Abstract
Background
Bevacizumab combined with modified FOLFOX6 is a standard regimen for colorectal cancer. The present study was to determine the efficacy and safety of bevacizumab-modified FOLFOX6 regimen in heavily pretreated patients with human epidermal growth factor receptor 2 (HER2/neu)-negative MBC.
Methods
Bevacizumab, 5 mg/kg every two weeks or 7.5 mg/kg every three weeks, was administered with modified FOLFOX6 (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, 5-FU 400 mg/m2 on day 1, followed by 5-FU 2400 mg/m2 intravenous infusion over 46 hours every 2 weeks) to patients who failed at least 1 chemotherapy regimen in the metastatic setting. The primary objective was progression free survival (PFS). Secondary objectives included objective response rate (ORR), clinical benefit rate (CBR), overall survival (OS), safety, and the change of tumor size and Eastern Cooperative Oncology Group (ECOG) performance status.
Results
69 patients were enrolled. The median PFS was 6.8 months (95% CI, 5.0 to 8.5 months), ORR was 50.0% and median OS was 10.5 months (95% CI, 7.9 to 13.1 months). Patients showing objective responses had a 4.2-month median PFS gain and 5.7-month median OS gain compared with those who did not (P < 0.05). Grade 3 or 4 adverse events occurring in more than one patient were neutropenia (53/69, 76.8%), leukopenia (36/69, 52.2%), thrombocytopenia (13/69, 18.8%), anemia (3/69, 4.3%) and hypertension (3/69, 4.3%).
Conclusions
Adding bevacizumab to modified FOLFOX6 does have significant anti-tumor activity and good safety profile in heavily pretreated HER2/neu-negative MBC patients. Further trials are required to confirm whether the high ORR can translate into a long-term PFS and even OS benefit.
Trial Registration
Introduction
A majority of metastatic breast cancer (MBC) patients will succumb to their disease within 2 years of diagnosis [1]. Despite significant efficacy of taxanes and anthracyclines, nearly all patients will eventually develop drug resistance, and subsequent chemotherapy regimens are frequently required. Oxaliplatin, 5-fluorouracil (5-FU) and leucovorin (LV) comprise a series of FOLFOX regimens for adjuvant or palliative treatment in colorectal cancer, with high efficacy and good safety profile. Data showed that those agents were well tolerated and potentially active in heavily pretreated MBC [2–4]. A phase II clinical trial in our institution demonstrated that modified FOLFOX6 (mFOLFOX6) served as a potentially effective salvage regimen with favorable toxicity in heavily pretreated MBC patients [5].
Bevacizumab, a humanized monoclonal antibody, produces angiogenesis inhibition by inhibiting vascular endothelial growth factor A (VEGF-A) [6]. Adding bevacizumab to the FOLFOX4 and mFOLFOX6 regimens are shown to be more effective for patients with metastatic colorectal cancer than FOLFOX4 and mFOLFOX6 regimens [7–9]. However, its long-term impact in breast cancer is still not clear. In the neoadjuvant setting, adding bevacizumab to chemotherapy significantly increases the pathological complete response rate in human epidermal growth factor receptor 2 (HER2/neu)-negative breast cancer [10–12]. In metastatic setting, bevacizumab combined with weekly paclitaxel for stage IV disease has a median progression free survival (PFS) of 10.4 to 11.8 months [13–15], which is listed as one of the first-line treatments by National Comprehensive Cancer Network (NCCN) guideline [16]. Although none of all published bevacizumab-based trials shows prolongation of overall survival (OS), its value in control of disease has been consistently confirmed whether combined with different chemotherapeutic agents or used in different clinical settings, like first- and second-line [17–19], and even later setting [20]. Further, a lot of studies are actively ongoing to explore bevacizumab maintenance therapy and drug resistance [21–23], other anti-angiogenesis agents, and relevant predictive biomarkers [24, 25].
Given the above encouraging data of bevacizumab and a series of FOLFOX regimens, the present phase II study was initiated to evaluate the efficacy and safety of combining bevacizumab with mFOLFOX6 (bevacizumab-mFOLFOX6) for patients with HER2/neu-negative MBC who had received one to six cytotoxic regimens in metastatic setting.
Patients and Methods
Patients
Inclusion criteria included patients with a histologically confirmed HER2/neu-negative MBC, age ≥ 18 years, more than 12-week of life expectancy, Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2 [26], and at least one extracranial measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [27], that had not been previously irradiated. Enrolled patients had to have at least 1 prior chemotherapy regimens for the metastatic disease, and were pretreated with anthracyclines and taxanes. Patients were required to complete all prior chemotherapy or radiotherapy at least 3 weeks before study entry.
Patients were excluded if they had no objective response to the prior treatments of oxaliplatin, capecitabine or continuous infusion of 5-FU. Patients, whose cumulative doses of doxorubicin and epirubicin exceeded 360 mg/m2 and 720 mg/m2 respectively, were also excluded.
