Abstract
Background
Targeting the mitochondria during ischemia/reperfusion (IR) can confer cardioprotection leading to improved clinical outcomes. The cardioprotective potential of (−)-epicatechin (EPI) during IR via modulation of mitochondrial function was evaluated.
Methods and Results
Ischemia was induced in rats via a 45 min occlusion of the left anterior descending coronary artery followed by 1 h, 48 h, or 3 weeks (wk) reperfusion. EPI (10 mg/kg) was administered IV 15 min prior to reperfusion for the single dose group and again 12 h later for the double dose group. Controls received water. Experiments also utilized cultured neonatal rat ventricular myocytes (NRVM) and myoblasts. A single dose of EPI reduced infarct size by 27% at 48 hours and 28% at 3 week. Double dose treatment further decreased infarct size by 80% at 48 h, and 52% by 3 weeks. The protective effect of EPI on mitochondrial function was evident after 1 hr of reperfusion when mitochondria demonstrated less respiratory inhibition, lower mitochondrial Ca2+ load, and a preserved pool of NADH that correlated with higher tissue ATP levels. Mechanistic studies in NRVM revealed that EPI acutely stimulated maximal rates of respiration, an effect that was blocked by inhibitors of the mitochondrial pyruvate carrier, nitric oxide synthase, or soluble guanylyl cyclase. In myoblasts, knockdown of components of the mitochondrial pyruvate carrier blocked EPI-induced respiratory stimulation.
Conclusions
IV EPI confers cardioprotection via preservation of mitochondrial function potentially through enhanced substrate provision. These provocative results document a novel mechanism of a natural product with potential clinical utility.
Keywords: myocardial ischemia-reperfusion injury, epicatechin, cardiac metabolism, mitochondrial Ca2+, mitochondrial pyruvate carrier
Introduction
Myocardial infarction (MI) continues to be a leading cause of death and disability. After an acute MI, prompt and successful reperfusion is the most effective strategy for reducing the extent of injury [1,2]. However, the return of blood flow to myocardium results in reperfusion injury for which effective therapies have proven elusive. The consumption of cacao-derived products appears to provide beneficial cardiovascular effects [3]. A recent meta-analysis associated moderate chocolate consumption with a 37% reduction in cardiovascular risks, including coronary heart disease and stroke [4]. These effects appear to be mediated by the flavanol (−)-epicatechin (EPI) [4]. We have embarked on studies to support the potential clinical utility of direct (rather than dietary) administration of EPI [5–8]. We, as well as others, previously reported on the capacity of prolonged EPI pre-treatment (≥10 days) to confer sustained cardioprotection in the setting of ischemia-reperfusion (IR) and permanent coronary occlusion by reducing infarct size and preventing adverse left ventricular (LV) remodeling [5,6,10]. Although many studies ascribe the effects of flavonoids to their antioxidant properties [11–13] the results of our pre-treatment studies [5,6] suggest more precise mechanisms involving a nitric oxide-dependent cytoplasmic signaling event .
The energetic requirements of cardiac myocytes are mainly met by mitochondria. This organelle also plays important roles in maintaining intracellular Ca2+ homeostasis and in regulating cell death [14]. With ischemia, the loss of O2 and consequent decrease in ATP levels disrupts cardiac myocyte ionic homeostasis resulting in depolarization and cytoplasmic Ca2+ accumulation [15]. Upon reperfusion and re-establishment of the mitochondrial membrane potential, excess Ca2+ enters the mitochondria and can trigger cardiac myocyte death by multiple mechanisms, including oxidative injury and opening of the mitochondrial permeability transition pore (MPTP) [2,16–18]. Of interest is that stabilizing mitochondrial structure/function during IR preserves myocyte viability and can lead to improved clinical outcomes [15].
A clinically relevant treatment paradigm would include administering the compound intravenously either just before or after reperfusion. This study examined the capacity of EPI to reduce infarct size and limit adverse left ventricular remodeling when given 15 min prior to reperfusion. In addition, we determined if the provision of a second dose of EPI 12 h after reperfusion further enhanced cardioprotection. We evaluated mitochondrial function early in the reperfusion period and identified a novel mechanism of action using cultured cardiomyocytes and myoblasts. We hypothesize that the cardioprotective effects of EPI are mediated through protection and enhancement of mitochondrial function, thus preserving myocardial bioenergetics and tissue integrity.
Materials and Methods
Materials
All chemicals used in the study were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. All cell culture reagents were purchased from Invitrogen (Carlsbad, CA). All tissue culture plates were purchased from Fisher Scientific (Pittsburgh, PA).
Experimental groups and (−)-epicatechin treatment
For in vivo studies, adult male Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 250–300 g were used. All procedures were approved by the IACUC Committee and conform to published NIH guidelines for animal research. There were six different groups (Table 1). For the single dose group, control animals received water and treated animals received EPI (10 mg/kg) IV via the tail vein 15 min prior to reperfusion. EPI was prepared fresh for each experiment, and dissolved in water (pH 7.4). For the double dose group, animals received the initial IV treatment and a second IV dose 12 h later. Short term and long term EPI effects were evaluated at 1 h, 48 h or 3 wk.
Table 1.
