Summary
Aberrant JAK2 signalling plays an important role in the aetiology of myeloproliferative neoplasms (MPNs). JAK2 inhibitors, however, do not readily eliminate neoplastic MPN cells and thus do not induce patient remission. Further understanding JAK2 signalling in MPNs may uncover novel avenues for therapeutic intervention. Recent work has suggested a potential role for cellular cholesterol in the activation of JAK2 by the erythropoietin receptor and in the development of an MPN-like disorder in mice. This study demonstrates for the first time that the MPN-associated JAK2-V617F kinase localizes to lipid rafts and that JAK2-V617F-dependent signalling is inhibited by lipid raft disrupting agents, which target membrane cholesterol, a critical component of rafts. We also show for the first time that statins, 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors, widely used to treat hypercholesterolaemia, induce apoptosis and inhibit JAK2-V617Fdependent cell growth. These cells are more sensitive to statin treatment than non-JAK2-V617F-dependent cells. Importantly, statin treatment inhibited erythropoietin-independent erythroid colony formation of primary cells from MPN patients, but had no effect on erythroid colony formation from healthy individuals. Our study is the first to demonstrate that JAK2-V617F signalling is dependent on lipid rafts and that statins may be effective in a potential therapeutic approach for MPNs.
Keywords: JAK2-V617F, statin, lipid raft, myeloproliferative neoplasms, cholesterol
Somatic mutations in the gene encoding the JAK2 tyrosine kinase are prevalent in myeloproliferative neoplasms (MPNs) (Baxter et al, 2005; James et al, 2005; Kralovics et al, 2005; Levine et al, 2005; Zhao et al, 2005; Scott et al, 2007), a group of haematopoietic stem cell diseases characterized in part by expansion of one or more lineages in the myeloid compartment (Levine et al, 2007). Classical MPNs include polycythaemia vera (PV), essential thrombocythaemia (ET), and primary myelofibrosis (PMF). Patients with PV have a defect in the erythroid lineage, leading to overproduction of red blood cells (RBCs). The main cellular defect in ET lies within the thrombocytic lineage, resulting in an overproduction of platelets. In PMF, excessive blood cell formation in the bone marrow results in fibrosis of the bone marrow, which can impede proper haematopoiesis (Levine et al, 2007). A recurrent activating mutation in JAK2, JAK2-V617F is found in c. 95% of PV patients and about 50% of ET and PMF patients (Oh & Gotlib, 2010). Some JAK2-V617F-negative MPN patients exhibit other mutations that alter JAK2 signalling. These include exon 12 mutations of JAK2, mutations of cytokine receptors that signal through JAK2, and mutations of other proteins that regulate JAK2 function. Importantly, many of these mutations can initiate an MPN-like syndrome in mouse models (Oh & Gotlib, 2010). Collectively, these data suggest that JAK2 dysregulation contributes to MPN formation.
While JAK2 inhibitors have had significant success in recent clinical trials due to their ability to reduce constitutional symptoms and thus relieve suffering of patients, they have not readily reduced the allele burden and thus do not induce remission in patients (Scherber & Mesa, 2011; Tefferi, 2012). Thus, alternative therapeutic approaches that enhance neoplastic cell killing are still needed for MPN patients. Further understanding the regulation of JAK2 signalling in MPN cells may uncover additional sites of potential therapeutic intervention that may be effective at treating MPNs.
JAK2 normally functions in signal transduction initiated by cytokine receptor activation. JAK2 associates with cytokine receptors and becomes activated following cytokine receptor stimulation by ligand (Jatiani et al, 2010). Cytokine binding to its receptor causes a conformational change in receptor-associated JAK2 proteins, which then trans-phosphorylate each other leading to their full activation (Lu et al, 2008). Activated JAKs then phosphorylate the cytokine receptor, creating binding sites for downstream mediators like signal transducer and activator of transcription (STAT) molecules. STATs are then phosphorylated on tyrosines by activated JAKs (Jatiani et al, 2010). Phosphorylated STATs function as transcription factors, promoting expression of genes involved in growth, survival, differentiation, etc. In the case of JAK2-V617F, the phenylalanine to valine substitution at amino acid residue 617, allows for dysregulated kinase activity through loss of an autoregulatory function of the pseudokinase domain (Lee et al, 2009). This mutation effectively leaves JAK2 primed for activation by circumventing the need for a conformational change of the kinase induced by cytokine receptor stimulation. However, even though JAK2-V617F does not require cytokine stimulation to be activated, a cytokine receptor is still necessary for JAK2-V617F-mediated signalling and cell transformation (Lu et al, 2005, 2008). Thus, it is thought that cytokine receptors provide a scaffolding function for JAK2-V617F-initiated signalling.
Erythropoietin receptor (EpoR) uses JAK2 to transduce signals initiated by erythropoietin (Epo) to promote RBC production (Witthuhn et al, 1993). We have recently shown that wild-type EpoR/JAK2 signalling requires lipid rafts (McGraw et al, 2012). Lipid rafts are microdomains of the plasma membrane that are enriched in cholesterol and sphingolipids (Galbiati et al, 2001). These microdomains are more rigid than the majority of the plasma membrane and have been shown to function in membrane trafficking, cytoskeletal arrangement (Simons & Ikonen, 1997), virus entry (Scheiffele et al, 1999; Waheed & Freed, 2009), and cellular signalling (Simons & Toomre, 2000). Protein compartmentalization in membrane rafts facilitates protein interactions that regulate signal transduction activation, especially for some receptor-initiated signals at the cell surface (Simons & Toomre, 2000).
While we have shown that wildtype EpoR/JAK2 signalling requires membrane rafts for proper signalling (McGraw et al, 2012), the role of cholesterol and membrane rafts in pathological signalling by JAK2-V617F in MPNs has never been reported, and this is what we explored in this study. We show for the first time that JAK2-V617F is localized to lipid rafts and JAK2-V617F-dependent signalling requires membrane cholesterol. By utilizing JAK2-V617F-dependent MPN model cell lines as well as primary cells from JAK2-V617F-positive MPN patients, we also show that JAK2-V617F-mediated transformation is sensitive to statins, inhibitors of the cholesterol-producing mevalonate pathway. Our data showing the requirement of cholesterol for JAK2-V617F-mediated signalling and the sensitivity of MPN cells to statins suggests that statins could potentially be incorporated into a therapeutic strategy for MPNs.
