Abstract
Background
Interventions during primary HIV infection (PHI) can modify the clinical course during the chronic phase. The long-term effect of structured treatment interruptions (STI) followed by low doses of interleukin-2 (IL-2) in treated PHI patients is unknown.
Methods
Twelve PHI patients with viral load (VL) <20 copies/mL, CD4 cells >500 cells/mm3, and CD4/CD8 ratio >1, on antiretroviral therapy (ART) initiated within the first 90 days of infection and continued for at least 12 months were included. They underwent four STI and were then allocated (week 0 of the study) to ART alone or ART plus low doses of IL-2. ART was stopped once VL <20 copies/mL ('final stop'). Primary endpoints were VL<3000 copies/mL and CD4 cells >500 cells/mm3 at 48 weeks; secondary endpoints were immune activation, inflammatory markers until 48 weeks and the time before resuming ART (CD4 <350 cells/mm3 or AIDS) after ‘final stop’, compared between groups.
Results
Ten out of 12 patients were males, median age was 35 years and the main risk was men-who-have-sex-with-men. Only one out of 12 patients (in the STI group) maintained VL<3000 copies/mL and CD4 cells >500 cells/mm3 without ART at 48 weeks. All other virological and immunological parameters were comparable between groups at week 0, 'final stop' and week 48. However, the proportion of CD8-CD38+ cells, tumor necrosis factor and srIL-2 were higher in the IL-2 group at 'final stop' and week 24. All these differences vanished during follow-up. At 5 years after the final stop 3 out of 6 patients in the IL-2 group and 6 out of 6 patients in the STI group have resumed ART (P = 0.19).
Conclusions
STI and IL-2 failed to achieve virological control after ART interruption. STI were not deleterious in long-term follow-up, an important issue for eradication and functional cure trials.
Trial Registration
ClinicalTrials.gov NCT02300623
Introduction
Potent combination antiretroviral treatment (ART) during primary HIV infection (PHI) results in strong viral suppression rates, rapid recovery of CD4 T cells[1, 2], and a reduction of viral reservoirs[3, 4]. Moreover, ART in this clinical phase preserves HIV-specific T-cell helper (Th) and cytotoxic T lymphocyte (CTL) immune responses, improves surrogate markers of disease progression[5–7] [8] and reduces HIV transmission[9, 10]. The fact that ART during PHI preserves HIV-specific CD4 helper T-cell response[5–7] is remarkable, as this response is usually absent in chronically-infected patients, even in those receiving successful ART since the early asymptomatic phase of chronic infection[5, 11]. In fact, ART-treated PHI patients exhibit HIV-specific Th and CTL immune responses in a similar manner as long-term non-progressors (LTNP) or “elite controllers” that spontaneously control HIV replication[12, 13].
Soon after the introduction of potent ART, observational case reports identified patients receiving ART during PHI that controlled HIV replication after ART discontinuation and reported that this control was associated with strong HIV-specific cell-mediated immune responses[14, 15]. These cases lead to the hypothesis that brief exposures to the autologous virus during supervised or structured treatment interruptions (STI) in patients receiving ART since PHI might act by boosting HIV-specific immune responses, which could provide immune control of viral replication. STI were also evaluated in several clinical trials during chronic HIV infection[16–18]. The encouraging results obtained in a small clinical trial using three STIs in subjects receiving ART since PHI[7] and similar experimental findings in primates[19] further fueled interest in this approach and in other possible immunotherapeutic procedures to induce/boost HIV-specific immune responses[20–22]. Unfortunately, however, the viral control after STI in the mentioned trial with ART-treated PHI patients was found to have a limited durability[23]. Nevertheless, recently, two reports have renewed further interest in administering ART very early during PHI as a considerable proportion of patients can control HIV replication after ART cessation[24, 25]. The proportion of these post-ART controllers was considerably high compared to that of spontaneous “elite” controllers, and the former lack some genetic characteristics that are overrepresented among the latter, suggesting that the key for controlling HIV viremia was the early initiation of ART[24, 25].
In our trial, we used IL-2, one of the first cytokines discovered to promote T-cell growth[26], as an adjunctive immunotherapy aimed at favoring the clonal expansion of HIV-specific Th and CTL responses. We utilized daily s.c. ultra-low IL-2 dose, which has been demonstrated to be not only nontoxic and safe but also effective in stimulating immunoreactivity in patients with AIDS and with AIDS-related malignancies[27–29].
These considerations provided the rationale for this pilot clinical trial to evaluate the impact of STI during PHI with or without the addition of low-dose recombinant IL-2 to boost HIV-1 specific immune responses and achieve a control of HIV viremia. Although STI in chronic HIV infection has shown to increase the risk of opportunistic infection and death [30], it can also enhance host immune control of viral replication [17] and no deleterious effect was shown during PHI in a recently published study[31]. This issue is particularly important in the context of the trials for HIV-functional cure, in which ART interruptions need to be performed to test the control of viremia by the immune system. We report here the virological, immunological and inflammatory markers up to 48 weeks of follow-up and the time to require resuming ART (CD4<350/μl or AIDS events) in the long-term follow-up.
Methods
Patient population
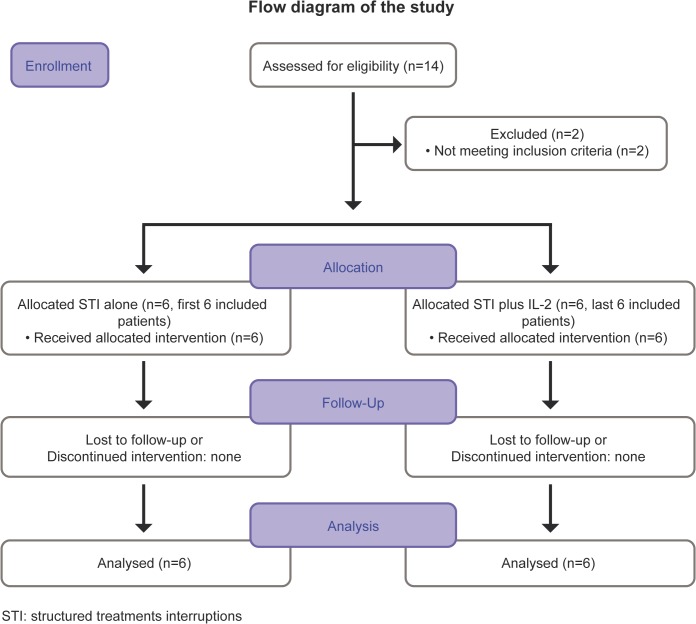
Enrolment started in March 2000 and finished in November 2001. Long-term follow-up for the last included patient finished in April 2012. All patients with PHI on stable effective ART for at least 12 months were invited to participate. The flow diagram of the study is shown in Fig 1.
