Abstract
Purpose
The risk for lymphedema (LE) after neoadjuvant chemotherapy (NCT) in breast cancer patients has not been fully understood thus far. This study is conducted to investigate the incidence and time course of LE after NCT.
Materials and Methods
A total of 313 patients with clinically node-positive breast cancer who underwent NCT followed by surgery with axillary lymph node (ALN) dissection from 2004 to 2009 were retrospectively analyzed. All patients received breast and supraclavicular radiation therapy (SCRT). The determination of LE was based on both objective and subjective methods, as part of a prospective database.
Results
At a median follow-up of 5.6 years, 132 patients had developed LE: 88 (28%) were grade 1; 42 (13%) were grade 2; and two (1%) were grade 3. The overall 5-year cumulative incidence of LE was 42%. LE first occurred within 6 months after surgery in 62%; 1 year in 77%; 2 years in 91%; and 3 years in 96%. In a multivariate analysis, age (hazard ratio [HR], 1.66; p < 0.01) and the number of dissected ALNs (HR, 1.68; p < 0.01) were independent risk factors for LE. Patients with both of these risk factors showed a significantly higher 5-year cumulative incidence of LE compared with patients with no or one risk factor (61% and 37%, respectively; p < 0.001). The addition of adjuvant chemotherapy did not significantly correlate with LE.
Conclusion
LE after NCT, surgery, and SCRT developed early after treatment, and with a high incidence rate. More frequent surveillance of arm swelling may be necessary in patients after NCT, especially during the first few years of follow-up.
Keywords: Breast neoplasms, Lymphedema, Neoadjuvant chemotherapy, Risk factors
Introduction
Lymphedema (LE) is the most common morbidity after breast cancer treatment. Recent data from a meta-analysis suggests that more than one in five patients who survived breast cancer will develop this complication [1]. LE and its associated symptoms, such as pain, heaviness, tightness, and decreased range of motion can cause functional and psychological impairment and degrade the quality of life.
We previously reported that the number of dissected axillary nodes (N-ALN), adjuvant chemotherapy (ACT), and ipsilateral axillary apex and supraclavicular radiation therapy (SCRT) were independent risk factors for the development of LE in the ACT setting [2]. The pathophysiology underlying LE is not fully understood; however, new data suggests that chronic inflammation induced by tumors has a potential role in the development of LE [3,4]. It is believed that the combination of physical alteration of the secondary and tertiary lymphatics via surgery, radiation of regional lymphatics, and chemotherapy agents that are commonly used to treat breast cancer can further exacerbate this inflammatory response [3].
Neoadjuvant chemotherapy (NCT) followed by definitive surgery and radiation therapy (RT) is a widely accepted strategy for locally advanced breast cancer [5]. The advantage of NCT is the possible assessment of disease response and increased rate of breast conserving surgery (BCS) for certain patients who would otherwise require a mastectomy. Despite the increasing use of NCT in breast cancer, relatively little attention has been given to LE in studies of NCT. The natural history of LE after NCT, including incidence, time course, and risk factors, has not been widely reported.
In this study, we analyzed the probability of LE after NCT by using graded scales, which combined the objective and subjective methods during a relatively long follow-up period. The main purpose of this study is to investigate the incidence and time course of LE after NCT. Risk factors for the development of LE are also examined.
Materials and Methods
1. Patients
Our patient database was used to select patients with clinically node-positive breast cancer who underwent NCT followed by modified radical mastectomy (MRM) or BCS with axillary lymph node dissection (ALND) and RT at the National Cancer Center of Korea, between 2004 and 2009. In total, we identified 406 patients. Of these patients, those with synchronous or metachronous contralateral breast cancer (n=3) and those who had not received RT (n=18) were excluded. Patients with a follow-up period of less than 3 years were also excluded (n=72). The remaining 313 patients were included in the present analysis, which was performed in accordance with the guidelines of our institutional review board.