Study Design and Objectives
This was an open-label, single-arm, phase II study (Trial Registration: http://www.clinicaltrials.gov NCT 01658033). The recruitment was from July 6, 2012 to November 29, 2013. Patients were treated with bevacizumab, 5 mg/kg every two weeks or 7.5 mg/kg every three weeks on day 1, and mFOLFOX6 every 2 weeks. It included oxaliplatin 85 mg/m2, LV 400 mg/m2, 5-Fu 400 mg/m2 intravenous bolus on day 1, followed by 5-Fu 2400 mg/m2 intravenous infusion over 46 hours. The bevacizumab-mFOLFOX6 regimen was repeated until progression disease (PD), unacceptable toxicity, death, or withdrawal of informed consent. Overall, two dose reductions for toxicities were allowed; if dose reduction was required for the third time, the treatment was discontinued. The primary objective was PFS. Secondary objectives included ORR, clinical benefit rate (CBR), OS, toxicity profiles, the change of tumor size and ECOG performance status from baseline. The follow-up time was from the date of first patient enrollment to March 10, 2015. This study was performed in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki (1996 version), applicable local regulatory requirements and laws. The study was approved by Fudan University Shanghai Cancer Center Ethic Committee for Clinical Investigation on July 2, 2014 and was carried out in Fudan University Shanghai Cancer Center. In the July, we initiated this trial and registered this trial on the web. There was no delay in the registration of this trial. Written informed consents were obtained from all patients prior to enrollment.
Assessments and Data Collection
Patients underwent clinical examination and radiographic assessment of measurable disease for tumor response every 2 cycles or clinically indicated. The cycle was counted by the treatment of mFOLFOX6 regimen and duration of one cycle was 28 days with twice medications of mFOLFOX6. PFS was defined as the time from enrollment to the first documented date of disease progression or death from any cause. OS was defined as the time from enrollment to the date of death from any cause. ORR was defined as the percentage of patients who achieved complete response (CR) and partial response (PR) by RECIST, version 1.1 [27]. CBR was defined as the percentage of patients who achieved CR, PR and stable disease (SD) ≥ 24 weeks by RECIST, version 1.1 [27]. The change of tumor size was defined as the change of the sum of longest dimensions for target lesions from baseline to maximal tumor shrinkage. Adverse events (AEs) were evaluated, graded and recorded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 [28]. The ECOG performance status was recorded at baseline and before every treatment cycle [26].
Statistics
Compared to the pilot mFOLFOX6 regimen without bevacizumab [5], the planned sample size of 62 patients treated with new bevacizumab-mFOLFOX6 regimen would allow detecting an increase of 2 months in median PFS, with a 5% significance level at 95% power and 10% patient dropout rate. PFS, OS and safety end points were analyzed in patients who received at least one dose of treatment. ORR and CBR were analyzed in patients who had at least one response evaluation data. Statistical analysis of 2×2 contingency tables of categorical variables was carried out using Fisher’s exact test. Median PFS, median OS and their 95% confidence intervals were calculated using Kaplan–Meier method. PFS and OS differences were calculated using Kaplan–Meier method with log-rank test. Factors with P<0.1 in the univariate analysis were examined with Cox proportional hazard model which defined independent predictive factors. The change of ECOG performance status after chemotherapy was analyzed by Wilcoxon signed-rank test. All statistical tests were 2-tailed, with significance defined as P < 0.05. The Statistical Package for the Social Sciences software (SPSS) version 19.0 was used for all statistical analyses [29].
Results
Characteristics of Patients at Baseline
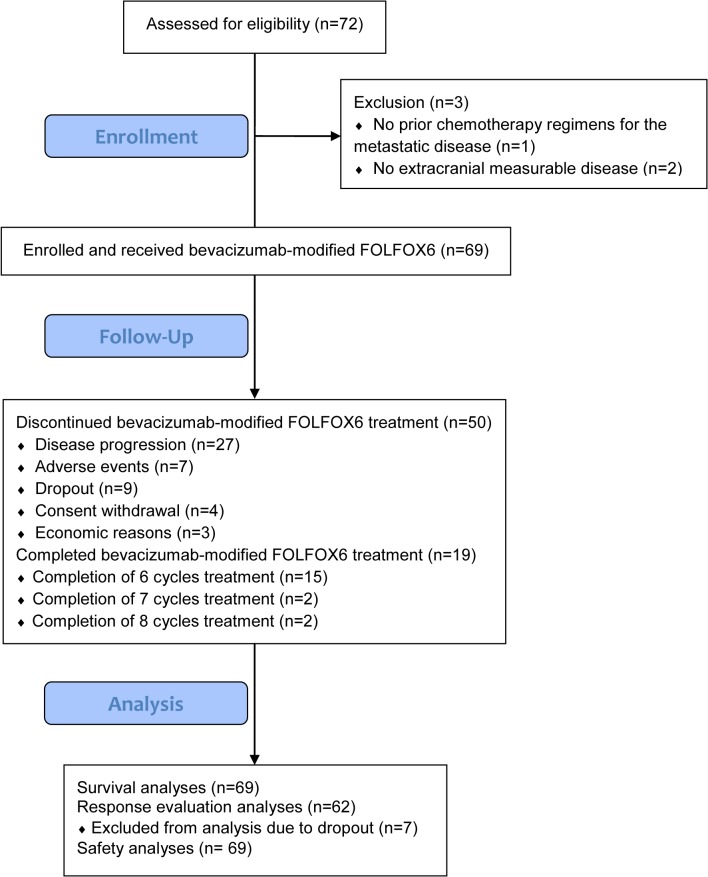
From July 2012 to November 2013, 72 heavily pretreated patients with HER2/neu-negative MBC were enrolled. Three patients were excluded from analysis because one had no prior chemotherapy regimens for the metastatic disease and the other two had no extracranial measurable disease according to the RECIST version 1.1 (Fig 1). The cutoff date for analysis was March 10, 2015, resulting in a median follow-up time of 10.5 months. The characteristics of patients at baseline are listed in Table 1.