Materials and methods
Immunofluorescence studies
HEL cells were treated with 10 mmol/l methyl-β-cyclodextrin (MBCD; Sigma-Aldrich, St Louis, MO, USA) for 30 min at 37°C and 5% CO2. Cells (2 × 106) were washed with chilled RPMI medium supplemented with 10% fetal bovine serum (FBS), followed by a 10-min incubation with 1 μg/ml cholera toxin B (CTB)-conjugate (Vybrant Lipid Raft Labeling Kit, Life Technologies, Carlsbad, CA, USA) in chilled RPMI/10% FBS for 10 min at 4°C. Cells were washed three times with chilled phosphate-buffered saline (PBS). For experiments in which Imgenex Corp. (San Diego, CA, USA) JAK2 antibody was utilized, anti-CTB antibody (Vybrant Alexa Fluor 594 Lipid Raft Labeling kit, component B) was then added for 10 min at 4°C. Cells (2·5–5 × 104) were cytospun onto glass microscopes slides, fixed using Cytofix Fixation Buffer (BD Biosciences, San Jose, CA, USA) for 10 min at 37°C and washed with room temperature (RT) PBS. Cells were then permeabilized for 5 min using 2 drops of 0·5% Triton X-100 in PBS. Slides were washed using RT PBS and subsequently blocked with 2% BSA in PBS for 30 min at RT and washed with RT PBS. Primary antibody incubation followed, using a 1:200 dilution (in 2% BSA/PBS) for JAK2 (D2E12, Cell Signaling Technology Inc., Boston, MA, USA) or (IMG-3007, Imgenex, Corp.) overnight at 4°C. Slides were washed with RT PBS. Secondary antibody for JAK2 ensued using Alexa® Fluor 488 goat-anti-rabbit (Life Technologies, #A11008) at 1:500 dilution (in 2% BSA/PBS) for 1 h at RT for the JAK2 (Cell Signaling Technology Inc.) primary, or DyLight 488 Conjugate donkey-anti goat secondary antibody (705-486147, Jackson Immunoresearch Laboratories, West Grove, PA, USA) at a 1:500 dilution for JAK2 (Imgenex, Corp.) primary. Slides were washed with RT PBS. Mounting media (ProLong® Gold antifade reagent with DAPI, Life Technologies ) was added to each slide and covered with a cover slip. Confocal microscopy with a Leica TCS SP5 AOBS laser scanning confocal microscope (Leica Microsystems, Mannheim, Germany) was used to image cells as previously described (McGraw et al, 2012). Definiens Developer version 1.5 (Definiens AG, Munich, Germany) was used to perform Pearson’s Correlation analysis for colocalization between lipid raft and JAK2 staining on an average of 102 cells per image of four or five images per sample. Briefly, the software was used to first segment lipid raft staining areas within each cell and then perform the colocalization analysis on each pixel within these areas.
Detergent-resistant membrane (DRM) isolation
SET-2 cells (12·5 × 106) were washed 3 times with chilled PBS and lysed in 250 μl of 0·75% Triton X-100 in TNE (25 mmol/l Tris pH 7, 150 mmol/l EDTA, 1 mmol/l dithiothreitol, 150 mmol/l NaCl) plus protease and phosphatase inhibitors (1 mmol/l sodium vanadate, 10 μg/ml leupeptin, 2 mmol/l sodium pyrophosphate, 2 μg/ml aprotinin, 1 mmol/l phenylmethylsulfonyl fluoride) and incubated on ice for 5 min. Cell lysate was sonicated three times for 10 s each (FS60 Fisher Scientific Sonicator, Thermo Scientific, Suwanee, GA, USA). Lysate (200 μl) was added to 400 μl of 60% OptiPrep™ (Sigma-Aldrich ) in TNE buffer, and this mixture was loaded into ultra clear ultracentrifuge tubes. Lower density OptiPrep™ solutions were loaded on top of 40% layer in decreasing order, 35%, 30%, 25%, 20% and 0%, final volume of 600 μl per layer. Samples were spun at 20 000 × g for 20 h at 4°C (Beckman Coulter Optima L-90K ultracentrifuge). Fractions (600 μl) were then removed from top to bottom of each gradient.
GM1 dot blots
Aliquots (5 μl) of each gradient fraction were dotted on nitrocellulose membrane, allowed to dry, and the membrane was washed with PBS. Membranes were blocked in 5% non-fat dry milk/PBS for 30 min at RT. GM1 detection in dot blots was performed using horseradish peroxidase conjugated CTB (Sigma-Aldrich, C3741) at a 1:10 000 dilution in 5% non-fat dry milk/PBS, and incubated overnight at 4°C. Dot blots were washed 3 times with 0·3% Tween-20/PBS and developed with ECL Plus (Thermo Scientific ).
Immunoblotting
For DRM experiments, 50 μl of fractions were analysed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For signalling studies, 2–5 × 106 cells were washed with chilled PBS and lysed in lysis buffer (25 mmol/l Tris, pH 7·4, 150 mmol/l NaCl, 25 mmol/l NaF, 1% Triton X-100, plus protease and phosphatase inhibitors). Lysed cells were centrifuged at 14 500 × g for 5 min at 4°C. Protein concentration was determined using Pierce BCA reagent (Thermo Scientific) and lysates run on SDS-PAGE. Primary antibodies utilized for immunoblotting included: JAK2 (Cell Signaling Technology Inc., #3230), phospho-(P-) JAK2 (pY-1007/1008; Santa Cruz Biotechnology, Santa Cruz, CA, USA, sc-16566), P-STAT5 (pY694; BD Biosciences, #611964), STAT5 (Santa Cruz Biotechnology, sc-835), Hsp90 (Santa Cruz Biotechnology, sc-7947), P-ERK (pT202/Y204; Cell Signaling Technology Inc., #4370), ERK (Santa Cruz Biotechnology, sc-93), P-AKT (pS473; Cell Signaling Technology Inc., #4060), AKT (Santa Cruz Biotechnology, sc-8312), and Flotillin-1 (Cell Signaling Technology Inc., #3253). Secondary antibodies were from Thermo Scientific. Immunoprecipitation experiments were done using JAK2 antibodies (Cell Signaling Technology Inc., #3230) and Protein-A agarose (Thermo Scientific). All blots were developed using West Pico Chemilluminescence, ECL Plus, or Super Signal West Femto Chemilluminescence (Thermo Scientific).
Cell growth curves
HEL or SET-2 cells were plated at 0·15 or 0·2 × 106 cells/ml and treated with dimethyl sulfoxide (DMSO) or simvastatin (Sigma-Aldrich, #S6196 ). DMSO content was kept constant at 0·1% for all samples. Total cells and viability were determined by trypan blue exclusion.
Annexin V staining
HEL cells (1 × 106) were treated with 1 or 5 μmol/l simvastatin for 24 and 48 h. Cells were washed with PBS and resuspended in 100 μl 5% BSA in PBS. Fifty μl of cells were added to 50 μl 2× Annexin V Binding Buffer (BD Biosciences ) and 11 μl staining solution (8 μl of 10ug/ml propidium iodide (BD Biosciences) plus 3 μl Annexin V-fluorescein isothiocyanate (FITC) (BD Biosciences). Cells and staining solution were incubated for 15 min at RT, followed by addition of 300 μl of 1× Annexin V Binding Buffer. Samples were analysed by flow cytometry.
Cholesterol measurement
Cells (2 × 106) were treated with 1 or 5 μmol/l simvastatin for 96 h. Non-viable cells were removed by ficoll centrifugation. Cholesterol was measured using Amplex Red Cholesterol Assay kit (Life Technologies ), per manufacturer’s directions. Fluorescence was measured on a Synergy HT fluorometer (Bio Tek Inc., Winooski, VT, USA) using 560/590 excitation/emission settings.
Colony formation assay
Peripheral blood mononuclear cells (MNCs) were isolated by ficoll separation. Cells (0·5–1 × 105) were then plated in methylcellulose containing recombinant human (rh) stem cell factor, rh interleukin 3, and rh granulocyte-macrophage colony-stimulating factor (Stem Cell Technologies, #H4534), with DMSO (0·1%) or 5 μmol/l simvastatin. For healthy controls, Epo was included at 3 u/ml. Cells were incubated for 12 d at 37°C with 5% CO2. Erythroid burst-forming unit (BFU-E) colonies were enumerated. All patients samples were obtained and utilized under informed consent through a Moffitt Cancer Center Scientific Review Committee approved protocol.
