Abstract
Murine NLR family, apoptosis inhibitory protein (Naip)1, Naip2, and Naip5/6 are host sensors that detect the cytosolic presence of needle and rod proteins from bacterial type III secretion systems and flagellin, respectively. Previous studies using human-derived macrophage-like cell lines indicate that human macrophages sense the cytosolic needle protein, but not bacterial flagellin. In this study, we show that primary human macrophages readily sense cytosolic flagellin. Infection of primary human macrophages with Salmonella elicits robust cell death and IL-1β secretion that is dependent on flagellin. We show that flagellin detection requires a full-length isoform of human Naip. This full-length Naip isoform is robustly expressed in primary macrophages from healthy human donors, but it is drastically reduced in monocytic tumor cells, THP-1, and U937, rendering them insensitive to cytosolic flagellin. However, ectopic expression of full-length Naip rescues the ability of U937 cells to sense flagellin. In conclusion, human Naip functions to activate the inflammasome in response to flagellin, similar to murine Naip5/6.
Introduction
Whereas certain aspects of innate immunity can be regarded as nonspecific, multicellular organisms have evolved specific recognition mechanisms as part of their defense system to respond to conserved bacterial structures and facilitate the clearance of invading pathogens (1). Flagella are surface appendages that facilitate bacterial motility and are required for the facultative intracellular pathogens Salmonella enterica serovars Typhimurium (S. Typhimurium) and Typhi (S. Typhi) to target host cells in the intestinal epithelium (2). During bacterial infections, conserved structures of the flagella-forming protein flagellin are recognized by several host pattern recognition receptors (1). Extracellular flagellin is recognized by the cell-surface sensor TLR5 (3), which promotes the activation of NF-κB and subsequent secretion of IL-8 (2). Flagellin monomers are also translocated into the host cell cytosol by a mechanism that requires bacterial secretion systems. For example, during Salmonella infections, flagellin is translocated into the host cell cytosol by the Salmonella pathogenicity island 1 (SPI-1) type III secretion system (T3SS) (4). In mice, this triggers the formation of the NLR family CARD domain–containing protein (NLRC)4 inflammasome (5, 6), which promotes two major caspase-1–dependent events: release of the proinflammatory cytokines IL-1β and IL-18, and the induction of a proinflammatory form of cell death termed pyroptosis (7). The NLRC4 inflammasome can also detect the rod (PrgJ) and needle (PrgI) proteins of the SPI-1 T3SS apparatus (8, 9).
The ability of mice to sense flagellin and components of the T3SS is facilitated by a group of receptors termed NLR family, apoptosis inhibitory proteins (Naips) that act functionally upstream of NLRC4. Mice use Naip1, Naip2, and Naip5/6 to detect the PrgI needle, the PrgJ rod subunit, and flagellin, respectively (10–12). There is evidence for direct interactions of Naip5 with the conserved N- and C-terminal helices of flagellin (13), and chimera studies of the Naip5 sensor revealed an ∼300-aa stretch as being important for oligomerization in response to flagellin (14). In contrast to mice, the human genome encodes a single genetic locus for Naip (15). Previous studies indicate that human Naip detects the PrgI needle protein, but does not detect flagellin (11, 12, 16).
In this study, we show that primary human macrophages respond rapidly to intracellular flagellin during Salmonella infection or when flagellin is delivered to the cytosol. This response to cytosolic flagellin is dependent on expression of a full-length Naip isoform. We demonstrate that the human monocytic tumor cells, U937, have a 30-fold reduction in levels of this full-length Naip transcript compared with primary human macrophages. These results reveal that the differential response to cytosolic flagellin between U937 cells and primary macrophages can be explained by the expression of full-length Naip. Therefore, primary macrophages that express this transcript are equipped with enhanced defense mechanisms.
Materials and Methods
Bacterial strains
Bacterial wild-type (WT) strains include S. Typhimurium SL1344 and S. Typhi Ty2. Salmonella mutant strains were generated via λ red recombination as described (17) and include the S. Typhimurium mutant ΔFla (fliC::Cm, fljAB::Kan) (18) and the S. Typhi mutants ΔFla (fliC::Kan, this study), ΔprgJ (prgJ::Kan, this study), and ΔprgI (prgI::Kan, this study).