2. Treatment
The NCT regimen was determined primarily based on prospective institutional trials during the treatment period [6,7]. For patients who were not included in the ongoing trials, the NCT regimen was selected by the physician. NCT consisted of four to six courses of anthracycline-based, taxane-based, or combined anthracycline-taxane therapy. Of the 313 patients, 180 (58%), who initially received four cycles of anthracycline-based NCT, received an additional taxane-based ACT after surgery. NCT was usually injected in the ipsilateral arm of breast cancer to preserve the contralateral arm for later use; however, the exact number of injected arms was not identified. The route of ACT administration after surgery was either intravenous in the contralateral arm or in an implanted port on the contralateral side for all patients.
Surgery of either MRM or BCS with ALND was performed within 3-4 weeks after NCT, unless there was evidence of disease progression. The general criteria for patient selection for BCS included a lack of initial extensive skin or chest wall involvement, absence of extensive microcalcifications or multifocal disease, anticipated adequacy of residual breast tissue following BCS, and absolute contraindication against breast irradiation. In all other patients, MRM was performed. Standard level I and II ALND was performed, with or without sentinel lymph node (SLN) biopsy in all patients.
All patients received ipsilateral breast (or chest wall) RT with SCRT in accordance to our institutional policy. Planning computed tomography (CT) scans were done, and RT was performed using conventional techniques. Typically, 50.4 Gy were delivered in 28 fractions to the ipsilateral breast or chest wall with tangential fields, using 6-MV photons. After BCS, all patients received an electron boost dose of 5.4-14.4 Gy with a daily 1.8 Gy to the lumpectomy cavity, using an appositional field (median, 10 Gy). Ipsilateral axillary apex and SCRT dose of 45-59.4 Gy with a daily 1.8 Gy was delivered to all patients (median, 45 Gy). The supraclavicular and axillary lymph nodes were contoured in planning CT scans, and the dose was prescribed to a supraclavicular target volume that encompassed a minimum of 95%. Daily posterior axillary boosts (PAB) were also delivered. Internal mammary nodal RT was administered to only 17 patients (5%) with clinically positive internal mammary nodes. The initial planned dose of RT was completed in all cases.
Adjuvant hormonal suppression therapy, using a tamoxifen or an aromatase inhibitor, was offered to 225 patients (72%) with estrogen receptor–positive or progesterone receptor– positive tumors. Following RT, trastuzumab (Herceptin) was administered for 1 year in 18 of 69 patients with c-erbB2–overexpressing tumors.
3. Measurement and assessment of LE
Determination of LE was based on both objective (circumference measurement) and subjective (patient perception of arm edema) assessments. The measurement and assessment of LE have been performed in all patients by a single physician (K.H.S) as part of a prospective database since 2004. Graded scales combining the subjective symptoms and Common Terminology Criteria for Adverse Events ver. 4.0 were used as the LE scoring systems; details of these methods have been described previously [2]. Briefly, a difference of 5%-10% in arm measurement or only self-perception of arm swelling with less than a 5% measurement difference was scored as grade 1, and arm measurement difference of 10%-30% or more than 30% was scored as grade 2 or 3, respectively. Assessments were made beginning at least 3 months postoperatively and regularly every 6 months until the last follow-up. Permanent LE was defined only when arm swelling was persistent at two consecutive followup examinations.
4. Statistical analysis
The rates of LE were calculated using a Kaplan-Meier method, and all statistics were measured from the date of surgery. Univariate Cox proportional hazards models were used to evaluate the risk factors associated with the development of LE. The factors included age (< 50 years vs. ≥ 50 years), body mass index (BMI; < 25 kg/m2 vs. ≥ 25 kg/m2), ypT classification (T0-T1 vs. T2-T4), ypN classification (N0-N1 vs. N2-N3), ypStage (pCR, I, or II vs. III or IV), type of surgery (BCS vs. MRM), N-ALNs (≤ 10 vs. > 10), and chemotherapy (NCT only vs. NCT and ACT). Variables that were shown to be significant or borderline significant (p < 0.1) in a univariate analysis were selected for multivariate analysis. Differences between the risk groups were estimated using a log-rank test. All statistical tests were two-sided, and statistical significance was defined at p < 0.05. Statistical analyses were performed using SPSS software ver. 17.0 (SPSS Inc., Chicago, IL).
Results
Patient and treatment characteristics are summarized in Table 1. The median N-ALN was 13 (range, 5 to 48).
Table 1.
