Abstract
Objective
To provide information about the role of liver fluke infection as a risk factor for hepatobiliary pathological changes and promote awareness among the people living in endemic areas, a systematic review and meta-analysis based on published studies was conducted to examine the association between liver fluke infection and hepatobiliary pathological changes.
Methods
Relevant original literature was searched in multiple literature databases, including PubMed, Cochrane, Clinical Evidence, Trip Database, Clinical Trials, Current Controlled Trials, Web of Science, the China National Knowledge Infrastructure (CNKI) database, and the Wanfang academic journal full-text database. Studies were selected based on strict screening with inclusion and exclusion criteria. Tests of heterogeneity, sensitivity and publication bias were performed with the Review Manager software, version 5.3, and meta-regression analyses were performed with the Stata software, version 11.0 (Stata Corporation, College Station, TX, USA). Pooled risk ratios (RRs) and odds ratios (ORs) with their 95% confidence intervals (95% CIs) were calculated and used to evaluate the risk of hepatobiliary pathological changes resulting from liver fluke infection. Linear trend analyses were conducted to determine the dose-response relationship using IBM SPSS Statistics 20.0.
Result
A total of 36 studies were included in the meta-analysis. Significant associations were found between liver fluke infection and cholangitis or cholecystitis (RR: 7.80, P<0.001; OR: 15.98, P<0.001), cholelithiasis (RR: 2.42, P = 0.03; OR: 4.96, P = 0.03), hepatocellular carcinoma (OR: 4.69, P<0.001) and cholangiocarcinoma (RR: 10.43, P<0.001; OR: 4.37, P<0.001). In addition, heavier infection was significantly associated with higher incidence of hepatobiliary pathological changes (P<0.05). However, cirrhosis was not significantly associated with liver fluke infection (RR: 3.50, P = 0.06; OR: 5.79, P = 0.08). The statistical heterogeneity was significant, no significant difference was observed in the sensitivity analysis, and no publication bias was found.
Conclusion
The meta-analysis found that liver fluke infection was significantly associated with cholangitis, cholecystitis, cholelithiasis, hepatocellular carcinoma and cholangiocarcinoma and that more severe infection was associated with higher incidence. However, the association between liver fluke infection and cirrhosis was not significant.
Introduction
At present, more than 750 million people throughout the world are at risk for infection with liver flukes, with an endemic concentration in southeast Asia and the western Pacific region[1]. The most important liver fluke species include Clonorchis sinensis, Fasciola spp. and Opisthorchis spp.[2]. The infectious metacercarial cyst stage is found in the meat of fish and shrimp as well as on the surfaces of water plants[3]. Once ingested, the metacercaria excysts in the duodenum, and the juvenile worm ascends the biliary tract through the ampulla of Vater[3]. The metabolites and mechanical stimulation of the liver fluke result in proliferation and inflammation in the epithelia of the biliary tracts as well as fibrosis and even cholangiocarcinoma[2, 4]. In humans, early and light infections may be asymptomatic or mild and are usually neglected. Infection by a large number of worms results in serious inflammation and leads to biliary tract obstruction, bile flux block and icterus[4]. However, the long-lived flukes cause chronic inflammation, which may be severe[5]. During chronic infection resulting from protracted episodes of re-infection over time, hepatic cells around the biliary ducts become denaturalized and putrescent, resulting in hepatic tissue atrophy and hepatocirrhosis[4, 6]. According to Keiser and Utzinger’s study, the global burden of food-born trematodiasis is 665,332 (479,496–859,051) DALYs (disability-adjusted life years). Moreover, they reported that food-borne trematode infections are among the most neglected of the so-called neglected tropical diseases[7, 8]. The awareness of liver fluke infection as a public health problem is insufficient because this infection impacts millions of people with severe morbidity and continues to emerge and expand. The increased infection rate of liver flukes may be due to factors such as the improved transportation and distribution systems to bring these aquatic foods to local and international markets[2, 8]. For example, in China, the current clonorchiasis rate is three times higher than that in the past decade[9, 10]. Findings of studies investigating the association between liver fluke infection and various hepatobiliary pathological changes have not been consistent, and systematic reviews and meta-analyses exploring the association have been even more limited. The present paper is based on a systematic review from cross-regional cohort studies and case-control studies to investigate the association between liver fluke infection and hepatobiliary pathological changes. This study will provide a more objective and comprehensive conclusion on this subject.
Materials and Methods
Search strategy
The study was performed using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)[11]. The PRISMA statement is available in the supplementary data (S1 Table). Relevant literature that reported an association between liver fluke infection and hepatobiliary pathological changes was identified and screened from databases, including PubMed, Cochrane, Clinical Evidence, Trip Database, Clinical Trials, Current Controlled Trials, Web of Science, the China National Knowledge Infrastructure (CNKI) database, and the Wanfang academic journal full-text database. The following Medical Subject Heading (MeSH) terms were used individually and in combination in the search: “Fasciola hepatica,” “Clonorchis sinensis,” “Opisthorchis,” “Case-Control Studies,” “Cohort Studies,” “Cross-Sectional Studies,” “Hepatobiliary pathological changes,” “Cholangitis,” “Cholecystitis,” “Cholelithiasis,” “Cirrhosis,” “Hepatocellular Carcinoma” and “Cholangiocarcinoma.” The literature search was not limited by language or geographical region. The references in all of the retrieved articles were reviewed to search for additional relevant studies.
Criteria for inclusion and exclusion
The inclusion criteria were as follows: (1) published full text available; (2) an observational study (a cohort study or a case-control study); (3) sufficient data reported to calculate the odds ratio (OR) with its 95% confidence interval (CI); and (4) the diagnosis of liver fluke infection based on (a) microscopy of liver fluke eggs in stool samples; (b) detection of worm-specific antibodies in serum samples or worm-specific antigens in serum or stool samples; (c) skin test with an intradermal injection of diluted crude live fluke antigen in veronal-buffered saline[12]; (d) observation of liver fluke eggs or parasites from bile, gallstones or intramural stones; (e) detection of diffuse dilatation of intrahepatic bile ducts in abdominal computed tomography (CT) or cholangiography; (f) results of molecular techniques such as polymerase chain reaction (PCR); or (g) history of liver fluke infection that could be confirmed by medical records. Studies were excluded if they were (1) comments, congresses, abstracts, reviews, or editorials without raw data or control subjects or (2) studies that included fewer than 10 participants.
Data extraction
The following information was independently extracted from all of the included studies: the name of the first author, publication year, country or geographical area, liver fluke species, diagnostic methods for liver fluke infection, sample size, the number of the exposure or outcome of interest for case-control or cohort studies, respectively, and the quality of each study.
Quality assessment
The quality of all of the included studies was assessed using The Newcastle-Ottawa Scale (NOS) (S2 Table). This scale involves a “star system” in which a study is judged on three broad perspectives: the selection of the study groups, the comparability of the groups and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies, respectively. Studies having more stars are considered to be of higher quality.