Experimental groups utilized in this study.
|
Infarct Size Determination |
Single Dose Groups |
Double Dose Groups | ||
|---|---|---|---|---|
| IR | IR | IR + Epicatechin | ||
| 1 h Reperfusion | n=5 | - | - | |
| 48 h Reperfusion | n=12 | n=12 | n=6 | |
| 3 wk Reperfusion | n=6 | n=6 | n=7 | |
| Hemodynamics | Single Dose Groups | |||
| Normal | Normal + Epicatechin | |||
| Short Term Effects | n=4 | n=4 | ||
| Mitochondrial | Single Dose Groups | |||
| Function | Sham | IR | IR + Epicatechin | |
| 1 h Reperfusion | See Figure Legends |
See Figure Legend | See Figure Legends | |
Surgical Procedures
Animals were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg; Zoetis Inc; Florham Park, NJ) and xylazine (10 mg/kg, Vedco Inc; Saint Joseph, MO), intubated, and positive-pressure ventilated. A left thoracotomy was performed to expose the heart. In IR animals, the left anterior descending coronary artery was ligated for 45 min, released and the suture left in place as a point of reference. Successful occlusion and reperfusion was verified by visual inspection of LV color. In animals undergoing 48 h or 3 wk reperfusion, the chest was closed in layers and animals allowed to recover. Sham animals underwent the surgical procedure described with the exception of the coronary ligation and ischemia.
Tissue collection and MI size determination
Following hemodynamic measurements, hearts were excised and weighed. The area at risk AAR was determined by the reocclusion of the snare and infusion of trypan blue into the cannulated aorta. Five 2 mm rings were taken from the middle of the LV and stained using triphenyltetrazolium chloride (TTC). Computer assisted image analysis was used to measure infarct area (IA) and (AAR). Results are expressed as IA/AAR. In one subgroup of 1 h animals, the hearts were perfused with cold saline to remove blood and the RV free wall was excised. Mitochondria were isolated from the remaining LV tissue as described below. In another subgroup of 1 h animals, hearts were excised and immediately flash frozen in liquid nitrogen and stored at −80°C for further biochemical analysis. The images of unfixed, stained rings were also used to measure internal and external chamber diameters, and anterior and septal wall thicknesses. Remaining tissue samples were flash frozen in liquid nitrogen and stored at −80°C.
Hemodynamic measurements
To evaluate for possible hemodynamic effects secondary to the acute administration of EPI studies were performed in normal animals that only underwent a neck dissection and IV infusion. Animals were treated via the tail vein with either vehicle (water, n=4 or 10 mg/kg EPI, n=4) and values were recorded before, immediately after, and 1 h after IV infusion. Anesthesia was induced with 5% isoflurane and maintained at 1–2% to sustain a steady control of hemodynamics during the procedure. The right carotid artery was exposed via a neck dissection. Carotid and LV pressures were acquired using a micromanometer (2 French, 140 cm; Millar instruments Inc.; Houston, TX) introduced via the carotid artery. Hemodynamic measurements were digitally recorded for subsequent analysis using WINDAQ software (version 2.15, DATAQ Instruments Inc; Akron, OH).
Electron Microscopy
Samples from the border area of the infarct region were fixed in 2% paraformaldehyde plus 2.5% glutaraldehyde and prepared for electron microscopy as previously described [18]. Images were taken with a JEOL 1200FX transmission EM (JEOL; Peabody, MA) operated at 80 kv and the negatives were digitized at 1800 dpi using a Nikon CoolScan system (Nikon Inc.; Melville, NY). A stereological analysis to ascertain the ratio of mitochondrial volume to cytoplasmic volume was performed with Adobe Photoshop (Adobe; San Jose, CA). Point counting was used to determine the mitochondrial volume densities by overlaying a grid on each digitized image. Mitochondria and cytoplasm lying under intercepts were counted. The relative volume of mitochondria was expressed as the ratio of intercepts coinciding with this organelle to the intercepts coinciding with cytoplasm.
Tissue ATP Measurements
Frozen LV tissue from the 1 h group was used to measure ATP levels. Tissue was homogenized in ice cold 20% trichloroacetic acid and incubated for 10 min at 4°C. Samples were centrifuged and the extracts (supernatant) stored at −80°C. Samples were also used to determine protein concentrations. Acid extracts were neutralized and ATP levels were measured by a commercially available kit (CellTiter-Glo 2.0 Assay # G9242; Promega; Madison, WI).
Mitochondrial Isolation
Tissues were minced and homogenized in isolation buffer (210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EGTA). Mitochondria were isolated by differential centrifugation as previously described [19]. The mitochondrial pellet was washed once and resuspended in isolation buffer. A Bradford assay was used to determine the protein concentration of samples.
Mitochondrial Oxygen (O2) consumption
Respiration in isolated mitochondria was measured with a temperature controlled Clark-type oxygen electrode (Oxytherm electrode control unit, Hansatech Instruments; Amesbury, MA). Mitochondria (0.125 mg/mL) were incubated at 37°C and continuously stirred in basal assay medium containing 125 mM KCl, 2 mM KH2PO4, 20 mM HEPES, and 1 mM MgCl2, (pH 7.2), plus 5 mM glutamate/5 mM malate, 5 mM pyruvate/0.1 mM malate, or 5 mM succinate/1 µM rotenone. State 3 (phosphorylating) respiration was measured by the addition of 125 µM ADP and State 4 (resting) respiration were measured by the addition of 1 µM oligomycin. The maximal rate of respiration (State 3u) was measured by titration with the uncoupler, p-trifluoromethoxyphenylhydrazone carbonyl cyanide (FCCP). Data is presented in rate of oxygen consumption (OCR).
Mitochondrial Ca2+ Levels
The total pool of sequestered calcium was measured in isolated ventricular mitochondria by a previously described approach [20,21]. Mitochondrial (500 µg) were incubated at 25°C in 2 mL of media (250 mM sucrose, 10 mM HEPES-KOH, 2 mM KH2PO4, pH 7.4) supplemented with 0.5 µM Calcium Green-5N. Alamethecin and FCCP were used to release the total pool of Ca2+ sequestered in mitochondria. Binding of released Ca2+ to the fluorophore was measured at 506/532 nm in an LS50B spectrofluorometer (Perkin Elmer; Waltham, MA).
