Abstract
Purpose
The purpose of this study was to assess the correlation of previous bladder cancer history with the recurrence and progression of patients with high-risk non–muscle-invasive bladder cancer treated with adjuvant Bacillus Calmette–Guérin (BCG) and to evaluate their natural history.
Materials and Methods
Patients were divided into two groups based on the existence of previous bladder cancer (primary, non-primary). A logistic regression analysis was used to identify the possible differences in the probabilities of recurrence and progression with respect to tumor history, while potential differences due to gender, tumor size (> 3 cm, < 3 cm), stage (pTa, T1), concomitant carcinoma in situ (pTis) and number of tumors (single, multiple) were also assessed. Univariate and multivariate models were employed. In addition, Kaplan-Meier survival analysis was used to compare recurrence- and progression-free survival between the groups.
Results
A total of 192 patients were included (144 with primary and 48 with non-primary tumors). The rates of recurrence and progression for patients with primary tumors were 27.8% and 12.5%, respectively. The corresponding percentages for patients with non-primary tumors were 77.1% and 33.3%, respectively. The latter group of patients displayed significantly higher probabilities of recurrence (p=0.000; 95% confidence interval [CI], 4.067 to 18.804) and progression (p=0.002; 95% CI, 1.609 to 7.614) in a univariate logistic regression analysis. Previous bladder cancer history remained significant in the multivariate model accounting for history, age, gender, tumor size , number of tumors, stage and concomitant pTis (p=0.000; 95% CI, 4.367 to 21.924 and p=0.002; 95% CI, 1.611 to 8.182 for recurrence and progression respectively). Kaplan-Meier curves revealed that the non-primary group hadreduced progression- and recurrence-free survival.
Conclusion
Previous non–muscle-invasive bladder cancer history correlates significantly with recurrence and progression in patients with high-risk non-muscle-invasive disease treated with adjuvant BCG.
Keywords: High-risk, Non-muscle-invasive bladder cancer, Previous bladder cancer history, Recurrence, Disease progression
Introduction
Bacillus Calmette–Guérin (BCG) is the most effective intravesical treatment for high-risk, non–muscle-invasive bladder cancer patients markedly meliorating their outcome [1,2]. However, a substantial proportion of patients do not respond well to intravesical BCG therapy, and their tumors persist or recur early after BCG treatment, which may become invasive and/or metastatic. The evaluation of these patients is challenging due to their heterogeneity and difficulty in their natural history prognosis [3,4].
Several prognostic parameters, including molecular pathological and immunological ones, have been evaluated in patients treated with BCG [5]. Some of these factors have been well studied however many of them have a controversial prognostic role. Traditional prognostic factors, such as tumor size, stage and grade, concomitant carcinoma in situ (pTis), tumor recurrence rate, and multiplicity, are broadly admitted to be clinically inaccurate for the purpose of determining the prognosis of recurrence and progression in high-risk, non–muscle-invasive patients treated with BCG. Therefore, there are many studies that attempt to identify the subset of patients who will not respond to BCG therapy and who could benefit from an early aggressive treatment [6-8].
We conducted a retrospective study in order to evaluate the role of previous non–muscle-invasive bladder cancer history as a risk factor for recurrence and progression in patients with high-risk, non–muscle-invasive bladder cancer, and to define their natural history via estimating the median time to recurrence and median time to progression.