Statistical analysis
Statistical heterogeneity among studies was calculated using the χ2 test, P values, and I2 statistics[13]. A random-effects model was used to estimate the overall relative risk (RR) or overall odds ratio (OR) when heterogeneity was significant (Q: P<0.1, or I2>50%); if the reverse was true, a fixed-effects model was used (Q: P>0.1, or I2>50%). The overall RRs and ORs and their 95% CIs were estimated (P<0.05 was considered significant), and forest plots were generated for each disease associated with liver fluke infection. A sensitivity analysis was conducted, and publication bias was evaluated using funnel plots[14]. Meta-regression analyses were generated to explore possible sources of heterogeneity (adjusted R2>50% and P<0.05 were considered significant.) [15, 16], such as geographical area, decade of publication, liver fluke species, diagnostic methods and study sample size. Linear trend analyses were performed to determine the relationship between infection intensity and incidences of hepatobiliary pathological changes. Risk estimates, tests of heterogeneity, sensitivity calculations and publication bias analyses were performed using the Review Manager software, version 5.3; meta-regression analysis was performed using the Stata software, version 11.0 (Stata Corporation, College Station, TX, USA); and linear trend analyses were performed using IBM SPSS Statistics 20.0.
Results
Study characteristics
A comprehensive search of databases provided 1881 potentially relevant citations, of which 10 cohort studies and 26 case-control studies met the study criteria and were included in the meta-analysis (Fig 1). Among the included studies, 14 were from mainland China[17–30], 1 was from Hong Kong[31], 2 were from Taiwan[32, 33], 7 were from Korea[34–40], and 11 were from Thailand[41–51]. The characteristics of the included studies with their quality are shown in Tables 1 and 2.
Fig 1. Flow chart of study selection.
Table 1. Characteristics of included cohort studies on liver fluke infection and the risk of hepatobiliary pathological changes.
| Infected | Uninfected | Quality | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathological changes | Author | Year | Area | Liver fluke species | Diagnosis | Sample size | Events | Total | Events | Total | Selection | Comparability | Outcome | Reference |
| Cholangitis or Cholecystitis | Zhu SH | 1982 | China | Clonorchis sinensis | Pathologic examination | 17603 | 381 | 2214 | 126 | 15389 | ★ | ★ | ★ | [17] |
| Mairiang E | 1992 | Thailand | Opisthorchis viverrini | Stool microscopy | 95 | 27 | 71 | 4 | 24 | ★★★ | ★ | ★ | [41] | |
| Chen ZZ | 1997 | China | Clonorchis sinensis | Stool microscopy | 5230 | 79 | 1315 | 31 | 3915 | ★★★ | ★ | ★ | [18] | |
| Cholelithiasis | Zhu SH | 1982 | China | Clonorchis sinensis | Pathologic examination | 17603 | 93 | 2214 | 46 | 15389 | ★ | ★ | ★ | [17] |
| Hou MF | 1989 | Taiwan | Clonorchis sinensis | Liver fluke history | 1091 | 89 | 947 | 8 | 144 | ★★★ | ★ | ★★ | [32] | |
| Mairiang E | 1992 | Thailand | Opisthorchis viverrini | Stool microscopy | 95 | 6 | 71 | 0 | 24 | ★★★ | ★ | ★ | [41] | |
| Choi MS | 2005 | China | Clonorchis sinensis | Stool microscopy | 1384 | 279 | 1215 | 9 | 169 | ★★★ | ★ | ★ | [19] | |
| Huang MH | 2005 | Taiwan | Clonorchis sinensis | Serologic test | 131 | 12 | 47 | 46 | 84 | ★★★ | ★ | ★ | [33] | |
| Kim HG | 2009 | Korea | Clonorchis sinensis | Several evidence lines | 3080 | 45 | 396 | 340 | 2684 | ★★★ | ★ | ★ | [34] | |
| Zhang X | 2010 | China | Clonorchis sinensis | Several evidence lines | 1326 | 352 | 682 | 78 | 644 | ★★★ | ★ | ★ | [20] | |
| Luo XB | 2013 | China | Clonorchis sinensis | Pathologic examination | 340 | 49 | 153 | 30 | 187 | ★★★ | ★ | ★ | [21] | |
| Cirrhosis | Zhu SH | 1982 | China | Clonorchis sinensis | Pathologic examination | 17603 | 128 | 2214 | 94 | 15389 | ★ | ★ | ★ | [17] |
| Huang MH | 2005 | Taiwan | Clonorchis sinensis | Serologic test | 131 | 3 | 47 | 3 | 84 | ★★★ | ★ | ★ | [33] | |
| Mairiang E | 2012 | Thailand | Opisthorchis viverrini | Stool microscopy | 3359 | 182 | 404 | 656 | 2955 | ★★★ | ★ | ★★ | [42] | |
| Cholangiocarcinoma | Zhu SH | 1982 | China | Clonorchis sinensis | Pathologic examination | 17603 | 5 | 2214 | 0 | 15389 | ★ | ★ | ★ | [17] |
| Mairiang E | 1992 | Thailand | Opisthorchis viverrini | Stool microscopy | 95 | 2 | 71 | 0 | 24 | ★★★ | ★ | ★ | [41] | |
| Huang MH | 2005 | Taiwan | Clonorchis sinensis | Serologic test | 131 | 1 | 47 | 0 | 84 | ★★★ | ★ | ★ | [33] | |
Table 2. Characteristics of included case-control studies on liver fluke infection and the risk of hepatobiliary pathological changes.