Fig 1. Flow diagram of the trial.
Diagnosis of PHI was defined by:
-A detectable plasma viral load (PVL) or p24 antigen detection coupled with a negative or indeterminate LIA assay (according to CDC criteria), or
-A negative HIV-1 EIA in the preceding 90 days, or
-A positive EIA and LIA assay with acute retroviral syndrome in the 90 days preceding the start of ART plus documented negative HIV-1 EIA within the previous year.
Date of infection was assumed to have occurred two weeks before the onset of acute symptoms. In asymptomatic patients this date was calculated as the midpoint between the last negative and first positive test.
All patients started stavudine, lamivudine and non-boosted-indinavir at usual doses, according to recommendations at that time, within 90 days of HIV exposure, and had to show good virological and immunological responses, defined as undetectable PVL (<20 copies/mL in the last two controls), CD4 T cells higher than 500 cells/mm3 and a CD4/CD8 ratio >1 in the 8 months prior to enrolment.
Study Design
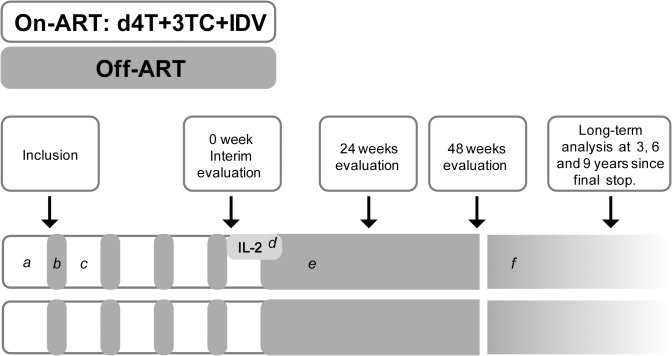
The study design included two phases (Fig 2). The first phase consisted of four STIs of 8 weeks each (off-ART), separated by at least 16 weeks of treatment–or the time necessary to return to PVL <20 copies/mL—(on-ART). At the end of 4th off-ART cycle (week 0), an interim evaluation was performed and the second phase initiated. During the second phase, the first 6 patients received ART until they reached PVL<20 copies/mL, discontinuing thereafter (final stop). The last 6 patients received ART and low doses of IL-2. ART was stopped after reaching PVL<20 copies/mL (final stop) and IL-2 after 6 months of treatment. IL-2 was prescribed at a dose of 750,000 UI/m2 daily and was self-administrated in all patients after training with a specialized nurse; administration was checked and verified during follow-up visits.
Fig 2. Design of the study.
Footnote: (a) Eligible individuals were patients treated within 90 days of HIV exposure on stable ART for at least 12 months. Patients had to show good virological and immunological responses (at least 2 viral load <20 copies/mL and CD4 T cells higher than 500 cells/mm3 with a CD4/CD8 ratio >1). All individuals started four structured treatment interruption cycles (b) of 8 week each (off-ART), followed by four cycles (c) of treatment (on-ART). After the last treatment interruption (week 0) 6 patients started self-administrated IL-2 at a dose of 750,000 UI/m2 daily for 6 months (d). Treatment was interrupted in both arms when patients reached viral load <20 copies/mL (e). Analyses were performed at 24 and 48 weeks and after a long term follow-up period (9 years). During this period (f) treatment was restarted in patients whose CD4 cell count dropped below 350 cell/mm3.
In both groups, ART was resumed in patients whose CD4 cell count dropped below 350 cells/mm3 in two consecutive determinations or in patients who developed opportunistic infections. Evaluations were performed at interim analysis (end of 4th off-ART, week 0) and 24 and 48 weeks after the end of 4th off-ART. A long-term follow-up analysis was also performed up to 9 years from the final stop. It included survival rate, clinical events, time to resume ART, CD4-CD8-CD4/CD8 ratio. This was performed, as standard of care, during follow-up visits.
Measurements and evaluation
At enrolment the investigators recorded patient medical histories and performed a clinical examination. During the follow-up, clinical status and adverse events were recorded. Safety parameters, plasma HIV-1 RNA load, and CD4 and CD8 T cell counts were obtained at weekly intervals during off-ART and until PVL lowered below 20 copies/mL and monthly thereafter. Viral reservoir (HIV proviral DNA) was not performed.
Laboratory methods
HIV serology was determined with a microparticulate enzyme immunoassay (MEIA) AxSYM system (Abbott Laboratories, North Chicago, IL) and confirmed with LIA (Inno-LIA HIV I/II Score. Innogenetics. Ghent, Belgium). PVL was determined using the Amplicor HIV-1 Monitor Ultra Sensitive Specimen Preparation Protocol Ultra Direct Assay (Roche Molecular Systems, Inc., Somerville, NJ) with a limit of detection of 20 copies/mL. Those samples below the detection limit were retested with a lower limit of detection of 5 HIV-1 RNA copies/mL as described[32]. HIV-1-RNA quantification in tonsillar tissue was performed in each patient at baseline as previously described[33]. Subpopulations of CD3+, CD4+, and CD8+ T cells, as well as proportion of CD8/CD38+ cells were determined by flow cytometry.
