Abstract
Objectives
Expression of strong nuclear STAT6 is thought to be a specific marker for solitary fibrous tumors (SFT). Little is known about subtle expression patterns in other mesenchymal lesions.
Methods
We performed immunohistochemical studies against the C-terminus of STAT6 in tissue microarrays and whole sections, comprising 2366 mesenchymal lesions.
Results
Strong nuclear STAT6 was expressed in 285/2021 tumors, including 206/240 SFT, 49/408 well/dedifferentiated liposarcomas, 8/65 unclassified sarcomas, and 14/184 desmoids, among others. Expression in SFT was predominately limited to the nucleus. Other positive tumors typically expressed both nuclear and cytoplasmic STAT6. Complete absence of STAT6 was most common in pleomorphic liposarcoma and alveolar soft part sarcoma (60% and 72% cases negative, respectively).
Conclusions
Strong nuclear STAT6 is largely specific for SFT. Physiologic low-level cytoplasmic/nuclear expression is common in mesenchymal neoplasia, and is of uncertain significance.
Keywords: STAT6, Solitary fibrous tumor, sarcoma, immunohistochemistry
INTRODUCTION
Signal transducers and activators of transcription (STATs) are a vital family of signaling intermediaries which are critical in cellular proliferation and immune response.1 STAT6 is required for inflammatory responses to interleukin-4 (IL-4) and IL-13.2, 3 In normal cells, STAT6 is shuttled continuously between the cytoplasm and nucleus.4 Tyrosine phosphorylation secondary to IL-4/ Il-13 stimulated receptor-tyrosine-kinase and Janus kinase (JAK) mediated signaling results in STAT6 dimerization. Dimerized STAT6 accumulates in the nucleus where it acts as a transcriptional transactivator.1
While STAT6 has long been studied for its roles in immune function, tumor immunosurveillance and lymphomagenesis,5-7 it has only been newly hypothesized to participate directly in solid tumorigenesis, including both carcinomas8-11 and mesenchymal tumors, such as rhabdomyosarcoma, where IL-4/IL-13 signaling via STAT6 was found to promote proliferation and metastasis.12 In the majority of reports, STAT6 activation in malignancy is subsequent to IL-4 signaling, with only a few series demonstrating mutations of uncertain significance in STAT6 itself.13 Recently, the first definitively oncogenic alteration of STAT6 was identified as a specific driver of tumorigenesis in solitary fibrous tumors.14, 15
Solitary fibrous tumors are uncommon mesenchymal tumors which demonstrate fibroblastic differentiation and may arise anywhere in the body.16 Solitary fibrous tumors may be classified as benign or malignant, although histologic criteria alone are rarely sufficient to accurately predict metastatic potential.17 Solitary fibrous tumors (including hemangiopericytomas of meninges and soft tissues) have been shown to harbor a recurrent paracentric inversion involving chromosome 12q1314, 15 forming a fusion of the genes NGFI-A binding protein 2 (NAB2) and STAT6, resulting in overexpression of a chimeric transactivation factor which drives tumor proliferation.14, 15
NAB2 is a transcriptional repressor involved in cellular differentiation and proliferation via the early growth response (EGR) family of transcription factors.18 In normal cells, early growth response 1 (EGR1) promotes NAB2 expression which in turn represses EGR1 in a tightly regulated feed-back loop. The fusion of NAB2 and STAT6 most commonly results in the replacement of the NAB2 repressor domain by either a full-length STAT6 protein (exon 4 to exon 2/3) or the fusion of a near complete NAB2 protein and the transactivation domain of STAT6 (exon 6 to exon 16/17).14, 19, 20 While normal cells typically express low levels of cytoplasmic and nuclear STAT6, the chimeric NAB2-STAT6 protein is detected at high levels in the nucleus of the neoplastic cells of solitary fibrous tumor.14, 21, 22
Although STAT6 expression has been proposed to play a role in prostate, breast and colon carcinoma,8, 23-25 little was known about its involvement in mesenchymal neoplasia until recently. Several studies have investigated the sensitivity and specificity of STAT6 immunohistochemistry in the diagnosis of solitary fibrous tumor, focusing primarily on entities in the usual differential diagnosis, or containing a wide variety of tumors, but only a few examples of each type.21, 22, 26, 27 These studies have reported high sensitivity and specificity of STAT6 nuclear expression for solitary fibrous tumor.
We were intrigued by the tangential observations in several of these studies as to the presence of weak nuclear and cytoplasmic STAT6 expression in several non-solitary fibrous tumor neoplasms. Because IL-4/IL-13 signaling is mediated by STAT6, and represents an understudied, potentially targetable pathway to treat sarcomas, we wished to further investigate the distribution of STAT6 expression in these neoplasms as a preliminary assessment of the integrity of this pathway and its involvement in sarcomagenesis. We therefore characterized the expression characteristics of STAT6 by immunohistochemistry in a large array of benign and malignant mesenchymal neoplasms, including solitary fibrous tumors, representing the largest dataset on this marker yet reported.
MATERIALS AND METHODS
Patients and tumor tissues
Acquisition of tissue specimens and clinical information and subsequent analyses were approved by the respective Institutional Review Boards (IRB) of The University of Texas M. D. Anderson Cancer Center (UTMDACC), Icahn School of Medicine at Mount Sinai (ISMMS), and the University of Michigan Medical Center (UMMC).