| Case | Control | Quality | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathological changes | Author | Year | Area | Liver fluke species | Diagnosis | Sample size | Exposure | Total | Exposure | Total | Selection | Comparability | Outcome | Reference |
| Cholangitis or Cholecystitis | Elkins DB | 1990 | Thailand | Opisthorchis viverrini | Stool microscopy | 53 | 10 | 12 | 28 | 41 | ★★ | ★ | ★★ | [43] |
| Itoh M | 1994 | Thailand | Opisthorchis viverrini | Serologic test | 69 | 29 | 49 | 0 | 20 | ★★★ | ★ | ★★ | [44] | |
| Zheng ZX | 1997 | China | Clonorchis sinensis | Serologic test | 53 | 6 | 14 | 1 | 39 | ★ | ★ | ★★ | [22] | |
| Chen MF | 2001 | China | Clonorchis sinensis | Serologic test | 117 | 12 | 38 | 1 | 79 | ★ | ★ | ★★ | [23] | |
| Cholelithiasis | Elkins DB | 1990 | Thailand | Opisthorchis viverrini | Stool microscopy | 47 | 5 | 6 | 28 | 41 | ★★ | ★ | ★★ | [43] |
| Zheng ZX | 1997 | China | Clonorchis sinensis | Serologic test | 53 | 6 | 14 | 1 | 39 | ★ | ★ | ★★ | [22] | |
| Chen MF | 2001 | China | Clonorchis sinensis | Serologic test | 117 | 12 | 38 | 1 | 79 | ★ | ★ | ★★ | [23] | |
| Huang MH | 2005 | Taiwan | Clonorchis sinensis | Serologic test | 252 | 9 | 131 | 1 | 121 | ★★ | ★ | ★★ | [33] | |
| Choi D | 2008 | Korea | Clonorchis sinensis | Stool microscopy | 134 | 3 | 67 | 1 | 67 | ★★★ | ★ | ★★ | [35] | |
| Choi D | 2008 | Korea | Clonorchis sinensis | Serologic test | 134 | 4 | 67 | 1 | 67 | ★★★ | ★★ | ★★ | [35] | |
| Choi D | 2008 | Korea | Clonorchis sinensis | Radiological examination | 134 | 10 | 67 | 16 | 67 | ★★★ | ★★ | ★★ | [35] | |
| Cirrhosis | Zheng ZX | 1997 | China | Clonorchis sinensis | Serologic test | 49 | 2 | 10 | 1 | 39 | ★ | ★ | ★★ | [22] |
| Chen MF | 2001 | China | Clonorchis sinensis | Serologic test | 129 | 12 | 50 | 1 | 79 | ★ | ★ | ★★ | [23] | |
| Sripa B | 2009 | Thailand | Opisthorchis viverrini | Stool microscopy | 328 | 46 | 200 | 20 | 128 | ★★★ | ★★ | ★★ | [45] | |
| Hepatocellular carcinoma | Chen HN | 1994 | China | Clonorchis sinensis | Liver fluke history | 246 | 9 | 123 | 2 | 123 | ★★★ | ★★ | ★ | [24] |
| Shin HR | 1996 | Korea | Clonorchis sinensis | Stool microscopy | 526 | 36 | 176 | 44 | 350 | ★★ | ★★ | ★★ | [36] | |
| Shin HR | 1996 | Korea | Clonorchis sinensis | Liver fluke history | 609 | 19 | 203 | 21 | 406 | ★★ | ★★ | ★ | [36] | |
| Zheng ZX | 1997 | China | Clonorchis sinensis | Serologic test | 111 | 16 | 72 | 1 | 39 | ★ | ★ | ★★ | [22] | |
| Chen MF | 2001 | China | Clonorchis sinensis | Serologic test | 98 | 4 | 19 | 1 | 79 | ★ | ★ | ★★ | [23] | |
| Tan SK | 2007 | China | Clonorchis sinensis | Liver fluke history | 1000 | 85 | 500 | 13 | 500 | ★ | ★ | ★ | [25] | |
| Tan SK | 2008 | China | Clonorchis sinensis | Serologic test | 944 | 73 | 444 | 12 | 500 | ★ | ★ | ★★ | [26] | |
| Cholangiocarcinoma | Gibson RB | 1971 | Hong Kong | Clonorchis sinensis | Stool microscopy | 1401 | 11 | 17 | 310 | 1384 | ★ | - | - | [31] |
| Kim YI | 1974 | Korea | Clonorchis sinensis | Stool microscopy | 1402 | 21 | 54 | 120 | 1348 | ★★ | - | - | [37] | |
| Chung CS | 1976 | Korea | Clonorchis sinensis | Stool microscopy | 595 | 19 | 36 | 88 | 559 | ★★ | - | - | [38] | |
| Kurathong S | 1985 | Thailand | Opisthorchis viverrini | Stool microscopy | 560 | 19 | 25 | 389 | 535 | ★★ | - | - | [46] | |
| Elkins DB | 1990 | Thailand | Opisthorchis viverrini | Stool microscopy | 49 | 8 | 8 | 28 | 41 | ★★ | ★ | ★★ | [43] | |
| Parkin DM | 1991 | Thailand | Opisthorchis viverrini | Stool microscopy | 202 | 43 | 101 | 9 | 101 | ★ | ★★ | - | [47] | |
| Elkins H | 1994 | Thailand | Opisthorchis viverrini | Stool microscopy | 1807 | 14 | 15 | 1383 | 1792 | ★★ | - | - | [48] | |
| Itoh M | 1994 | Thailand | Opisthorchis viverrini | Serologic test | 67 | 42 | 47 | 0 | 20 | ★★★ | ★ | ★★ | [44] | |
| Shin HR | 1996 | Korea | Clonorchis sinensis | Stool microscopy | 386 | 12 | 36 | 44 | 350 | ★★ | ★★ | ★★ | [36] | |
| Shin HR | 1996 | Korea | Clonorchis sinensis | Liver fluke history | 447 | 3 | 41 | 21 | 406 | ★★ | ★★ | ★ | [36] | |
| Chen MF | 2001 | China | Clonorchis sinensis | Serologic test | 85 | 3 | 6 | 1 | 79 | ★ | ★ | ★★ | [23] | |
| Honjo S | 2005 | Thailand | Opisthorchis viverrini | Serologic test | 253 | 65 | 126 | 8 | 127 | ★ | ★★ | ★★ | [49] | |
| Choi D | 2006 | Korea | Clonorchis sinensis | Stool microscopy | 244 | 3 | 122 | 5 | 122 | ★★ | ★★ | ★★ | [39] | |
| Choi D | 2006 | Korea | Clonorchis sinensis | Pathologic examination | 148 | 13 | 74 | 8 | 74 | ★★ | ★★ | ★★ | [39] | |
| Choi D | 2006 | Korea | Clonorchis sinensis | Serologic test | 328 | 25 | 164 | 11 | 164 | ★★ | ★★ | ★★ | [39] | |
| Choi D | 2006 | Korea | Clonorchis sinensis | Skin test | 276 | 19 | 138 | 12 | 138 | ★★ | ★★ | ★★ | [39] | |
| Choi D | 2006 | Korea | Clonorchis sinensis | Radiological examination | 370 | 156 | 185 | 57 | 185 | ★★ | ★★ | ★★ | [39] | |
| Lee TY | 2008 | Korea | Clonorchis sinensis | Stool microscopy | 2869 | 26 | 619 | 9 | 2250 | ★ | ★★ | ★★ | [40] | |
| Poomphakwaen K | 2009 | Thailand | Opisthorchis viverrini | Stool microscopy | 145 | 29 | 76 | 17 | 69 | ★★★ | ★★ | ★★ | [50] | |
| Cai WK | 2011 | China | Clonorchis sinensis | Not mentioned | 921 | 4 | 313 | 1 | 608 | ★ | ★★ | ★ | [27] | |
| Peng NF | 2011 | China | Clonorchis sinensis | Not mentioned | 294 | 18 | 98 | 19 | 196 | ★★ | ★★ | ★ | [28] | |
| Wang XP | 2012 | China | Clonorchis sinensis | Not mentioned | 302 | 6 | 102 | 3 | 200 | ★ | ★★ | ★ | [29] | |
| Gao LB | 2013 | China | Clonorchis sinensis | Liver fluke history | 640 | 2 | 128 | 2 | 512 | ★ | ★ | ★ | [30] | |
| Manwong M | 2013 | Thailand | Opisthorchis viverrini | Serologic test | 246 | 110 | 123 | 99 | 123 | ★★ | ★★ | ★★ | [51] | |
The risk of hepatobiliary pathological changes associated with liver fluke infection
Cholangitis or cholecystitis
Several studies have reported a close association between liver fluke infection and cholangitis or cholecystitis [34, 52]. The overall RR with its 95% CI was extracted from the 3 included cohort studies[17, 18, 41], and the overall OR with its 95% CI was extracted from the 4 included case-control studies[22, 23, 43, 44]. The statistical heterogeneities of both the cohort studies and case-control studies were significant (I2 = 95%, P<0.001 and I2 = 55%, P = 0.08, respectively); hence, the overall RR for the cohort studies and the overall OR for the case-control studies were estimated using a random-effects model. The analysis of the cohort studies and case-control studies revealed that liver fluke infection was significantly associated with cholangitis and cholecystitis. (RR: 7.80, 95% CI: 2.69–22.59, P<0.001; OR: 15.98, 95% CI: 3.17–80.63, P<0.001) (Figs 2 and 3).