Materials and Methods
1. Patients and treatment
During the enrollment period, between August 2001 and December 2010, we included patients with transurethrally resected, histologically proven, primary or not, single or multiple, non–muscle-invasive (carcinoma in situ, pTis, pTa, pT1), high-grade (according to the 2004 World Health Organization [WHO] classification) bladder cancer. The follow-up period for each individual patient was calculated starting from the day of first postoperative follow-up cystoscopy until last encounter with the patient or death, or in every other case, December 2011. Patients with a present or previous history of upper urinary tract carcinoma or patients with muscle invasive disease were excluded. Within 6 hours after transurethral resection, patients received one intravesical instillation of epirubicin 50 mg. The instillation was omitted in every case of postoperative clinically significant bleeding, fever, and perforation. In addition, random multiple biopsies of the bladder were performed in the event of a Tis lesion suspicion. After 3 weeks of resection (either first or repeated) all patients received an adjuvant induction course of 6 weekly intravesical BCG instillations. Furthermore, they received maintenance BCG therapy. As maintenance therapy, we applied a modification of the procedure proposed by Lamm et al. [9]. Patients received three weekly intravesical BCG instillations administered at 3, 6, 12, 18, 24, 30, and 36 months after the last inductive instillation. Our study was approved by the Ethics Committee—Scientific Board of the University Hospital of Larissa and it adheres to the provisions of the Declaration of Helsinki (as revised in Tokyo 2008).
2. Assessment
Whenever the biopsy specimen lacked muscle or the resection was incomplete, a transurethral resection was repeated within 4-6 weeks. More than 80% of our pathological specimens were evaluated by the same uropathologist. All patients underwent a cystoscopy every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Any visible lesion had to be resected. When cystoscopy was performed, a cytology examination was additionally performed. Patients had an imaging examination of the upper urinary tract (intravenous urogram) every two years.
Recurrence was established only by a histologic report. We considered a progression as every change from Ta to at least T1, and from T1 to at least T2 stage, as well as the presence of Tis at any point in histologic reports derived from patients with no previous Tis diagnosis. Patients were classified in the following two groups: group A, patients with primary tumors; and group B, patients with non-primary tumors, who had a positive history of histologically diagnosed high-risk, non–muscle-invasive bladder cancer, in our institution or not, prior to the beginning of the enrollment period.
Primary end point of our study was the evaluation of differences in the potential prognostic factors associated with recurrence and progression (age, gender, bladder cancer history, stage, number of tumors, concomitant Tis, and tumor size) for all patients, and the secondary point was the investigation of the natural history of these tumors by calculating the median time to recurrence and progression.
3. Statistical analysis
A logistic regression analysis was used in order to identify the possible differences in the probabilities of recurrence and progression with respect to tumor history (primary, non-primary). The potential differences due to gender, tumor size (> 3 cm, < 3 cm), stage (Ta, T1), concomitant Tis, and number of tumors (single, multiple) were assessed, following the same approach both in univariate and multivariate models. Kaplan-Meier survival analysis was employed to compare recurrence- and progression-free survival between the primary and non-primary tumors. A statistical analysis was implemented using the SPSS ver. 19 (IBM Co., Armonk, NY). Two-sided p-values < 0.05 were considered to be statistically significant.
Results
1. Patients
A total of 192 patients were enrolled in our study; 160 of whom (83.3%) were male and 32 (16.7%) were female. The mean age at diagnosis was 69.5 years, and the mean follow-up was 33.5 months. Taking into consideration of the previous history of high-risk, non–muscle-invasive bladder cancer histologically diagnosed, 144 patients (75%) had primary tumors and 48 patients (25%) had non-primary tumors. In total, 163 patients (84.9%) received a single postoperative intravesical instillation of epirubicin 50 mg. The instillation was omitted in 29 patients (15.1%) due to postoperative clinically significant bleeding or fever. We proceeded to take random multiple biopsies from the bladder wall of 44 patients (22.9%), due to a suspicion for the presence of carcinoma in situ lesion. A transurethral resection was repeated within 4-6 weeks after the first one in 29 patients (15.1%) since the biopsied specimen did not contain muscle or the initial transurethral resection was incomplete (4 patients from group A and 1 patient from group B). In groups A and B, 121 patients (84%) and 39 patients (81.3%), respectively, completed at least one induction course of BCG (6 consecutive weekly instillations). The frequency distribution of the primary and non-primary tumors with respect to the demographic and pathological features of the 192 subjects enrolled in our study is presented in Table 1.
Table 1.