Fig 1. The flow diagram of the present phase II clinical study.
Table 1. Patient Characteristics at baseline (N = 69).
Abbreviations: ECOG, Eastern Cooperative Oncology Group; MBC, metastatic breast cancer.
| Characteristics | No. | % |
|---|---|---|
| Age (Median, range) | 49, 28–73 | |
| < 60 years | 56 | 81.2 |
| ≥ 60 years | 13 | 18.8 |
| Menstruation status | ||
| Post-menopausal | 43 | 62.3 |
| Pre-menopausal | 26 | 37.7 |
| ECOG performance status | ||
| 0 | 2 | 2.9 |
| 1 | 61 | 88.4 |
| 2 | 6 | 8.7 |
| Molecular subtype | ||
| Luminal A | 5 | 7.2 |
| Luminal B | 32 | 46.4 |
| Triple-negative | 32 | 46.4 |
| Number of metastatic sites | ||
| < 3 | 25 | 36.2 |
| ≥ 3 | 44 | 63.8 |
| Metastatic sites | ||
| Lymph nodes | 49 | 71.0 |
| Bone | 42 | 60.9 |
| Liver | 40 | 58.0 |
| Lung | 36 | 52.3 |
| Chest wall recurence | 30 | 43.5 |
| Pleura | 16 | 23.2 |
| Brain | 7 | 10.1 |
| Contralateral breast | 3 | 4.3 |
| Others | 2 | 2.9 |
| Visceral metastasis | ||
| Yes | 58 | 84.1 |
| No | 11 | 15.9 |
| Disease-free interval* | ||
| > 12 months | 47 | 74.6 |
| ≤ 12 months | 16 | 25.4 |
| Prior Adjuvant Chemotherapy | ||
| Anthracyclines | 62 | 89.9 |
| Taxanes | 44 | 63.8 |
| Prior chemotherapy regimens for MBC (Median, range) | 2, 1–6 | |
| ≥ 3 | 31 | 44.9 |
| 2 | 17 | 24.6 |
| 1 | 21 | 30.4 |
| Prior chemotherapy drug | ||
| Taxanes | 68 | 98.6 |
| Anthracyclines | 65 | 94.2 |
| Gemcitabine | 48 | 69.6 |
| Capecitabine | 32 | 46.4 |
| Vinorelbine | 31 | 44.9 |
* There were no disease-free interval data for 6 patients, because they had metastatic sites when first diagnosed and did not receive radical surgery.
Treatment Exposure
69 patients received at least one dose of assigned medical treatment. At the last follow-up on March 10, 2015, patients received a median of 4.0 treatment cycles (range, 0.5 to 8.0). Seven cases had their treatment discontinued due to AEs, including 4 cases with grade 3 or 4 thrombocytopenia who had not recovered within additional two weeks of delays; these 4 cases received 1.0, 2.0, 4.5 and 5.0 cycles of bevazicumab-mFOLFOX6 regimen, respectively; 2 cases with cardiac events (1 with 18% decrease in left ventricular ejection fraction compared with baseline; 1 with moderate supraventricular ectopic beats with ST segment depression and T wave changes); these 2 patients had 0.5 and 2.0 treatment cycles before therapy was discontinued; 1 case with grade 3 wound healing complications and the patient received 2 treatment cycles before therapy was discontinued. The relative dose intensity was 99.9% for bevacizumab (range, 81.6% to 110.1%), 99.1% for 5-FU (range, 82.6% to 104.82%), 99.1% for LV (range, 87.6% to 103.8%), and 98.1% for oxaliplatin (range, 71.8% to 105.0%), respectively.