Specimens included in the study included whole tumor sections at 4 μm obtained from UMMC and ISMMS (n= 33 and 3 respectively, including 33 solitary fibrous tumors and 3 other sarcomas), sections from a diverse array of previously published sarcoma-specific tissue microarrays, including: soft tissue and uterine leiomyosarcomata,28, 29 miscellaneous, predominately complex karyotype sarcomas,30, 31 desmoid tumors,32, 33 malignant peripheral nerve sheath tumors,34 angiosarcomas,35, alveolar soft part sarcomas, 36 epithelioid sarcoma,37 myxoid liposarcoma,38 pleomorphic liposarcoma,39 well-differentiated/ de-differentiated liposarcoma,40 and 5 previously unpublished tumor-specific tissue microarrays, including: solitary fibrous tumors (2 arrays, from UTMDACC and ISMMS, respectively, including meningeal hemangiopericytomas), clear cell sarcoma, an additional well-differentiated/de-differentiated liposarcoma array, and neurofibromatosis-associated malignant peripheral nerve sheath tumors.
Tissue microarray construction
Tumor specific tissue microarrays were constructed at UTMDACC or ISMMS. All available formalin-fixed, paraffin-embedded specimens from the relevant tumor type collected between 1993 and 2010 were retrieved from UTMDACC pathology archives, as were all available solitary fibrous tumors in the ISMMS archives from 1996-2012. Hematoxylin and eosin (H&E)-stained sections were reviewed to confirm diagnoses, define areas of viable tumor, and select one or more areas for inclusion in tissue microarray. For UTMDACC micorarrays, an automated tissue microarray apparatus (ATA-27, Beecher Instruments, Sun Prairie, WI) was used to obtain and format paired 1.2 mm punch samples from each selected area of tumor into recipient blocks. ISMMS tissue microarray was constructed using a manual tissue microarray apparatus (ATA-100, Chemicon International, Temecula, CA) and paired 1 mm punch samples. H&E staining of 4 μm tissue microarray sections was used to verify all samples.
Immunohistochemistry
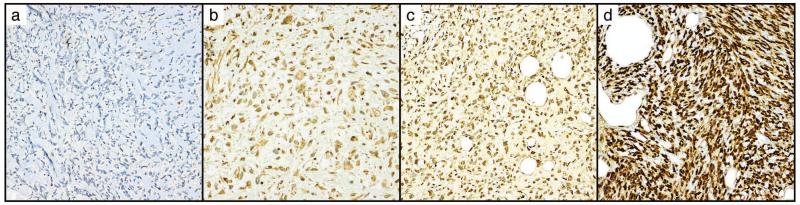
STAT6 immunohistochemistry was performed on all slides using antibody specific to the C-terminus (S-20, SC-621, 1:200 dilution [Santa Cruz, Dallas, TX]) using Benchmark XT automated staining system (Ventana/Roche, USA).21, 22, 26, 27 Immunohistochemical staining was performed using Ultraview Universal DAB detection kit (Ventana/Roche#760-500) following the manufacturer’s instructions for antigen retrieval and detection conditions. Sections were counterstained with hematoxylin (Cat# 790-2208 Ventana/Roche). Nuclear and cytoplasmic staining were qualitatively scored as 0 (absent to minimal blush, or staining of < 10% of cells, any intensity), or present in ≥ 10% of cells with intensity scored as 1+ (weak physiologic stain equivalent to intensity seen in intratumoral lymphocytes), 2+ (moderate overexpression) or 3+ (high level overexpression). For cases represented on multiple tissue microarrays, or for which more than one area was sampled, STAT6 scoring represented the aggregate data from all available cores. For the purposes of data analysis, scores of 2-3+ were considered strong positive. Stains were scored twice and those which showed discrepant reads between weak and strong (e.g. 1+, 2+) were considered to be equivocal (Figure 1).
Figure 1.
Scoring of nuclear STAT6 immunohistochemical staining.
A. Score 0, complete absence of nuclear and cytoplasmic expression. B. Score 1+ nuclear, 1+ cytoplasmic weak positivity. C. Score 2+ nuclear stain, D. 3+ nuclear stain. All images at 200×
Statistics
Comparisons between solitary fibrous tumors based on STAT6 nuclear positivity were performed using the Mann-Whitney U-test for median case age, or 2-tailed Chi-squared test for age-stratified data. Alpha of 0.05 was considered significant.
RESULTS
Tissue microarrays and whole sections comprised 2366 unique tumors/tissues, of which STAT6 immunostain was interpretable in 2082 (88%). The other 284 cases were not able to be scored due to loss of cores, absence of tumor tissue in cores, or staining artifact. Tissues successfully scored for STAT6 expression included 240 solitary fibrous tumors, 1781 other neoplasms (Table 1), and 61 non-neoplastic tissues (Table 2). In the majority of cases, staining intensity was uniform throughout the tissue with all tumor cells showing similar levels of nuclear and cytoplasmic expression.
Table 1.
Nuclear STAT6 Expression in Mesenchymal Tumors
| Diagnosis | N= | Nuclear expression |
||
|---|---|---|---|---|
| 0-1+ | Equivocal | 2-3+ | ||
| Solitary fibrous tumor | 240 | 15 | 19 | 206 (86%) |
| Unclassified sarcoma | 65 | 55 | 2 | 8 (12%) |
| ALT/WDL/DDLPS | 409 | 359 | 1 | 49 (12%) |
| Desmoid Tumor | 184 | 162 | 8 | 14 (8%) |
| Neurofibroma | 60 | 57 | 0 | 3 (5%) |
| Clear cell sarcoma | 19 | 18 | 0 | 1 (5%) |
| Myxoid liposarcoma | 108 | 106 | 0 | 2 (2%) |
| Undifferentiated pleomorphic sarcoma | 173 | 171 | 0 | 2 (1%) |
| Alveolar soft part sarcoma | 25 | 25 | 0 | 0 |
| Angiofibroma, cellular | 1 | 1 | 0 | 0 |
| Angiofibroma, nasopharyngeal | 2 | 2 | 0 | 0 |
| Angiosarcoma | 66 | 66 | 0 | 0 |
| DFSP +/− FS | 6 | 6 | 0 | 0 |
| Epithelioid sarcoma | 30 | 30 | 0 | 0 |
| Fibrosarcoma | 3 | 3 | 0 | 0 |
| GIST | 26 | 25 | 1 | 0 |
| Hemangioma | 1 | 1 | 0 | 0 |
| Leiomyoma | 9 | 9 | 0 | 0 |
| Leiomyosarcoma | 391 | 391 | 0 | 0 |
| LGFMS | 6 | 5 | 1 | 0 |
| MPNST | 111 | 111 | 0 | 0 |
| Myofibroma | 1 | 0 | 1 | 0 |
| Myxoma | 1 | 1 | 0 | 0 |
| Osteosarcoma | 2 | 2 | 0 | 0 |
| Pleomorphic liposarcoma | 55 | 55 | 0 | 0 |
| Sinonasal glomangiopericytoma | 4 | 3 | 1 | 0 |
| Synovial sarcoma | 23 | 22 | 1 | 0 |
|
| ||||
| Total | 2021 | 1701 | 35 | 285 (14%) |
Abbreviations: DFSP – dermatofibrosarcoma protuberans; FS – fibrosarcomatous transformation; GIST – gastrointestinal stromal tumor; LGFMS – Low grade fibromyxoid sarcoma; MPNST – Malignant peripheral nerve sheath tumor; ALT – atypical lipomatous tumor; WDL – well-differentiated liposarcoma; DDLPS – dedifferentiated liposarcoma.