Fig 2. Forest plot of cohort studies on the relationship between liver fluke infection and various hepatobiliary pathological changes.
Fig 3. Forest plot of case-control studies on the relationship between liver fluke infection and various hepatobiliary pathological changes.
Cholelithiasis
Liver fluke infection has been investigated as a risk factor for cholelithiasis[21]. In total, 8 cohort studies[17, 19–21, 32–34, 41] and 5 case-control studies[22, 23, 33, 35, 43] were used to perform the respective meta-analyses using a random-effects model (I2 = 97%, P<0.001 and I2 = 75%, P<0.001, respectively). The analyses yielded an RR of 2.42 (95% CI: 1.07–5.46, P = 0.03) and an OR of 4.96 (95% CI: 1.19–20.56, P = 0.03), indicating that infection with liver flukes is a risk factor for cholelithiasis and that the association is significant (Figs 2 and 3).
Cirrhosis
In total, 3 cohort studies[17, 33, 42] and 3 case-control studies[22, 23, 45] on cirrhosis and liver fluke infection were identified and used to perform meta-analyses. A random-effects model was applied to the analyses (I2 = 98%, P<0.001 and I2 = 74%, P = 0.02, respectively). However, the result did not reveal a significant association between liver fluke infection and cirrhosis. For cohort studies, the overall RR of cirrhosis between infection with liver fluke and without infection was 3.50 (95% CI: 0.95–12.89, P = 0.06); for case-control studies, the overall OR of exposure to liver fluke infection between the case group and control group was 5.79 (95% CI: 0.83–40.28, P = 0.08) (Figs 2 and 3).
Hepatocellular carcinoma
Liver fluke infection has also been regarded as a risk factor for hepatocellular carcinoma [53]. Analysis of data from 6 case-control studies [22–26, 36] yielded inconsistent findings. The statistical heterogeneity was significant (I2 = 79%, P<0.001); thus, a random-effects model was applied. According to the analysis of the case-control studies, hepatocellular carcinoma was significantly associated with liver fluke infection with an OR of 4.69 (95% CI: 2.32–9.49, P<0.001) (Fig 3).
Cholangiocarcinoma
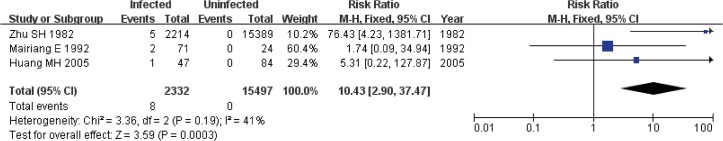
The association between cholangiocarcinoma and liver fluke infection has been identified in articles over the last several decades [54, 55]. In our meta-analysis, 3 cohort studies[17, 33, 41] and 19 case-control studies[23, 27–31, 36–40, 43, 44, 46–51] were included. A fixed-effects model was used in the analysis of the cohort studies (I2 = 41%, P = 0.19), and a random-effects model was used (I2 = 77%, P<0.001) in the analysis of the case-control studies. The overall RR for the association between liver fluke infection and cholangiocarcinoma was 10.43 (95% CI: 2.90–37.47, P<0.001), and the association was significant. The overall OR for the association of cholangiocarcinoma with liver fluke infection was 4.37 (95% CI: 2.84–6.72, P<0.001), which indicated that liver fluke infection was a risk factor for cholangiocarcinoma (Figs 3 and 4).
Fig 4. Forest plot of cohort studies on the relationship between liver fluke infection and cholangiocarcinoma.
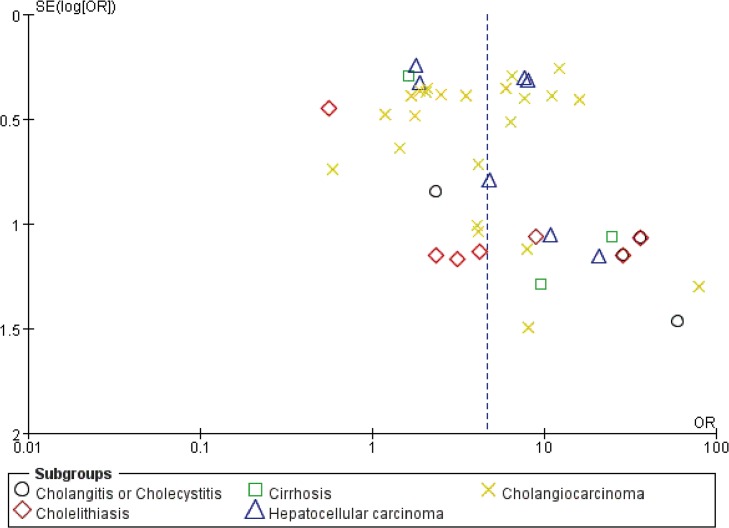
Sensitivity analysis
A sensitivity analysis was performed to identify whether the results of the meta-analysis were significantly affected by the exclusion of any individual study or the study with the highest quality or the greatest weight in the results. There was no significant impact observed in the overall ORs and 95% CIs.
Publication bias
Funnel plots of the studies in the meta-analysis were generated to evaluate publication bias (Figs 5 and 6). For both cohort studies and case-control studies, the plots approximately resembled a symmetrical funnel, and no publication bias was found.
Fig 5. Funnel plot of cohort studies to detect publication bias.
Fig 6. Funnel plot of case-control studies to detect publication bias.