Patient characteristics and treatment information (n=313)
| Characteristic | Classification | No. of patients (%) |
|---|---|---|
| Age (yr) | 46 (26-76) | |
| Body mass index (kg/m2) | 24 (17-36) | |
| ypT classification | T0 | 54 (17) |
| T1 | 127 (41) | |
| T2 | 97 (31) | |
| T3-4 | 35 (11) | |
| ypN classification | N0 | 97 (31) |
| N1 | 103 (33) | |
| N2-3 | 113 (36) | |
| ypStage | pCR | 50 (16) |
| I-II | 142 (45) | |
| III | 121 (39) | |
| Type of surgery | Breast-conserving surgery | 219 (70) |
| Mastectomy | 94 (30) | |
| No. of dissected axillary nodes | 5-10 | 108 (35) |
| 11-20 | 169 (54) | |
| ≥ 21 | 36 (11) | |
| Chemotherapy | Neoadjuvant only | 133 (42) |
| Neoadjuvant and adjuvant | 180 (58) |
Values are presented as median (range) or number (%). pCR, pathologic complete response.
1. Incidence and time course of LE
The median follow-up duration from the date of surgery was 5.6 years (range, 3.0 to 9.1 years). Initially, a total of 184 patients (59%) had been reported to have episodes of subjective or objective LE during the follow-up. Among these 184 patients, 52 patients with the arm edema that resolved spontaneously and showed no more LE episodes at the next 6-month follow-up were defined as presenting transient LE. The remaining 132 patients (42%) with persistent arm swelling at two consecutive follow-up examinations were classified as having permanent LE; in accordance to our gradig scale, 88 (28%) were grade 1, 42 (13%) were grade 2, and two (1%) were grade 3. The initial grade at the first swelling episode was grade 1 in 123 (39%) and grade 2 in nine (3%) patients. A total of 37 of 123 patients who were initially grade 1 progressed to grade 2, and one patient progressed to grade 3. One of nine patients who were initially grade 2 progressed to grade 3, and three patients showed an improvement to grade 1 at the subsequent follow-up exam. Of the 88 permanent grade 1 patients, 39 had only a subjective self-perception of arm edema with a less than 5% measurement difference. The median interval from surgery to initial swelling in patients with permanent LE was 4 months (range, 0.1 to 5.4 years). The overall 5-year cumulative incidence of LE was 42.2% (Fig. 1). Among the 132 affected patients, LE first occurred within 6 months after surgery in 62%; 1 year in 77%; 2 years in 91%; and 3 years in 96% of patients.
Fig. 1.
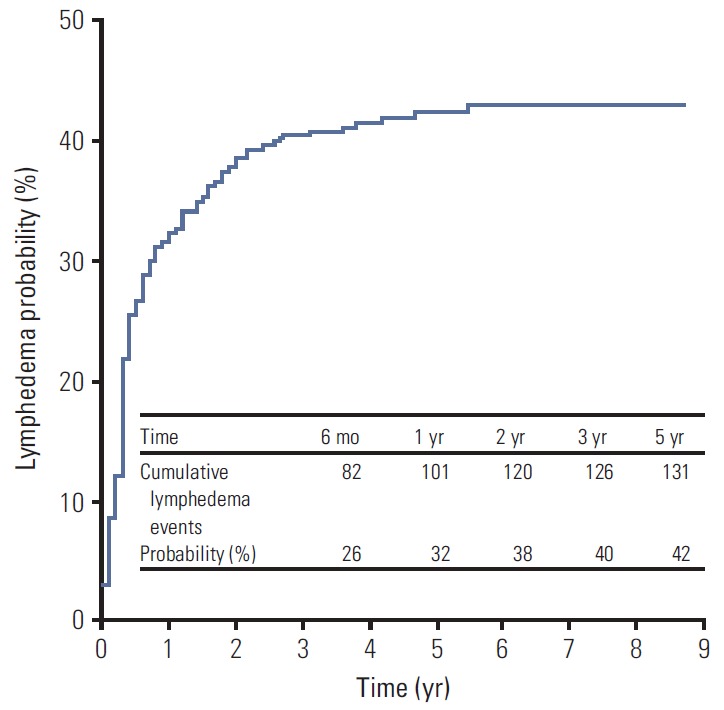
Kaplan-Meier plots of the cumulative incidence of breast cancer–related lymphedema.