Mitochondrial NADH Measurements
Mitochondria (500 µg) were incubated in basal assay medium at 25°C supplemented with either 5 mM glutamate and 5 mM malate, or 5 mM pyruvate and 0.1 mM malate. NADH was measured by fluorescence at 340/460 nm in an LS50B spectrofluorometer (Perkin Elmer; Waltham, MA) and calibrated using 0.5 µM FCCP to induce a maximally oxidized state (0% NADH) and 2 uM rotenone to induce a maximally reduced state (100% NADH).
Cell culture
Neonatal rat ventricular cardiomyoctes (NRVM) were isolated and cultured as previously described [22] in order to evaluate EPI’s mechanisms of mitochondrial protection. Briefly, hearts were obtained from 1–2 day old Sprague Dawley rat pups, digested with collagenase, and myocytes purified Percoll gradient separation and by pre-plating techniques. Myocytes were maintained overnight in Medium 199 supplemented with 10% fetal bovine serum, 2 mM glutaMAX and antibiotics. Cells were then washed and switched to high glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM glutaMAX, and antibiotics. Cells were serum starved for 24 h prior to the experiments.
Cellular O2 consumption
An XF24 extracellular flux analyzer (Seahorse Biosciences, North Billerica, MA) was used to measure the rates of oxygen consumption in monolayers of NRVM under a variety of controlled conditions. Respiration was measured after 24 h serum starvation in unbuffered, serum free DMEM supplemented with 10 mM glucose, 10 mM pyruvate, and 2 mM glutaMAX (pH7.4) at 37°C unless otherwise noted. Rates of respiration were measured before (endogenous rates) and after the addition of vehicle or EPI, therefore acute effects of EPI on bioenergetics were assessed. State 4 (resting) respiration was measured following the addition of 2 µM oligomycin. To measure maximal respiration (State 3u), a titrated concentration of 300 nM carbonyl cyanide 4-(trifluromethoxy)phenylhydrazone (FCCP) was added, followed by measurement of non-mitochondrial O2 consumption in the presence of 200 nM rotenone and 4 µM antimycin A.
For studies examining EPI’s effect on mitochondrial substrate utilization, an inhibitor of monocarboxylate transport α-cyano-4-hydroxycinnamic acid (CIN) was used at 400 µM to more selectively inhibit mitochondrial pyruvate transport or 40 µM etomoxir (ETO) to inhibit entry of fatty acylcarnitines into mitochondria. The inhibitors were added to their respective wells 1 h prior to addition of EPI. For studies testing EPI’s effect on cell signaling, 200 µM N-nitro-L-arginine methyl ester (L-NAME) or 10µM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-1-one (ODQ) was added to their respective wells 2 h prior to addition of treatment. Data is presented in rate of oxygen consumption (OCR) and was normalized to basal rates of respiration using Seahorse software.
Lentiviral-mediated shRNA knockdown of MPC1 and MPC2, RT-PCR, and respirometry of transduced C2C12 myoblasts
C2C12 myoblasts were used to allow for stable gene modification while exploring the mechanistic effects of EPI. MISSION lentiviral shRNA plasmids were obtained from Sigma-Aldrich (St. Louis, MO). For viral packaging, HEK293LTV were transfected with either pLKO.1-Control shRNA, pLKO.1-Brp44 shRNA (NM_027430.2-474s21c1; CCGGTTGGAGTTTGTTCGCTGTTAACTCGA GTTAACAGCGAACAAACTCCAATTTTTG), or pLKO.1-Brp44-Like shRNA (NM_018819.3-336s1c1;CCGGCAAACGAAGTAGCTCAGCTCACTCGAGTGAGCTGAGCTACTTCGTTTGTTT TTT G) together with the pSMD2.G and psPAX-2 (Addgene; Cambridge, MA) packaging vectors using Fugene (Roche; San Francisco, CA). Virus-containing media was harvested 48 hr post-transfection. Supernatant of a 3,000×g centrifugation was collected and mixed with 8 µg/mL polybrene. C2C12 cells (ATCC; Manassas, VA) were then transduced with the lentivirus. The media was changed after 18 hr, and cells were passaged 2 days later. Cells were selected with 5 µg/mL puromycin.
For measurement of MPC mRNA, 1 µg of mRNA was used to prepare cDNA with an iScript cDNA synthesis kit (BioRAD; Hercules, CA). MPC1 and MPC2 expression levels were determined by qRT-PCR using SyBR Green and an ABI 7500 with β-actin as an endogenous control. The fold change in expression was determined using the ΔΔCT method, with shRNA control cells serving as the reference sample.
Transduced C2C12 myoblasts were plated in XF96 Seahorse plates at 2×104 cells/well the day prior to experimentation, and respiratory rates were measured as described above.
Statistical Analysis
Results are expressed as mean ± SEM. Comparisons between means were analyzed, as appropriate, by student’s t- tests or one-way ANOVA followed by Bonferroni t-test. A value of p<0.05 was considered statistically significant.