Meta-regression analyses
Meta-regression analyses were generated to explore possible sources of heterogeneity. Our meta-regression showed that geographical area, decade of publication, liver fluke species and diagnostic method did not contribute significantly to the heterogeneity (Adjusted R2<50% or P>0.05) for either cohort studies or case-control studies. In contrast, for cohort studies only, the study sample size did have a contribution (Adjusted R2 = 73.13%, P<0.001). The results of the meta-regression analyses are shown in Table 3.
Table 3. Results of the meta-regression analyses.
| Study type | Factor | Adjusted R2 | P |
|---|---|---|---|
| Cohort studies | Area | 39.57% | 0.009 |
| Decade of publication | 28.85% | 0.023 | |
| Liver fluke species | -3.20% | 0.475 | |
| Diagnostic methods | 32.05% | 0.015 | |
| Study sample size | 73.13% | <0.001 | |
| Case-control studies | Area | 10.92% | 0.007 |
| Decade of publication | -2.46% | 0.491 | |
| Liver fluke species | -3.20% | 0.705 | |
| Diagnostic methods | -5.21% | 0.822 | |
| Study sample size | -6.80% | 0.75 |
Linear trend analyses of the dose-response relationship
In total, 2 cohort studies[19, 41] and 3 case-control studies[25, 43, 50] with intensity groups (≥3) of liver fluke infection were included in the linear trend analysis to examine the relationship between infection intensity and incidences of hepatobiliary pathological changes (Table 4). The results revealed a significant trend toward increasing incidences of hepatobiliary pathological changes with increasing intensity of liver fluke infection (P<0.05).
Table 4. Linear trend analysis.
| Test of linear trend | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study type | Pathological changes | Author | Year | Infection intensity | Events | Total | Incidence | Value | Sig. (2-sided) | Reference |
| Cohort studies | Cholangitis or cholecystitis | Mairiang | 1992 | 1 (EPGa = 0) | 4 | 20 | 20.0% | 16.598 | < 0.001 | [41] |
| 2 (EPG = 200 to 1000) | 4 | 16 | 25.0% | |||||||
| 3 (EPG = 2000 to 7000) | 11 | 16 | 68.8% | |||||||
| 4 (EPG > 10000) | 12 | 15 | 80.0% | |||||||
| Cholelithiasis | Mairiang | 1992 | 1 (EPG = 0) | 0 | 16 | 0.0% | 4.983 | 0.026 | [41] | |
| 2 (EPG = 200 to 1000) | 2 | 14 | 14.3% | |||||||
| 3 (EPG = 2000 to 7000) | 3 | 8 | 37.5% | |||||||
| 4 (EPG > 10000) | 1 | 4 | 25.0% | |||||||
| Choi | 2005 | 1 (EPG = 0) | 9 | 169 | 5.3% | 150.063 | < 0.001 | [19] | ||
| 2 (EPG = 1 to 500) | 54 | 532 | 10.2% | |||||||
| 3 (EPG = 501 to 2000) | 74 | 322 | 23.0% | |||||||
| 4 (EPG ≥ 2001) | 151 | 361 | 41.8% | |||||||
| Cholangiocarcinoma | Mairiang | 1992 | 1 (EPG = 0) | 0 | 16 | 0.0% | 7.827 | 0.005 | [41] | |
| 2 (EPG = 200 to 1000) | 0 | 12 | 0.0% | |||||||
| 3 (EPG = 2000 to 7000) | 0 | 5 | 0.0% | |||||||
| 4 (EPG > 10000) | 2 | 5 | 40.0% | |||||||
| Case-control studies | Cholangitis or cholecystitis | Elikins | 1990 | 1 (EPG = 0) | 2 | 15 | 13.3% | 5.321 | 0.021 | [43] |
| 2 (EPG = 1 to 500) | 2 | 14 | 14.3% | |||||||
| 3 (EPG = 501 to 2500) | 2 | 11 | 18.2% | |||||||
| 4 (EPG = 2501 to 10000) | 1 | 4 | 25.0% | |||||||
| 5 (EPG > 10000) | 5 | 9 | 55.6% | |||||||
| Cholelithiasis | Elikins | 1990 | 1 (EPG = 0) | 1 | 14 | 7.1% | 4.711 | 0.03 | [43] | |
| 2 (EPG = 1 to 500) | 1 | 13 | 7.7% | |||||||
| 3 (EPG = 501 to 2500) | 0 | 9 | 0.0% | |||||||
| 4 (EPG = 2501 to 10000) | 1 | 4 | 25.0% | |||||||
| 5 (EPG > 10000) | 3 | 7 | 42.9% | |||||||
| Hepatocellular carcinoma | Tan | 2007 | 1 (Yearsb = 0) | 415 | 902 | 46.0% | 57.423 | < 0.001 | [25] | |
| 2 (Years < 10) | 39 | 48 | 81.3% | |||||||
| 3 (Years ≥ 10) | 46 | 50 | 92.0% | |||||||
| Cholangiocarcinoma | Elikins | 1990 | 1 (EPG = 0) | 0 | 13 | 0.0% | 12.306 | < 0.001 | [43] | |
| 2 (EPG = 1 to 500) | 0 | 12 | 0.0% | |||||||
| 3 (EPG = 501 to 2500) | 2 | 11 | 18.2% | |||||||
| 4 (EPG = 2501 to 10000) | 2 | 5 | 40.0% | |||||||
| 5 (EPG > 10000) | 4 | 8 | 50.0% | |||||||
| Poomphakwean | 2009 | 1 (EPG = 0) | 47 | 99 | 47.5% | 4.353 | 0.037 | [50] | ||
| 2 (EPG = 1 to 1000) | 13 | 24 | 54.2% | |||||||
| 3 (EPG > 1000) | 16 | 22 | 72.7% | |||||||
Discussion
Several published studies [52, 56, 57] have reported an association between liver fluke infection and various hepatobiliary pathological changes, including cholangitis, cholecystitis, cholelithiasis, cirrhosis, hepatocellular carcinoma and cholangiocarcinoma. However, these published studies have not identified consistent findings for the risk of these hepatobiliary pathological changes and liver fluke infection. In this systematic review and meta-analysis of cohort studies and case-control studies, significant associations were found between liver fluke infection and cholangitis or cholecystitis (RR: 7.80, 95% CI: 2.69–22.59, P<0.001; OR: 15.98, 95% CI: 3.17–80.63, P<0.001), cholelithiasis (RR: 2.42, 95% CI: 1.07–5.46, P = 0.03; OR: 4.96, 95% CI: 1.19–20.56, P = 0.03), hepatocellular carcinoma (OR: 4.69, 95% CI: 2.32–9.49, P<0.001) and cholangiocarcinoma (RR: 10.43, 95% CI: 2.90–37.47, P<0.001; OR: 4.37, 95% CI: 2.84–6.72, P<0.001). However, cirrhosis was not significantly associated with liver fluke infection (RR: 3.50, 95% CI: 0.95–12.89, P = 0.06; OR: 5.79, 95% CI: 0.83–40.28, P = 0.08). The observed statistical heterogeneity was significant, although sensitivity analysis did not alter the overall RR, overall OR, or their 95% CIs, and there was no evidence of publication bias.