Genotypic mutations of both reverse transcriptase (RT) and protease (PR) genes from viral RNA were tested using the ViroSeq HIV Genotyping System v.2 (Abbott Laboratories, North Chicago, IL) and ABI3100 sequencer. Samples for genotype were drawn at baseline and when patients presented PVL higher than 1,000 copies/mL after the final ART discontinuation. Resistance mutations were re-interpreted according the HIV2007 IAS consensus. Viral subtype was inferred comparing the “fasta sequence” in REGA HIV-1 Subtyping Tool—Version 2.0[34]. The HLA class I genotype was determined by reverse sequence-specific oligonucleotide (SSO) (RELIDynal, Madrid, Spain). Allele definition was automatically assigned by the RELI SSO Pattern-matching Program software and was manually supervised. Tumoral necrosis factor (TNF) and soluble receptor for interleukin 2 (srIL2) levels were measured by EIA based techniques (Immunotech, France).
Lymphocyte Proliferation Assays
Freshly isolated PBMCs were washed twice and resuspended at 2 × 106/mL in a serum-free medium X-VIVO 10. Cultures were plated in triplicate at 2 × 105/well in 7-day assays, in 96 round-bottomed microplates (TPP, Trasadingen, Switzerland). Cells were cultured in the absence or presence of Pokeweed mitogen 10 μg/mL (Sigma) and 5 μg/mL of HIV-1 recombinant proteins gp160 and p24 (Protein Sciences, Meriden, CT). Incorporation of tritium-labeled thymidine was assessed for the last 18 hours of culture (Betaplate LKB, Wallac, Sweden). Results were expressed as mean counts per minute (cpm). The stimulation index (SI) was calculated for each sample using the formula: SI = mean cpm for cells with stimulus/mean cpm for cells without stimulus. Positive antigen-specific responses were defined as >3,000 cpm and SI >3. For analytical purposes, results were expressed as ‘positive’ or ‘negative’ CD4 proliferative responses to HIV-1 P24 protein.
HIV-1-Specific CD8+ T-Cell Responses
An ELISPOT assay (enzyme-linked immuno spot assay) was used to measure HIV epitope-specific CD8+ T-cell interferon-release from cryopreserved PBMC samples[6]. A mean of 16 (range: 3–27) different HLA class I–restricted synthetic peptides from gag, pol, env, and nef proteins were tested in each individual according to the HLA genotype. Results were expressed as total Spot Forming Cells (SFC)/10^6 PBMC and considered as positive with more than 500 SFC/determination.
Statistical analysis
The primary endpoint was the proportion of patients who maintained a PVL <3,000 copies/mL and CD4 cells >500/μL at 48 weeks from the end of the 4th STI (allocation to ART alone or ART plus IL-2, week 0). An interim analysis was performed at week 0 and at week 24.
Virological and immunological parameters during follow-up and changes from baseline were summarized by median and interquartile range (IQR) values. For the purpose of analysis, undetectable PVLs (<5 and <20 copies/mL) were considered equivalent to 5 and 20 copies/mL, respectively. The PVL values underwent log10 transformation before analysis.
The χ2 test and the Fischer exact test were used, as appropriate, to compare categorical variables. Continuous variables were compared between subgroups using the Mann-Whitney test. For long-term analysis and time free of ARV after final stop, Kaplan-Meier curves and the log-rank test were used. All p were considered significant at p<0.05. For missing values related to endpoints, the most recent available determination for this variable was considered and specifically mentioned in the results section. All statistical analyses were performed with Stata and SPSS software.
Ethics Statement
The study was approved by the Hospital Clínic Ethics Committee Board, and by the Spanish Regulatory Agency (‘Agencia Española del Medicamento’). All participants gave their written informed consent before enrolment. Registration of the trial in clinicalTrails.gov (NCT02300623) was retrospectively performed (registration was not compulsory at the time of the study conception).
Results
Patient selection
Fourteen patients were enrolled (Fig 1). Two patients were prematurely discontinued. Twelve patients completed the protocol and were included in the final analysis. All patients included in this analysis completed the four STIs and the follow-up period up to 8 years from the 48 weeks analysis. The STI periods started on 3/27/2000 for the first patient. The last patient completed the 48 weeks of follow-up on 4/20/2004.
PHI characteristics
Of the 12 patients, 10 were male, median age was 35 years (IQR 28.5–2.54) and the main risk category was homosexual/bisexual intercourse. PHI was symptomatic in 9 of 12 patients, and the median interval between the estimated date of infection and ART initiation was 11 weeks (IQR 5.5–12), median VL was 103,500 copies/mL (IQR 23,379–207,000), median CD4 cell count 536.5 cells/mm3 (IRQ 391–612.5) and CD4/CD8 ratio 0.735 (IQR 0.425–0.970), all variables being comparable between the two groups. All patients were infected with HIV-1 B subtype virus, none had evidence of transmitted antiretroviral drug resistance and all patients were in Fiebig 4 phase at the time of ART initiation. None of the patients showed genetic variation in the CCR5 co-receptor gene, HBV or HCV co-infection.
Characteristics at inclusion
The median time of treatment was 22.5 months (range 12–45), RNA-VIH-1 was below 5 copies/mL in 11/12 patients (patient #14 had 12 copies/mL), and the median CD4 count was 1,067 cells/mm3, (IQR 848–1328) with a median increase of 487 cells/mm3 since PHI. All but one patient had tonsillar RNA-HIV-1 below 40 copies/mg (patient #17 had 104 copies/mg). Baseline data at inclusion are shown in Table 1.