Results
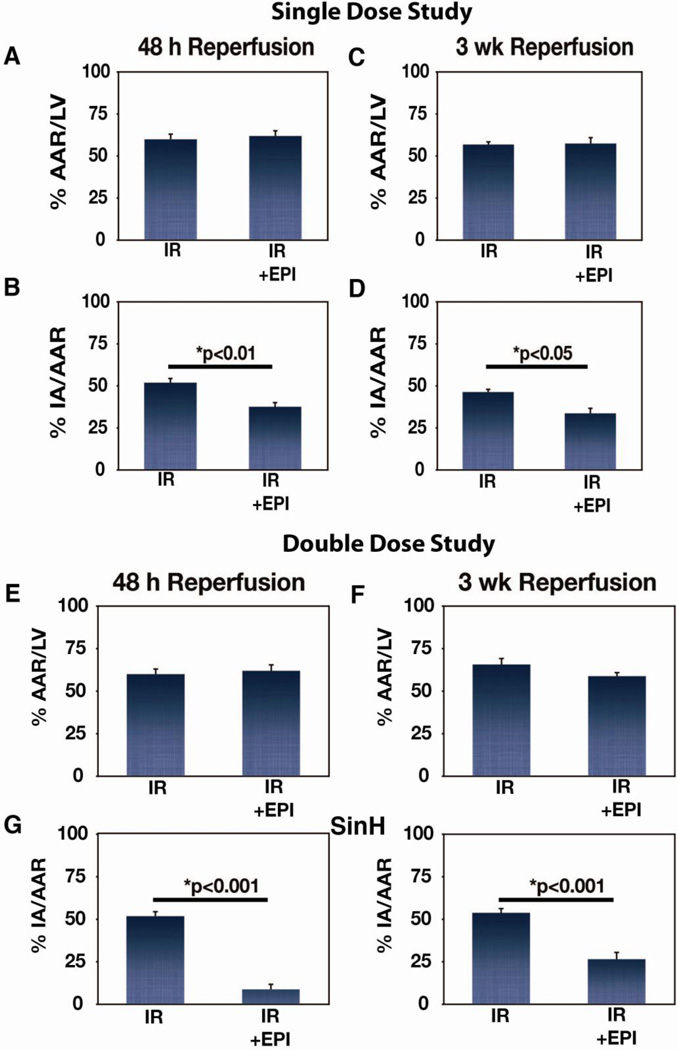
Ischemia was induced in rats via a 45 min occlusion of the left anterior descending coronary artery followed by 1 h, 48 h, or 3 weeks (wk) reperfusion in an experimental scheme depicted in Fig. 1. EPI (10 mg/kg) was administered IV 15 min prior to reperfusion for the single dose group and again 12 h later for the double dose group (n=6–8 per group per time point unless otherwise stated). The ability of IV administration of EPI to reduce infarct size is shown in Fig. 2. The area at risk (AAR) in IR and IR + EPI at 48 h (Fig. 2A) and at 3 wk (Fig. 2C) was not different. A single dose of EPI led to a 27% reduction in infarct size (p<0.01; Fig. 2B) at 48 h and a 28% reduction at 3 wk (p<0.05; Fig. 2D). At 48 h, a double dose of EPI decreased infarct size by ~80% (p<0.001; Fig. 2G), and by 52% at 3 wk (p<0.001; Fig. 2H) while AAR at 48 h (Fig. 2E) or 3 wk (Fig. 2F) were similar.
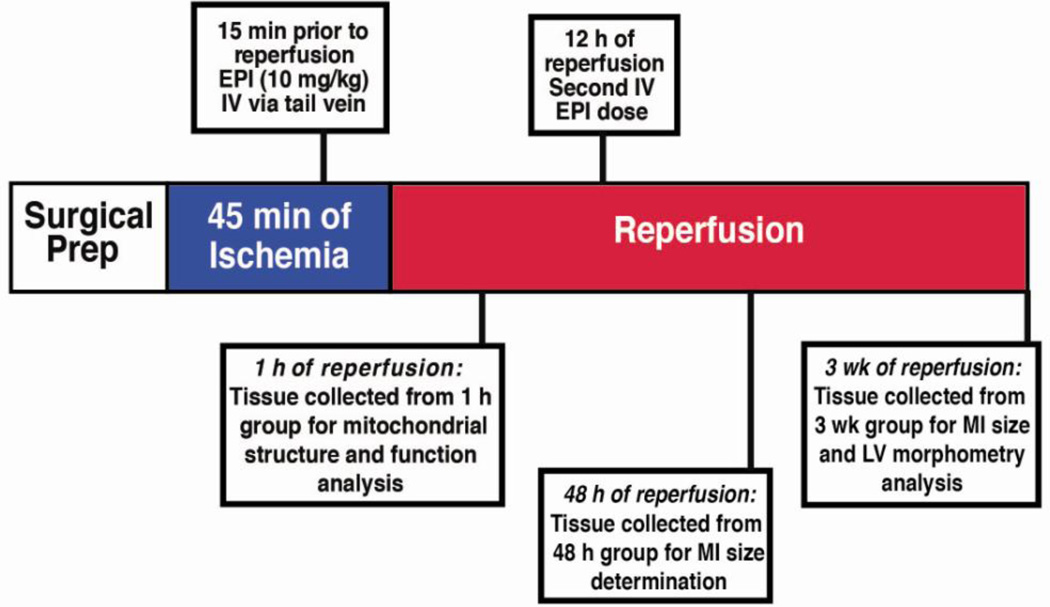
Figure 1. Experimental Design.
Timeline of epicatechin dosing scheme and tissue collection used throughout the study.
Figure 2. Effects of single and double dose epicatechin (EPI) treatment on MI size.