Efficacy
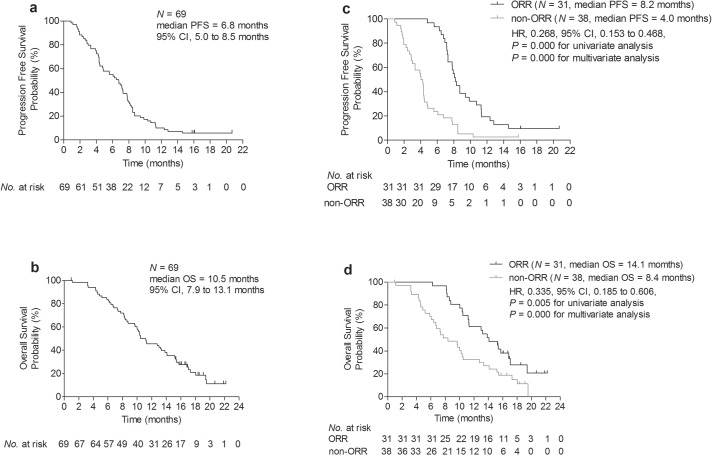
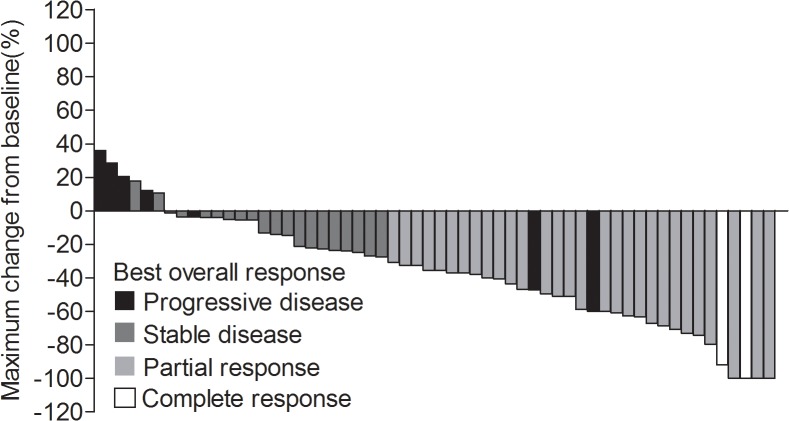
Of the 69 enrolled patients, 94.2% patients (65/69) had had PD and 79.7% patients (55/69) had died at data cutoff. As shown in Fig 2A and 2B, the median PFS was 6.8 months (95% confidence interval [CI], 5.0 to 8.5 months) and the median OS was 10.5 months (95% CI, 7.9 to 13.1 months). Fig 2C and 2D showed that patients showing objective responses had a longer PFS and OS than those who did not (P < 0.05). Among 62 patients eligible for response evaluation, an ORR was achieved in 31 (50.0%) patients including 2 complete response and 29 partial responses. The CBR was achieved in 35 (56.5%) patients including 2 CR, 29 PR and 4 SD ≥24 weeks. The change in tumor size (sum of longest dimensions for target lesions) from baseline to maximal tumor shrinkage was shown in Fig 3.
Fig 2. Kaplan–Meier plot of PFS (a) and OS (b) for all patients and Kaplan-Meier plot of PFS (c) and OS (d) in patients with ORR versus non-ORR.
Fig 3. Waterfall plot of the maximum change in tumor size showed best overall responses in each patient.
Factors predicting clinical outcome
According to the clinical significance and literature published [24, 30, 31], age, ECOG performance status, disease free interval, molecular subtype, menopausal status, metastatic sites at baseline (visceral metastases, yes vs. no), number of metastasis sites (< 3 vs. ≥ 3), objective response status (ORR vs. non-ORR), lines of bevacizumab-mFOLFOX6 regimen treatment (2 vs. ≥ 3), prior chemotherapy drug, hypertension and proteinuria were included for analysis (S1 Table and S2 Table). In univariate analysis, PFS and OS was significantly longer among patients achieving objective response (for PFS, ORR vs. non-ORR, 8.2 month vs. 4.0 month, P = 0.000; for OS, ORR vs. non-ORR, 14.1 month vs. 8.4 month, P = 0.005) [Fig 2C and 2D]. In multivariable analysis, independent factors for PFS with statistical significance were objective response (HR, 0.268, 95% CI: 0.153 to 0.468, P = 0.000), non-TNBC pathology (HR, 0.484, 95% CI: 0.281 to 0.833, P = 0.009) and number of metastasis sites (HR, 1.210, 95% CI: 1.004 to 1.459, P = 0.045). Independent factors for OS with statistical significance were objective response (HR, 0.335, 95% CI: 0.185 to 0.606, P = 0.000), older age (HR, 0.954, 95% CI: 0.926 to 0.983, P = 0.002) and number of metastasis sites (HR, 1.420, 95% CI: 1.156 to 1.743, P = 0.001).
Safety
AEs are presented in Table 2. Most AEs were grade 1 or 2. Grade 3 or 4 AEs occurring in more than one patient were neutropenia (53/69, 76.8%), leukopenia (36/69, 52.2%), thrombocytopenia (13/69, 18.8%), anemia (3/69, 4.3%), hypertension (3/69, 4.3%) and febrile neutropenia (2/69, 2.9%). There were no severe AEs due to bevacizumab-mFOLFOX6 regimen, and specifically no severe AEs related patient deaths, neither during treatment nor within 1 month after treatment. The common AEs, which were reported previously and may be related to bevacizumab [8, 32–35], were mostly mild (grade 1 or 2) and included bleeding (23/69, 33.3%), cardiac events (22/69, 31.9%) and proteinuria (11/69, 15.9%). The bleeding was primarily limited to minor mucosal oozing that did not require medical intervention. Cardiac events and proteinuria were limited to clinical documented laboratory reports with no symptoms. There were only one case of grade 3 wound healing complications and three cases of grade 3 hypertension. Furthermore, the ECOG performance status had not increased substantially over cycles of chemotherapy (P = 0.414).
Table 2. AEs (N = 69).