Tissue culture infections and cytosolic delivery of flagellin and needle protein
Human primary monocyte–derived macrophages (MDM) were prepared by adherence from whole-blood buffy coat fractions from healthy donors. Primary monocytes were cultured in RPMI 1640 with 10% FBS and treated with 30 ng/ml human M-CSF for 6 d. Human U937 monocytes were differentiated in RPMI 1640 with 10% FBS and 50 ng/ml PMA for 48 h. Salmonella infections were carried out under SPI-1–inducing conditions (18), centrifuged for 15 min at 1500 rpm, and placed at 37°C, 5% CO2 for 1 h (30 min for bacterial entry assay). Recombinant and endotoxin-free Bacillus anthracis lethal factor–coupled proteins (LFn-FlaA, LFn-FliC, LFn-PrgI, LFn-FlaAAAA, LFn-FliCAAA) or the LFn domain alone were generated and purified from Escherichia coli as previously described (19). B. anthracis protective Ag (PA, 1 μg/ml; EMD Millipore) in macrophage medium was used with 4 μg/ml B. anthracis lethal factor–coupled protein for 5 h to induce IL-1β release and cell death, respectively.
Cytokine measurement and cell death assay
IL-1β (R&D Systems) was measured by ELISA. Cell death was calculated from lactate dehydrogenase activity in the supernatant via the CytoTox 96 assay (Promega).
Immunoblotting
Protein extracts were separated by SDS-PAGE and membranes were exposed to goat anti-human caspase-1 (R&D Systems, AF6215) or goat anti–β-actin (R&D Systems, AF-201-NA) and subsequently incubated with horseradish-coupled secondary Ab (Promega). Proteins were detected using ECL films (Amersham Hyperfilm, Fisher Scientific).
Real-time quantitative PCR analysis
Total RNA was isolated from MDM, THP-1, or U937 cells using an RNeasy mini kit (Qiagen) on column DNase treated (Promega) and reverse transcribed using Invitrogen’s SuperScript III First Strand Synthesis System. Quantitative PCR was performed using FastStart Universal SYBR Green Master mix (Roche). The following primers were used: Naip*, forward, 5′-CTG GAT AAG TTC CTG TGC CTG A-3′, reverse, 5′-AGG ATC ATA CTC AGC TGA AAT TTG G-3′; GAPDH, forward, 5′-TGC ACC ACC AAC TGC TTA GC-3′, reverse, 5′-GGC ATG GAC TGT GGT CAT GAG-3′. The amounts of mRNA analyzed were normalized to GAPDH.
Transient transfections
U937 cells were transfected with empty vector control or the human Naip cDNA (in this study, Naip*, BIRC1 isoform 1, NM_004536.2) in pCMV6-XL5 (OriGene) using Lipofectamine 2000 (Invitrogen) according to the manufacturers’ instructions.
Stable short hairpin RNA knockdown
The plasmid pLKO.1_puro (Addgene) harboring a human Naip-specific short hairpin RNA (shRNA; TRCN0000063733) upstream of a puromycin-resistant cassette was transduced into U937 cells by lentiviral infection. Puromycinr U937 cells were differentiated for functional analysis.
Statistical analysis
Prism 6.0 (GraphPad Software) was used for statistical analysis and significance was determined as indicated in the figure legends.