(A) Percent area at risk (AAR)/total LV area in IR (n=12) and IR + EPI (n=8) animals subjected to 48 h reperfusion, (B) Percent infarct area (IA) as a function of AAR (IA/AAR) in IR (n=12) and IR + EPI (n=8) animals subjected to 48 h reperfusion, (C) AAR in IR (n=6) and IR + EPI (n=8) animals subjected to 3 wk reperfusion, (D) IA as a function of AAR (IA/AAR) in IR (n=6) and IR + EPI (n=8) animals subjected to 3 wk reperfusion. (E) Percent area at risk (AAR)/total LV area in IR (n=12) and IR + EPI (n=6) animals subjected to 48 h reperfusion, (F) Percent infarct area (IA) as a function of AAR (IA/AAR) in IR (n=12) and IR + EPI (n=6) animals subjected to 48 h reperfusion, (G) AAR in IR (n=6) and IR + EPI (n=7) animals subjected to 3 wk reperfusion, (H) IA as a function of AAR (IA/AAR) in IR (n=6) and IR + EPI (n=7) animals subjected to 3 wk reperfusion.
The effects of single or double dose EPI treatment on LV remodeling related endpoints at 3 wk are shown in Table 2. In control IR animals, the anterior wall thickness was significantly thinner than the septal wall demonstrating the occurrence of wall thinning. In the single and double EPI dose groups, no wall thinning was observed. No differences were observed in any of the other parameters measured.
Table 2.
Morphometry data obtained from animals undergoing 45 min ischemia and 3 weeks of reperfusion.
| Single Dose Group | ||
|---|---|---|
| IR | IR + Single Dose Epicatechin | |
| Group Size | 6 | 8 |
| HW/BW | 4.6 ± 0.2 | 4.6 ± 0.2 |
| Outer LV Diameter (cm) | 1.20 ± 0.04 | 1.19 ± 0.03 |
| Inner LV Diameter (cm) | 0.36 ± 0.05 | 0.37 ± 0.05 |
| AW Thickness (cm) | 0.27 ± 0.03* | 0.37 ± 0.02 |
| SW Thickness (cm) | 0.38 ±0.02 | 0.37 ± 0.01 |
| Double Dose Group | ||
| IR | IR + Double Dose Epicatechin | |
| Group Size | 6 | 7 |
| HW/BW | 5.1 ± 0.3 | 5.3 ± 0.3 |
| Outer LV Diameter (cm) | 1.47 ±0.04 | 1.51 ±0.04 |
| Inner LV Diameter (cm) | 0.54 ±0.05 | 0.58 ±0.06 |
| AW Thickness (cm) | 0.26 ± 0.01* | 0.34 ±0.03 |
| SW Thickness (cm) | 0.42 ±0.01 | 0.38 ± 0.02 |
p<0.01 vs. IR SW.
HW/BW = heart weight/body weight, AW = anterior wall, SW = septal wall.
To ascertain for possible changes in heart rate and blood pressure with EPI treatment, hemodynamic parameters were measured in sham animals receiving a single dose of water or EPI (10 mg/kg). EPI-treated sham animals had no significant differences in heart rate, LV systolic and end diastolic pressure and mean arterial pressure compared to controls before or after IV infusion (data not shown). Thus, cardioprotective effects of EPI are independent from changes in hemodynamics.
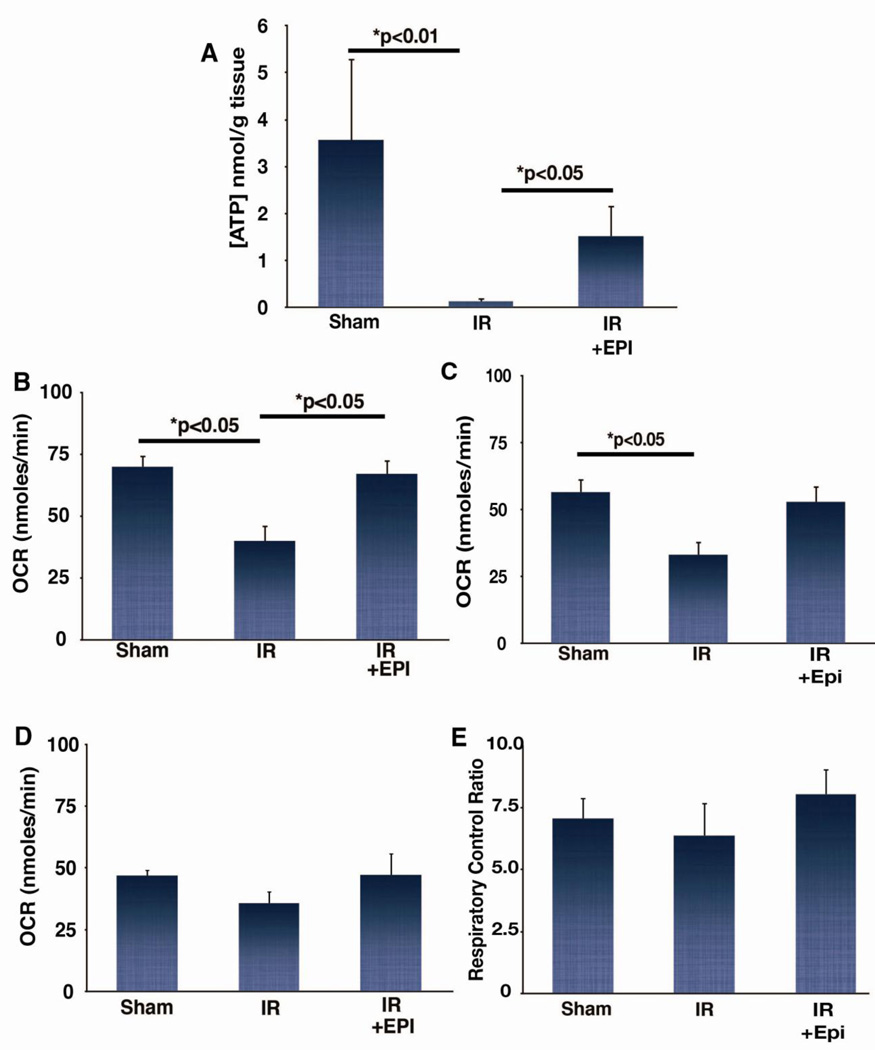
We assessed EPI’s ability to modulate myocardial bioenergetics 1 h after reperfusion, as events early in reperfusion are critical to determining the extent of tissue injury [23]. IR significantly reduced tissue ATP levels in sham animals (p<0.01) (Fig. 3A), while EPI treatment preserved ATP levels (1.5±0.04 nmol/g; p<0.05). We then determined if improvements in the myocardium’s bioenergetic status were associated with protection of mitochondrial structure and function. IR-mediated injury to mitochondrial electron transport and oxidative phosphorylation systems may limit tissue recovery [24]. Oxygen consumption rates in mitochondria isolated from the left ventricle of IR animals were significantly reduced compared to shams in the presence of the complex I-linked substrates pyruvate/malate (Fig. 3B) or glutamate/malate (Fig. 3C). Using either substrate, EPI treatment preserved mitochondrial respiration to levels similar to shams. While there were no differences between groups in complex II-dependent respiration on succinate/rotenone (Fig. 3D) or respiratory control ratio (Fig. 3E), they follow the same pattern seen in Fig. 3B and 3C.