Results
JAK2-V617F co-localizes with lipid rafts
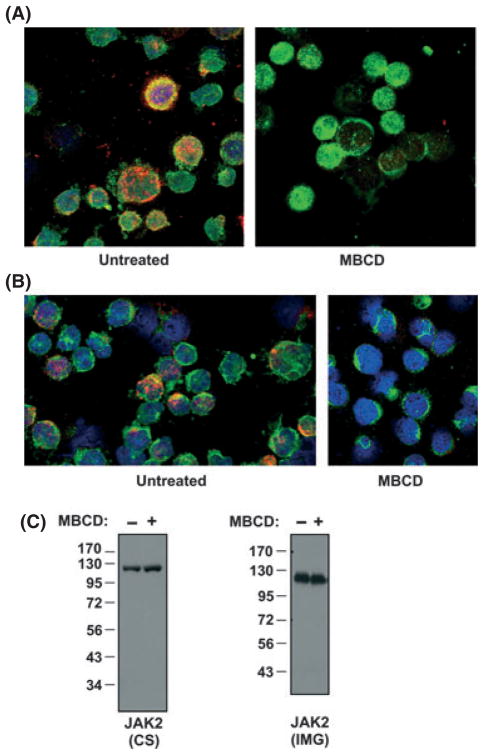
HEL and SET-2 cells are widely used as MPN model cell lines to study JAK2-V617F-mediated transformation in MPNs. Each of these patient-derived cell lines expresses endogenous JAK2-V617F and requires this activated JAK2 for growth (Walz et al, 2006; Jedidi et al, 2009). We first assessed if JAK2-V617F co-localized with lipid rafts in cells by utilizing immunofluorescence. We stained HEL cells, which are homozygous for JAK2-V617F (Quentmeier et al, 2006), for GM1 ganglioside (red fluorescence), a lipid raft-associating lipid and JAK2 (green fluorescence) and used single z plane images from confocal microscopy to visualize localization. Yellow in HEL cell images represented green and red fluorescence overlap, suggesting JAK2 co-localization with lipid rafts (Fig 1A). The lipid raft disrupting agent MBCD acts by binding to cholesterol and removing it from the membrane (Ostermeyer et al, 1999). When HEL cells were treated with MBCD and stained for JAK2 and lipid rafts, single z plane images from confocal microscopy showed disruption of red staining, indicative of lipid raft disruption and thus confirming our raft staining (Fig 1A). To reduce the possibility of false-positive staining, we used a second JAK2 antibody and obtained similar results (Fig 1B). To ensure the antibodies used in staining JAK2 in Fig 1A and 1B were specific for JAK2, we immunoblotted HEL cell lysates with the same JAK2 antibodies (Cell Signaling Technology, Inc. used in Fig 1A and Imgenex, Corp. in Fig 1B). Only a single band at the expected molecular weight for JAK2 (c. 125 kDa) was detected, demonstrating the JAK2 specificity of the antibodies (Fig 1C). These same two JAK2 antibodies were also used in a recent study that utilized immunofluorescence to study JAK2 subcellular localization in MPN cells (Dawson et al, 2009).
Fig. 1.
Mutant JAK2 co-localizes with lipid rafts in JAK2-V617F-positive cells. (A) HEL cells, untreated (left) or treated with MBCD (10 mmol/l, 30 min, right), were stained with antibodies that recognize JAK2 (Cell Signaling Technology Inc.) (green) and lipid rafts were detected by CTB, which binds to the lipid raft lipid GM1 ganglioside (red). Co-localization is demonstrated by merging green and red images, creating yellow. 4′, 6-diamidino-2-phenylindole (DAPI) was used to stain the nucleus (blue). Cells were analysed by confocal microscopy and single z plane images are shown. (B) The experiment in (a) was repeated using a different JAK2 antibody (Imgenex Corp.) (C) Immunoblot analyses showing total JAK2 expression in HEL cells using the two different JAK2 antibodies, Cell Signaling Technology Inc. (CS) (left blot) and Imgenex Corp. (IMG) (right blot), used in (A) and (B) respectively, are shown. Molecular weights are indicated in kDa. Immunoblots demonstrate antibody specificity to JAK2 (C. 125 kDa).
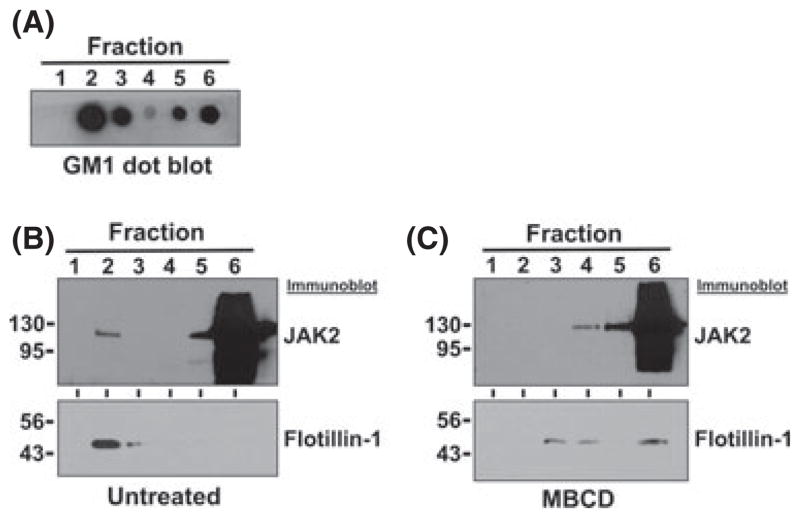
We next employed a second method to detect the presence of JAK2V617F protein in lipid rafts. Lipid rafts are resistant to Triton X-100 solubilization and are referred to as DRMs because of these properties. They can be isolated by ultra-centrifugation based on their differential buoyant density compared to other membranes and cellular constituents (Simons & Toomre, 2000). We utilized an iodixanol gradient to isolate DRMs from Triton X-100 solubilized SET-2 whole cell lysate. After centrifugation, fractions were removed from the top of the gradient, resulting in lower density fractions being present in the lower numbered fractions. Equal volumes of each fraction were analysed by dot blot analysis to identify the fractions containing the resident raft lipid marker GM1 ganglioside (Fig 2A). GM1 was detected predominantly in fraction 2, as well as in fractions 3, 5, and 6. Separation of GM1 between fractions 3 (lower buoyant density) and 5 (higher buoyant density) suggests that DRMs separated from the whole cell lysate (fractions 5 and 6), and identifies the lower buoyant density fractions as raft-containing fractions (fractions 2 and 3) (Fig 2A). We then analysed each fraction by immunoblotting for JAK2. Although the majority of JAK2 was present in the lower/cell lysate fraction (fraction 6), JAK2 was detectable in the raft fraction (fraction 2) in the untreated SET-2 cells (Fig 2C). A resident raft protein, Flotillin-1 was primarily detected in fraction 2, supporting our raft fraction designation. However, when SET-2 cells were treated with MBCD, JAK2 was no longer found in the lower buoyant density fraction 2, but solely in the higher buoyant density non-raft fractions (higher numbered fractions), suggesting that raft disruption by MBCD altered JAK2 protein localization (Fig 2C). MBCD treatment also shifted the raft marker Flotillin-1 from raft fractions to higher density buoyant fractions (higher fraction numbers), demonstrating effective disruption of lipid rafts (Fig 2C). Based on our immunofluorescence and DRM isolation data, we conclude that JAK2-V617F can be detected in lipid rafts, and the lipid raft-disrupting agent, MBCD, can abrogate this sub-cellular localization.
Fig. 2.
