Stokin K, Korzus E, Nagaoka-Yasuda R, Mayford M. Nuclear calcium/calmodulin regulates memory consolidation. J Neurosci. 2004;24:10858–10867. doi: 10.1523/JNEUROSCI.1022-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vieira PA, Corches A, Lovelace JW, Westbrook KB, Mendoza M, Korzus E. Prefrontal NMDA receptors expressed in excitatory neurons control fear discrimination and fear extinction. Neurobiol Learn Mem. 2015;119C:52–62. doi: 10.1016/j.nlm.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vieira PA, Lovelace JW, Corches A, Rashid AJ, Josselyn SA, Korzus E. Prefrontal consolidation supports the attainment of fear memory accuracy. Learn Mem. 2014;21:394–405. doi: 10.1101/lm.036087.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrett RM, Malvaez M, Kramar E, et al. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology. 2011;36:1545–1556. doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maddox SA, Watts CS, Schafe GE. p300/CBP histone acetyltransferase activity is required for newly acquired and reactivated fear memories in the lateral amygdala. Learn Mem. 2013;20:109–119. doi: 10.1101/lm.029157.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Q, Hutchins AE, Doyle CM, Lundblad JR, Kwok RP. Acetylation of cAMP-responsive element-binding protein (CREB) by CREB-binding protein enhances CREB-dependent transcription. J Biol Chem. 2003;278:15727–15734. doi: 10.1074/jbc.M300546200. [DOI] [PubMed] [Google Scholar]
- 62.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang XJ. Lysine acetylation and the bromodomain: a new partnership for signaling. Bioessays. 2004;26:1076–1087. doi: 10.1002/bies.20104. [DOI] [PubMed] [Google Scholar]
- 65.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Kimura A, Matsubara K, Horikoshi M. A decade of histone acetylation: marking eukaryotic chromosomes with specific codes. J Biochem. 2005;138:647–662. doi: 10.1093/jb/mvi184. [DOI] [PubMed] [Google Scholar]
- 67.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]