Frequency distribution of primary and non-primary tumors with respect to all studied variables
| History |
Total | Chi-squared (p-value) | ||
|---|---|---|---|---|
| Primary | Non-primary | |||
| Gender (male/female) | 118/26 | 42/6 | 160/32 | 0.371 |
| No. of tumors (single/multiple) | 63/81 | 24/24 | 87/105 | 0.451 |
| Size of tumor (> 3 cm/< 3 cm) | 113/31 | 32/16 | 145/47 | 0.099 |
| Stage (Ta/T1) | 32/112 | 15/33 | 47/145 | 0.208 |
| Concomitant Tis (no/yes) | 97/47 | 32/16 | 129/63 | 0.929 |
2. Recurrence
The 3-year-recurrence rate for patients with history of primary tumor (group A) was 27.8%; while for patients with non-primary tumors (group B) was 77.1%. The latter group of patients exhibited significantly higher probabilities of recurrence (p=0.000; 95% confidence interval [CI], 4.067 to 18.804) in the univariate logistic regression analysis. Bladder cancer history remained significant in the multivariate model (Table 2), accounting for history, age, gender, size of tumor, number of tumors, stage and concomitant Tis (p=0.000; 95% CI, 4.367 to 21.924). The median time to recurrence for non-primary patients was 11.9 months (range, 3.3 to 39.6 months) versus 21.2 months (range, 5.3 to 87.8 months) for primary ones. Kaplan-Meier curves revealed that the group of patients with non-primary tumors had a statistically significant reduced recurrence-free survival (log rank p-value=0.000) (Fig. 1).
Table 2.
Multivariate logistic regression analysis for recurrence
| p-value | Odds ratio | 95% CI | |
|---|---|---|---|
| History | 0.000 | 9.785 | 4.367-21.924 |
| Age | 0.632 | 0.992 | 0.959-1.026 |
| Gender | 0.695 | 0.824 | 0.312-2.172 |
| No. of tumors (single/multiple) | 0.750 | 1.113 | 0.576-2.150 |
| Size of tumor (> 3 cm/< 3 cm) | 0.322 | 0.654 | 0.283-1.514 |
| Stage (Ta/T1) | 0.348 | 1.460 | 0.663-3.217 |
| Concomitant Tis (no/yes) | 0.433 | 1.315 | 0.663-2.611 |
CI, confidence interval.
Fig. 1.
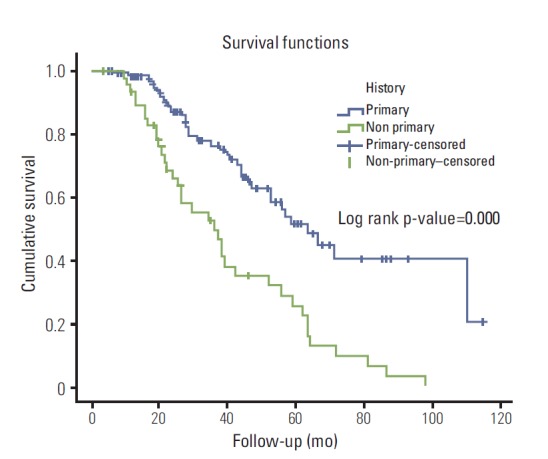
Kaplan-Meier survival curves for the difference in recurrence free survival between patients with primary (blue) and non-primary (green) tumors.
3. Progression
The 3-year-progression rate for patients with history of primary tumor (group A) was 12.5%, and the corresponding percentage for patients with non-primary tumors (group B) was 33.3%. Patients in group B displayed significantly higher probabilities of progression (p=0.002; 95% CI, 1.609 to 7.614) in a univariate logistic regression analysis. Bladder cancer history remained significant in the multivariate model (Table 3), accounting for history, age, gender, size of tumor, number of tumors, stage and concomitant Tis (p=0.002; 95% CI, 1.611 to 8.182). The median time to progression was 16.8 months (range, 5.3 to 97.2 months) for primary and 20.2 months (range, 4.4 to 41.4 months) for non-primary patients. Kaplan-Meier curves revealed that the group of patients with non-primary tumors had a statistically significant reduced progression-free survival (log rank p-value=0.004) compared to the group of patients with primary tumors (Fig. 2).