JAK2 is present in fractions containing detergent resistant membranes. (A) SET-2 cell lysates were analysed by density buoyant gradient fractionation. Fractions were removed from the top of the gradient and thus lower fraction numbers correspond to lower buoyant density fractions. Each fraction was analysed by dot blot for GM1 ganglioside, a resident raft lipid, using CTB as a probe. Separation of GM1 into lower and higher buoyant fractions suggests separation of DRMs. Highest detection of GM1 is present in fraction 2, thus designating fraction 2 as the raft fraction. (B) Immunoblot analyses of gradient fractions of untreated cells to detect JAK2 or the resident lipid raft marker, Flotillin-1. (C) Immunoblot analyses for JAK2 and Flotillin-1 as in (B), but utilizing MBCD-treated SET-2 cells. MBCD treatment redistributed JAK2 and Flotillin-1 to the higher density non-raft fractions. Molecular weights are indicated in kDa.
Lipid raft disrupting agents downregulate JAK2/STAT5 activation in JAK2V617F-dependent cell lines
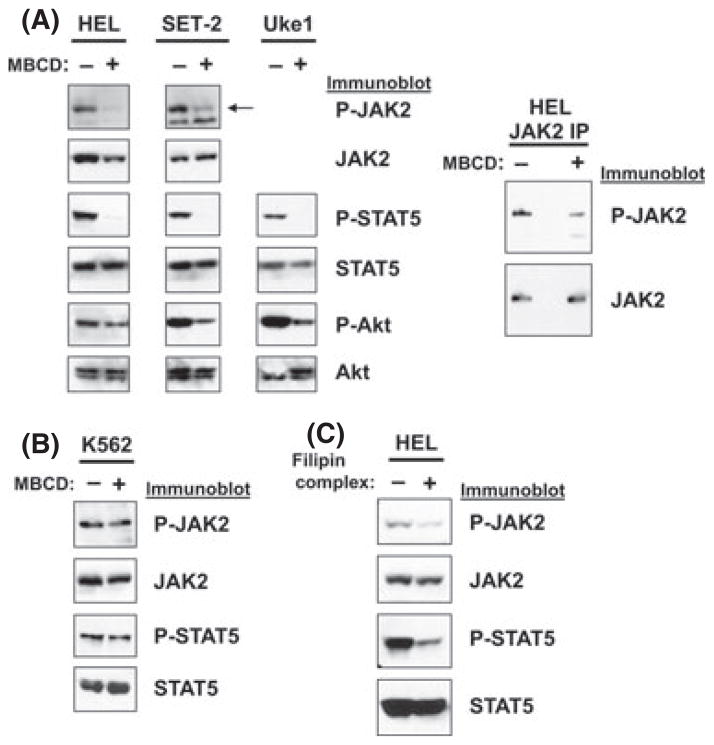
Because JAK2-V617F localization in lipid rafts was abrogated by the raft-disrupting agent, MBCD, we next investigated the effect lipid raft disrupting agents had on signalling induced by JAK2-V617F. To test this we treated the JAK2-V617F-dependent patient-derived cell lines HEL, SET-2, and Uke1 with lipid raft disrupting agents, which function by affecting membrane cholesterol. These cell lines express JAK2-V617F and JAK2 signalling, including STAT5 activation, is dependent on JAK2-V617F (Jedidi et al, 2009). MBCD treatment of HEL cells decreased P-JAK2 as shown by immunoblotting for P-JAK2 in total cell lysates (Fig 3A) and in JAK2 immunoprecipitations (Fig 3A, right panel). Activation/phosphorylation of STAT5 was effectively eliminated by MBCD treatment. Additionally, MBCD treatment resulted in a marginal decrease in P-Akt, another downstream effector of JAK2-V617F activity, in HEL cells (Fig 3A). Similar results were seen in SET-2 cells where MBCD treatment significantly decreased P-JAK2, P-STAT5, and P-Akt (Fig 3A). Likewise, MBCD treatment decreased P-STAT5 and P-Akt levels in Uke1 cells (Fig 3A). To test the effect of lipid raft disruption in a non-JAK2-V617F transformed myeloid cell line, we treated K562 cells, which display constitutive JAK2/STAT5 signalling due to the activated BCR-ABL1 tyrosine kinase, with MBCD. MBCD treatment did not significantly affect activation of JAK2 or STAT5 in K562 cells (Fig 3B),
Fig. 3.
Lipid raft disrupting agents downregulate signalling in JAK2-V617F-dependent MPN model cell lines. (A) The JAK2-V617F-dependent cell lines HEL, SET-2, and Uke1 were left untreated (−) or were treated with MBCD (10 mmol/l) for 30 min (+). Lysates were analysed by immunoblotting with antibodies that recognize phosphorylated/activated (P-) JAK2, P-STAT5, and P-Akt, as well as total JAK2, STAT5, and Akt, as indicated. Arrow indicates mobility of JAK2 (125 kDa) in SET-2 cells. (B) K562 cells, a BCR-ABL1-positive CML cell line that has wild-type JAK2 but constitutive JAK2/STAT5 activation, were left untreated (−) or were treated with MBCD (+) as in (A) and analysed by immunoblotting with antibodies that recognize P-JAK2, JAK2, P-STAT5, and STAT5, as indicated. (C) HEL cells were left unreated (−) or were treated with filipin complex (1 μg/ml) for 15 min (+). Lysates were analysed by immunoblotting with antibodies that recognize P-JAK2, JAK2, PSTAT5, and STAT5 as indicated.
Filipin complex is a lipid raft disrupting agent that functions through a different mechanism than MBCD. While MBCD removes cholesterol from the membrane, filipin complex binds to cholesterol in the membrane thereby interfering with proper lipid raft integrity (Brown, 2006). Filipin complex is a weaker lipid raft disruptor than MBCD (Awasthi-Kalia et al, 2001; Monastyrskaya et al, 2005). Filipin complex treatment of HEL cells also led to a decrease in JAK2/STAT5 activation, with a modest effect on P-JAK2 but a significant reduction of P-STAT5 (Fig 3C).