2. Risk factors correlated with LE
The results of the univariate analysis of patient-, disease-, and treatment-related factors associated with the development of permanent LE are displayed in Table 2. Age ≥ 50 years (p=0.01) and N-ALN > 10 (p=0.03) were significantly correlated with an increased rate of LE development. With regard to age, the LE rates were 38% in patients aged < 50 years and 52% in those aged ≥ 50 years. The LE rate was 32% in patients with N-ALN ≤ 10 and 47% in patients with N-ALN > 10. The ypN2-N3 (p=0.08), ypStage III-IV (p=0.053), and mastectomy (p=0.08) showed borderline significance with LE development. BMI (p=0.13) and additional ACT (p=0.61) did not affect LE development.
Table 2.
Univariate analysis of risk factors associated with breast cancer–related lymphedema
| Variable | Classification | No. of patients |
p-valuea) | |
|---|---|---|---|---|
| Total | Lymphedema (%) | |||
| Age (yr) | < 50 | 214 | 81 (38) | 0.01 |
| ≥ 50 | 99 | 51 (52) | ||
| Body mass index (kg/m2) | < 25 | 213 | 84 (39) | 0.13 |
| ≥ 25 | 100 | 48 (48) | ||
| ypT classification | T0-1 | 181 | 75 (41) | 0.72 |
| T2-4 | 132 | 57 (43) | ||
| ypN classification | N0-1 | 200 | 77 (39) | 0.08 |
| N2-3 | 113 | 55 (49) | ||
| ypStage | pCR, I-II | 192 | 73 (38) | 0.053 |
| III | 121 | 59 (49) | ||
| Type of surgery | Breast-conserving surgery | 219 | 86 (39) | 0.08 |
| Mastectomy | 94 | 46 (49) | ||
| No. of dissected axillary nodes | ≤ 10 | 108 | 35 (32) | 0.03 |
| > 10 | 205 | 97 (47) | ||
| Chemotherapy | Neoadjuvant only | 133 | 58 (44) | 0.61 |
| Neoadjuvant and adjuvant | 180 | 74 (41) | ||
pCR, pathologic complete response.
Cox proportional hazards model.
In a multivariate analysis that used a stepwise backward selection procedure, age ≥ 50 years (hazard ratio [HR], 1.66; p < 0.01) and N-ALN > 10 (HR, 1.68; p < 0.01) were independent risk factors for LE development (Table 3). The ypStage, ypN classification, and mastectomy did not significantly affect the development of LE in a multivariate model.
Table 3.
Multivariate analysis of risk factors associated with breast cancer–related lymphedema
| Variable | Hazard ratio (95% CI) | p-valuea) |
|---|---|---|
| Age (< 50 yr vs. ≥ 50 yr) | 1.66 (1.16-2.36) | < 0.01 |
| No. of dissected axillary nodes (≤ 10 vs. > 10) | 1.68 (1.14-2.48) | < 0.01 |
| Type of surgery (breast conserving surgery vs. mastectomy) | 1.42 (0.99-2.04) | 0.056 |
CI, confidence interval.
Cox proportional hazards model.
The 5-year LE rates according to the two risk factors that showed a significant difference by both univariate and multivariate analyses (age and N-ALN) are displayed in Table 4. The 5-year LE rate in patients with no risk factors (age < 50 and N-ALN ≤ 10) was 31%. In patients with one risk factor, the 5-year LE rates were 35% (age ≥ 50 and N-ALN ≤ 10; p=0.69) and 41% (age < 50 and N-ALN > 10; p=0.24), respectively, and these were not significantly different from patients with no risk factors. Patients with both risk factors (age ≥ 50 and N-ALN > 10) showed an LE rate as high as 61% at 5 years (p=0.001), which was significantly higher than 37% of 251 patients with no or one risk factor (p < 0.001) (Fig. 2).
Table 4.