Note: This table included AEs occurring between the date of the first dose and 30 days following the last dose of study treatment. Abbreviations: AEs, adverse events; NA, not applicable.
| AEs | Grade | |||||
|---|---|---|---|---|---|---|
| 1 or 2 | 3 or 4 | Total | ||||
| No. | % | No. | % | No. | % | |
| Non-hematologic | ||||||
| Nausea | 30 | 43.5 | 0 | 0.0 | 30 | 43.5 |
| Sensory neuropathy | 28 | 40.6 | 0 | 0.0 | 28 | 40.6 |
| Vomiting | 25 | 36.2 | 0 | 0.0 | 25 | 36.2 |
| Bleeding | 23 | 33.3 | 0 | 0.0 | 23 | 33.3 |
| Cardiac events | 22 | 31.9 | 0 | 0.0 | 22 | 31.9 |
| Fatigue | 19 | 27.5 | 1 | 1.4 | 20 | 28.9 |
| Diarrhea | 18 | 26.1 | 0 | 0.0 | 18 | 26.1 |
| Mucositis | 10 | 14.5 | 1 | 1.4 | 11 | 15.9 |
| Proteinuria | 11 | 15.9 | 0 | 0.0 | 11 | 15.9 |
| Abdominal pain | 10 | 14.5 | 0 | 0.0 | 10 | 14.5 |
| Liver dysfunction | 9 | 13.0 | 0 | 0.0 | 9 | 13.0 |
| Hypertension | 5 | 7.2 | 3 | 4.3 | 8 | 11.6 |
| Fever | 8 | 11.6 | 0 | 0.0 | 8 | 11.6 |
| Cough | 5 | 7.2 | 0 | 0.0 | 5 | 7.2 |
| Alopecia | 5 | 7.2 | NA | NA | 5 | 7.2 |
| Anorexia | 4 | 5.8 | 0 | 0.0 | 4 | 5.8 |
| Rash | 4 | 5.8 | 0 | 0.0 | 4 | 5.8 |
| Hand-foot syndrome | 3 | 4.3 | 0 | 0.0 | 3 | 4.3 |
| Myalgia | 3 | 4.3 | 0 | 0.0 | 3 | 4.3 |
| Vertigo | 3 | 4.3 | 0 | 0.0 | 3 | 4.3 |
| Infection | 3 | 4.3 | 0 | 0.0 | 3 | 4.3 |
| Arthralgia/Bone pain | 2 | 2.9 | 0 | 0.0 | 2 | 2.9 |
| Febrile neutropenia | NA | NA | 2 | 2.9 | 2 | 2.9 |
| Constipation | 2 | 2.9 | 0 | 0.0 | 2 | 2.9 |
| Headache | 1 | 1.4 | 0 | 0.0 | 1 | 1.4 |
| Abdominal distension | 1 | 1.4 | 0 | 0.0 | 1 | 1.4 |
| Hoarseness | 1 | 1.4 | 0 | 0.0 | 1 | 1.4 |
| Anaphylaxis | NA | NA | 1 | 1.4 | 1 | 1.4 |
| Nail discoloration | 1 | 1.4 | NA | NA | 1 | 1.4 |
| Tinnitus | 1 | 1.4 | 0 | 0.0 | 1 | 1.4 |
| Allergic reaction | 1 | 1.4 | 0 | 0.0 | 1 | 1.4 |
| Palpitations | 1 | 1.4 | NA | NA | 1 | 1.4 |
| Wound healing complications | 0 | 0.0 | 1 | 1.4 | 1 | 1.4 |
| Hematologic | ||||||
| Leukopenia | 29 | 42.0 | 36 | 52.2 | 65 | 94.2 |
| Neutropenia | 11 | 15.9 | 53 | 76.8 | 64 | 92.8 |
| Thrombocytopenia | 24 | 34.8 | 13 | 18.8 | 37 | 53.6 |
| Anemia | 31 | 44.9 | 3 | 4.3 | 34 | 49.3 |
Discussion
This open label, single-arm, phase II study evaluated the efficacy and safety of bevacizumab-mFOLFOX6 regimen in patients with HER-2/neu-negative MBC. Although all patients were heavily pretreated, the bevacizumab-mFOLFOX6 regimen was tolerated reasonably well, with an ORR of 50.0% and CBR of 56.5%. The median PFS was 6.8 months, 1.8-month longer than the anticipated median PFS of 5-month, a result meeting our primary endpoint. Moreover, longer PFS and OS were found in patients with ORR, which was an independent predictor in multivariable analyses. When compared with our previously published study, adding bevacizumab to mFOLFOX6 regimen resulted in 32% improvement in ORR, 3.8-month improvement in median PFS [5].
Compared with other regimens for stage IV disease, our regimen produces comparable or possibly better results. Two trials restricted to second- or third-line treatment showed a median PFS of shorter than 5 months and an ORR of less than 30% [20, 36]. von Minckwitz et al reported an ORR of 21% and a median PFS was 6.3 month with bevacizumab-based regimen as second-line treatment [22], but our trials included a population with poorer prognosis (eg, patients with more than 84% having visceral disease, about 63% having ≥ 3 metastatic sites, 45% undergoing fourth-line therapy, 98% pretreated with taxane therapy and 94% with anthracyclines therapy). Eribulin, one standard drug in later setting, is still not commercially available in China. In terms of OS, bevacizumab-mFOLFOX6 regimen were comparable, but had stronger anti-tumor activity than single-agent eribulin (ORR, 14.1%; median PFS, 2.6 months; S3 Table) [37, 38]. With other chemotherapeutic agents including gemcitabine, vinorelbine, vinflunine and paclitaxel, patients in our trial showed a 2 to 4 months improvement in PFS, and 27% to 50% improvement in ORR (S3 Table) [39–45].