Statins inhibit growth and viability of JAK2-V617F-dependent cells
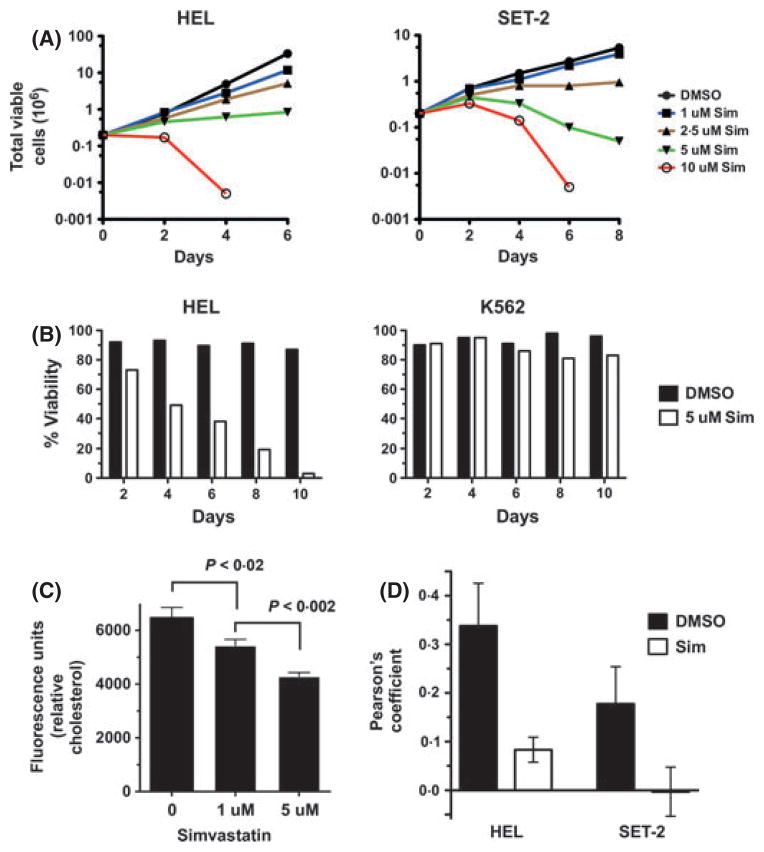
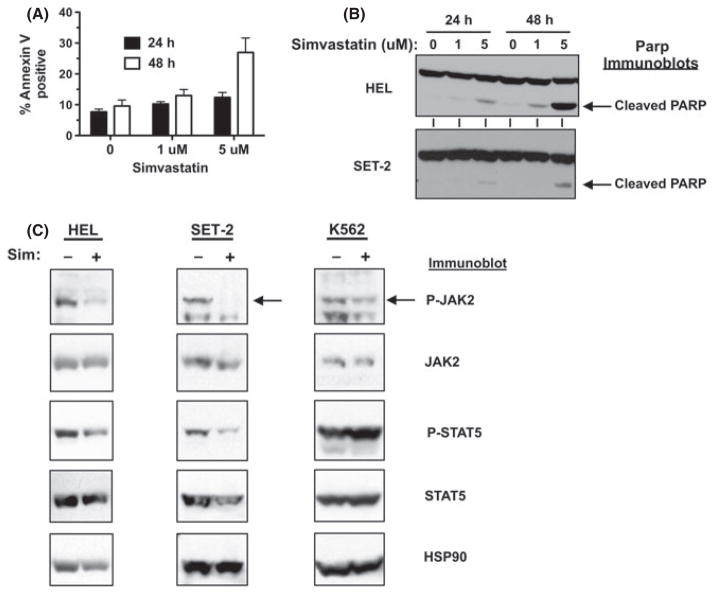
HEL, SET-2, and Uke1 cells require JAK2-V617F signalling for growth (Walz et al, 2006; Jedidi et al, 2009) and our data indicates lipid raft disruption has a negative effect on JAK2-V617F-dependent signalling (Fig 3). We next wanted to disrupt JAK2-V617F localization in lipid rafts in a longer term of study in order to analyse effects on growth and survival of these JAK2-V617F dependent cells. Statins inhibit the rate-limiting enzyme, HMG-CoA reductase, in the mevalonate pathway, which leads to cholesterol biosynthesis (Weng et al, 2010) and can also be used to alter cholesterol-rich lipid rafts (Allen et al, 2007). In addition, statins have been shown to alter the localization of cytokine receptors (e.g. EpoR) to the plasma membrane, which could also affect JAK2-dependent signalling in lipid rafts (Hamadmad & Hohl, 2007). Simvastatin treatment of HEL cells, as well as SET-2 cells, led to a dose-dependent reduction in total viable cell numbers over time (Fig 4A). Similar results were seen with lovastatin and atorvastatin (data not shown). Simvastatin treatment reduced the viability of HEL cells, while the viability of K562 cells was not significantly affected (Fig 4B). Given that we utilized statins to target cholesterol, we wanted to confirm cholesterol levels were indeed affected. We determined that the low dose of simvastatin utilized in this study did indeed decrease cholesterol levels in HEL cells (Fig 4C), with 5 uM simvastatin reducing cellular cholesterol by c. 34% after 4 d of treatment. Similarly, cholesterol reduction was observed with lovastatin and atorvastatin treatment (not shown). Finally, statin treatment inhibited the localization of JAK2-V617F to lipid rafts as determined by immunofluorescence (Fig 4D), however it did not disrupt lipid raft formation (data not shown).
Fig. 4.
Simvastatin reduces JAK2-V617F-dependent cell viability and growth. (A) HEL (left graph) or SET-2 (right graph) cells were treated with 0 (0·1% dimethyl sulfoxide, DMSO) to 10 μmol/l simvastatin (Sim). Trypan blue exclusion was used to enumerate total viable cells over time. (B) Percent viability of HEL (left graph) and K562 (right graph) cells was determined by trypan blue exclusion over time following either DMSO (0·1%) or 5 μmol/l simvastatin (Sim) treatment. Data shown is representative of three independent experiments. (C) Cholesterol was measured in HEL cells after 4 d of 0 (DMSO, 0·1%), 1 μmol/l, and 5 μmol/l simvastatin treatment. Error bars indicate standard deviation and p value was determined by T-test (GRAPHPAD Software, Inc.). This experiment is representative of three independent experiments. (D) Colocalization of JAK2-V617F and lipid rafts in HEL and SET-2 cells was performed as in Fig 1A and Pearson’s correlation analysis for colocalization was determined using Definiens Developer software. Data represent the average (with standard deviation) correlation coefficient for four to five images for each condition for each cell line (see methods).
Simvastatin induces apoptosis and downregulates JAK2/STAT5 activation in JAK2-V617F-dependent cell lines
As we observed a decrease in cell viability with statin treatment, we next assessed if statins could induce apoptosis of JAK2-V617F-dependent cell lines. Simvastatin treatment (5 μmol/l) induced apoptosis of HEL cells as measured by annexin V staining (Fig 5A). Poly (ADP-ribose) polymerase (PARP) cleavage after simvastatin treatment of HEL cells for 24 and 48 h also demonstrated simvastatin-induced apoptosis in a dose and time-dependent manner, even at the very low dose of 1 μmol/l (Fig 5B). Induction of PARP cleavage was also seen in SET-2 cells treated with simvastatin (Fig 5B). Simvastatin treatment of HEL cells reduced the activated/phosphorylated levels of JAK2 and, to a less significant extent, STAT5 (Fig 5C). Similar results were seen in SET-2 cells (Fig 5C). However, JAK2/STAT5 activation in K562 cells was less sensitive to simvastatin treatment than in JAK2-V617F-dependent cells (Fig 5C).
Fig. 5.
Simvastatin induces apoptosis and downregulates JAK2/STAT5 activation in JAK2-V617F-dependent cells. (A) HEL cells were treated with 0 (DMSO, 0·1%), 1 μmol/l, and 5 μmol/l simvastatin for 24 and 48 h and stained with Annexin V and analysed by flow cytometry to detect Annexin V-positive cells. Error bars indicate standard deviation of triplicate samples. This experiment was performed four times with similar results. (B) Immunoblot analysis to detect PARP cleavage after 0 (DMSO, 0·1%), 1 μmol/l, and 5 μmol/l simvastatin treatment of HEL (top blot) and SET-2 (bottom blot) cells for 24 and 48 h. Arrows indicate migration of cleaved PARP. (C) HEL, SET-2, and K562 cells were treated with 5 μmol/l simvastatin for 4 d and cellular lysates were analysed by immunoblotting for P-JAK2, JAK2, P-STAT5, STAT5, and HSP90 (as an additional loading control), as indicated.