Table 1. Baseline characteristics at inclusion and CD4 T cells and viral load during STI.
| Total (n = 12) | STI (n = 6) | IL-2 (n = 6) | p value* | |||||
|---|---|---|---|---|---|---|---|---|
| PHI characteristics | n | % | n | % | n | % | ||
| Risk Category | 0.545 | |||||||
| MSM/bisexual | 8 | 67 | 5 | 83 | 3 | 50 | ||
| Heterosexual | 3 | 25 | 1 | 17 | 2 | 33 | ||
| IDU | 1 | 8 | 1 | 17 | ||||
| Gender | 1 | |||||||
| Male | 10 | 83 | 5 | 83 | 5 | 83 | ||
| Female | 2 | 17 | 1 | 17 | 1 | 17 | ||
| median | IQR | median | IQR | median | IQR | p value** | ||
| Age (years) | 35 | 28.5; 42.5 | 36.5 | 28; 47 | 32.5 | 29; 36 | 0.52 | |
| CD4 T cells/mm3 | 1,067 | 848; 1328 | 1,064 | 566; 1415 | 1,067.5 | 1012; 1097 | 0.973 | |
| CD4/CD8 ratio | 1.59 | 1.31; 1.80 | 1.64 | 1.58; 1.78 | 1.31 | 1.10; 1.86 | 0.648 | |
| CD4 T cells and viral load during STI | ||||||||
| CD4 T cells (Nadir during STI) cells/mm3 | ||||||||
| 1st STI | 641 | 606; 776 | 666 | 626; 804 | 637 | 550; 749 | 0.485 | |
| 2nd STI | 546 | 443; 899 | 718 | 383; 870 | 597 | 114; 667 | 0.486 | |
| 3rd STI | 681 | 448; 870 | 586 | 465; 1040 | 738 | 106; 823 | 0.699 | |
| 4th STI | 592 | 347; 916 | 592 | 433; 968 | 528 | 204; 730 | 0.485 | |
| HIV RNA (Peak during STI) copies/mL | ||||||||
| 1st STI | 150,500 | 25,650; 364,500 | 78,250 | 21,100; 596,000 | 363,500 | 30,200; 182,000 | 0.394 | |
| 2nd STI | 23,250 | 5,260; 50,450 | 7,780 | 130; 26,000 | 42,000 | 10,400; 197,000 | 0.1 | |
| 3rd STI | 19,200 | 1,655; 112,200 | 14,400 | 2,020; 19,200 | 98,350 | 1,290; 558,000 | 0.31 | |
| 4th STI | 20,000 | 1,360; 54,650 | 13,740 | 1,240; 42,400 | 22,100 | 1,480; 197,000 | 0.485 | |
*Fisher's exact test
**Wilcoxon Rank Sum test
Primary endpoint
Only one out of 12 patients (in the STI group) maintained VL<3000 copies/mL and CD4 cells >500 cells/mm3 without ART at 48 weeks.
Viral dynamics during the STI cycles and follow-up period
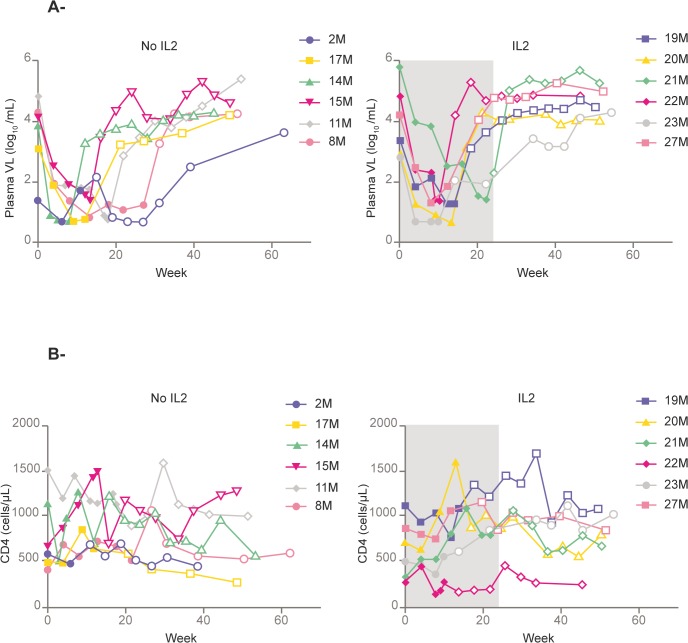
Viral load rebounded during STI and at follow-up in all patients except for patient #2, who maintained viral load <5 copies/mL until 48 week after the ART interruption. The peak of PVL rebound was highest in the first STI, PVR rose to 150,500 copies/mL (IQR, 25,650–364,500 copies/mL) and decreased to stable levels during the following off-ART cycles: 23,250 (5,260–50,450) copies/mL in 2nd, 19,200 (1,655–112,200) copies/mL in 3rd and 20,000 (1,360–54,650) in 4th (Table 1). There was a trend to an increased doubling time of PVL during the off-ART cycles (11.07 days in the 1st off-ART cycle and 24.45 days in the 4th p = 0.09). After resuming ART, all patients showed rapid declines in PVL in each treatment phase. The number of patients fulfilling Responder criteria was 2 out of 12, 4 out of 12, 5 out of 12 and 4 out of 12 patients during 1st to 4th off-ART cycle respectively. After 24 weeks of follow-up, 2/6 patients in the IL-2 arm and 2/6 patients in the non-IL-2 arm maintained PVL <3,000 c/mL and after 48 weeks only one (in the STI group, although reported at 40 weeks) out of 12 patients did. Between weeks 40 and 60, all patients presented VL around log4. VL dynamics after 4th STI are shown in Fig 3 and Table 2.
Fig 3. A) Plasma HIV Viral Load evolution after 4th STI cycle. B) CD4+ T cell evolution after 4th STI cycle. Footnote: The period of IL-2 administration is shown in grey.
The filled points represent periods on ART. Empty points are determinations without ART.