A random-effects model was used in all of the analyses (except the analysis of cohort studies in cholangiocarcinoma) because significant heterogeneity was observed. Meta-regression analyses showed that the study sample size contributed significantly to the heterogeneity of the cohort studies (Adjusted R2 = 73.13%, P<0.001); as interpreted, the study sample size could explain 73.13% of the heterogeneity. In contrast, geographical area, decade of publication, liver fluke infection and diagnostic methods did not contribute to the heterogeneity. This result is most likely related to the limited information included in the studies, such as study design, the stages of pathological changes, and other demographic characteristics.
In this systematic review and meta-analysis, we found that liver fluke infection was significantly associated with increased risk of cholangitis and cholecystitis. The liver fluke secretes metabolites while invading, some of which are highly immunogenic, stimulating a strong humoral immune response that can be measured in the serum and bile[58]. Another study revealed that Opisthorchis antigens were observed along with an inflammatory cell infiltration, and the antigens were not only in the fluke itself but also in the biliary epithelium and surrounding tissue, which might then activate host immune responses[59].
Our study confirmed that liver fluke infection was significantly associated with cholelithiasis. The cause of clonorchiasis was most likely related to changes in the concentration of bilirubin, cholesterol, phospholipids, bile acid and the core of the gallstone formed from parasite debris or epithelial cells from the biliary ducts[60]. The metaplasia of bile duct epithelial cells into goblet cells and mucin secretion occurs in clonorchiasis and promotes a favorable environment for secondary bacterial infection[61].
A positive association was found between hepatocellular carcinoma and liver fluke infection. The mechanism of hepatocellular carcinoma in patients with liver fluke infection remains unknown. One possible mechanism is that epithelial ulceration and hyperplasia induced by the suckers of liver flukes induce stimulation of metabolites from the worms[62]. Secondary bacterial infection gives rise to periductal adenomatous hyperplasia and mucin secretion, which may result in hepatocellular carcinoma[62]. Another possible mechanism is that severin, a liver fluke excretory/secretory product, plays a key role in inhibiting apoptosis in human hepatocellular carcinoma cell lines and exacerbates hepatocellular carcinoma[63].
Our systematic review and meta-analysis confirm a significant relationship between infection with liver flukes and cholangiocarcinoma. The mechanisms by which liver flukes contribute to cholangiocarcinoma are multi-factorial[56] and include mechanical damage caused by the activities and movements of the worms, chronic inflammation, and the effects of parasite secretions[57].
This study confirms not only the relationship between liver fluke infection and various hepatobiliary pathological changes, such as cholangitis, cholecystitis, cholelithiasis, hepatocellular carcinoma and cholangiocarcinoma, but also the relationship between intensity of liver fluke infection and incidences of the hepatobiliary pathological changes. We found a significant trend toward increasing incidences of hepatobiliary pathological changes with increasing intensity of liver fluke infection. The ordinal intensity of liver fluke infection was analyzed by linear trend analyses instead of meta-analyses due to the limited sample size and the different ordinal scales used among the included studies. Additionally, information was too limited to generate analyses of the association between the intensity of liver fluke infection and the severity of pathological changes. However, in our included studies [41, 43], most cases of cholangiocarcinoma were identified from heavily infected patients, which supports the hypothesis that high pathogenicity relates to heavy parasite infection. The pathogenesis is due to the mechanical irritation by the flukes and some toxic substances produced by them[64].
Although published studies provided evidence to support the hypothesis that liver fluke infection is associated with cirrhosis [45], our analysis failed to provide sufficient evidence for this association. This inconsistency likely occurred because the studies that identified a relationship between cirrhosis and liver fluke were limited to animals, such as cattle, goats and sheep [65, 66]. In addition, most cirrhosis is associated with Fasciola hepatica infection [66, 67], which was not included in our analysis because of the absence of eligible studies.
Several limitations of our study deserve mention. First, non-English, non-Chinese studies were not included in our meta-analyses, which might have an impact on the overall results. Second, because of the limited number of studies involved and limited information on the studies, our study was not powered to perform subgroup analyses, which might provide reasons for the significant heterogeneity as well.
Conclusion
In conclusion, our systematic review and meta-analysis found that liver fluke infection is associated with an increased risk of cholangitis, cholecystitis, cholelithiasis, hepatocellular carcinoma and cholangiocarcinoma, and more severe infection is associated with higher incidence. However, no significant evidence was found for the association between liver fluke infection and cirrhosis.
Supporting Information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the funding of National Natural Science Foundation of China (no. 81271866), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2014) to HJP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Santiago M-C, Jitra W, H CJ, Darwin MK, Paul A, Suvanee S. Human fascoliasis: Epidemiological patterns in human endemic areas of south America, Africa and Asia. Southeast Asian journal of tropical medicine and public health. 2004;35(2):1–11. [Google Scholar]
- 2.Keiser J, Utzinger J, Uuml RG. Emerging foodborne trematodiasis2005. 1507–14 p.