Table 2.
STAT6 expression in selected non-neoplastic mesenchymal tissues
| Tissue | N= | Nuclear expression |
||
|---|---|---|---|---|
| 0-1+ | Equivocal | 2-3+ | ||
| Scar | 17 | 11 | 2 | 4 (24%) |
| Adipose Tissue | 24 | 22 | 0 | 2 (8%) |
| Bowel muscularis | 4 | 4 | 0 | 0 |
| Nerve | 10 | 10 | 0 | 0 |
| Uterine myometrium | 6 | 6 | 0 | 0 |
Solitary Fibrous Tumors
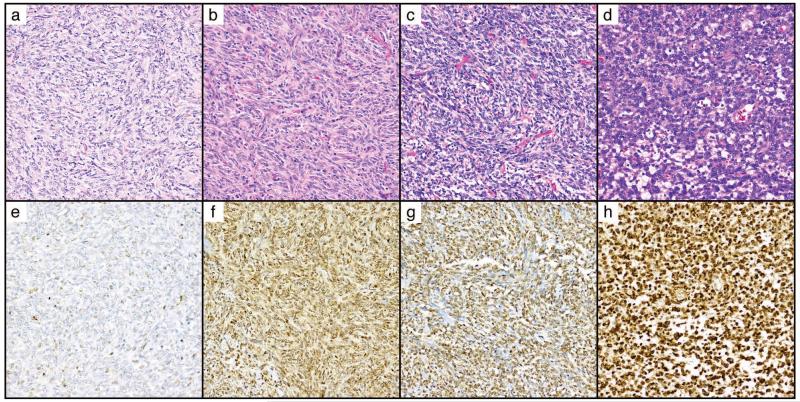
Strong nuclear positivity for STAT6 was seen in 206/ 240 (86%) solitary fibrous tumors (Figure 2), including 3 cases with at least focal dedifferentiation. Equivocal staining was seen in an additional 19 cases. Strong nuclear STAT6 expression in SFT was frequently seen in the absence of cytoplasmic expression (89/206, 43%), with only 26/205 (13%) of cases having both 2-3+ nuclear and cytoplasmic expression. Negative to equivocal nuclear staining for STAT6 was associated with older cases (median case age for negative 12 years vs. 9 years positive, p=0.015). When stratified by case age (where known), 95% (39/41) of solitary fibrous tumors resected within the past five years (<5 years old) were positive for strong nuclear STAT6, compared to 88% (60/68) of cases ≥5 and <10 years of age, and only 78% (80/102) of those resected ≥10 years ago (p=0.027). Among cases ≤5 years old, there were no differences in staining frequency or intensity between meningeal hemangiopericytomas (7/8 [88%] 2-3+) and solitary fibrous tumors of all other sites (42/46 [91%] 2-3+).
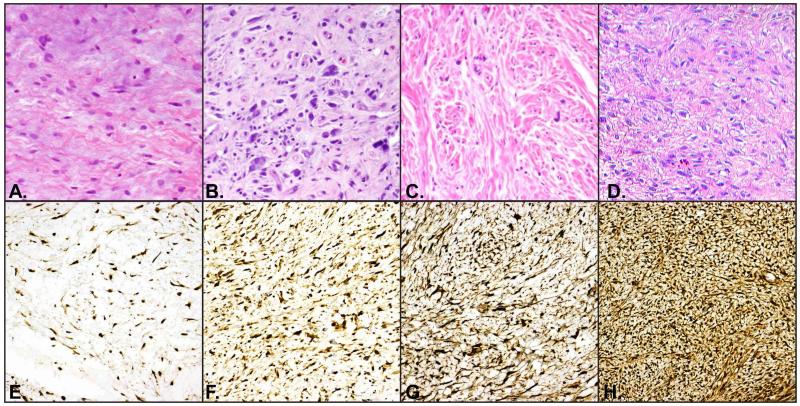
Figure 2.
Variable STAT6 staining was seen in solitary fibrous tumors.
A-D. H&E demonstrating histologic features of representative solitary fibrous tumors with E. negative-to-focal-weak nuclear STAT6, F. 2+ nuclear and cytoplasmic expression, G. 2+ nuclear expression, H. 3+ nuclear STAT6. All images at 200×.
Other tumors with strong STAT6 expression
Strong nuclear STAT6 staining was very limited in other mesenchymal tumors (4% overall), and 36% demonstrated complete absence of nuclear STAT6.