Results and Discussion
Salmonella flagellin triggers inflammasome activation in primary human macrophages
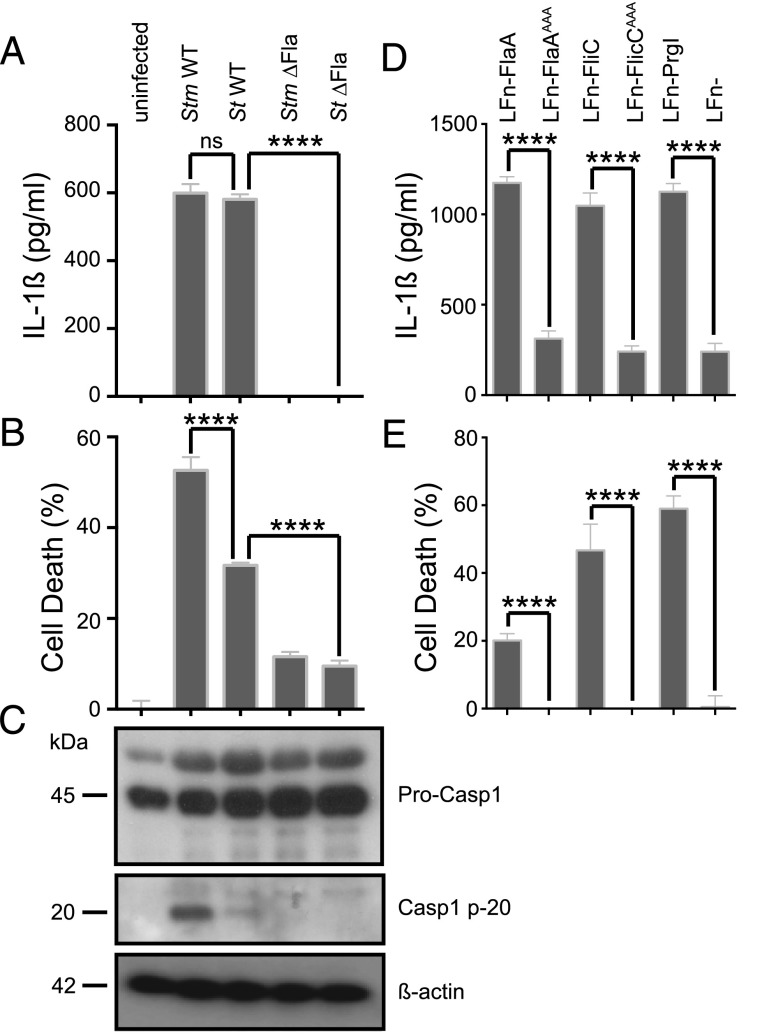
Mouse bone marrow–derived macrophages sense flagellin via the intracellular Naip5-NLRC4 sensor apparatus (10, 11). Humans encode a single Naip gene, previously shown to detect the T3SS needle protein, PrgI, but not flagellin (11, 12, 16). This seems counterintuitive, as flagellin is a very potent trigger of acute inflammatory processes (20) and the human host is routinely challenged with flagellated pathogens. We predicted that human primary MDM from healthy donors could have a more effective molecular sensor arsenal to detect cytosolic flagellin than macrophage-like immortalized cell lines. To test this notion, we infected primary MDM from healthy donors with WT and nonflagellated S. Typhimurium and S. Typhi (ΔFla) strains. Importantly, infection conditions were chosen such that primary MDM were infected with identical levels of intracellular S. Typhimurium or S. Typhi (Supplemental Fig. 1A). WT S. Typhimurium and S. Typhi strains induced a significant increase in IL-1β secretion by MDM compared with flagellin-deficient strains and uninfected control cells (Fig. 1A). However, IL-6 release was not affected by the presence of flagellin (Supplemental Fig. 1B). Additionally, WT S. Typhimurium and S. Typhi induced significantly more death of infected MDM compared with the isogenic flagellin-deficient strains (Fig. 1B). S. Typhi has more effective immune evasion strategies, which explains the reduction in pyroptosis during its infection (21).
FIGURE 1.
Flagellin stimulates inflammasome activation in primary human macrophages. Human MDM were primed with 100 ng/ml LPS and infected with log phase WT Serovars S. Typhimurium (Stm) and S. Typhi (St) or the respective nonflagellated (ΔFla) variant (multiplicity of infection of 50). IL-1β secretion (A) and cytotoxicity (B) were determined 1 h after infection. (C) Activation of pyroptotic caspase-1 (Casp1) processing was visualized by immunoblotting (kDa). (D and E) IL-1β secretion and cell death caused by lethal factor–coupled protein variants were tested as described above for (A) and (B). Graphs show mean and SD of six replicate wells and are representative of at least three independent experiments. Significance was calculated using an unpaired Student t test. ****p < 0.0001. ns, p = 0.1–0.9999.
Pyroptosis and IL-1β release is characterized by activation of the protease caspase-1 (7). As previously published, we observed processing of pro–caspase-1 upon infection with S. Typhimurium as determined by Western blotting for the caspase-1 p20 subunit in the culture supernatant (Fig. 1C). The extent of caspase-1 processing correlated with the level of MDM cell death. However, when macrophages were infected with nonflagellated strains, the caspase-1 p20 subunit was not detected (Fig. 1C). We have previously published that infection of primary bone marrow–derived macrophages with WT S. Typhimurium induces the formation of a single cytoplasmic focus of the adaptor molecule ASC (22). We investigated whether ASC structures might form in primary MDM infected with the human-specific pathogen S. Typhi. Importantly, primary MDM infected with WT S. Typhi contained >20-fold more ASC foci compared with cells infected with the flagellin-deficient strain (Supplemental Fig. 1C).