- 3. Saijuntha W, Sithithaworn P, Kaitsopit N, Andrews RH, Petney TN. Liver flukes: Clonorchis and Opisthorchis. Advances in experimental medicine and biology. 2014;766:153–99. Epub 2014/06/07. 10.1007/978-1-4939-0915-5_6 . [DOI] [PubMed] [Google Scholar]
- 4.Kujawa M. Control of Foodborne Trematode Infections. WHO Technical Report Series 849. 157 Seiten, 4 Tabellen. World Health Organization, Geneva 1995. Preis: 26,—Sw.fr. Food / Nahrung. 1996;40(3):166-. 10.1002/food.19960400342 [DOI]
- 5. Lim JH. Liver flukes: the malady neglected. Korean journal of radiology. 2011;12(3):269–79. Epub 2011/05/24. 10.3348/kjr.2011.12.3.269 ; PubMed Central PMCID: PMCPmc3088844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villegas F, Angles R, Barrientos R, Barrios G, et al. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano2012. e1720 p. [DOI] [PMC free article] [PubMed]
- 7. Fürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2012;12(3):210–21. 10.1016/S1473-3099(11)70294-8 [DOI] [PubMed] [Google Scholar]
- 8.Utzinger J, Uuml RG, de Savigny D. Control of neglected tropical diseases: integrated chemotherapy and beyond2006. e112 p. [DOI] [PMC free article] [PubMed]
- 9.Li T, He S, Zhao H, Zhao G, Zhu X-Q. Major trends in human parasitic diseases in China2010. 264–70 p. [DOI] [PubMed]
- 10.Lun Z-R, Gasser RB, Lai D-H, Li A-X, Zhu X-Q, Yu X-B, et al. Clonorchiasis: a key foodborne zoonosis in China2005. 31–41 p. [DOI] [PubMed]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement2009 2009-07-21 10:46:49. [PMC free article] [PubMed]
- 12.Min DY. Remarks on the diagnosis of Clonorchis sinensis infection1984. 1153–6 p. [PubMed]
- 13.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis2002. 1539–58 p. [DOI] [PubMed]
- 14.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis2001. 1046–55 p. [DOI] [PubMed]
- 15. Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. Journal of health services research & policy. 2002;7(1):51–61. Epub 2002/02/02. . [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Statistics in medicine. 2004;23(11):1663–82. Epub 2004/05/26. 10.1002/sim.1752 . [DOI] [PubMed] [Google Scholar]
- 17. Zhu SH. Clonorchiasis with its comorbidities. Guangdong Medical Journal. 1982;(01):1–3. [Google Scholar]
- 18. Chen ZZ. The Relationship between Clonorchisis sinensis Infection and Acute Cholangitis—A Survey of Clonorchis sinesis Epidemic Areas and Analysis of Clinical Data. Journal of Pathogen Biology. 1997;(01):33–5. [Google Scholar]
- 19. Choi MS, Choi D, Choi MH, Ji Z, Li Z, Cho SY, et al. Correlation between sonographic findings and infection intensity in clonorchiasis. The American journal of tropical medicine and hygiene. 2005;73(6):1139–44. Epub 2005/12/16. 16354827. [PubMed] [Google Scholar]
- 20. Zhang X. Analysis of the Relationship between the Cholelithiasis and Clonorchiasis Diagnosed by Ultrasonography. Pratical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2010;(11):1585–6. [Google Scholar]
- 21. Luo XB, Qiao T, Ma RH, Zheng PM, Luo ZL, Cai HY, et al. Clonorchis sinensis infection—a risk factor for the patients with intramural gallstones complicated with gallbladder stones. Chinese Journal OF Zoonoses. 2013;29(11):1084–9. 10.3969/cjz.j.issn.1002-2694.2013.11.011 [DOI] [Google Scholar]
- 22. Zheng ZX. Analysis of Positive of Antibody to Clonorchiasis sinensis in Hepatobiliary Disease. Guangdong Journal of Health and Epidemic Prevention. 1997;(03):35–6. [Google Scholar]
- 23. Chen MF, Zhang JR. Relationship between positive of antibody of liver fluke and hepatobiliary disease. Guangdong Journal of Health and Epidemic Prevention. 2001;27(1):67–8. 10.3969/j.issn.1671-5039.2001.01.029 [DOI] [Google Scholar]
- 24. Chen HN, Hu MX, Zheng SA, Guo YQ. The Analysis of Risk Factors of Primary Hepatocellular Carcinoma with the Model of Conditional Logistic Regression. Chinese Journal of Cancer. 1994;(02):128–30. [Google Scholar]
- 25.Tan SK. To investigate the relation between social, psychological, behavial and environmental fhctors and Primary hepatic carcinoma [M.Sc. Thesis]2007.
- 26. Tan SK, Qiu XQ, Yu HP, Zeng XY, Zhao MN, Hu L. Evaluation of the risk of clonorchiasis inducing primary hepatocellular carcinoma. Chinese Journal of Hepatology. 2008;16(2):114–6. [PubMed] [Google Scholar]
- 27. Cai WK, Sima H, Chen BD, Yang GS. Risk factors for hilar cholangiocarcinoma: a case-control study in China. World journal of gastroenterology: WJG. 2011;17(2):249–53. 10.3748/wjg.v17.i2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peng NF, Li LQ, Qin X, Guo Y, Peng T, Xiao KY, et al. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of hepatitis B virus. Annals of surgical oncology. 2011;18(5):1258–66. Epub 2011/01/06. 10.1245/s10434-010-1458-5 . [DOI] [PubMed] [Google Scholar]
- 29. Wang XP. Risk factors and pathogenesis of intrahepatic cholangiocarcinoma. China Modern Doctor. 2012;50(16):75–7. 10.3969/j.issn.1673-9701.2012.16.034 [DOI] [Google Scholar]
- 30. Gao LB. Analysis of Risk Factors in Hilar Cholangiocarcinoma. Hebei Medicine. 2013;19(1):93–7. 10.3969/j.issn.1006-6233.2013.01.036 [DOI] [Google Scholar]
- 31.Gibson RB. Parasites, liver disease, and liver cancer. (IARC Scientific Publication no 1) Lyon. 1971.
- 32. Hou MF, Ker CG, Sheen PC, Chen ER. The ultrasound survey of gallstone diseases of patients infected with Clonorchis sinensis in southern Taiwan. The Journal of tropical medicine and hygiene. 1989;92(2):108–11. Epub 1989/04/01. . [PubMed] [Google Scholar]
- 33. Huang MH, Chen CH, Yen CM, Yang JC, Yang CC, Yeh YH, et al. Relation of hepatolithiasis to helminthic infestation. Journal of gastroenterology and hepatology. 2005;20(1):141–6. 10.1111/j.1400-1746.2004.03523.x . [DOI] [PubMed] [Google Scholar]
- 34. Kim H-G. Prevalence of clonorchiasis in patients with gastrointestinal disease: A Korean nationwide multicenter survey. World Journal of Gastroenterology. 2009;15(1):86 10.3748/wjg.15.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, et al. Gallstones and Clonorchis sinensis infection: a hospital-based case-control study in Korea. Journal of gastroenterology and hepatology. 2008;23(8 Pt 2):e399–404. 10.1111/j.1440-1746.2007.05242.x . [DOI] [PubMed] [Google Scholar]
- 36.Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea1996. 933–40 p. [DOI] [PubMed]
- 37. Kim YI. Relationship between Clonorchis sinensis infestation and cholangiocarcinoma of the liver in Korea. Seoul J Med 1974;(15):247–55. [Google Scholar]
- 38. Chung CS. An epidemiological study of primary liver carcinomas in Busan area with special reference to clonorchis. Korean J Pathol 1976;(10):33–46. [Google Scholar]
- 39. Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, et al. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. Journal of hepatology. 2006;44(6):1066–73. 10.1016/j.jhep.2005.11.040 . [DOI] [PubMed] [Google Scholar]