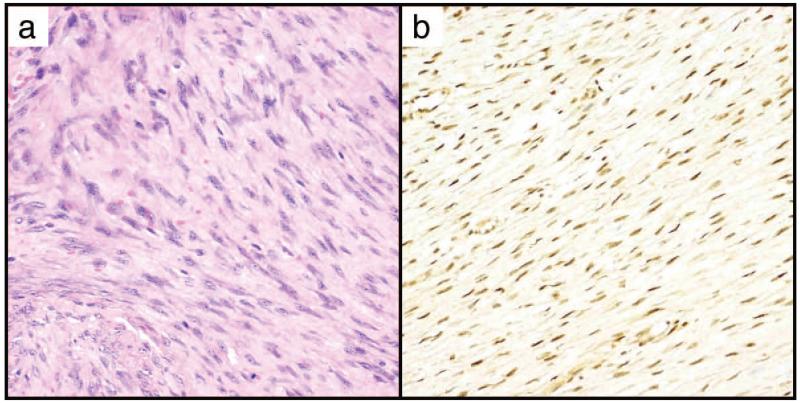
Weak nuclear STAT6 was common in desmoid-type fibromatosis (67%) usually in conjunction with an equivalent level of cytoplasmic expression, with equivocal positivity in an additional 4%, and 2+ nuclear staining present in 8% of cases. All cases with strong nuclear positivity were accompanied by weak cytoplasmic STAT6 expression (Figure 3).
Figure 3.
Moderate STAT6 nuclear expression may occur in desmoid tumors.
A. H&E, B. STAT6, 2+ nuclear, 1+ cytoplasmic staining. Both panels at 200×.
Although only 2 of 173 classic undifferentiated pleomorphic sarcoma were positive for strong nuclear STAT6 (1% of cases), a high proportion of unclassified spindle cell/ epithelioid sarcomas, (8/65, 12%) were strongly positive for nuclear STAT6. Of these eight, two were tumors for which solitary fibrous tumors had been considered to be in the differential diagnosis, but which lacked classical, diagnostic features. The remaining 6 cases were all spindle cell sarcomas that were felt to be histologically incompatible with a diagnosis of solitary fibrous tumor. Strong nuclear STAT6 in unclassified spindle cell sarcomas was accompanied by strong cytoplasmic expression in 5/8 cases, and by 1+ cytoplasmic staining in the other 3.
Second to unclassified sarcomas, well-differentiated/ de-differentiated liposarcomas were the next most frequent sarcoma to express strong nuclear STAT6 (49/409 cases, 12%). As with the unclassified sarcomas, strong nuclear expression in well/de-differentiated liposarcoma was most commonly seen in conjunction with strong cytoplasmic positivity (26/49, 53%). Only rare cases of well-differentiated liposarcoma (6/49, 12%) displayed strong nuclear STAT6 in the absence of cytoplasmic expression.
Non-tumor tissues
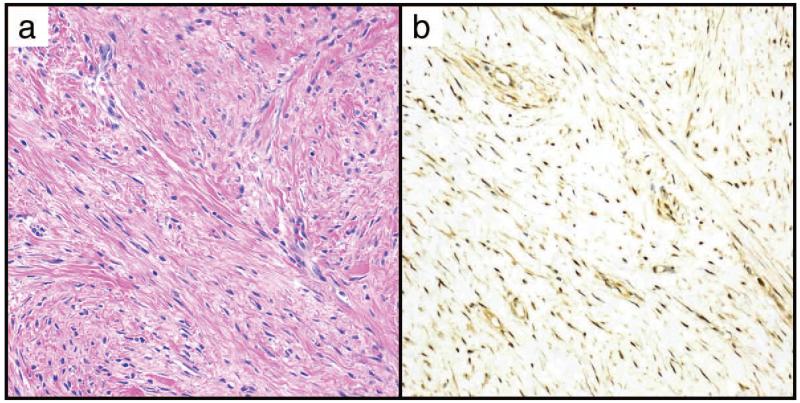
Scar tissue was commonly seen to have at least 1+ nuclear and cytoplasmic STAT6 (12/17, 71%), with 2+ nuclear stain present in 4/17 (24%) of cases, (Figure 5, Table 2). Normal fat showed nuclear positivity in 2/24 (8%) of cases, but was completely negative for STAT6 in the other 18 (92%) (Table 2). Other non-neoplastic tissues including nerve and smooth muscle from myometrium and bowel muscularis propria did not demonstrate high levels of nuclear STAT6, but frequently showed 1+ nuclear and cytoplasmic expression (16/20 cases, 80%).
Figure 5.
STAT6 expression was common in scar tissue.
A. Representative H&E, B. Representative STAT6 showing 2+ cytoplasmic and nuclear expression. Both panels at 200×.
Patterns of low-level STAT6 expression in mesenchymal neoplasia
In tumors with ≤1+ nuclear STAT6, a weak cytoplasmic blush was frequently present (1164/1701 cases, 68%). Strong cytoplasmic stain in the absence of nuclear expression was detected in 5 cases (<1%). Weak nuclear expression without cytoplasmic expression was present in 122 tumors (6%), and was most common in leiomyosarcoma (44/391, 11%). Low level nuclear and cytoplasmic staining, similar to that seen in non neoplastic inflammatory cells was common, particularly in normal tissues (nerve, scar) as well as certain neoplasms, including neurofibroma, low grade fibromyxoid sarcoma, undifferentiated pleomorphic sarcoma, epithelioid sarcoma and synovial sarcoma.
Complete absence of STAT6 in either nucleus or cytoplasm was seen in 418/2021 (21%) of all cases. Nonreactivity for STAT6 was most common in alveolar soft part sarcoma (18/25, 72%), pleomorphic liposarcoma (33/55, 60%), dermatofibrosarcoma protuberans (3/6, 50%) and myxoid liposarcoma (52/108, 48%).
DISCUSSION
Our study represents the largest dataset of mesenchymal tumors evaluated for STAT6 yet reported, and confirms previous reports as to the specificity of high nuclear expression of STAT6 for the detection of solitary fibrous tumors. Nevertheless, we also found that the majority of mesenchymal tumors and tissues do express at least low levels of nuclear and cytoplasmic STAT6.