Table 3.
Multivariate logistic regression analysis for progression
| p-value | Odds ratio | 95% CI | |
|---|---|---|---|
| History | 0.002 | 3.630 | 1.611-8.182 |
| Age | 0.675 | 1.008 | 0.970-1.048 |
| Gender | 0.842 | 1.114 | 0.388-3.200 |
| No. of tumors (single/multiple) | 0.465 | 1.344 | 0.608-2.968 |
| Size of tumor (> 3 cm /< 3 cm) | 0.251 | 1.662 | 0.698-3.954 |
| Stage (Ta/T1) | 0.438 | 1.461 | 0.561-3.807 |
| Concomitant Tis (no/yes) | 0.561 | 1.275 | 0.562-2.893 |
CI, confidence interval.
Fig. 2.
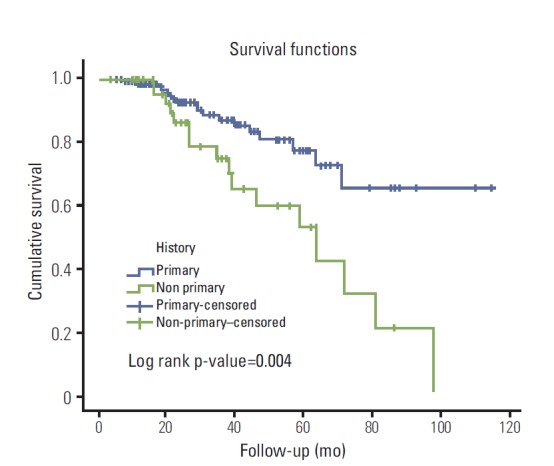
Kaplan-Meier survival curves for the difference in progression free survival between patients with primary (blue) and non-primary (green) tumors.
Discussion
In the literature, a few studies have evaluated the prognostic value of non–muscle-invasive bladder cancer history in high-risk patients treated with adjuvant BCG, and their results are conflicting. Fernandez-Gomez et al. [7] highlighted the importance of a previous bladder cancer history in the prognosis of patients with non–muscle-invasive bladder cancer treated with adjuvant BCG in regards to recurrence and progression. This multivariate analysis of four randomized CUETO trials included data from 1,062 patients with primary and relapsing high- and intermediate-risk tumors, showing a notable correlation between prior bladder cancer resections and recurrence [7]. Furthermore, Takashi et al. [10] evaluated the prognostic significance of various clinicopathological factors that affect recurrence and progression in 146 patients with superficial bladder cancer undergoing BCG therapy and reported that patients with a past history of bladder cancer experienced significantly earlier recurrence than those without such history [10]. In the same way, Losa et al. [11] found a connection between previous resections and recurrence in patients with non–muscle-invasive bladder tumor treated with adjuvant BCG immunotherapy.
On the other hand, there are many studies that failed to demonstrate any association between prior bladder cancer history and recurrence; however, they found a significant correlation with progression. For instance, Alkhateeb et al. [12] compared the outcome of 95 primary and 96 non-primary high-risk, non–muscle-invasive bladder cancer patients. This study focused its results on pT1 tumors, as they believed that patients with a history of prior resections for Ta or Tis, before progressing to T1, had a higher risk for progression after adjuvant BCG than patients with primary tumors [12]. Recently, a large retrospective study by Thomas et al. [13] with a long follow-up (59 months) compared the clinical outcomes of 1,061 patients with primary, progressive, and recurrent high-risk tumors. They showed that the latter had higher progression, disease-specific mortality, and the overall mortality rates than those with primary cancers [13]. Finally, Martinez-Pineiro et al. [14] and Hurle et al. [15] in a multivariate analysis also found that patients with previous tumor resections are at higher risk for progression.