Continuous VEGF inhibition is necessary to maximize the benefit of bevacizumab [46], and recent studies provided evidences, showing that bevacizumab maintenance improved both PFS and OS [47]. In the context of no OS benefit with bevacizumab in other trials, it is risingly important to characterize validated biomarkers predicting bevacizumab treatment outcome. In our present study, the heavily-pretreated HER2/neu-negative breast cancer patients achieving objective responses had a 4.2-month median PFS gain (P = 0.000) and 5.7-month median OS gain (P = 0.005) when compared with those who did not achieve objective responses. Other clinicopathological factors favorably linked to longer PFS included non-TNBC pathology and number of metastasis sites in our cohort. Older age, objective response status and number of metastasis sites were predictive factors for longer OS. It was reported that TNBC patients with particularly prominent VEGFA amplification was associated with poor outcomes when treated with paclitaxel with or without bevacizumab [48]. Therefore, future larger studies were required to confirm that if these encouraging benefits of bevazcizumab-mFOLFOX6 regimen would translate into a long-term survival gains in patients with HER2/neu-negative MBC.
With regard to safety, AE profile of bevacizumab-mFOLFOX6 regimen did not change significantly compared to the previous data published for mFOLFOX6 alone [5] and the increase of grade 3–4 toxicities was restricted to neutropenia (S4 Table) [5]. When compared with the same bevacizumab-mFOLFOX6 regimen in colorectal cancer, our present study in MBC resulted in a somehow higher hematological toxicities (S5 Table) [9, 49–51]. However, febrile neutropenia and dose discontinuation rates were very low in our trial (S5 Table) [9, 49–51], and patients’ ECOG performance status did not worsen over cycles of bevazicumab-mFOLFOX6 treatment (P = 0.414). These results were in line with other clinical trials showing that hematological AEs increase with the bevacizumab treatment [17–20, 52, 53], and these toxicities are manageable.
There were several limitations with our study. First, it was a single-arm phase II clinical trial with limited statistical power. Second, recruited patients were rather heterogeneous in terms of prior chemotherapy regimens, with other likely weaknesses in patient’s characteristics inherent to a phase II design. Notwithstanding, it is unlikely that any of these limitations would substantially alter the qualitative nature of our conclusions.
In summary, our study was the first to evaluate bevacizumab-mFOLFOX6 regimen in heavily pretreated patients with HER2/neu-negative MBC, who were in a situation with very limited additional treatment options. For patients who were in good performance status at the study start, bevacizumab-mFOLFOX6 regimen showed high efficacy and good safety profile with promising objective responses rates, PFS and OS. For all these reasons, a robust controlled phase III trial based on large number of patients is guaranteed to confirm our results of the bevacizumab-mFOLFOX6 combination.
Supporting Information
(DOC)
(PDF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the patients who shared their experiences with our oncologists, as well as the network of investigators, research nurses, study coordinators and operations staff. In particular, we would like to acknowledge Yunhua Lu for helping us with data collection and Minhao Fan for statistical advice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This is an investigator-initiated clinical trial (IIT). All of the work including study design, running of the trial and data collection was done by the investigators. The cost for protocol review and revision as well as statistics came from the grant of Natural Science Foundation of China (grant number: 81372846; the URL: http://www.nsfc.gov.cn/). The medical insurance for recruited patients in the trial was provided by the Fudan University Shanghai Cancer Center, the authors’ institution. No financial support was from pharmaceuticals. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chia SK, Speers CH, D'Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007; 110: 973–979. [DOI] [PubMed] [Google Scholar]
- 2. Pectasides D, Pectasides M, Farmakis D, Bountouroglou N, Nikolaou M, Koumpou M, et al. Oxaliplatin plus high-dose leucovorin and 5-fluorouracil in pretreated advanced breast cancer: a phase II study. Ann Oncol. 2003; 14: 537–542. [DOI] [PubMed] [Google Scholar]