To selectively activate a flagellin-sensing NLRC4 inflammasome, we delivered Legionella pneumophila and S. Typhimurium flagellin bound to the N-terminal domain of B. anthracis lethal factor (LFn-FlaA and LFn-FliC, respectively) (11, 19) to the cytosol via the anthrax PA channel. Delivery of Legionella and Salmonella flagellin into the cytosol triggered robust IL-1β secretion and cell death in primary human macrophages (Fig. 1D, 1E). The S. Typhimurium PrgI needle protein (LFn-PrgI) also triggered IL-1β secretion and cell death in MDM, similar to what was previously described for monocytic U937 and THP-1 cells (11, 16). Delivery of the N-terminal B. anthracis lethal factor domain alone had no impact on IL-1β secretion or cell death (Fig. 1D, 1E). Additionally, we show that a Salmonella mutant that expresses PrgI but not flagellin stimulates inflammasome activation in MDM at later time points (e.g., 8–24 h) compared with strains that express flagellin (Supplemental Fig. 1E–G). Full activation of the murine Naip5-NLRC4 inflammasome requires three C-terminal leucine residues (5). Alanine substitution of these residues (LFn-FlaAAAA and LFn-FliCAAA) significantly abolished the ability of both intracellular Legionella and Salmonella flagellin to induce IL-1β release and cell death in primary human macrophages (Fig. 1D, 1E). The different LFn fusion proteins did not affect IL-6 secretion (Supplemental Fig. 1D). This indicates that intracellular flagellin is detected with high specificity in both murine cells and primary human macrophages and that the intracellular receptors detect the same conserved region of flagellin, suggesting that they may function by a similar mechanism.
The abundance of a human Naip isoform in primary macrophages and macrophage-like cells reflects differences in Salmonella-induced cell death
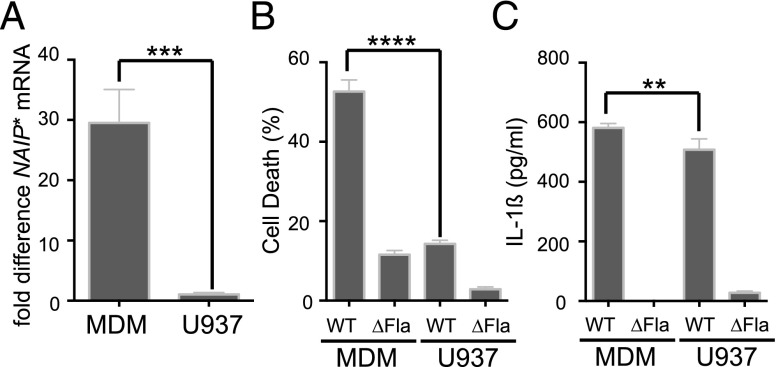
Our data indicate that during a Salmonella infection human macrophages rapidly detect flagellin, which induces caspase-1–dependent pyroptosis and secretion of IL-1β. Why has the inflammasome-activating potential of flagellin in human macrophages not been demonstrated previously? One possibility is that primary human MDM are better equipped to detect flagellin than previously tested macrophage-like cell lines. Interestingly, the human Naip gene has several annotated transcript variants that encode unique NAIP isoforms. One transcript encodes an isoform used in previous studies (Naip) (11, 16) with 65% protein identity to murine Naip5 (Supplemental Fig. 2A). A second full-length transcript encodes an isoform with slightly higher identity to murine Naip5. Naip transcript variant 1 (Naip*) has 68% protein identity to the mouse flagellin sensor Naip5 (Supplemental Fig. 2A). The NAIP* transcript variant contains a nucleotide sequence (nt 3990–4160) that is absent in NAIP mRNA, allowing us to measure specific expression of this isoform in MDM and U937 cells (Supplemental Fig. 2B). With the exception of this internal deletion, the encoded amino acid sequence of NAIP and NAIP* is identical. Fully differentiated MDM contain ∼30-fold more intracellular NAIP* than do differentiated U937 macrophage-like cells (Fig. 2A). These differences in NAIP* mRNA expression correlated with the levels of flagellin-dependent cell death in MDM compared with U937 cells and suggest that human macrophages detect Salmonella flagellin with Naip* (Fig. 2B). Surprisingly, S. Typhimurium infection elicited high amounts of IL-1β secretion in both MDM