- 40. Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. The American journal of gastroenterology. 2008;103(7):1716–20. 10.1111/j.1572-0241.2008.01796.x . [DOI] [PubMed] [Google Scholar]
- 41. Mairiang E, Elkins DB, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, et al. Relationship between intensity of Opisthorchis viverrini infection and hepatobiliary disease detected by ultrasonography. Journal of gastroenterology and hepatology. 1992;7(1):17–21. Epub 1992/01/01. . [DOI] [PubMed] [Google Scholar]
- 42. Mairiang E, Laha T, Bethony JM, Thinkhamrop B, Kaewkes S, Sithithaworn P, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitology International. 2012;61(1):208–11. 10.1016/j.parint.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elkins DB, Haswell-Elkins MR, Mairiang E, Mairiang P, Sithithaworn P, Kaewkes S, et al. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84(5):715–9. Epub 1990/09/01. . [DOI] [PubMed] [Google Scholar]
- 44. Itoh M, Pairojkul C, Thamawit W, Sithithaworn P, Tiwawech D, Uttaravicien T, et al. Association of antibodies to Opisthorchis viverrini with hepatobiliary disease in northeastern Thailand. The American journal of tropical medicine and hygiene. 1994;51(4):424–9. Epub 1994/10/01. . [PubMed] [Google Scholar]
- 45. Sripa B, Mairiang E, Thinkhamrop B, Laha T, Kaewkes S, Sithithaworn P, et al. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology. 2009;50(4):1273–81. 10.1002/hep.23134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kurathong S. Opisthorchis viverrini infection and cholangiocarcinoma: A prospective, case-controlled study. Gastroenterology. 1985;(89):151–6. [DOI] [PubMed] [Google Scholar]
- 47. Parkin DM, Srivatanakul P, Khlat M, Chenvidhya D, Chotiwan P, Insiripong S, et al. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. International journal of cancer Journal international du cancer. 1991;48(3):323–8. Epub 1991/05/30. . [DOI] [PubMed] [Google Scholar]
- 48. Haswell-Elkins MR. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma within a high risk area in Northeast Thailand. International Journal of Cancer 1994;(59):505–9. [DOI] [PubMed] [Google Scholar]
- 49. Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. International journal of cancer Journal international du cancer. 2005;117(5):854–60. 10.1002/ijc.21146 . [DOI] [PubMed] [Google Scholar]
- 50.Poomphakwaen K, Promthet S, Kamsa-Ard S, Vatanasapt P, Chaveepojnkamjorn W, Klaewkla J, et al. Risk factors for cholangiocarcinoma in Khon Kaen, Thailand: a nested case-control study2009. 251–8 p. [PubMed]
- 51. Manwong M, Songserm N, Promthet S, Matsuo K. Risk factors for cholangiocarcinoma in the lower part of Northeast Thailand: a hospital-based case-control study. Asian Pacific journal of cancer prevention: APJCP. 2013;14(10):5953–6. Epub 2013/12/03. . [DOI] [PubMed] [Google Scholar]
- 52. Huang JY, Fang XH. Relationship between Clonorchis sinensis infection and Liver and Gallbladder disease. Journal of Tropical Medicine. 2010;10(2):226–8. [Google Scholar]
- 53. Parkin DM. The global health burden of infection-associated cancers in the year 2002. International journal of cancer Journal international du cancer. 2006;118(12):3030–44. 10.1002/ijc.21731 . [DOI] [PubMed] [Google Scholar]
- 54. Pao-Chang H, Shu-Chao P. Chorionepithelioma: An analytical study of 28 necropsied cases, with special reference to the possibility of spontaneous retrogression. The Journal of Pathology and Bacteriology. 1956;72(1):95–104. 10.1002/path.1700720113 [DOI] [PubMed] [Google Scholar]
- 55. Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS medicine. 2007;4(7):e201 Epub 2007/07/12. 10.1371/journal.pmed.0040201 ; PubMed Central PMCID: PMCPmc1913093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, et al. The tumorigenic liver fluke Opisthorchis viverrini—multiple pathways to cancer. Trends in parasitology. 2012;28(10):395–407. Epub 2012/09/06. 10.1016/j.pt.2012.07.006 ; PubMed Central PMCID: PMCPmc3682777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smout MJ, Sripa B, Laha T, Mulvenna J, Gasser RB, Young ND, et al. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Molecular bioSystems. 2011;7(5):1367–75. Epub 2011/02/12. 10.1039/c0mb00295j ; PubMed Central PMCID: PMCPmc3739706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wongratanacheewin S, Bunnag D, Vaeusorn N, Sirisinha S. Characterization of Humoral Immune Response in the Serum and Bile of Patients with Opisthorchiasis and Its Application in Immunodiagnosis. The American journal of tropical medicine and hygiene. 1988;38(2):356–62. [DOI] [PubMed] [Google Scholar]
- 59. Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. International Journal for Parasitology. 2000;30(6):735–40. 10.1016/S0020-7519(00)00054-0 [DOI] [PubMed] [Google Scholar]
- 60. Lim JH. Oriental cholangiohepatitis: pathologic, clinical, and radiologic features. AJR American journal of roentgenology. 1991;157(1):1–8. Epub 1991/07/01. 10.2214/ajr.157.1.2048504 . [DOI] [PubMed] [Google Scholar]
- 61. Lin AC, Chapman SW, Turner HR, Wofford JD Jr. Clonorchiasis: an update. Southern medical journal. 1987;80(7):919–22. Epub 1987/07/01. . [DOI] [PubMed] [Google Scholar]
- 62. Zeng SQ, Liu HJ, Wang H, Wang Q, Hu SQ. Surgical Diagnosis and Treatment for 135 Patients with Complication of Clonorchiasis. Journal of Tropical Medicine. 2005;(03):350–2. [Google Scholar]
- 63. Chen X, Li S, He L, Wang X, Liang P, Chen W, et al. Molecular characterization of severin from Clonorchis sinensis excretory/secretory products and its potential anti-apoptotic role in hepatocarcinoma PLC cells. PLoS neglected tropical diseases. 2013;7(12):e2606 Epub 2013/12/25. 10.1371/journal.pntd.0002606 ; PubMed Central PMCID: PMCPmc3868641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Harinasuta T, Riganti M, Bunnag D. Opisthorchis viverrini infection: pathogenesis and clinical features. Arzneimittel-Forschung. 1984;34(9b):1167–9. Epub 1984/01/01. . [PubMed] [Google Scholar]
- 65. Alvarez Rojas CA, Ansell BR, Hall RS, Gasser RB, Young ND, Jex AR, et al. Transcriptional analysis identifies key genes involved in metabolism, fibrosis/tissue repair and the immune response against Fasciola hepatica in sheep liver. Parasites & vectors. 2015;8:124 Epub 2015/04/18. 10.1186/s13071-015-0715-7 ; PubMed Central PMCID: PMCPmc4382932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marcos LA, Bussalleu A, Terashima A, Espinoza JR. Detection of antibodies against Fasciola hepatica in cirrhotic patients from Peru. Journal of helminthology. 2009;83(1):23–6. Epub 2008/09/27. 10.1017/s0022149x08067205 . [DOI] [PubMed] [Google Scholar]
- 67. Marcos LA, Terashima A, Yi P, Andrade R, Cubero FJ, Albanis E, et al. Mechanisms of liver fibrosis associated with experimental Fasciola hepatica infection: roles of Fas2 proteinase and hepatic stellate cell activation. The Journal of parasitology. 2011;97(1):82–7. Epub 2011/02/26. 10.1645/ge-2420.1 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.