Table 2. Inflammation, immune activation, immunological and virological evolution following the end of 4th STI (week 0) until week 48 of follow-up.
| Week 0 (end 4th STI) | End of ART (final STOP) | Week 24 | Week 48 | |||||
|---|---|---|---|---|---|---|---|---|
| CD4 (cells/mm3) | ||||||||
| STI group | 622 | (514–1,167) | 1,116 | (669–1,297) | 928 | (601–1,079) | 668 | (514–1,033) |
| IL-2 group | 613 | (278–864) | 933 | (559–1092) | 865 | (832–906) | 690 | (669–936) |
| CD4/CD8 ratio | ||||||||
| STI group | 1.05 | (0.90–1.60) | 1.55 | (0.80–1.60) | 1.35 | (1.00–1.55) | 1.05 | (0.75–1.30) |
| IL-2 group | 0.83 | (0.77–1.00) | 1.1 | (0.92–1.87) | 0.93 | (0.67–1.28) | 0.77 | (0.48–0.87) |
| CD8/CD28+ (%) | ||||||||
| STI group | 57 | (37–60) | 56 | (39–65) | 59 | (33–61) | 52 | (34–62) |
| IL-2 group | 49 | (36–64) | 38 | (34–58) | 47 | (26–50) | 51 | (42–53) |
| CD8/CD38+ (%) | ||||||||
| STI group | 57 | (52–64) | 47 | (52–64)* | 47 | (52–64) | 53 | (44–63) |
| IL-2 group | 46 | (36–57) | 61 | (53–67)* | 56 | (53–69) | 47 | (44–59) |
| HIV Viral Load (log10/mL) | ||||||||
| STI group | 4.13 | (3.64–4.64) | <1.30 | 3.36 | (2.89–3.93) | 4.26 | (4.15–4.60) | |
| IL-2 group | 3.98 | (2.96–4.83) | <1.30 | 4.52 | (3.76–5.08) | 4.58 | (4.19–5.01) | |
| HIV Viral Load<3000 copies/mL | ||||||||
| STI group | 2 (33%) | N/A | 2(33%) | 1(17%) | ||||
| IL-2 group | 3 (50%) | N/A | 2(33%) | 0 | ||||
| TNF levels (pg/mL) | ||||||||
| STI group | 30 | (29–33) | 24 | (21–26)* | 26 | (16–29)* | 19 | (17–32) |
| IL-2 group | 36 | (21–42) | 39 | (37–49)* | 42 | (27–73)* | 44 | (29–64) |
| srIL-2 (pM) | ||||||||
| STI group | 61 | (61–82) | 59 | (35–60)* | 60 | (39–67)* | 76 | (39–91) |
| IL-2 group | 76 | (42–104) | 124 | (116–249)* | 124 | (101–127)* | 114 | (101–132) |
| Specific CD4 responses (positive antigen-specific anti-P24 protein responses) number of patients/total patients | ||||||||
| STI group | 2 out of 6 | 1 out of 6 | none | |||||
| IL-2 group | 1 out of 6 | 2 out of 6 | 1 out of 6 | |||||
| Specific CD8 responses (>500 SFC/10E6 PBMC) number of patients/total patients | ||||||||
| STI group | 4 out of 6* | 1 out of 6 | none | |||||
| IL-2 group | none* | none | none | |||||
Values are median and IQR
*p<0.05
ART: Antiretroviral treatment
STI: structured treatment interruptions
srIL-2: seric receptor for IL-2
SFC: Spot Forming Cells
PBMC: Peripheral blood mononuclear cells
Lymphocyte dynamics and cytokine levels during STI cycles and follow-up period
CD4 cells dropped in every STI but never below 500 T CD4 cells/μl. Immunological, virological and cytokine evolution at the end of 4th STI (interim evaluation), the end of ART (Final Stop), week 24 and week 48 is shown in Table 2. Briefly, CD4 cell count, CD4/CD8 ratio and CD8-CD28+ cells were all comparable for both groups at the end of 4th STI, the end of ART (final stop), week 24 and week 48. However, the proportion of CD8-CD38+ cells was significantly higher at the end of ART (final stop) for the IL-2 group. TNF and srIL-2 were also higher in the IL-2 group and the end of ART (final stop) and at 24 weeks of follow-up. These differences vanished at 48 weeks of follow-up. CD4 cell dynamics after 4th STI and until week 48 are shown in Fig 3 and Table 2.
Specific CD4 and CD8 immune responses
None of the 12 patients presented specific CD4 responses before STI. This response increased to 5/6 patients at 3rd STI cycle for the STI group and to 4/6 patients at 2nd STI for the IL-2 group. However, they decreased progressively for both groups (2/6 for the STI group and 1/6 for the IL-2 group at week 0-end of 4th STI-), and, at week 48, all but one patient in the IL-2 group had lost these HIV-specific CD4 responses.
Regarding HIV-specific CD8 responses, 1/12 presented specific CD8 responses before STIs (in the IL-2 group), which also increased in both groups during STI. The number of patients presenting HIV-specific CD8 responses was comparable in both groups in 1st, 2nd and 3rd STI (p = 0.558, p = 1, p = 0.558 respectively). However, at week 0-end of 4th STI- these responses decreased to zero patients for the IL-2 group compared to 4 in the STI group (p = 0.014), and at 48 weeks of follow-up, they were absent in all 12 patients (Table 2).
Considering the 12 patients together, there were no statistically significant differences in the VL doubling time among those patients eliciting a strong CD8 response compared to those eliciting a weak CD8 response (p = 0.530, p = 0.180, p = 1, p = 0.283 for 1st, 2nd, 3rd, 4th STI respectively).
Development of ART resistance mutations
After the 4 STI cycles, only one patient developed K70R resistance mutation.
Low IL-2 dose tolerability
Overall injections were well tolerated, but all patients presented mild injection-site reactions. One patient presented cellulitis. No adverse effects on liver tests, red blood cells, white blood cells or lipid profile were seen (data not shown).