Simvastatin inhibits Primary MPN Cell Growth
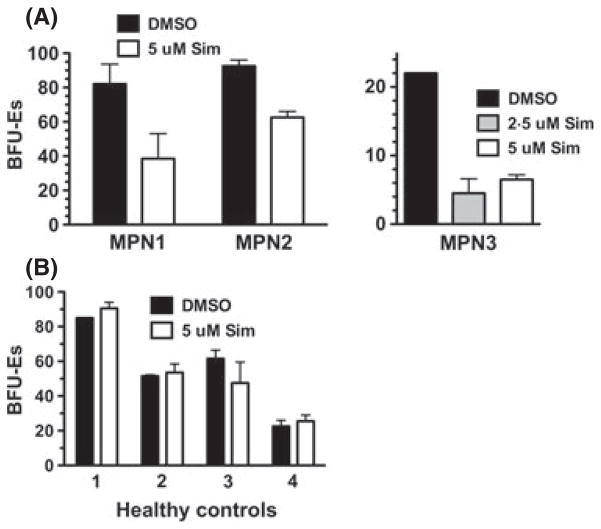
Haematopoietic progenitor cells from MPN patients form erythroid colonies in methylcellulose medium lacking Epo (Prchal & Axelrad, 1974). To test the effects of statin treatment on primary MPN cells we performed colony formation assay using mononuclear cells (MNCs) from peripheral blood of two JAK2-V617F-positive MPN patients. Thus, JAK2-V617F-positive erythroid progenitors will proliferate and differentiate to form erythroid colonies in the absence of Epo. Simvastatin reduced Epo-independent erthyroid colony formation of cells from three independent MPN patients tested (Fig 6A). These patients included a JAK2-V617F-positive PV patient (MPN 1), a JAK2-V617F-positive MF patient (MPN 2), and a JAK2-V617F-positive post-PV/MF patient (MPN 3). Inhibition of colony formation was seen with 5 μmol/l simvastatin. We utilized simvastatin at 2·5 μmol/l in the experiment with MPN 3 and this lower dose inhibited colony formation to a similar extent (c. 75%). Similar experiments performed with cells from normal healthy controls (n = 4) suggested erythroid colony formation from normal progenitor cells is unaffected by statin treatment at the same dose that showed efficacy at reducing colony formation of cells from MPN patients (Fig 6B).
Fig. 6.
Simvastatin reduces erythroid colony formation of primary MPN cells. (A) Colony formation assay performed using mononuclear cells (MNCs) isolated from peripheral blood of MPN patients (n = 3). MNCs were plated in cytokine-containing methylcellulose medium without Epo and Epo-independent erythroid colonies [as erythroid burst-forming units (BFU-Es)] were enumerated after 12 d of incubation. The experiment was performed with either 0·1% DMSO, 2·5 μmol/l simvastatin (Sim) or 5 μmol/l simvastatin in the medium, as indicated. Data is presented as number of BFU-Es per 105 cells plated. MPN Patients 1, 2 and 3 are a JAK2-V617F-positive PV patient, a JAK2-V617F-positive MF patient, and a JAK2-V617F-positive post-PV/MF patient, respectively. (B) The same experiment as in (A) was performed with cells from healthy controls (n = 4) and with erythropoietin in the medium. All error bars represent the standard deviation of replicate plates.
Discussion
JAK inhibitor therapy was recently approved for the treatment of MF patients. JAK inhibitors have proven to be effective at improving constitutional symptoms and reducing spleen size in MPN patients. However, they do not appreciably decrease disease allele burden and thus do not induce remission in patients (Pardanani et al, 2011; Tefferi, 2012). JAK inhibitors can block the aberrant JAK2 and JAK1 signalling induced by the cytokine storm associated with MPNs, and this may be the basis for improvement in MPN patients’ constitutional symptoms (Pardanani et al, 2011; Tefferi, 2012). With the inability of JAK inhibitors to decrease the allele burden in MPN patients, exploration of alternative therapeutic approaches for MPN patients continues.
We initiated our studies to further our understanding of the requirements for JAK2-V617F-mediated signal transduction in an effort to uncover novel avenues for therapeutic intervention for MPNs. We recently demonstrated that EpoR/JAK2 signalling requires lipid raft formation (McGraw et al, 2012) and thus wanted to determine the potential role of lipid rafts in deregulated JAK2 signalling in MPNs. While previous studies support the notion that JAK2 functions in lipid rafts (Sehgal, 2003; McGraw et al, 2012), our studies are the first to demonstrate that the MPN driver JAK2-V617F co-localizes with lipid rafts (Figs 1 and 2). Localization of this tyrosine kinase to lipid rafts is not seen in all cells, largely because not all cells exhibit raft staining (Fig 1). This may be due to the dynamic nature of lipid rafts, which is influenced by factors such as variability in raft size and half-life (Harder & Simons, 1997; Kurzchalia & Parton, 1999; Pralle et al, 2000; Edidin, 2001; Anderson & Jacobson, 2002). In addition, only a minor fraction of JAK2 was associated with DRMs. This is not surprising for multiple reasons. First, rafts are dynamic in nature and all cells did not display raft staining. Second, JAK2 is a cytoplasmic protein and more recently has been found in the nucleus of cells, including MPN cells (Dawson et al, 2009; Rinaldi et al, 2010). Third, our hypothesis is that JAK2-V617F is associated with a transmembrane receptor, such as a cytokine receptor (e.g. EpoR). Therefore, JAK2-V617F is not physically present in rafts per se, but rather associated with a protein in rafts. DRM isolation experiments utilized an overnight ultracentrifigation spin and it is likely that some JAK2 protein would not maintain its interaction with raft-associated proteins during this protocol and thus fractionate with the remainder of the JAK2, which is non-raft associated.
Using agents that disrupt cholesterol in the plasma membrane, we found that JAK2 and STAT5 activation in JAK2-V617F-dependent cells were dependent on cholesterol in the plasma membrane, while JAK2 and STAT5 activation in K562 cells, which express wildtype JAK2, were not (Fig 3). JAK2-V617F requires cytokine receptors for activation (Lu et al, 2005, 2008) while wildtype JAK2 activation in K562 cells is probably induced by the BCR-ABL1 tyrosine kinase (Xie et al, 2001; Hantschel et al, 2012). We believe that JAK2 activation by mechanisms that involve a cell surface receptor may be more sensitive to lipid raft disruption than activation of JAK2 by non-receptor mechanisms, such as BCR-ABL1. The BCR-ABL1-induced constitutive JAK/STAT signalling may not rely on lipid rafts because the cytoplasmic BCR-ABL1 tyrosine kinase may activate or signal to these molecules directly (Xie et al, 2001; Hantschel et al, 2012). Lipid rafts may play an integral role in the receptor scaffolding function for JAK2-V617F activation by coordinating the proper molecular complexes at the cell surface (Simons & Toomre, 2000; Lu et al, 2005, 2008).
MPN model cell lines are also more sensitive to statin treatment than BCR-ABL1 positive K562 cells. We find MPN cells are sensitive to single digit micromolar statins, which is similar to certain acute myeloid leukaemia cell lines, but significantly less than cells from a variety of solid tumours (Dimitroulakos et al, 1999). This may, in part, be due to the inherent driving oncogenic lesions in these cells, compared to other cancers. Statin treatment also inhibited the growth of primary MPN cells. Importantly, the growth of primary MPN cells is more sensitive to statins than cells from healthy controls (Fig 6). This is in agreement with other studies looking at the effect of statins on normal and neoplastic haematopoietic cell growth, where normal haematopoietic cells are not sensitive to statins until high doses are achieved (Newman et al, 1997; Dimitroulakos et al, 1999; Dai et al, 2007). This suggests statins may be considered as a potential therapeutic agent for MPNs, although the effect of statins on JAK2-V617F-negative MPNs needs to be determined.