and U937 cells (Fig. 2C). Recent single-cell analysis of caspase-1 dynamics revealed that dead macrophages are the main source of IL-1β secretion within cell populations (23). In contrast to MDM, U937 cells have a prolonged increase in IL-1 gene transcription in response to LPS with a gradual accumulation of cytosolic IL-1 gene products, including IL-1β (24), which explains the high IL-1β secretion despite reduced cell death. However, we decided to also test the monocytic THP-1 cell line for a correlation of NAIP* abundance and inflammasome activation (Supplemental Fig. 2C–E). Similar to U937 cells, THP-1 cells harbor significantly less NAIP* transcript with a direct effect on cell death (>50% reduction compared with MDM) and IL-1β release (>5-fold reduction compared with MDM) after infection with S. Typhimurium. An alternative primer sequence has been used previously to determine intracellular transcript levels of the already described human Naip isoform (11). Quantitative PCR analysis using the respective oligonucleotides revealed that MDM contain significantly more NAIP transcript than do THP-1 or U937 cells (Supplemental Fig. 2F). The significantly reduced steady-state levels of NLRC4 transcript in THP-1 and U937 cells compared with MDM also reflects this trend and suggests an overall deficiency of human monocytic cell lines in inflammasome sensor molecules (Supplemental Fig. 2F).
FIGURE 2.
Flagellin-dependent inflammasome activation correlates with the expression level of a human Naip isoform. (A) Quantitative RT-PCR was performed to assess the amounts of transcript isoform NAIP* expressed in primary macrophages (MDM) and differentiated U937 cells and normalized to GAPDH. (B and C) MDM and differentiated U937 cells were tested for their ability to undergo Salmonella-induced cell death and secretion of IL-1β (as described). Results are representative of at least two or more independent experiments. Significance was determined using an unpaired Student t test. **p < 0.05, ***p < 0.001, ****p < 0.0001.
Complementation with Naip* isoform in U937 monocytes increases cell death during Salmonella infection
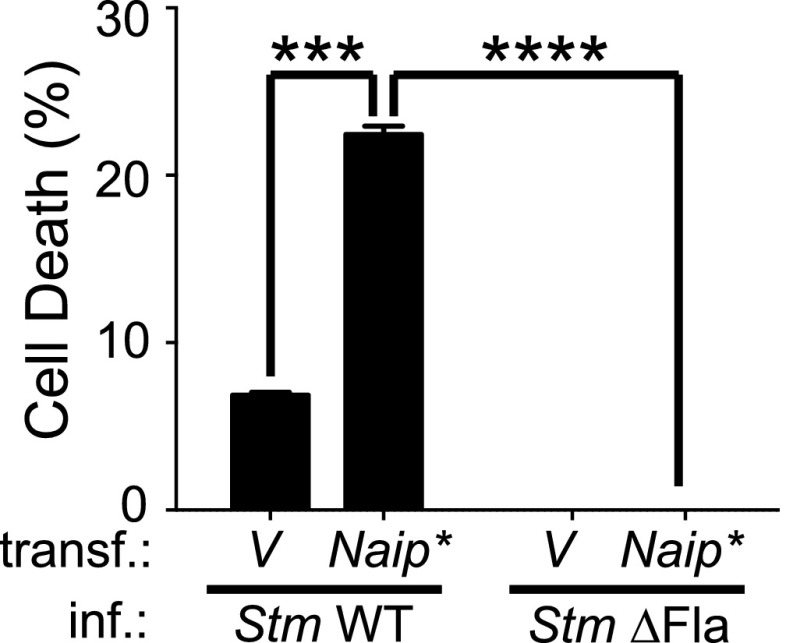
Our data suggest a correlation between high levels of intracellular human Naip transcript (NAIP*) and increased flagellin-induced cell death in human macrophages. NAIP* is poorly expressed in U937 cells as determined by quantitative PCR analysis (Fig. 2A). Thus, we hypothesized that U937 cells expressing increased levels of NAIP* would restore the ability of this cell line to undergo cell death in response to Salmonella flagellin. Indeed, transfection of undifferentiated U937 cells with a vector ectopically expressing Naip* significantly enhanced cell death induced by WT S. Typhimurium and S. Typhi (Fig. 3, Supplemental Fig. 2G). Importantly, and in agreement with our previous results, enhanced death in the presence of Naip* was only triggered by infection with WT S. Typhimurium and S. Typhi but not after infection with the respective ΔFla mutant strains (Fig. 3, Supplemental Fig. 2G). Similarly, deletion of the T3SS components PrgI or PrgJ resembled the phenotype of a flagellin-deficient strain (Supplemental Fig. 2G). To ensure that similar levels of bacteria invaded both cell types, we quantified intracellular invasion at the time of infection and found that S. Typhimurium and S. Typhi ΔFla variants as well as the corresponding isogenic WT strains invaded MDM and U937 cells to the same extent (Supplemental Fig. 1A).