The differences of our study in comparison to the literature are that firstly, we showed a statistically significant association of non-primary tumors with both recurrence and progression; and secondly, our group of patients consisted of those with only high-risk non–muscle-invasive tumors treated with BCG. Our study evaluated the prognostic significance of non-primary tumors of the urinary bladder because we believe that these patients have a different prognosis than those with primary tumors. Indeed, we found that patients who were presented with a history of non-primary tumors at diagnosis of non–muscle-invasive, high-risk bladder cancer had a significantly higher frequency of recurrence and progression compared to those with no previous history. Furthermore, we tried to explore the natural history of the disease in these patients because they form a subset with a clinical course, which is difficult to be prognosed. To this end, we determined median time to recurrence and median time to progression for patients with primary bladder cancer tumors (group A) and non-primary tumors (group B).
Among all prognostic factors we have evaluated, positive bladder cancer history at diagnosis of non–muscle-invasive high-risk disease seems to be the most important factor to take into account in the evaluation of patients treated with BCG, postoperatively. Other parameters associated with recurrence and progression, such as age, gender, stage, concomitant Tis, number of tumors, and tumor size (> 3 cm or < 3 cm), appear to be less helpful for the natural history prognosis of the disease in this particular patient group. This observation may help in identifying patients who need a more aggressive treatment, most probably an early cystectomy, due to their higher likelihood of recurrence and progression.
The mechanism that leads bladder cancer to recur and progress more quickly in patients with prior resections remains elusive. Schrier et al. [16] gave an explanation for this situation; based on the assumption that in high-risk, non–muscle-invasive bladder cancer, there are cells sensitive and non-sensitive to BCG treatment. Thus, BCG intravesical therapy may destroy the sensitive cells, leaving the refractory and more aggressive ones to grow [16]. Therefore, according to that hypothesis, patients with a history of resections progress earlier, because most of them, as well as in our study, have been exposed at least one cycle of BCG. Another hypothesis comes from El-Abbady et al. [17], who observed that patients with previous resections had a more aggressive local spread into the bladder. As a consequence, the relapsing tumors may not be treated appropriately, and thereafter, may progress faster than the non-relapsing tumors.
Limitations of our study seem to be its retrospective nature which is an inherent weak point, and the duration of the follow-up period. Indeed the mean follow-up period was 33.5 months, which is relatively short. Nevertheless, our follow-up indicates the trend of recurrence and progression for this specific group of patients. Based on our findings, as well as the results from other studies, we believe that in patients with a positive history of bladder cancer, before BCG treatment, recurrence and progression begin earlier and more often, when compared to those with a negative history. This fact must be taken into account in the management of patients with high-risk, non–muscle-invasive bladder cancer. Previous studies have proposed several prognostic factors of response to adjuvant BCG; however, a practical marker with good independent prognostic value has not been identified yet. Therefore, our study indicates the importance of prior history of non–muscle-invasive bladder cancer as a risk factor for progression and recurrence. Using real life data, our observation can become useful to many urologists in identifying patients who will not respond to BCG therapy.