Long-term clinical and immunological follow-up
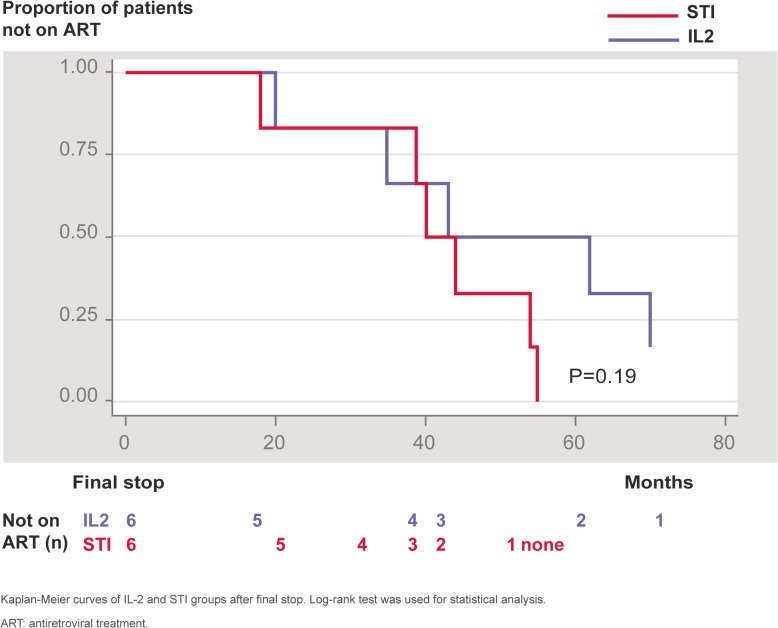
One patient in the IL-2 group died at 7 years and 10 months following the final stop due to metastatic colon cancer, aged 60. Overall 11/12 patients were alive at 9 years after the final stop. No other patient presented an AIDS-related opportunistic infection or neoplasm or other non-AIDS defining cancer. All patients in the STI group resumed ART at the end of the long-term follow-up and 5 out of 6 in the IL-2 group (one patient did not need to resume ART during the 9 years following the final stop, keeping CD4 cells consistently between 600 and 1100 cells/μl and VL to range around log4). Most of the patients in both groups resumed ART with a NNRTI (efavirenz)-based regimen. At the time of resuming ART, CD4 cells were comparable: 291/mm3 for the STI group and 325/mm3 for the IL-2 group (p = 0.855); CD4 percentage and CD4/CD8 ratios were also comparable for both groups. Immunological parameters remained comparable during long-term follow-up. Nine years after final stop the number of CD4 and CD8 cells and CD4/CD8 ratio were not different between groups: CD4 cells = 769.5/mm3 for the STI group and 844.5/mm3 for the IL-2 group (p = 1), CD4 percentage = 38.88 for the STI group and 38.7 for the IL-2 group (p = 0.394) and CD4/CD8 ratio = 1.20 for the STI group and 0.95 for the IL-2 group (p = 0.522). Time to resume ART after the final stop is shown in Fig 4. There was a trend to delayed resuming of ART in the IL-2 group. At 60 months after final stop, 3 out of 6 patients in the IL-2 group were not on ART compared to none in the STI group, but the difference did not reach statistical significance (p = 0.19).
Fig 4. Proportion of patients not receiving ART after the final stop.
Discussion
The most recently published reports suggest that a favorable immunological outcome is obtained when ART is initiated very early in the course of primary HIV infection [25, 35, 36]. Moreover, in recent years it has been shown to have had a big impact in transmission for patients with PHI/recent HIV infection, adding epidemiological aspects to the arguments for ART during this phase[35, 37, 38]. ART in primary infection may also improve patients’ quality of life[39]. However, although all guidelines suggest treating symptomatic PHI patients, the indication is less clear for those who are asymptomatic. When ART is initiated, most guidelines recommend lifelong therapy due to the risk of viral rebound (and, probably, loss of any advantage) if treatment is stopped. In cases where ART is stopped, several immunological strategies have been evaluated as having impact on the clinical and immunological evolution of HIV infection and delaying the resuming of ART. Among these strategies, STI might boost HIV immunity, and several studies have analyzed this approach, including the use of STI alone or associated with other immune modulating therapies. A recently published study evaluated the impact of Peg-IFN and STI on PHI, but apart from lower peaks of HIV viremia during STI, no favorable immunological differences were seen in the long-term follow-up[31]. In our study, unfortunately, we also failed to prove any immunological benefit: at 48 weeks of follow-up all patients in both groups had lost the specific anti-HIV immune responses. According to our definition criteria of Responder, only one patient in the STI group had less than 3,000 copies of HIV-1 RNA, thus failing to show control of viral replication in both groups. We have seen no differences in the CD4 levels and viral load between groups. Thus, our 12 patients rebounded during the follow-up, with a relative decrease in the PVL with the following interruptions, but none of them evolved as a post-treatment controller, which has been calculated to occur in around 10% of the patients treated during PHI[25]. However, all our patients evolved clinically well when ART was resumed, thus there was not a deleterious long-term effect of STI. This is particularly important for the design of HIV functional-cure trials, in which, sooner or later, ART must be stopped to test spontaneous viral control but without harming patients not showing such viral control. However, we did not measured viral reservoirs, which could have been increased with STIs.
The IL-2 group showed higher immune activation and higher levels of inflammatory markers during the initial 24 weeks of follow-up. It is not clear whether this higher immune activation and inflammation impact the clinical outcome negatively in the long-term follow-up, but the only death, due to a non-AIDS defining cancer, was noted in the IL-2 group. Chronic inflammation and immune activation, among other factors, have been associated with non-AIDS defining cancers among HIV-infected patients[40]. However, in the studies conducted with IL-2 in chronic HIV-infection, with more than 3,000 patients, no increased incidence of cancer was seen[41]. On the other hand, there was a trend to delayed initiation of ART after the final stop in the IL-2 group but the numbers are too small to draw definite conclusions. The delayed resuming of ART did not prevent achieving equal immunological parameters (CD4, CD4 proportion and CD4/CD8 ratio) 9 years after the final stop between groups. However, in a life-long treatment scenario, this has very little clinical relevance. Indeed, nowadays most guidelines recommend an earlier initiation of ART, regardless of CD4 cell count.