Figure 3. Effects of epicatechin (EPI) on tissue ATP levels and isolated mitochondria respiration rates following IR.
(A) ATP levels measured in tissue collected from sham (n=3), IR (n=8), and IR + EPI (n=7) animals subjected to 1 h reperfusion. (B) Oxygen consumption rates (OCR) measured in sham (n=4), IR (n=4), and IR +EPI (n=4) in the presence of 5 mM pyruvate and 0.1 mM malate, (C) OCR measured in sham (n=8), IR (n=8), and IR + EPI (n=8) in the presence of 5 mM glutamate and 5 mM malate, (D) OCR measured in sham (n=4), IR (n=4), and IR + EPI (n=4) in the presence of 5 mM succinate and 2 mM rotenone, (E) respiratory control ratio measured in sham (n=8), IR (n=8), and IR + EPI (n=8) in the presence of 5 mM pyruvate and 0.1 mM malate.
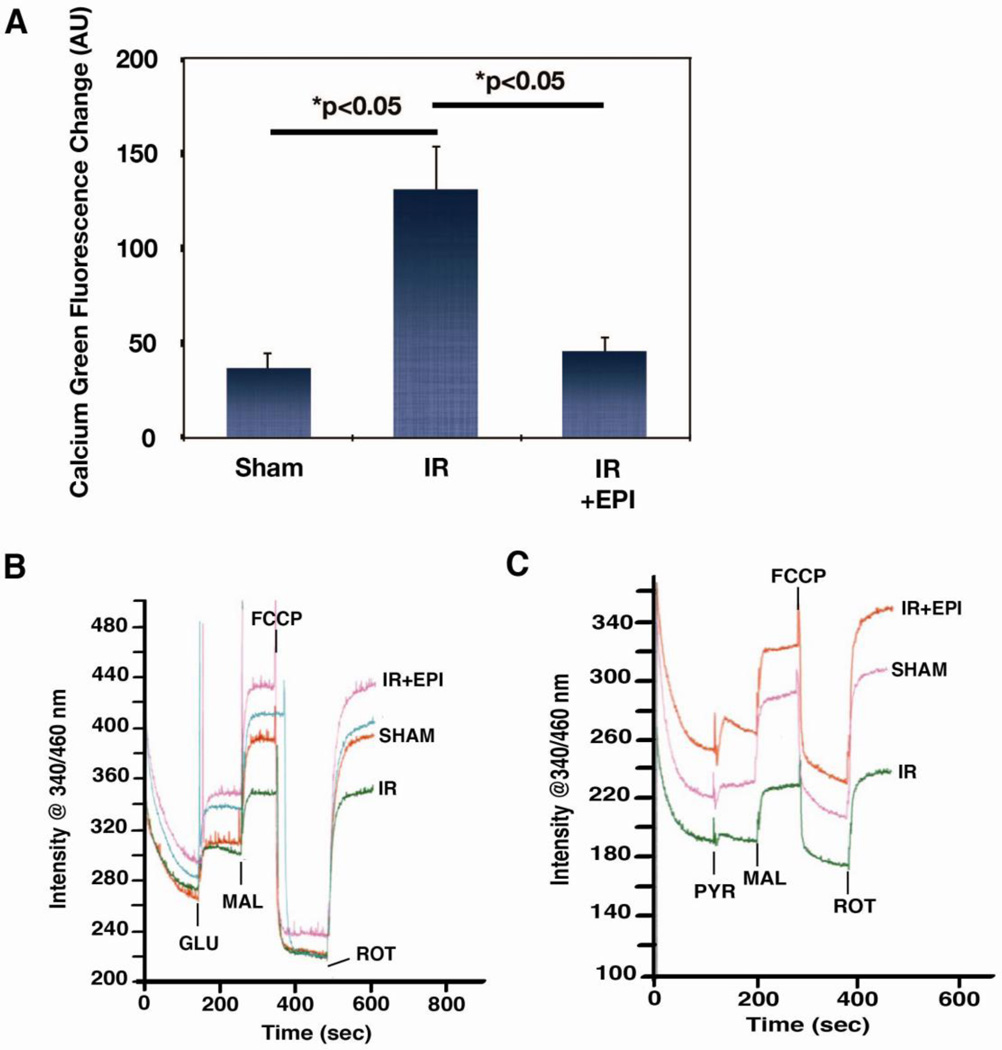
Excessive mitochondrial Ca2+ accumulation early in reperfusion is a critical aspect of the injury process [25]. Therefore, the quantity of sequestered Ca2+ after 1 h of reperfusion was measured in isolated ventricular mitochondria. Fig. 4A demonstrates that the Ca2+ load in mitochondria rises 2.5 fold after IR (p<0.05) and EPI significantly mitigated this accumulation (p<0.05). Retention of pyridine nucleotides within the matrix is associated with bioenergetic recovery during reperfusion [26] and pyridine nucleotides can be lost as a function of the permeability transition [27]. Thus, the NADH levels in the isolated mitochondria were also measured. Representative tracings of fluorometric measurements of the NADH levels in isolated mitochondria are shown in Fig. 4B and C. Using an uncoupler and rotenone to maximally oxidize and reduce mitochondrial NADH, respectively, we observed that mitochondria from IR animals had an overall decrease in the NADH pool vs. shams, whereas EPI helped to preserve the pool, and this was evident with either glutamate/malate or pyruvate/malate as substrates.