FIGURE 3.
Expression of human Naip isoform that responds to flagellin in U937 monocytes increases cell death during Salmonella infection. Undifferentiated U937 cells were transfected with either an empty vehicle (V) or a construct containing the Naip* isoform (Naip*) under control of a CMV promoter. Twenty-four hours later the cells were infected with log phase S. Typhimurium (Stm) variants as indicated and the amount of cell death was determined by lactate dehydrogenase assay. Statistics were done using an unpaired Student t test. ***p < 0.001, ****p < 0.0001.
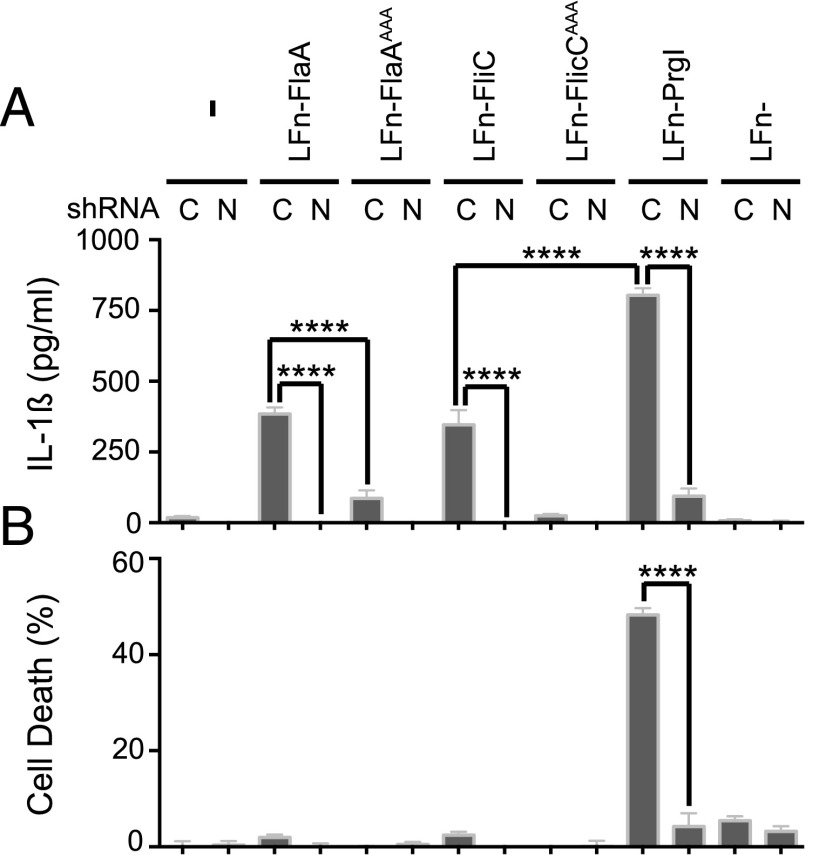
Depleting cells of human Naip isoform reduced flagellin-dependent inflammasome activation
As described earlier, U937 cells express lower amounts of NAIP* transcript compared with primary MDM. However, the level of Naip* sensor in the U937 cells was still sufficient to cause inflammasome activation in a flagellin-dependent manner (Fig. 2B, 2C). We tested whether U937 cells that are selectively depleted of Naip* would be impaired in their ability to detect cytoplasmic flagellin. We knocked down Naip expression in U937 cells by stably expressing a Naip-directed shRNA (Supplemental Fig. 2H) followed by treatment with the lethal factor–coupled fusions as described earlier (Fig. 1D, 1E). Strikingly, the depletion of human NAIP* dramatically reduced inflammasome activation in response to intracellular flagellin delivered via the anthrax PA pore and resulted in significantly reduced release of IL-1β (Fig. 4A). As expected, U937 control cells expressing a nonfunctional shRNA treated with PA plus LFn-FlaA or LFn-FliC secreted robust amounts of IL-1β (Fig. 4A). Consistent with what we saw in primary MDM (Fig. 1D, 1E), the mutant flagellin constructs that are lacking the C-terminal leucine residues did not activate the inflammasome (Fig. 4A). In good agreement with previously published data (11, 16), the U937 control cells released IL-1β after treatment with the LFn-PrgI fusion. Furthermore, LFn-PrgI but not LFn-FlaA or LFn-FliC induced robust cell death in U937 cells expressing the control shRNA (Fig. 4B). One possible explanation for the differences in PrgI- and flagellin-dependent inflammasome activation could be due to differences in the amounts of PrgI needle (80 aa) delivered through the PA pore compared with the amounts of flagellin fusion proteins (FlaA, 475 aa; FliC, 494 aa) delivered into the cytosol. Finally, knockdown of NAIP* resulted in drastic loss of PrgI-induced IL-1β release and cell death (Fig. 4), which is similar to previously published data (16). Collectively, our findings reveal that human-derived cells are able to sense both cytosolic flagellin and needle protein