It seems that the window to obtain an immunological advantage when treating PHI is very narrow, as suggested by a recent cohort study[36] in which patients reached 900 CD4 cells/μl more frequently when they started ART within 4 months of primary infection. In another recently published study, Goujard et al. suggest that the precocity of ART is not the only important factor: baseline immune response against HIV is a key factor to control HIV replication efficiently in a long-term follow-up after stopping ART[35]. In another study, however, apart from early ART during PHI, no other factor was found to be associated with long-term control of viremia[24]. The PRIMO-SHM trial investigated the impact of 24 or 60 weeks of ART compared to no-ART during PHI, and they concluded that a short cycle of ART might delay the indication for treatment later[42]. In the SPARTAC study, the conclusions were similar, but the time gained to resume ART was approximately the same for patients who were on treatment during PHI, thus the benefit was very limited[43]. Therefore, it seems clear that ART should be administered as early as possible to obtain the maximum immunological advantage, but whether the addition of another immunomodulating strategy (such as IL-2) may help ART in this setting remains unknown.
As far as we know, this study is the first to evaluate the effect of daily low-dose IL-2 in association with STI during PHI. It is also one of the few studies reporting such a long period of follow-up, giving a reliable view of the intervention effect. It has, however, several limitations. First, the small size of patients in both arms probably prevented any statistically significant difference being found in the time to resume ART. Secondly, the indication to resume ART was outside of the originally selected period of follow-up of the study, and it was driven by the clinical decision of the attending physician; however, CD4 cells at resuming were not statistically different among both groups, allowing us to compare them. Third, at the time of the design of this study, a VL lower than 3000 copies/mL and a CD4 cell count higher than 500 cells/mm3 was not considered an indication for ART. Nowadays most clinicians would treat these patients, since indications for ART have evolved and most guidelines recommend ART as of diagnosis, regardless of CD4 cell count or VL. Finally, due to the lack of clinical benefit in large clinical trials, the interest on IL-2 administration in HIV infection has significantly decreased.
In conclusion, in the short term, STI proved able to boost specific immune responses, but these responses were lost 48 weeks after stopping ART. IL-2 failed to boost the specific anti-HIV T-cell immune responses. There was an increased immune activation and inflammation in the IL-2 group that also vanished during follow-up. In the long-term follow-up, there was a trend to delayed need to resume ART in the IL-2 arm, not reaching statistically significant differences. Although considering that the potential delay of ART in a lifetime treatment might not be very relevant, this small study suggests that immunological interventions during PHI may impact the long-term outcome. Probably these results cannot be translated into clinical practice, but they may encourage further research in this topic. Larger studies are needed to prove whether IL-2 or other immune modulating strategies may be useful in the setting of PHI.
Supporting Information
(DOC)
(DOC)
(DOC)
Acknowledgments
Presented in part at the XV International AIDS Conference, Bangkok (Thailand). July 11–16, 2004. Abstract WePeB5690, and at V National Congress GeSIDA, Sitges (Spain), November 19–22, 2013. Abstract 145.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project was supported in part by grants from the Spanish Foundation for AIDS Research and Prevention (FIPSE), Grant 00/3128, Madrid (Spain) awarded to JM and by the “Fondo de Investigaciones Sanitarias” (FIS) grant 04/0363 from the “Instituto de Salud Carlos III”, Madrid, Spain awarded to JM. Interlekin-2 (Macrolin®) was provided free-of-charge by Chiron, Madrid, Spain. JA developed this work in the frame of a ‘Juan de la Cierva 2012’ post-doctoral program, Ministerio de Competitividad, Spain. DN developed this work in the frame of a a post-residency Scholarship Ajuts a la Recerca ‘Josep Font’ 2014, Hospital Clinic, Barcelona, Spain, FA held a Rio Hortega Research Grant from the “Instituto de Salud Carlos III” and the “Ministerio de Economia and Competitividad”, Madrid (Spain) whilst this work was being developed. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kaufmann GR, Zaunders JJ, Cunningham P, Kelleher AD, Grey P, Smith D, et al. Rapid restoration of CD4 T cell subsets in subjects receiving antiretroviral therapy during primary HIV-1 infection. AIDS 2000; 14(17):2643–2651. [DOI] [PubMed] [Google Scholar]
- 2. Zaunders JJ, Cunningham PH, Kelleher AD, Kaufmann GR, Jaramillo AB, Wright R, et al. Potent antiretroviral therapy of primary human immunodeficiency virus type 1 (HIV-1) infection: partial normalization of T lymphocyte subsets and limited reduction of HIV-1 DNA despite clearance of plasma viremia. J Infect Dis 1999; 180(2):320–329. [DOI] [PubMed] [Google Scholar]
- 3. Yerly S, Perneger TV, Vora S, Hirschel B, Perrin L. Decay of cell-associated HIV-1 DNA correlates with residual replication in patients treated during acute HIV-1 infection. AIDS 2000; 14(18):2805–2812. [DOI] [PubMed] [Google Scholar]
- 4. Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 2013. [DOI] [PubMed] [Google Scholar]
- 5. Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med 2001; 193(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oxenius A, Price DA, Easterbrook PJ, O'Callaghan CA, Kelleher AD, Whelan JA, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A 2000; 97(7):3382–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL, et al. Immune control of HIV-1 after early treatment of acute infection. Nature 2000; 407(6803):523–526. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 1997; 278(5342):1447–1450. [DOI] [PubMed] [Google Scholar]