Five-year rate of breast cancer.related lymphedema according to the number of risk factors
| No. of risk factors | Risk factor |
No. | Five-year lymphedema rate (%) | p-valuea) | |
|---|---|---|---|---|---|
| Age (yr) | N-ALN | ||||
| 0 | < 50 | ≤ 10 | 71 | 31 | - |
| 1 | ≥ 50 | ≤ 10 | 37 | 35 | 0.69 |
| 1 | < 50 | > 10 | 143 | 41 | 0.24 |
| 2 | ≥ 50 | > 10 | 62 | 61 | 0.001 |
N-ALN, number of dissected axillary lymph nodes
Log-rank test; p-values were determined vs. 0 risk factors.
Fig. 2.
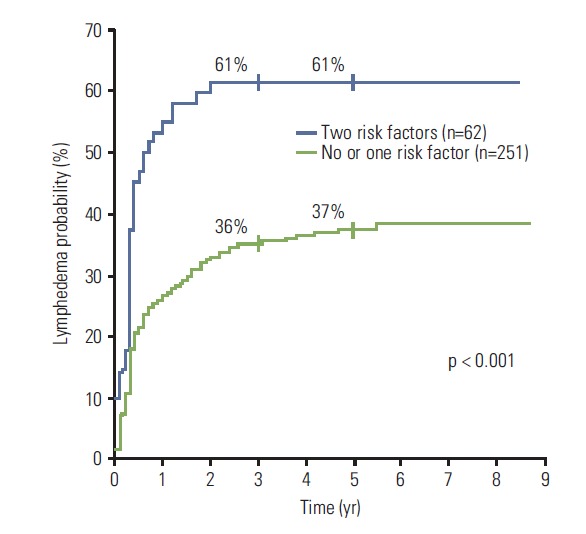
Kaplan-Meier plots of the cumulative rates of breast cancer–related lymphedema in accordance to the number of risk factors (age ≥ 50 years and > 10 dissected axillary nodes).
Discussion
A few data for breast cancer-related LE in NCT setting have been reported. Huang et al. [8] evaluated the risk factors for LE in 230 breast cancer patients and found that the risk of LE was decreased by 0.62 times (p=0.04) in patients receiving NCT. The overall 5-year cumulative LE rate of 42% after NCT in the present study seems higher than the previously reported rates in the literature in an ACT setting, which includes our previous study that showed 17% [2]. Nonetheless, a direct comparison of LE rates between NCT and ACT was not attempted, and thus, it is unclear whether NCT itself is a significant risk factor for LE. Several points could be considered in explaining why the incidence of LE is high in this study. All patients received NCT and surgery with ALND, followed by whole breast or chest wall RT with SCRT, all of which have been considered as significant risk factors for LE development [2,9,10]. The 5-year LE rate in our previous study of patients with all three treatment-related risk factors (N-ALN > 10, ACT, and SCRT) was 38%, and it was 24% among those with two factors (N-ALN ≤ 10, ACT, and SCRT) [2]. In the present study, the LE rates for patients with N-ALN > 10 and N-ALN ≤ 10 were 47% and 32%, respectively. The second point is that we were not able to identify the arm in which NCT was administered due to the retrospective nature of the present study. Hospital recommendations for NCT were to use the ipsilateral arm in order to preserve the contralateral arm for later use. Clark et al. [11] reported a 2.44-fold higher risk for LE in patients undergoing intentional intravenous infusion of nonchemotherapy solutions ipsilateral to the previous surgery for breast cancer. The number of cycles of chemotherapy infusion in the ipsilateral arm has been reported by Bevilacqua et al. [12] as an independent risk factor for developing LE. Another point to consider is that more aggressive axillary surgery might have been performed on patients in the current study because they all had initial clinically node-positive breast cancer. The proportion of patients with N-ALN >10 in this study was 65%, whereas it was 50% in our previous study [2]. However, notwithstanding these factors that affect LE, it remains unclear whether NCT itself increases the risk of LE. In a recent study by Specht et al. [13], the incidence of LE was compared between breast cancer patients who received NCT and those who received ACT, based on the hypothesis that NCT may decrease the risk of LE associated with ALND for patients with node-positive breast cancer by reducing the number of positive nodes. However, they found no statistically significant difference in LE risk between NCT and ACT patients.