In solitary fibrous tumors, immunohistochemical studies for STAT6 using antibodies against the C-terminus identify the NAB2-STAT6 fusion protein which is localized to the nucleus, while wild-type STAT6 distributes evenly between the nucleus and cytoplasmic compartments due to continuous protein shuttling.1, 22, 41 As this phenomenon was incompletely explored in earlier studies (Table 3),21, 22, 26, 27, 41 and because STAT6 signaling may play a yet-to-be-elucidated role in mesenchymal tumorigenesis, we performed an in-depth analysis of the patterns of nuclear and cytoplasmic STAT6 in both tumors with strong nuclear staining and those without.
Table 3.
Reported STAT6 Expression in Mesenchymal and Spindle Cell Neoplasms.
| Tumor type | Total number | STAT6 Positive† | % Positive |
|---|---|---|---|
| Solitary fibrous tumor | 208 | 204 | 98% |
| Tumors of the central nervous system | |||
| Glioblastoma | 10 | 0 | 0 |
| Gliosarcoma* | 12 | 0 | 0 |
| Hemangioblastoma | 12 | 0 | 0 |
| Meningeal sarcoma/unclassified meningeal tumor | 7 | 4 | 57% |
| Meningioma* | 98 | 0 | 0 |
| Tumors of the pleura/ lung/ mediastinum | |||
| Sarcomatoid lung carcinoma* | 5 | 0 | 0 |
| Sarcomatoid mesothelioma* | 14 | 0 | 0 |
| Synovial sarcoma* | 86 | 0 | 0 |
| Thymoma (type A)* | 2 | 0 | 0 |
| Adipocytic tumors | |||
| Dedifferentiated liposarcoma* | 134 | 12 | 9% |
| Myxoid liposarcoma | 46 | 1 | 2% |
| Pleomorphic liposarcoma | 11 | 0 | 0 |
| Spindle cell lipoma* | 14 | 0 | 0 |
| Well-differentiated liposarcoma | 10 | 0 | 0 |
| Aggressive/ malignant tumors of soft tissue | |||
| Dermatofibrosarcoma protuberans* | 40 | 0 | 0 |
| Desmoid tumor* | 41 | 0 | 0 |
| Extraskeletal myxoid chondrosarcoma | 9 | 0 | 0 |
| Gastrointestinal stroma tumor* | 56 | 0 | 0 |
| Leiomyosarcoma* | 39 | 0 | 0 |
| Low grade endometrial stroma sarcoma* | 4 | 0 | 0 |
| Low grade fibromyxoid sarcoma* | 23 | 2 | 9% |
| Low grade myofibroblastic sarcoma | 18 | 0 | 0 |
| Malignant peripheral nerve sheath tumor | 70 | 0 | 0 |
| Mesenchymal chondrosarcoma | 6 | 0 | 0 |
| Myxofibrosarcoma | 8 | 0 | 0 |
| Undifferentiated pleomorphic sarcoma* | 140 | 2 | 1% |
| Alveolar soft part sarcoma | 8 | 0 | 0 |
| Angiosarcoma | 12 | 0 | 0 |
| Clear cell sarcoma | 4 | 0 | 0 |
| Epithelioid sarcoma | 8 | 0 | 0 |
| Ewing sarcoma* | 6 | 0 | 0 |
| Benign soft tissue tumors | |||
| Cellular angiofibroma | 10 | 0 | 0 |
| Dermatofibroma/benign fibrous histiocytoma* | 26 | 1 | 4% |
| Desmoplastic fibroblastoma | 5 | 0 | 0 |
| Fibroma of tendon sheath | 4 | 0 | 0 |
| Hemangioma | 25 | 0 | 0 |
| Leiomyoma of deep soft tissue* | 2 | 0 | 0 |
| Myofibroma/myopericytoma* | 5 | 0 | 0 |
| Neurofibroma | 4 | 0 | 0 |
| Nodular fasciitis* | 66 | 1 | 2% |
| Ovarian fibroma* | 2 | 1 | 50% |
| Schwannoma* | 43 | 0 | 0 |
| Sinonasal glomangiopericytoma | 6 | 0 | 0 |
| Soft tissue angiofibroma* | 1 | 0 | 0 |
| Soft tissue perineurioma* | 15 | 0 | 0 |
| Total non-solitary fibrous tumors | 1167 | 24 | 2% |
Positive STAT6 expression was defined as strong nuclear expression in the absence of cytoplasmic staining.
Indicates tumors for which combined nuclear/cytoplasmic positivity has been reported. Not all reports distinguished between this pattern and negative stain.
Previous reports found an aggregate sensitivity of nuclear STAT6 for solitary fibrous tumors of 98%, successfully staining 204/208 reported cases.21, 22, 26, 27, 42 Among our cases we found an overall lower sensitivity of only 87%. However, further analysis revealed that among cases resected within the past 5 years, STAT6 immunohistochemistry displayed 97% sensitivity for solitary fibrous tumors, equivalent to the previously published findings. Older cases progressively lost antigenicity over time. We therefore agree that strong nuclear staining for STAT6 is a useful prospective diagnostic marker for solitary fibrous tumors. Unfortunately, if using STAT6 in a retrospective analysis to confirm or re-evaluate case diagnoses, the risk of loss of antigenicity should be kept in mind. Care must be taken to interpret negative results carefully and to not to let a negative stain exclude the diagnosis of solitary fibrous tumor, especially in dealing with older cases.