- 9. Ambrosioni J, Calmy A, Hirschel B. HIV treatment for prevention. J Int AIDS Soc 2011; 14:28 10.1186/1758-2652-14-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365(6):493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plana M, Garcia F, Gallart T, Miro JM, Gatell JM. Lack of T-cell proliferative response to HIV-1 antigens after 1 year of highly active antiretroviral treatment in early HIV-1 disease. Immunology Study Group of Spanish EARTH-1 Study. Lancet 1998; 352(9135):1194–1195. [DOI] [PubMed] [Google Scholar]
- 12. Shasha D, Walker BD. Lessons to be Learned from Natural Control of. Front Immunol 2013; 4:162 10.3389/fimmu.2013.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zaunders J, van BD. Innate and Adaptive Immunity in Long-Term Non-Progression in HIV Disease. Front Immunol 2013; 4:95 10.3389/fimmu.2013.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med 1999; 340(21):1683–1684. [DOI] [PubMed] [Google Scholar]
- 15. Ortiz GM, Nixon DF, Trkola A, Binley J, Jin X, Bonhoeffer S, et al. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Invest 1999; 104(6):R13–R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fagard C, Oxenius A, Gunthard H, Garcia F, Le BM, Mestre G, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med 2003; 163(10):1220–1226. [DOI] [PubMed] [Google Scholar]
- 17. Jacobson JM, Pat BR, Spritzler J, Saag MS, Eron JJ Jr, Coombs RW, et al. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis 2006; 194(5):623–632. [DOI] [PubMed] [Google Scholar]
- 18. Papasavvas E, Kostman JR, Mounzer K, Grant RM, Gross R, Gallo C, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med 2004; 1(3):e64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lori F, Lewis MG, Xu J, Varga G, Zinn DE Jr, Crabbs C, et al. Control of SIV rebound through structured treatment interruptions during early infection. Science 2000; 290(5496):1591–1593. [DOI] [PubMed] [Google Scholar]
- 20. Allen TM, Kelleher AD, Zaunders J, Walker BD. STI and beyond: the prospects of boosting anti-HIV immune responses. Trends Immunol 2002; 23(9):456–460. [DOI] [PubMed] [Google Scholar]
- 21. Autran B, Carcelain G. AIDS. Boosting immunity to HIV—can the virus help? Science 2000; 290(5493):946–949. [DOI] [PubMed] [Google Scholar]
- 22. Gandhi RT, Walker BD. Promises and pitfalls in the reconstitution of immunity in patients who have HIV-1 infection. Curr Opin Immunol 2002; 14(4):487–494. [DOI] [PubMed] [Google Scholar]
- 23. Kaufmann DE, Lichterfeld M, Altfeld M, Addo MM, Johnston MN, Lee PK, et al. Limited durability of viral control following treated acute HIV infection. PLoS Med 2004; 1(2):e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hocqueloux L, Prazuck T, Avettand-Fenoel V, Lafeuillade A, Cardon B, Viard JP, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 2010; 24(10):1598–1601. [DOI] [PubMed] [Google Scholar]
- 25. Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9(3):e1003211 10.1371/journal.ppat.1003211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith KA. Interleukin-2: inception, impact, and implications. Science 1988; 240(4856):1169–1176. [DOI] [PubMed] [Google Scholar]
- 27. Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci U S A 1996; 93(19):10405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobson EL, Pilaro F, Smith KA. Interleukin-2 infusions in HIV-infected patients. N Engl J Med 1997; 336(17):1260–1261. [DOI] [PubMed] [Google Scholar]
- 29. Smith KA. Low-dose daily interleukin-2 immunotherapy: accelerating immune restoration and expanding HIV-specific T-cell immunity without toxicity. AIDS 2001; 15 Suppl 2:S28–S35. [DOI] [PubMed] [Google Scholar]
- 30. El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355(22):2283–2296. [DOI] [PubMed] [Google Scholar]
- 31. Goujard C, Emilie D, Roussillon C, Godot V, Rouzioux C, Venet A, et al. Continuous versus intermittent treatment strategies during primary HIV-1 infection: the randomized ANRS INTERPRIM Trial. AIDS 2012; 26(15):1895–1905. [DOI] [PubMed] [Google Scholar]
- 32. Schockmel GA, Balavoine JF, Yerly S, Perrin L. Use of highly sensitive assays for the evaluation of post-exposure HIV prophylaxis. AIDS 1998; 12(13):1726–1727. [PubMed] [Google Scholar]
- 33. Alos L, Navarrete P, Morente V, Garcia F, Garrido M, Plana M, et al. Immunoarchitecture of lymphoid tissue in HIV-infection during antiretroviral therapy correlates with viral persistence. Mod Pathol 2005; 18(1):127–136. [DOI] [PubMed] [Google Scholar]
- 34.REGA HIV-1 Subtyping Tool—Version 2.0 Available: http://dbpartners.stanford.edu/RegaSubtyping/. Accessed: May 2008.
- 35. Goujard C, Girault I, Rouzioux C, Lecuroux C, Deveau C, Chaix ML, et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther 2012; 17(6):1001–1009. 10.3851/IMP2273 [DOI] [PubMed] [Google Scholar]
- 36. Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368(3):218–230. 10.1056/NEJMoa1110187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ambrosioni J, Junier T, Delhumeau C, Calmy A, Hirschel B, Zdobnov E, et al. Impact of highly active antiretroviral therapy on the molecular epidemiology of newly diagnosed HIV infections. AIDS 2012; 26(16):2079–2086. 10.1097/QAD.0b013e32835805b6 [DOI] [PubMed] [Google Scholar]
- 38. Brenner BG, Roger M, Moisi DD, Oliveira M, Hardy I, Turgel R, et al. Transmission networks of drug resistance acquired in primary/early stage HIV infection. AIDS 2008; 22(18):2509–2515. 10.1097/QAD.0b013e3283121c90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grijsen M, Koster G, van VM, van KM, Kootstra G, Steingrover R, et al. Temporary antiretroviral treatment during primary HIV-1 infection has a positive impact on health-related quality of life: data from the Primo-SHM cohort study. HIV Med 2012; 13(10):630–635. 10.1111/j.1468-1293.2012.01020.x [DOI] [PubMed] [Google Scholar]
- 40. Cutrell J, Bedimo R. Non-AIDS-defining cancers among HIV-infected patients. Curr HIV/AIDS Rep 2013; 10(3):207–216. 10.1007/s11904-013-0166-8 [DOI] [PubMed] [Google Scholar]
- 41. Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 2009; 361(16):1548–1559. 10.1056/NEJMoa0903175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grijsen ML, Steingrover R, Wit FW, Jurriaans S, Verbon A, Brinkman K, et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: the randomized Primo-SHM trial. PLoS Med 2012; 9(3):e1001196 10.1371/journal.pmed.1001196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fidler S, Porter K, Ewings F, Frater J, Ramjee G, Cooper D, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med 2013; 368(3):207–217. 10.1056/NEJMoa1110039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.