Figure 4. Effects of epicatechin (EPI) on mitochondrial calcium and NADH levels following IR.
(A) Levels of calcium measured in mitochondria isolated from sham (n=6), IR (n=14), and IR + EPI (n=7) animals subjected to 1 h reperfusion. Alamethicin was used as a pore-inducing agent to release sequestered calcium. Representative tracing of NADH measurements from one sham, IR, and IR + EPI animal in the presence of 5 mM glutamate/5 mM malate (B) and 5 mM pyruvate/0.1 mM malate (C).
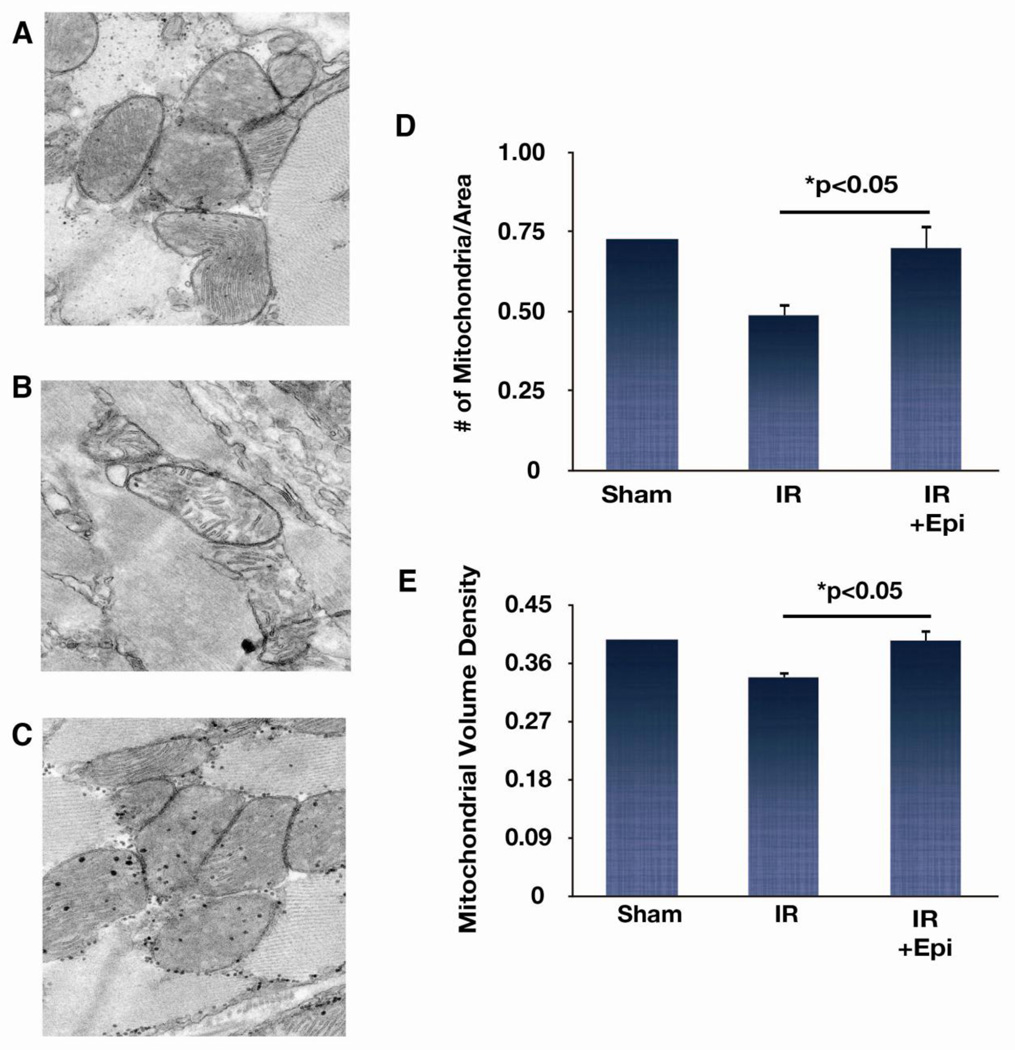
Electron microscopy was used to investigate ultrastructural modifications to the myocardium after 1 h of reperfusion. High-resolution images comprising heart tissue taken from the border area were examined from a sham animal, IR (n=4), and IR + EPI (n=3) animals. Representative images from each group are shown in Fig. 5. EPI treatment protected against the reductions in the number of mitochondria and mitochondrial volume densities in IR animals compared to sham. Together, these results suggest that EPI preserves mitochondrial structure and function early in the injury period, and this correlates with increased viability of ischemic myocardium measured at later time points.