Although a requirement for lipid rafts in JAK2 signalling could provide a mechanistic rationale for the use of statins to inhibit MPN cells, cholesterol is not the only end product of the mevalonate pathway (Demierre et al, 2005). While we have not obtained evidence that statins inhibit lipid raft formation in our cell systems, we showed that statins do appear to inhibit the localization of JAK2-V617F to lipid rafts (Fig 4D), which in effect also targets the requirement of rafts for signalling. Importantly, statins also inhibit protein prenylation by inhibiting the production of farnesyl pyrophosphate and geranygeranyl pyrophosphate, two other end products of the mevalonate pathway downstream of HMG-CoA reductase. Interestingly, it has been shown that EpoR cell surface expression requires protein geranylgeranylation (Hamadmad & Hohl, 2007). It is possible that the effects of statins in MPN cells may be mediated through protein prenylation, perhaps through inhibition of a requisite cytokine receptor for JAK2-V617F-mediated signalling. In our efforts to ascertain further details regarding the mechanism of statin affects on MPN cells, we have determined that adding back geranylgeranyl pyrophosphate to cells can reduce the statin-induced loss of viability of cells, but does not significantly restore proliferation of cells (not shown). Thus, while the mechanistic details by which statins inhibit MPN cell growth are probably complex and remain to be elucidated, our data suggest that statins may be a candidate to be used as a potential therapeutic strategy to target MPN cells. While these details will be the focus of future studies, it is important to note that statins induce MPN cell apoptosis (Fig 5A and B). This is significant because JAK2 inhibitors fail to decrease allele burden in patients and additional therapeutic approaches to complement JAK2 inhibitors are needed, especially ones that can contribute to an apoptotic response in MPN cells.
While our work is the first to directly investigate the role of lipid rafts and cholesterol in MPN cells, there is additional evidence that suggests cellular cholesterol levels could play a role in MPN cell biology. Mice deficient in cholesterol efflux transporters ABCA1 and ABCG1 display an MPN-like phenotype (Yvan-Charvet et al, 2010). This suggests that an increase in cellular cholesterol in haematopoietic cells can lead to an MPN-like phenotype. In fact, Yvan-Charvet et al (2010) demonstrated that haematopoietic stem and progenitor cells from these mice displayed aberrant proliferation, and that removal of cholesterol from these cells restored a normal proliferative phenotype. These studies clearly indicate that cellular cholesterol can regulate growth control pathways of haematopoietic stem and progenitor cells, and that increasing cholesterol levels can lead to aberrant myeloproliferation. Thus, cellular cholesterol may play an important role in the development of human MPNs. Our work, showing that alteration of membrane cholesterol with lipid raft disrupting agents inhibits JAK2-V617F signalling, together with the results reported by Yvan-Charvet et al (2010), suggests that altering cholesterol in haematopoietic stem and progenitor cells may affect cell signalling that leads to JAK2-V617F-driven myelopoiesis. Thus, altering cellular cholesterol or inhibiting localization of JAK2-V617F to lipid rafts, perhaps through the use of statins, may be an effective approach to target the aberrant myelopoiesis associated with MPNs.
The use of statins to treat MPN patients has been previously rationally suggested (Hasselbalch & Riley, 2006; Hasselbalch, 2012). This hypothesis is based on the antithrombotic, antiproliferative, proapoptotic, and antiangiogenic effects of statins and the role thrombohaemorrhagic complications play in MPNs. The use of statins in the treatment of MPNs has been discussed in the context of the potential role of chronic inflammation in the development of MPNs. The antiinflammatory effects of statins may be advantageous to MPN patients as chronic inflammation may be a driving force toward clonal evolution as well as a deadly myelofibrotic state (Hasselbalch & Riley, 2006; Hasselbalch, 2012). For example, tumour necrosis factor α (TNFα) may contribute to clonal expansion of MPN cells (Fleischman et al, 2011) and simvastatin lowers TNFα expression in myeloid cells in patients (Ferro et al, 2000). Also, MPN patients have an increased risk of developing both haematological and non-haematological secondary cancers and this may be due to the elevated inflammation associated with MPNs (Vannucchi et al, 2009; Frederiksen et al, 2011). Thus, in addition to the potential direct effects of statins on MPN cells, statins may also contribute to the amelioration of disease through their antiinflammatory effects.
In summary, we found that JAK2-V617F is associated with lipid rafts and that signalling by this constitutively activated kinase is dependent on proper lipid raft formation. Statins reduce JAK2 localization to lipid rafts, induce apoptosis of MPN cells, and inhibit colony formation of primary cells from MPN patients. Given that JAK inhibitors have not had success at reducing allele burden in MPN patients, additional therapeutic approaches are needed in order to induce remission in these patients. Our work suggests that statins might be an effective component of a therapeutic strategy for MPN patients. Additional studies are needed to investigate the potential efficacy of statins, alone and in combination with JAK inhibitors, as a potential therapeutic option for MPNs.
Acknowledgments
We thank Susumu Kobayashi (Harvard Medical School) for SET-2 cells, Ross Levine (Memorial Sloan Kettering Cancer Center) for HEL and Uke1 cells, and Channing Der (University of North Carolina at Chapel Hill) for K562 cells. We also thank Devon Roll, Que Lambert and Justine Clark for technical assistance and helpful discussions. This work has been supported in part by the Analytic Microscopy and Flow Cytometry Core Facilities at the H. Lee Moffitt Cancer Center & Research Institute, a comprehensive cancer centre designated by the National Cancer Institute.
Footnotes
Author contributions
LNG, KLM, and JOJ performed the research. LNG and GWR designed the research and wrote the paper. LNG, JOJ, and GWR analysed the data. AFL provided guidance and advice on the project as well as essential experimental expertise.