All our patients received SCRT with PAB; therefore, it is not possible to determine whether the addition of PAB increased the risk of LE compared to SCRT alone. Hayes et al. [14] evaluated the risk of LE from regional node irradiation. Overall, there was no significant difference between SCRT and SCRT with PAB. However, in a subgroup analysis, an addition of PAB increased the risk of LE by 4.5-fold over the whole breast with SCRT alone in N2 patients. Another study by Warren et al. [15] indicated that the 2-year cumulative incidence of LE was 21.9% and 21.1% for breast/chest wall with SCRT and breast/chest wall with SCRT+PAB, respectively. The addition of PAB to SCRT did not increase the risk of LE in comparison to SCRT alone.
The time of onset of LE after NCT determined in the current study seems to be earlier than that of previous reports. Most patients (96%) developed their first swelling of permanent LE within the first 3 years, and LE occurred within 6 months after surgery in 62; within 1 year in 77%; and within 2 years in 91%. Norman et al. [16] reported that 80% of patients first developed LE within 2 years of diagnosis and 89% within 3 years. In patients who received ACT in our previous study, LE first occurred within 6 months after surgery in only 16%; 43% within 1 year; 76% within 2 years; and 91% within 3 years [2]. The median interval from surgery to initial swelling in patients with permanent LE was 0.3 year in the present study, but was 1.2 years in our previous adjuvant study [2]. More frequent surveillance immediately after surgery throughout the first 2 years appears to be necessary in patients with NCT.
Increased age is often reported to be an important risk factor for LE [12,17,18]. This finding was also observed in this study, where the incidence of LE was 38% for those aged < 50 years and 52% for those aged ≥ 50 years, which is a statistically significant difference. The higher incidence of LE in older patients may be attributable to progressive loss of lymphovenous anastomoses, which results from an increased pressure of the lymph system and can bridge the lymph and blood circulation systems as an alternate route for lymph drainage [10].
As long as the treatment of the axilla is necessary in breast cancer treatment, LE remains a potential complication. Risk-reduction practices to prevent LE development have been attempted through changes in the treatment- or patient-related factors. First, SLN surgery, rather than ALND, may be instrumental in reducing the risk for LE. To date, it is unclear whether SLN surgery should be applied to patients with clinically node-positive disease before NCT. The results from the ACOSOG Z1071 trial may support the use of SLN as an alternative to ALND [19]. Second, RT, especially reional lymph node RT, has been shown to be a major independent risk factor for the development of LE, with a 2- to 4.5-fold increase in the risk for LE [1,2,9,10]; therefore, optimal omission of regional lymph node RT after NCT may also reduce the risk for LE. Daveau et al. [20] reported that no significant impact on clinical outcome was observed when regional lymph node irradiation was withheld in stage II and III breast cancer patients with ypN0 after NCT and BCS. Le Scodan et al. [21] also reported that the omission of postmastectomy radiotherapy was acceptable in stage II-III breast cancer patients with ypN0 after NCT. Patient enrollment has begun for a prospective randomized trial National Surgical Adjuvant Breast and Bowel Project (NSABP) B-51/Radiation Therapy Oncology Group (RTOG) 1304 to evaluate the omission of RT in node-negative patients after NCT.
Limitations of our study include those inherent to any retrospective analysis. As mentioned above, the lack of data regarding the arm used for the injection of chemotherapy might have affected the calculated incidence of LE. Patient-related factors (arm infection, injury, and excessive hand use) could not be tested or included in the risk groups because we did not use questionnaires that included such factors. However, estimations of the risk for LE after NCT for patients with no or one risk factor (37%) and two risk factors (60%) may help clinicians to educate patients concerning the possibility of modifying patient-related factors. Additionally, although none of the 313 study patients recalled symptoms of arm edema or an arm injury event that preceded surgery in their questionnaire responses, the lack of arm measurements before NCT is a potential limitation.
Conclusion
In conclusion, our study showed that more than 40% of breast cancer patients who received NCT followed by surgery and SCRT suffered permanent LE, in which the onset time was during the early follow-ups. Risk factors were age ≥ 50 years and N-ALN > 10. More frequent surveillance of arm swelling appears to be necessary in patients after NCT, especially during the first few years of follow-up. Clinical trials addressing SLN surgery or omission of regional lymph node RT after NCT may assist in strategizing a method to reduce LE risk.