Doyle et al (2014) recently reported the expression of moderate-to-strong STAT6 in up to 12% of dedifferentiated liposarcomas, and determined that STAT6 expression was due to gene locus inclusion in the 12q13~15 amplicon characteristic of this tumor.41 We found similar rates of high nuclear STAT6 expression in both well-differentiated (14%) and de-differentiated (8%) liposarcomas in our cohort. As with their study, nuclear expression of STAT6 was usually seen in conjunction with an equivalent, or near-equivalent degree of cytoplasmic staining, and is compatible with their interpretation that elevated expression is likely to be due to amplification and subsequent overexpression of full-length STAT6. Intimal sarcomas also possess 12q15 amplification,43, 44 and thus may rarely also express strong nuclear and cytoplasmic STAT6 (unpublished clinical observation by EGD). Fortunately, most cases of atypical lipomatous tumor/ well-differentiated liposarcoma / intimal sarcoma have histological, clinical and radiological features which allow them to be distinguished from solitary fibrous tumors. Rarely, lipomatous solitary fibrous tumor may mimic well-differentiated liposarcoma, while both dedifferentiated liposarcoma and intimal sarcoma can have bizarre morphology which may be difficult to distinguish from dedifferentiated solitary fibrous tumor without clinical information or in small biopsy specimens. In the event of STAT6 expression in such cases, both may be distinguished from solitary fibrous tumor by the pattern of elevated cytoplasmic and nuclear STAT6, as well as by the co-expression of MDM2, demonstrable by immunohistochemistry or molecular evidence of gene amplification.
Despite the occasional presence of strong STAT6 in well/de-differentiated liposarcoma, when taken as a whole, tumors with adipocytic differentiation and normal fat were among the most likely tissues to be completely negative for any STAT6, with 33% of all liposarcoma types and 75% of normal fat demonstrating absence of nuclear or cytoplasmic STAT6 expression, compared to 16% of all other tumors and tissues. Although STAT6 does not appear to be involved in regulation of normal adipogenesis,45 it is possible that dysregulation is somehow important in the genesis of fatty malignancies. Further study is needed.
We found high levels of nuclear +/− cytoplasmic STAT6 in 8 unclassified sarcomas and undifferentiated pleomorphic sarcomas. Two of these cases had been considered to represent possible dedifferentiated or atypical solitary fibrous tumors prior to the study, among other diagnoses considered, and expressed nuclear STAT6 only. Thus, positivity for STAT6 in these 2 cases was able to resolve the diagnostic dilemma and confirm a suspected diagnosis of solitary fibrous tumor. The remaining 6 cases were histologically incompatible with a diagnosis of solitary fibrous tumor, and most expressed high levels of cytoplasmic STAT6 as well as nuclear. In light of the above observations for liposarcoma, these 6 cases may also represent previously undiagnosed de-differentiated liposarcoma or dedifferentiated solitary fibrous tumors where conventional areas are no longer seen or sampled. Unfortunately, we were not able to investigate further based on available information.
Nuclear STAT6 (weak or strong) was frequently seen in both desmoid tumors and scar tissue. IL-4/IL-13 mediated signaling is actively involved in wound healing and fibrosis, and is thought to contribute to the activation of fibroblasts.46 Thus, the expression of increased levels of STAT6 in scar tissue is likely part of the normal healing response. In desmoid tumors however, the role of Il-4/IL-13 signaling is not well studied, and it is possible that alterations in STAT6-mediated signaling may play a role in mediating the distinctive histologic features of this scar-like neoplasm.
In conclusion, we report on the expression of STAT6 in the largest series of mesenchymal neoplasms yet evaluated, with over 2000 tumors, representing 26 different entities. We confirm the high sensitivity and specificity of strong nuclear STAT6 for solitary fibrous tumors, and propose that further functional study is needed to better understand the role STAT6 may play in the biology of liposarcomas and desmoid tumors.
Figure 4.
Strong nuclear and cytoplasmic STAT6 expression is rarely seen in unclassified sarcomas.
A-D, H&E, E-H corresponding STAT6 stains showing strong nuclear and cytoplasmic expression. All images at 200×.
ACKNOWLEDGEMENTS
Thanks to the Icahn School of Medicine Histology Shared Resource Facility for tissue microarray construction, and to Kim Anh-Vu for her expert assistance in creating the figures. PWH is a recipient of the Dermatopathology Research Career Development Award from the Dermatology Foundation.
N.P. receives research funding from Ventana/Roche; this funding did not play a part in development of the STAT6 immunohistochemical assay for this project.
Footnotes
Disclosures: We have no conflicts of interest to declare.
Data in this report was previously presented as a poster at the 2014 annual meeting of the United State and Canadian Academy of Pathology, San Diego, CA on March 4, 2014.