- 3. Zelek L, Cottu P, Tubiana-Hulin M, Vannetzel JM, Chollet P, Misset JL, et al. Phase II study of oxaliplatin and fluorouracil in taxane- and anthracycline-pretreated breast cancer patients. J Clin Oncol. 2002; 20: 2551–2558. [DOI] [PubMed] [Google Scholar]
- 4. Garufi C, Nistico C, Brienza S, Vaccaro A, D'Ottavio A, Zappala AR, et al. Single-agent oxaliplatin in pretreated advanced breast cancer patients: a phase II study. Ann Oncol. 2001; 12: 179–182. [DOI] [PubMed] [Google Scholar]
- 5. Sun S, Wang LP, Zhang J, Yang XY, Zhang QL, Jia Z, et al. Phase II study of oxaliplatin plus leucovorin and 5-fluorouracil in heavily pretreated metastatic breast cancer patients. Med Oncol. 2012; 29: 418–424. 10.1007/s12032-011-9839-6 [DOI] [PubMed] [Google Scholar]
- 6. Vaziri SA, Kim J, Ganapathi MK, Ganapathi R. Vascular endothelial growth factor polymorphisms: role in response and toxicity of tyrosine kinase inhibitors. Curr Oncol Rep. 2010; 12: 102–108. 10.1007/s11912-010-0085-4 [DOI] [PubMed] [Google Scholar]
- 7. Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26: 2013–2019. 10.1200/JCO.2007.14.9930 [DOI] [PubMed] [Google Scholar]
- 8. Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007; 25: 1539–1544. [DOI] [PubMed] [Google Scholar]
- 9. Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008; 26: 3523–3529. 10.1200/JCO.2007.15.4138 [DOI] [PubMed] [Google Scholar]
- 10. von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012; 366: 299–309. 10.1056/NEJMoa1111065 [DOI] [PubMed] [Google Scholar]
- 11. Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015; 33: 13–21. 10.1200/JCO.2014.57.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bear HD, Tang G, Rastogi P, Geyer CJ, Robidoux A, Atkins JN, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012; 366: 310–320. 10.1056/NEJMoa1111097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007; 357: 2666–2676. [DOI] [PubMed] [Google Scholar]
- 14. Lang I, Brodowicz T, Ryvo L, Kahan Z, Greil R, Beslija S, et al. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer: interim efficacy results of the randomised, open-label, non-inferiority, phase 3 TURANDOT trial. Lancet Oncol. 2013; 14: 125–133. 10.1016/S1470-2045(12)70566-1 [DOI] [PubMed] [Google Scholar]
- 15. Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Mayer EL, et al. CALGB 40502/NCCTG N063H: Randomized phase III trial of weekly paclitaxel (P) compared to weekly nanoparticle albumin bound nab-paclitaxel (NP) or ixabepilone (Ix) with or without bevacizumab (B) as first-line therapy for locally recurrent or metastatic breast cancer (MBC). J Clin Oncol. 2012; 30: abstract CRA1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast cancer, version 1.2015. Available: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 17. Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010; 28: 3239–3247. 10.1200/JCO.2008.21.6457 [DOI] [PubMed] [Google Scholar]
- 18. Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011; 29: 1252–1260. 10.1200/JCO.2010.28.0982 [DOI] [PubMed] [Google Scholar]
- 19. Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O'Neill V, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011; 29: 4286–4293. 10.1200/JCO.2010.34.1255 [DOI] [PubMed] [Google Scholar]
- 20. Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005; 23: 792–799. [DOI] [PubMed] [Google Scholar]
- 21. Miller K, M OA, Dang CT, Northfelt DW, Gradishar WJ, Goldstein LJ, et al. Bevacizumab (Bv) in the adjuvant treatment of HER2-negative breast cancer: Final results from Eastern Cooperative Oncology Group E5103. J Clin Oncol. 2014; 32:5s (suppl; abstr 500). [Google Scholar]
- 22. von Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15: 1269–1278. 10.1016/S1470-2045(14)70439-5 [DOI] [PubMed] [Google Scholar]
- 23. Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga JY, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014; 15: 1351–1360. 10.1016/S1470-2045(14)70444-9 [DOI] [PubMed] [Google Scholar]
- 24. Fan M, Zhang J, Wang Z, Wang B, Zhang Q, Zheng C, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat. 2014; 143: 141–151. 10.1007/s10549-013-2793-6 [DOI] [PubMed] [Google Scholar]
- 25. Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014; 135: 1961–1969. 10.1002/ijc.28829 [DOI] [PubMed] [Google Scholar]
- 26. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5: 649–655. [PubMed] [Google Scholar]
- 27. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228–247. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 U.S.A: National Institutes Health Publication; 2010. [Google Scholar]
- 29.SPSS Inc. IBM SPSS Advanced Statistics 19. Armonk, NY; 2010.