References
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nature Reviews Neuroscience. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- Awasthi-Kalia M, Schnetkamp PP, Deans JP. Differential effects of filipin and methylbeta-cyclodextrin on B cell receptor signaling. Biochemical and Biophysical Research Communications. 2001;287:77–82. doi: 10.1006/bbrc.2001.5536. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- Dai Y, Khanna P, Chen S, Pei XY, Dent P, Grant S. Statins synergistically potentiate 7-hydroxystaurosporine (UCN-01) lethality in human leukemia and myeloma cells by disrupting Ras farnesylation and activation. Blood. 2007;109:4415–4423. doi: 10.1182/blood-2006-09-047076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nature Reviews Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- Dimitroulakos J, Nohynek D, Backway KL, Hedley DW, Yeger H, Freedman MH, Minden MD, Penn LZ. Increased sensitivity of acute myeloid leukemias to lovastatin-induced apoptosis: a potential therapeutic approach. Blood. 1999;93:1308–1318. [PubMed] [Google Scholar]
- Edidin M. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends in Cell Biology. 2001;11:492–496. doi: 10.1016/s0962-8924(01)02139-0. [DOI] [PubMed] [Google Scholar]
- Ferro D, Parrotto S, Basili S, Alessandri C, Violi F. Simvastatin inhibits the monocyte expression of proinflammatory cytokines in patients with hypercholesterolemia. Journal of the American College of Cardiology. 2000;36:427431. doi: 10.1016/s0735-1097(00)00771-3. [DOI] [PubMed] [Google Scholar]
- Fleischman AG, Aichberger KJ, Luty SB, Bumm TG, Petersen CL, Doratotaj S, Vasudevan KB, LaTocha DH, Yang F, Press RD, Loriaux MM, Pahl HL, Silver RT, Agarwal A, O’Hare T, Druker BJ, Bagby GC, Deininger MW. TNFalpha facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118:6392–6398. doi: 10.1182/blood-2011-04-348144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Farkas DK, Christiansen CF, Hasselbalch HC, Sorensen HT. Chronic myeloproliferative neoplasms and subsequent cancer risk: a Danish population-based cohort study. Blood. 2011;118:6515–6520. doi: 10.1182/blood-2011-04-348755. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Hamadmad SN, Hohl RJ. Lovastatin suppresses erythropoietin receptor surface expression through dual inhibition of glycosylation and geranylgeranylation. Biochemical Pharmacology. 2007;74:590–600. doi: 10.1016/j.bcp.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Warsch W, Eckelhart E, Kaupe I, Grebien F, Wagner KU, Superti-Furga G, Sexl V. BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nature Chemical Biology. 2012;8:285–293. doi: 10.1038/nchembio.775. [DOI] [PubMed] [Google Scholar]
- Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Current Opinion in Cell Biology. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119:3219–3225. doi: 10.1182/blood-2011-11-394775. [DOI] [PubMed] [Google Scholar]
- Hasselbalch HC, Riley CH. Statins in the treatment of polycythaemia vera and allied disorders: an antithrombotic and cytoreductive potential? Leukemia Research. 2006;30:1217–1225. doi: 10.1016/j.leukres.2005.12.018. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Jatiani SS, Baker SJ, Silverman LR, Reddy EP. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes and Cancer. 2010;1:979–993. doi: 10.1177/1947601910397187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedidi A, Marty C, Oligo C, Jeanson-Leh L, Ribeil JA, Casadevall N, Galy A, Vainchenker W, Villeval JL. Selective reduction of JAK2V617F-dependent cell growth by siRNA/shRNA and its reversal by cytokines. Blood. 2009;114:1842–1851. doi: 10.1182/blood-2008-09-176875. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England Journal of Medicine. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Current Opinion in Cell Biology. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- Lee TS, Ma W, Zhang X, Giles F, Kantarjian H, Albitar M. Mechanisms of constitutive activation of Janus kinase 2-V617F revealed at the atomic level through molecular dynamics simulations. Cancer. 2009;115:1692–1700. doi: 10.1002/cncr.24183. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nature Reviews Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, Gilliland DG, Lodish H. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proceedings of the National Academy of Sciences USA. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Huang LJ, Lodish HF. Dimerization by a cytokine receptor is necessary for constitutive activation of JAK2V617F. The Journal of Biological Chemistry. 2008;283:5258–5266. doi: 10.1074/jbc.M707125200. [DOI] [PubMed] [Google Scholar]
- McGraw KL, Fuhler GM, Johnson JO, Clark JA, Caceres GC, Sokol L, List AF. Erythropoietin receptor signaling is membrane raft dependent. PLoS ONE. 2012;7:e34477. doi: 10.1371/journal.pone.0034477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastyrskaya K, Hostettler A, Buergi S, Draeger A. The NK1 receptor localizes to the plasma membrane microdomains, and its activation is dependent on lipid raft integrity. The Journal of Biological Chemistry. 2005;280:71357146. doi: 10.1074/jbc.M405806200. [DOI] [PubMed] [Google Scholar]
- Newman A, Clutterbuck RD, Powles RL, Catovsky D, Millar JL. A comparison of the effect of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors simvastatin, lovastatin and pravastatin on leukaemic and normal bone marrow progenitors. Leukemia & Lymphoma. 1997;24:533–537. doi: 10.3109/10428199709055590. [DOI] [PubMed] [Google Scholar]
- Oh ST, Gotlib J. JAK2 V617F and beyond: role of genetics and aberrant signaling in the pathogenesis of myeloproliferative neoplasms. Expert Review of Hematology. 2010;3:323–337. doi: 10.1586/ehm.10.28. [DOI] [PubMed] [Google Scholar]
- Ostermeyer AG, Beckrich BT, Ivarson KA, Grove KE, Brown DA. Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells. methyl-beta-cyclodextrin does not affect cell surface transport of a GPI-anchored protein. The Journal of Biological Chemistry. 1999;274:34459–34466. doi: 10.1074/jbc.274.48.34459. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Vannucchi AM, Passamonti F, Cervantes F, Barbui T, Tefferi A. JAK inhibitor therapy for myelofibrosis: critical assessment of value and limitations. Leukemia. 2011;25:218–225. doi: 10.1038/leu.2010.269. [DOI] [PubMed] [Google Scholar]
- Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. Journal of Cell Biology. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchal JF, Axelrad AA. Letter: bone-marrow responses in polycythemia vera. The New England Journal of Medicine. 1974;290:1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- Quentmeier H, MacLeod RA, Zaborski M, Drexler HG. JAK2 V617F tyrosine kinase mutation in cell lines derived from myeloproliferative disorders. Leukemia. 2006;20:471–476. doi: 10.1038/sj.leu.2404081. [DOI] [PubMed] [Google Scholar]
- Rinaldi CR, Rinaldi P, Alagia A, Gemei M, Esposito N, Formiggini F, Martinelli V, Senyuk V, Nucifora G, Pane F. Preferential nuclear accumulation of JAK2V617F in CD34+ but not in granulocytic, megakaryocytic, or erythroid cells of patients with Philadelphia-negative myeloproliferative neoplasia. Blood. 2010;116:6023–6026. doi: 10.1182/blood-2010-08-302265. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. The Journal of Biological Chemistry. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- Scherber R, Mesa RA. Future therapies for the myeloproliferative neoplasms. Current Hematologic Malignancy Reports. 2011;6:22–27. doi: 10.1007/s11899-010-0068-4. [DOI] [PubMed] [Google Scholar]
- Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, Futreal PA, Erber WN, McMullin MF, Harrison CN, Warren AJ, Gilliland DG, Lodish HF, Green AR. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. The New England Journal of Medicine. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal PB. Plasma membrane rafts and chaperones in cytokine/STAT signaling. Acta Biochimica Polonica. 2003;50:583–594. [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nature Reviews Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Tefferi A. JAK inhibitors for myeloproliferative neoplasms: clarifying facts from myths. Blood. 2012;119:2721–2730. doi: 10.1182/blood-2011-11-395228. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Masala G, Antonioli E, Chiara Susini M, Guglielmelli P, Pieri L, Maggi L, Caini S, Palli D, Bogani C, Ponziani V, Pancrazzi A, Annunziato F, Bosi A. Increased risk of lymphoid neoplasms in patients with Philadelphia chromosome-negative myeloproliferative neoplasms. Cancer Epidemiology Biomarkers and Prevention. 2009;18:2068–2073. doi: 10.1158/1055-9965.EPI-09-0353. [DOI] [PubMed] [Google Scholar]
- Waheed AA, Freed EO. Lipids and membrane microdomains in HIV1 replication. Virus Research. 2009;143:162–176. doi: 10.1016/j.virusres.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz C, Crowley BJ, Hudon HE, Gramlich JL, Neuberg DS, Podar K, Griffin JD, Sattler M. Activated Jak2 with the V617F point mutation promotes G1/S phase transition. The Journal of Biological Chemistry. 2006;281:18177–18183. doi: 10.1074/jbc.M600064200. [DOI] [PubMed] [Google Scholar]
- Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. Journal of Clinical Pharmacy and Therapeutics. 2010;35:139–151. doi: 10.1111/j.1365-2710.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Xie S, Wang Y, Liu J, Sun T, Wilson MB, Smithgall TE, Arlinghaus RB. Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene. 2001;20:6188–6195. doi: 10.1038/sj.onc.1204834. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, Zhao ZJ. Identification of an acquired JAK2 mutation in polycythemia vera. The Journal of Biological Chemistry. 2005;280:22788– 22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]