References
- 1.Reich NC. STATs get their move on. JAKSTAT. 2013;2:e27080. doi: 10.4161/jkst.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 3.Izuhara K, Heike T, Otsuka T, Yamaoka K, Mayumi M, Imamura T, Niho Y, Harada N. Signal transduction pathway of interleukin-4 and interleukin-13 in human B cells derived from X-linked severe combined immunodeficiency patients. J Biol Chem. 1996;271:619–622. doi: 10.1074/jbc.271.2.619. [DOI] [PubMed] [Google Scholar]
- 4.Chen HC, Reich NC. Live cell imaging reveals continuous STAT6 nuclear trafficking. J Immunol. 2010;185:64–70. doi: 10.4049/jimmunol.0903323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrand-Rosenberg S, Sinha P, Clements V, Dissanayake SI, Miller S, Davis C, Danna E. Signal transducer and activator of transcription 6 (Stat6) and CD1: inhibitors of immunosurveillance against primary tumors and metastatic disease. Cancer Immunol Immunother. 2004;53:86–91. doi: 10.1007/s00262-003-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinnider BF, Elia AJ, Gascoyne RD, Patterson B, Trumper L, Kapp U, Mak TW. Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2002;99:618–626. doi: 10.1182/blood.v99.2.618. [DOI] [PubMed] [Google Scholar]
- 7.Carey GB, Semenova E, Qi X, Keegan AD. IL-4 protects the B-cell lymphoma cell line CH31 from anti-IgM-induced growth arrest and apoptosis: contribution of the PI-3 kinase/AKT pathway. Cell Res. 2007;17:942–955. doi: 10.1038/sj.cr.2007.90. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Roth CP, Wasson LM, Vishwanatha JK. Signal transducer and activator of transcription-6 (STAT6) is a constitutively expressed survival factor in human prostate cancer. Prostate. 2007;67:1550–1564. doi: 10.1002/pros.20640. [DOI] [PubMed] [Google Scholar]
- 9.Xu SB, Liu XH, Li BH, Zhang Y, Yuan J, Yuan Q, Li PD, Yang XZ, Li F, Zhang WJ. DNA methylation regulates constitutive expression of Stat6 regulatory genes SOCS-1 and SHP-1 in colon cancer cells. J Cancer Res Clin Oncol. 2009;135:1791–1798. doi: 10.1007/s00432-009-0627-z. [DOI] [PubMed] [Google Scholar]
- 10.Wang CG, Ye YJ, Yuan J, Liu FF, Zhang H, Wang S. EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis. World J Gastroenterol. 2010;16:2421–2427. doi: 10.3748/wjg.v16.i19.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das S, Shetty P, Valapala M, Dasgupta S, Gryczynski Z, Vishwanatha JK. Signal transducer and activator of transcription 6 (STAT6) is a novel interactor of annexin A2 in prostate cancer cells. Biochemistry. 2010;49:2216–2226. doi: 10.1021/bi9013038. [DOI] [PubMed] [Google Scholar]
- 12.Hosoyama T, Aslam MI, Abraham J, Prajapati SI, Nishijo K, Michalek JE, Zarzabal LA, Nelon LD, Guttridge DC, Rubin BP, Keller C. IL-4R drives dedifferentiation, mitogenesis, and metastasis in rhabdomyosarcoma. Clin Cancer Res. 2011;17:2757–2766. doi: 10.1158/1078-0432.CCR-10-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank DA. STAT6 in PMBL: pathogenic or passenger? Blood. 2009;114:1133–1134. doi: 10.1182/blood-2009-05-221770. [DOI] [PubMed] [Google Scholar]
- 14.Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, Iyer MK, Roychowdhury S, Siddiqui J, Pienta KJ, Kunju LP, Talpaz M, Mosquera JM, Singer S, Schuetze SM, Antonescu CR, Chinnaiyan AM. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chmielecki J, Crago AM, Rosenberg M, O’Connor R, Walker SR, Ambrogio L, Auclair D, McKenna A, Heinrich MC, Frank DA, Meyerson M. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45:131–132. doi: 10.1038/ng.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher CDM, Bridge JA, Lee J-C. Extrapleural solitary fibrous tumor. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. World Health Organization of Tumors of Soft Tissue and Bone. IARC Press; Lyon: 2013. pp. 80–82. [Google Scholar]
- 17.Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, Lazar AJ, Wang WL. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298–1306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya S, Fang F, Tourtellotte W, Varga J. Egr-1: new conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis) J Pathol. 2013;229:286–297. doi: 10.1002/path.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohajeri A, Tayebwa J, Collin A, Nilsson J, Magnusson L, von Steyern FV, Brosjo O, Domanski HA, Larsson O, Sciot R, Debiec-Rychter M, Hornick JL, Mandahl N, Nord KH, Mertens F. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer. 2013;52:873–886. doi: 10.1002/gcc.22083. [DOI] [PubMed] [Google Scholar]
- 20.Barthelmess S, Geddert H, Boltze C, Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A, Agaimy A, Haller F. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol. 2014;184:1209–1218. doi: 10.1016/j.ajpath.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Gock T, Jones DT, Mawrin C, Schittenhelm J, Becker A, Heim S, Simon M, Herold-Mende C, Mechtersheimer G, Paulus W, Konig R, Wiestler OD, Pfister SM, von Deimling A. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125:651–658. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 22.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2013;27:390–395. doi: 10.1038/modpathol.2013.164. [DOI] [PubMed] [Google Scholar]
- 23.Ni Z, Lou W, Lee SO, Dhir R, DeMiguel F, Grandis JR, Gao AC. Selective activation of members of the signal transducers and activators of transcription family in prostate carcinoma. J Urol. 2002;167:1859–1862. [PubMed] [Google Scholar]
- 24.Gooch JL, Christy B, Yee D. STAT6 mediates interleukin-4 growth inhibition in human breast cancer cells. Neoplasia. 2002;4:324–331. doi: 10.1038/sj.neo.7900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Zhou Y, Xie C, Zhou F, Chen Y, Han G, Zhang WJ. STAT6 specific shRNA inhibits proliferation and induces apoptosis in colon cancer HT-29 cells. Cancer Lett. 2006;243:38–46. doi: 10.1016/j.canlet.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, Asamura H, Kushima R. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38:552–559. doi: 10.1097/PAS.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 27.Koelsche C, Schweizer L, Renner M, Warth A, Jones DT, Sahm F, Reuss DE, Capper D, Knosel T, Schulz B, Petersen I, Ulrich A, Renker EK, Lehner B, Pfister SM, Schirmacher P, von Deimling A, Mechtersheimer G. Nuclear relocation of STAT6 reliably predicts NAB2/STAT6 fusion for the diagnosis of Solitary Fibrous Tumour. Histopathology. 2014 doi: 10.1111/his.12431. [DOI] [PubMed] [Google Scholar]