- 30. Khoja L, Kumaran G, Zee YK, Murukesh N, Swindell R, Saunders MP, et al. Evaluation of hypertension and proteinuria as markers of efficacy in antiangiogenic therapy for metastatic colorectal cancer. J Clin Gastroenterol. 2014; 48: 430–434. 10.1097/MCG.0b013e3182a8804c [DOI] [PubMed] [Google Scholar]
- 31. Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009; 20: 227–230. 10.1093/annonc/mdn637 [DOI] [PubMed] [Google Scholar]
- 32. Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011; 29: 632–638. 10.1200/JCO.2010.31.9129 [DOI] [PubMed] [Google Scholar]
- 33. Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur J Cancer. 2010; 46: 439–448. 10.1016/j.ejca.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 34. Mir O, Coriat R, Cabanes L, Ropert S, Billemont B, Alexandre J, et al. An observational study of bevacizumab-induced hypertension as a clinical biomarker of antitumor activity. Oncologist. 2011; 16: 1325–1332. 10.1634/theoncologist.2010-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cortes J, Caralt M, Delaloge S, Cortes-Funes H, Pierga JY, Pritchard KI, et al. Safety of bevacizumab in metastatic breast cancer patients undergoing surgery. Eur J Cancer. 2012; 48: 475–481. 10.1016/j.ejca.2011.11.021 [DOI] [PubMed] [Google Scholar]
- 36. Polyzos A, Kalbakis K, Kentepozidis N, Giassas S, Kalykaki A, Vardakis N, et al. Salvage treatment in metastatic breast cancer with weekly paclitaxel and bevacizumab. Cancer Chemother Pharmacol. 2011; 68: 217–223. 10.1007/s00280-010-1475-x [DOI] [PubMed] [Google Scholar]
- 37. Aogi K, Iwata H, Masuda N, Mukai H, Yoshida M, Rai Y, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2012; 23: 1441–1448. 10.1093/annonc/mdr444 [DOI] [PubMed] [Google Scholar]
- 38. Cortes J, Vahdat L, Blum JL, Twelves C, Campone M, Roche H, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010; 28: 3922–3928. 10.1200/JCO.2009.25.8467 [DOI] [PubMed] [Google Scholar]
- 39. Seo HY, Lee HJ, Woo OH, Park KH, Woo SU, Yang DS, et al. Phase II study of vinorelbine monotherapy in anthracycline and taxane pre-treated metastatic breast cancer. Invest New Drugs. 2011; 29: 360–365. 10.1007/s10637-009-9357-y [DOI] [PubMed] [Google Scholar]
- 40. Fumoleau P, Cortes-Funes H, Taleb AB, Chan S, Campone M, Pouget JC, et al. Phase 2 study of single-agent IV vinflunine as third-line treatment of metastatic breast cancer after failure of anthracycline-/taxane-based chemotherapy. Am J Clin Oncol. 2009; 32: 375–380. 10.1097/COC.0b013e31818f2d2f [DOI] [PubMed] [Google Scholar]
- 41. Modi S, Currie VE, Seidman AD, Bach AM, Panageas KS, Theodoulou M, et al. A phase II trial of gemcitabine in patients with metastatic breast cancer previously treated with an anthracycline and taxane. Clin Breast Cancer. 2005; 6: 55–60. [DOI] [PubMed] [Google Scholar]
- 42. Rha SY, Moon YH, Jeung HC, Kim YT, Sohn JH, Yang WI, et al. Gemcitabine monotherapy as salvage chemotherapy in heavily pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005; 90: 215–221. [DOI] [PubMed] [Google Scholar]
- 43. Smorenburg CH, Bontenbal M, Seynaeve C, van Zuylen C, de Heus G, Verweij J, et al. Phase II study of weekly gemcitabine in patients with metastatic breast cancer relapsing or failing both an anthracycline and a taxane. Breast Cancer Res Treat. 2001; 66: 83–87. [DOI] [PubMed] [Google Scholar]
- 44. Rivera E, Holmes FA, Frye D, Valero V, Theriault RL, Booser D, et al. Phase II study of paclitaxel in patients with metastatic breast carcinoma refractory to standard chemotherapy. Cancer. 2000; 89: 2195–2201. [DOI] [PubMed] [Google Scholar]
- 45. Abrams JS, Vena DA, Baltz J, Adams J, Montello M, Christian M, et al. Paclitaxel activity in heavily pretreated breast cancer: a National Cancer Institute Treatment Referral Center trial. J Clin Oncol. 1995; 13: 2056–2065. [DOI] [PubMed] [Google Scholar]
- 46. Bagri A, Berry L, Gunter B, Singh M, Kasman I, Damico LA, et al. Effects of anti-VEGF treatment duration on tumor growth, tumor regrowth, and treatment efficacy. Clin Cancer Res. 2010; 16: 3887–3900. 10.1158/1078-0432.CCR-09-3100 [DOI] [PubMed] [Google Scholar]
- 47. Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga JY, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014; 15: 1351–1360. 10.1016/S1470-2045(14)70444-9 [DOI] [PubMed] [Google Scholar]
- 48. Schneider BP, Gray RJ, Radovich M, Shen F, Vance G, Li L, et al. Prognostic and predictive value of tumor vascular endothelial growth factor gene amplification in metastatic breast cancer treated with paclitaxel with and without bevacizumab; results from ECOG 2100 trial. Clin Cancer Res. 2013; 19: 1281–1289. 10.1158/1078-0432.CCR-12-3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol. 2012; 30: 3588–3595. 10.1200/JCO.2012.42.5355 [DOI] [PubMed] [Google Scholar]
- 50. Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Colangelo LH, Lopa SH, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009; 27: 3385–3390. 10.1200/JCO.2009.21.9220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013; 14: 1278–1286. 10.1016/S1470-2045(13)70490-X [DOI] [PubMed] [Google Scholar]
- 52. Huang H, Zheng Y, Zhu J, Zhang J, Chen H, Chen X. An updated meta-analysis of fatal adverse events caused by bevacizumab therapy in cancer patients. PLoS One. 2014; 9: e89960 10.1371/journal.pone.0089960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Geiger-Gritsch S, Stollenwerk B, Miksad R, Guba B, Wild C, Siebert U. Safety of bevacizumab in patients with advanced cancer: a meta-analysis of randomized controlled trials. Oncologist. 2010; 15: 1179–1191. 10.1634/theoncologist.2009-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.