from pathogens and that intracellular detection in human cells requires a full-length isoform of the Naip sensor. We show that the full-length Naip* isoform as well as the NLRC4 sensor are abundant in primary MDM from healthy donors but are dramatically reduced in monocytic U937 and THP-1 cells, rendering them less sensitive to bacterial flagellin. Resembling their murine counterparts (5), the human Naip-NLRC4 sensor complex targets leucine residues in the C-terminal region of flagellin. This raises further interesting questions about the evolution of the inflammasome in different mammalian hosts. The cytosolic pathogen recognition system in mice is based on a division of labor by four Naip proteins expressed from four different genes (10–12). It is tantalizing to speculate that the human cytosolic-sensor toolbox is based on receptor variations made from a single gene. Indeed, the human Naip gene acquired multiple tissue-specific promoters by recruitment of endogenous retroviral elements and has undergone extensive genomic rearrangements resulting in expression of several Naip paralogs (25). There is also evidence of increased Naip gene duplications in populations with high exposure to flagellated pathogens (26). In the future, it will be important to investigate how the constant exposure to flagellated pathogens shaped the genomic context of the human Naip sensor and which Naip isoforms are specialized in detection of specific bacterial agonists.
FIGURE 4.
Depletion of the human Naip sensor decreases inflammasome activation in response to flagellin. Fully differentiated U937 cells stably expressing either a scrambled control shRNA or Naip-specific shRNA were treated with either PA alone (−) or in combination with LFn-fusion proteins as indicated. IL-1β secretion (A) and cell death (B) were determined following each stimulus. Results are representative of at least two or more independent experiments. Statistics were done using an unpaired Student t test. ****p < 0.0001. C, control shRNA; N, Naip-specific shRNA.
Supplementary Material
Acknowledgments
We thank Lilian Lam, Kelly Storek, and Nicholas A. Eisele for comments and suggestions on the manuscript, all members of the Monack laboratory for constant support during the course of this work, and Russel E. Vance and Jeannette Tenthorey for providing the LFn-FlaA expression construct.
This work was supported by German Research Foundation Postdoctoral Fellowship Ko 4584/1-1 (to J.K.), National Institutes of Health Training Fellowship 5 T32 AI 7328-27 (to S.W.B.), and National Institute of Allergy and Infectious Diseases Grants AI095396 and AI08972 (to D.M.M.).
The online version of this article contains supplemental material.