- 28.Demicco EG, Boland GM, Brewer Savannah KJ, Lusby K, Young ED, Ingram D, Watson KL, Bailey M, Guo X, Hornick JL, van de Rijn M, Wang WL, Torres KE, Lev D, Lazar AJ. Progressive Loss Of Myogenic Differentiation In Leiomyosarcoma Has Prognostic Value. Histopathology. 2014 doi: 10.1111/his.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lusby K, Savannah KB, Demicco EG, Zhang Y, Ghadimi MP, Young ED, Colombo C, Lam R, Dogan TE, Hornick JL, Lazar AJ, Hunt KK, Anderson ML, Creighton CJ, Lev D, Pollock RE. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution’s experience. Ann Surg Oncol. 2013;20:2364–2372. doi: 10.1245/s10434-012-2834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahat G, Tuvin D, Wei C, Wang WL, Pollock RE, Anaya DA, Bekele BN, Corely L, Lazar AJ, Pisters PW, Lev D. Molecular prognosticators of complex karyotype soft tissue sarcoma outcome: a tissue microarray-based study. Ann Oncol. 2010;21:1112–1120. doi: 10.1093/annonc/mdp459. [DOI] [PubMed] [Google Scholar]
- 31.Lahat G, Zhang P, Zhu QS, Torres K, Ghadimi M, Smith KD, Wang WL, Lazar AJ, Lev D. The expression of c-Met pathway components in unclassified pleomorphic sarcoma/malignant fibrous histiocytoma (UPS/MFH): a tissue microarray study. Histopathology. 2011;59:556–561. doi: 10.1111/j.1365-2559.2011.03946.x. [DOI] [PubMed] [Google Scholar]
- 32.Lazar AJ, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, Warneke CL, Lopez-Terrada D, Pollock RE, Lev D. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo C, Foo WC, Whiting D, Young ED, Lusby K, Pollock RE, Lazar AJ, Lev D. FAP-related desmoid tumors: a series of 44 patients evaluated in a cancer referral center. Histol Histopathol. 2012;27:641–649. doi: 10.14670/HH-27.641. [DOI] [PubMed] [Google Scholar]
- 34.Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, Zhang W, McCutcheon IE, Slopis JM, Lazar AJ, Pollock RE, Lev D. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 35.Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD, Phung TL, Pollock RE, Benjamin R, Hunt KK, Lazar AJ, Lev D. Angiosarcoma: clinical and molecular insights. Ann Surg. 2010;251:1098–1106. doi: 10.1097/SLA.0b013e3181dbb75a. [DOI] [PubMed] [Google Scholar]
- 36.Lazar AJ, Lahat G, Myers SE, Smith KD, Zou C, Wang WL, Lopez-Terrada D, Lev D. Validation of potential therapeutic targets in alveolar soft part sarcoma: an immunohistochemical study utilizing tissue microarray. Histopathology. 2009;55:750–755. doi: 10.1111/j.1365-2559.2009.03436.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakharpe A, Lahat G, Gulamhusein T, Liu P, Bolshakov S, Nguyen T, Zhang P, Belousov R, Young E, Xie X, Rao P, Hornick JL, Lazar AJ, Pollock RE, Lev D. Epithelioid sarcoma and unclassified sarcoma with epithelioid features: clinicopathological variables, molecular markers, and a new experimental model. Oncologist. 2011;16:512–522. doi: 10.1634/theoncologist.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demicco EG, Torres KE, Ghadimi MP, Colombo C, Bolshakov S, Hoffman A, Peng T, Bovee JV, Wang WL, Lev D, Lazar AJ. Involvement of the PI3K/Akt pathway in myxoid/round cell liposarcoma. Mod Pathol. 2012;25:212–221. doi: 10.1038/modpathol.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghadimi MP, Liu P, Peng T, Bolshakov S, Young ED, Torres KE, Colombo C, Hoffman A, Broccoli D, Hornick JL, Lazar AJ, Pisters P, Pollock RE, Lev D. Pleomorphic liposarcoma: clinical observations and molecular variables. Cancer. 2011;117:5359–5369. doi: 10.1002/cncr.26195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang P, Bill K, Liu J, Young E, Peng T, Bolshakov S, Hoffman A, Song Y, Demicco EG, Terrada DL, Creighton CJ, Anderson ML, Lazar AJ, Calin GG, Pollock RE, Lev D. MiR-155 is a liposarcoma oncogene that targets casein kinase-1alpha and enhances beta-catenin signaling. Cancer Res. 2012;72:1751–1762. doi: 10.1158/0008-5472.CAN-11-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doyle LA, Tao D, Marino-Enriquez A. STAT6 is amplified in a subset of dedifferentiated liposarcoma. Mod Pathol. 2014 doi: 10.1038/modpathol.2013.247. [DOI] [PubMed] [Google Scholar]
- 42.Agaimy A, Barthelmess S, Geddert H, Boltze C, Moskalev E, Koch M, Wiemann S, Hartmann A, Haller F. Phenotypic And Molecular Distinctness of Sinonasal Hemangiopericytoma compared to Solitary Fibrous Tumor of the Sinonasal Tract. Histopathology. 2014 doi: 10.1111/his.12452. [DOI] [PubMed] [Google Scholar]
- 43.Bode-Lesniewska B, Zhao J, Speel EJ, Biraima AM, Turina M, Komminoth P, Heitz PU. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 2001;438:57–65. doi: 10.1007/s004280000313. [DOI] [PubMed] [Google Scholar]
- 44.Neuville A, Collin F, Bruneval P, Parrens M, Thivolet F, Gomez-Brouchet A, Terrier P, de Montpreville VT, Le Gall F, Hostein I, Lagarde P, Chibon F, Coindre JM. Intimal sarcoma is the most frequent primary cardiac sarcoma: clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am J Surg Pathol. 2014;38:461–469. doi: 10.1097/PAS.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 45.Harp JB, Franklin D, Vanderpuije AA, Gimble JM. Differential expression of signal transducers and activators of transcription during human adipogenesis. Biochem Biophys Res Commun. 2001;281:907–912. doi: 10.1006/bbrc.2001.4460. [DOI] [PubMed] [Google Scholar]
- 46.Barron L, Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastrointest Liver Physiol. 2011;300:G723–728. doi: 10.1152/ajpgi.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]