Conclusion
A few studies have reported the prognostic factors for high-risk, non–muscle-invasive bladder cancer patients treated only with adjuvant BCG. Our analysis, in determining progression-free survival, recurrence-free survival, median time to progression, and median time to recurrence, revealed that patients with previous relapses recur and progress earlier in comparison to those with primarily diagnosed high-risk tumors. Additionally, a prior, non-muscle-invasive bladder-tumor history may play a significant role in the evaluation of patients receiving postoperative BCG immunotherapy. The latter observation is important, because it could be used, in combination with other prognostic factors, in counseling patients who are at high risk for progression, and to whom BCG treatment does not seem to offer clinical benefit.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Sylvester RJ, Brausi MA, Kirkels WJ, Hoeltl W, Calais Da Silva F, Powell PH, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57:766–73. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee CT, Dunn RL, Ingold C, Montie JE, Wood DP., Jr Early-stage bladder cancer surveillance does not improve survival if high-risk patients are permitted to progress to muscle invasion. Urology. 2007;69:1068–72. doi: 10.1016/j.urology.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166:1296–9. [PubMed] [Google Scholar]
- 5.Saint F, Salomon L, Quintela R, Cicco A, Hoznek A, Abbou CC, et al. Do prognostic parameters of remission versus relapse after Bacillus Calmette-Guerin (BCG) immunotherapy exist?: analysis of a quarter century of literature. Eur Urol. 2003;43:351–60. doi: 10.1016/s0302-2838(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–77. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Gomez J, Solsona E, Unda M, Martinez-Pineiro L, Gonzalez M, Hernandez R, et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: multivariate analysis of data from four randomized CUETO trials. Eur Urol. 2008;53:992–1001. doi: 10.1016/j.eururo.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Zachos I, Tzortzis V, Mitrakas L, Samarinas M, Karatzas A, Gravas S, et al. Tumor size and T stage correlate independently with recurrence and progression in high-risk non-muscle-invasive bladder cancer patients treated with adjuvant BCG. Tumour Biol. 2014;35:4185–9. doi: 10.1007/s13277-013-1547-8. [DOI] [PubMed] [Google Scholar]
- 9.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent Ta, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 10.Takashi M, Wakai K, Hattori T, Furuhashi K, Ono Y, Ohshima S, et al. Multivariate evaluation of factors affecting recurrence, progression, and survival in patients with superficial bladder cancer treated with intravesical bacillus Calmette-Guerin (Tokyo 172 strain) therapy: significance of concomitant carcinoma in situ. Int Urol Nephrol. 2002;33:41–7. doi: 10.1023/a:1014444601158. [DOI] [PubMed] [Google Scholar]
- 11.Losa A, Hurle R, Lembo A. Low dose bacillus Calmette-Guerin for carcinoma in situ of the bladder: long-term results. J Urol. 2000;163:68–71. [PubMed] [Google Scholar]
- 12.Alkhateeb SS, Van Rhijn BW, Finelli A, van der Kwast T, Evans A, Hanna S, et al. Nonprimary pT1 nonmuscle invasive bladder cancer treated with bacillus Calmette-Guerin is associated with higher risk of progression compared to primary T1 tumors. J Urol. 2010;184:81–6. doi: 10.1016/j.juro.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Thomas F, Noon AP, Rubin N, Goepel JR, Catto JW. Comparative outcomes of primary, recurrent, and progressive high-risk non-muscle-invasive bladder cancer. Eur Urol. 2013;63:145–54. doi: 10.1016/j.eururo.2012.08.064. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Pineiro JA, Flores N, Isorna S, Solsona E, Sebastian JL, Pertusa C, et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guerin with a reduced dose of 27 mg in superficial bladder cancer. BJU Int. 2002;89:671–80. doi: 10.1046/j.1464-410x.2002.02722.x. [DOI] [PubMed] [Google Scholar]
- 15.Hurle R, Losa A, Manzetti A, Lembo A. Intravesical bacille Calmette-Guerin in Stage T1 grade 3 bladder cancer therapy: a 7-year follow-up. Urology. 1999;54:258–63. doi: 10.1016/s0090-4295(99)00116-8. [DOI] [PubMed] [Google Scholar]
- 16.Schrier BP, Hollander MP, van Rhijn BW, Kiemeney LA, Witjes JA. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol. 2004;45:292–6. doi: 10.1016/j.eururo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 17.El-Abbady AA, Shoukry MS, Hanno AG, Younis LK, Abdel-Rahman M. Repeated transurethral resection of recurrent superficial bladder tumors: does it affect the spread and stage of the tumor? Scand J Urol Nephrol. 2002;36:60–4. doi: 10.1080/003655902317259382. [DOI] [PubMed] [Google Scholar]