- MDM
- monocyte-derived macrophage
- Naip
- NLR family, apoptosis inhibitory protein
- NLRC
- NLR family CARD domain–containing protein
- PA
- protective Ag
- shRNA
- short hairpin RNA
- SPI-1
- Salmonella pathogenicity island 1
- S. Typhi
- Salmonella enterica serovar Typhi
- S. Typhimurium
- Salmonella enterica serovar Typhimurium
- T3SS
- type III secretion system
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Broz P., Ohlson M. B., Monack D. M. 2012. Innate immune response to Salmonella typhimurium, a model enteric pathogen. Gut Microbes 3: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fàbrega A., Vila J. 2013. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin. Microbiol. Rev. 26: 308–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen-Nissen E., Smith K. D., Bonneau R., Strong R. K., Aderem A. 2007. A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin. J. Exp. Med. 204: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y. H., Rolán H. G., Tsolis R. M. 2007. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J. Biol. Chem. 282: 33897–33901. [DOI] [PubMed] [Google Scholar]
- 5.Lightfield K. L., Persson J., Brubaker S. W., Witte C. E., von Moltke J., Dunipace E. A., Henry T., Sun Y. H., Cado D., Dietrich W. F., et al. 2008. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 9: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchi L., Amer A., Body-Malapel M., Kanneganti T. D., Ozören N., Jagirdar R., Inohara N., Vandenabeele P., Bertin J., Coyle A., et al. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat. Immunol. 7: 576–582. [DOI] [PubMed] [Google Scholar]
- 7.Mariathasan S., Monack D. M. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7: 31–40. [DOI] [PubMed] [Google Scholar]
- 8.Miao E. A., Mao D. P., Yudkovsky N., Bonneau R., Lorang C. G., Warren S. E., Leaf I. A., Aderem A. 2010. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl. Acad. Sci. USA 107: 3076–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao E. A., Warren S. E. 2010. Innate immune detection of bacterial virulence factors via the NLRC4 inflammasome. J. Clin. Immunol. 30: 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kofoed E. M., Vance R. E. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y., Yang J., Shi J., Gong Y. N., Lu Q., Xu H., Liu L., Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 596–600. [DOI] [PubMed] [Google Scholar]
- 12.Rayamajhi M., Zak D. E., Chavarria-Smith J., Vance R. E., Miao E. A. 2013. Cutting edge: mouse NAIP1 detects the type III secretion system needle protein. J. Immunol. 191: 3986–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halff E. F., Diebolder C. A., Versteeg M., Schouten A., Brondijk T. H., Huizinga E. G. 2012. Formation and structure of a NAIP5-NLRC4 inflammasome induced by direct interactions with conserved N- and C-terminal regions of flagellin. J. Biol. Chem. 287: 38460–38472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenthorey J. L., Kofoed E. M., Daugherty M. D., Malik H. S., Vance R. E. 2014. Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol. Cell 54: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diez E., Lee S. H., Gauthier S., Yaraghi Z., Tremblay M., Vidal S., Gros P. 2003. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 33: 55–60. [DOI] [PubMed] [Google Scholar]
- 16.Yang J., Zhao Y., Shi J., Shao F. 2013. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc. Natl. Acad. Sci. USA 110: 14408–14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broz P., Newton K., Lamkanfi M., Mariathasan S., Dixit V. M., Monack D. M. 2010. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207: 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Moltke J., Trinidad N. J., Moayeri M., Kintzer A. F., Wang S. B., van Rooijen N., Brown C. R., Krantz B. A., Leppla S. H., Gronert K., Vance R. E. 2012. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liaudet L., Szabó C., Evgenov O. V., Murthy K. G., Pacher P., Virág L., Mabley J. G., Marton A., Soriano F. G., Kirov M. Y., et al. 2003. Flagellin from gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock 19: 131–137. [DOI] [PubMed] [Google Scholar]
- 21.Winter S. E., Winter M. G., Atluri V., Poon V., Romão E. L., Tsolis R. M., Bäumler A. J. 2015. The flagellar regulator TviA reduces pyroptosis by Salmonella enterica serovar Typhi. Infect. Immun. 83: 1546–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broz P., von Moltke J., Jones J. W., Vance R. E., Monack D. M. 2010. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T., Yamaguchi Y., Shirasaki Y., Shikada K., Yamagishi M., Hoshino K., Kaisho T., Takemoto K., Suzuki T., Kuranaga E., et al. 2014. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Reports 8: 974–982. [DOI] [PubMed] [Google Scholar]
- 24.Ucla C., Roux-Lombard P., Fey S., Dayer J. M., Mach B. 1990. Interferon gamma drastically modifies the regulation of interleukin 1 genes by endotoxin in U937 cells. J. Clin. Invest. 85: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romanish M. T., Lock W. M., van de Lagemaat L. N., Dunn C. A., Mager D. L. 2007. Repeated recruitment of LTR retrotransposons as promoters by the anti-apoptotic locus NAIP during mammalian evolution. PLoS Genet. 3: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boniotto M., Tailleux L., Lomma M., Gicquel B., Buchrieser C., Garcia S., Quintana-Murci L. 2012. Population variation in NAIP functional copy number confers increased cell death upon Legionella pneumophila infection. Hum. Immunol. 73: 196–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.