Acknowledgments
This research was supported by National Cancer Center Grant NCC-1210181-2 by the National Cancer Center, Republic of Korea.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 2.Kim M, Kim SW, Lee SU, Lee NK, Jung SY, Kim TH, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:498–503. doi: 10.1016/j.ijrobp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Shah C, Wilkinson JB, Baschnagel A, Ghilezan M, Riutta J, Dekhne N, et al. Factors associated with the development of breast cancer-related lymphedema after whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2012;83:1095–100. doi: 10.1016/j.ijrobp.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 4.Redon CE, Dickey JS, Nakamura AJ, Kareva IG, Naf D, Nowsheen S, et al. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proc Natl Acad Sci U S A. 2010;107:17992–7. doi: 10.1073/pnas.1008260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 6.Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008;109:481–9. doi: 10.1007/s10549-007-9672-y. [DOI] [PubMed] [Google Scholar]
- 7.Lee KS, Ro J, Lee ES, Kang HS, Kim SW, Nam BH, et al. Primary systemic therapy with intermittent weekly paclitaxel plus gemcitabine in patients with stage II and III breast cancer: a phase II trial. Invest New Drugs. 2010;28:83–90. doi: 10.1007/s10637-009-9229-5. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Zhou J, Zeng Q. Secondary lymphoedema after breast cancer surgery: a survival analysis. Int J Nurs Pract. 2012;18:589–94. doi: 10.1111/ijn.12005. [DOI] [PubMed] [Google Scholar]
- 9.Coen JJ, Taghian AG, Kachnic LA, Assaad SI, Powell SN. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiat Oncol Biol Phys. 2003;55:1209–15. doi: 10.1016/s0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- 10.Sakorafas GH, Peros G, Cataliotti L, Vlastos G. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15:153–65. doi: 10.1016/j.suronc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. QJM. 2005;98:343–8. doi: 10.1093/qjmed/hci053. [DOI] [PubMed] [Google Scholar]
- 12.Bevilacqua JL, Kattan MW, Changhong Y, Koifman S, Mattos IE, Koifman RJ, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. 2012;19:2580–9. doi: 10.1245/s10434-012-2290-x. [DOI] [PubMed] [Google Scholar]
- 13.Specht MC, Miller CL, Skolny MN, Jammallo LS, O'Toole J, Horick N, et al. Residual lymph node disease after neoadjuvant chemotherapy predicts an increased risk of lymphedema in node-positive breast cancer patients. Ann Surg Oncol. 2013;20:2835–41. doi: 10.1245/s10434-012-2828-y. [DOI] [PubMed] [Google Scholar]
- 14.Hayes SB, Freedman GM, Li T, Anderson PR, Ross E. Does axillary boost increase lymphedema compared with supraclavicular radiation alone after breast conservation? Int J Radiat Oncol Biol Phys. 2008;72:1449–55. doi: 10.1016/j.ijrobp.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 15.Warren LE, Miller CL, Horick N, Skolny MN, Jammallo LS, Sadek BT, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys. 2014;88:565–71. doi: 10.1016/j.ijrobp.2013.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–7. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman SA, Localio AR, Kallan MJ, Weber AL, Torpey HA, Potashnik SL, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010;19:2734–46. doi: 10.1158/1055-9965.EPI-09-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26:3536–42. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 19.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daveau C, Stevens D, Brain E, Berges O, Villette S, Moisson P, et al. Is regional lymph node irradiation necessary in stage II to III breast cancer patients with negative pathologic node status after neoadjuvant chemotherapy? Int J Radiat Oncol Biol Phys. 2010;78:337–42. doi: 10.1016/j.ijrobp.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 21.Le Scodan R, Selz J, Stevens D, Bollet MA, de la Lande B, Daveau C, et al. Radiotherapy for stage II and stage III breast cancer patients with negative lymph nodes after preoperative chemotherapy and mastectomy. Int J Radiat Oncol Biol Phys. 2012;82:e1–7. doi: 10.1016/j.ijrobp.2010.12.054. [DOI] [PubMed] [Google Scholar]