Figure 5. Effects of epicatechin (EPI) on mitochondrial structure.
Representative electron microscope images of heart tissue taken from sham (A), IR (B), and IR + EPI (C) animals subjected to 1 h reperfusion. Images were taken at 6000× magnification. Average volume density (E) analyses were done for each group (IR,n=4;IR + EPI,n=3). Number of mitochondria per area (micron−2) (D) and mitochondrial volume density (fraction of cytoplasm occupied by mitochondria—myofibers included) (E) analyses showed that IR caused loss of mitochondria and this loss was prevented by EPI treatment.
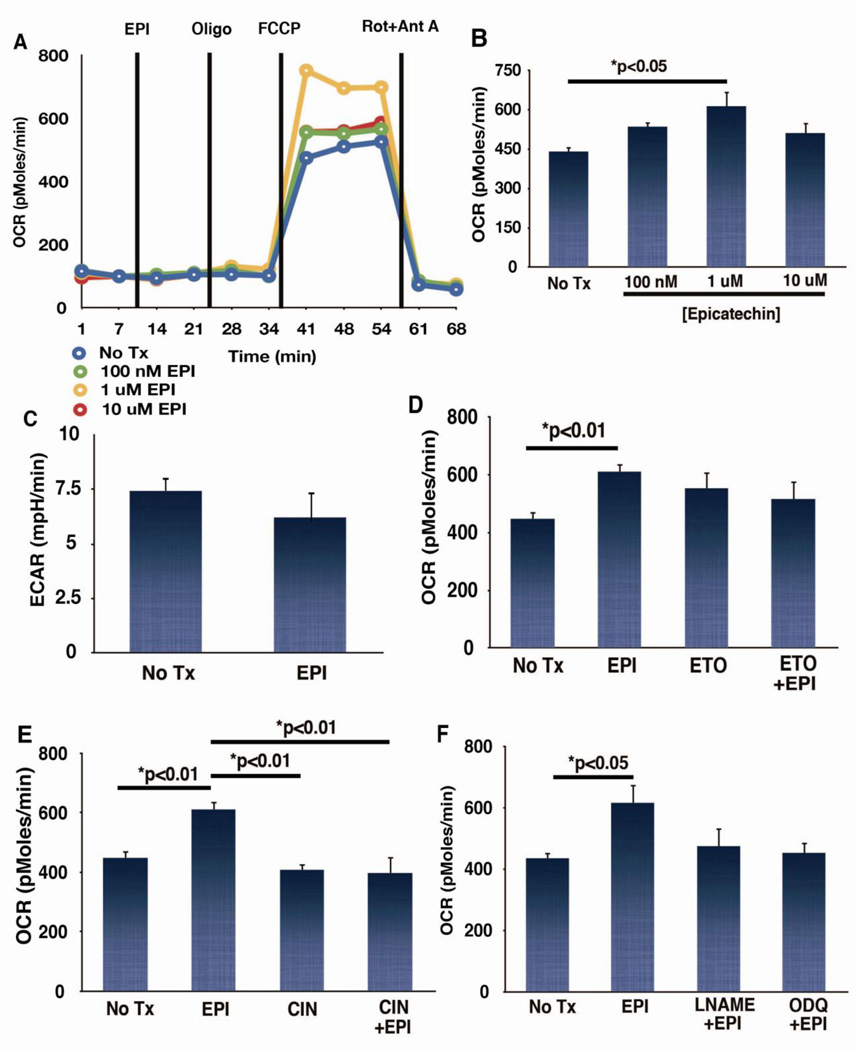
To further identify mechanisms involved in EPI-mediated protection of mitochondrial bioenergetics, studies in two cultured myocyte models were conducted. NRVM were treated with EPI for 1 h and rates of O2 consumption were then measured using the XF24 system in the presence of glucose, pyruvate, and glutamine as oxidizable substrates. EPI acutely increased maximal rates of respiration measured by the addition of the respiratory uncoupler FCCP by ~30% (Fig. 6). There appeared to be no effects of EPI on rates of endogenous (basal) respiration, or State 4 respiration in the presence of oligomycin (Fig. 6A), suggesting a lack of effect on rates of ATP turnover or on the extent of uncoupling.
Figure 6. Epicatechin (EPI) effects on neonatal rat ventricular cardiomyocyte (NRVM) respiration, extracellular acidification and respiration in the presence of α-cyano-4-hydroxycinnamic acid (CIN), etomoxir (ETO), of N-Nitro-L-arginine methyl ester (L-NAME) or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-1-one (ODQ).
Oxygen consumption rate were measured in NRVM following addition of increasing doses of EPI using a XF24 extracellular flux analyzer. (A) Representative tracing from one experiment. Lines represent the time drug additions took place. (B) Maximal rates of respiration following addition of the uncoupler FCCP (n=4). (C) Extracellular acidification rates were measured as a reflection of glycolytic lactate production in the presence of 2 µM oligomycin and 2 µM rotenone in the presence of 10 mM glucose as oxidizable substrate. (D) Oxygen consumption rate (OCR) was measured in unbuffered, serum free media supplemented with 10 mM pyruvate, and 2 mM glutaMAX. No glucose was present in the extracellular media for these experiments. (B) Maximal rates of respiration in the presence of 1 µM EPI and 40 µM ETO, an inhibitor of the mitochondrial carnitine palmitoyltransferase 1 protein; (n=4). (E) Maximal rates of respiration in the presence of 1 µM EPI and 400 µM CIN, an inhibitor of the mitochondrial pyruvate carrier; (n=4). (F) Maximal rates of respiration in NRCM in the presence of 1 µM EPI, 200 µM L-NAME, an inhibitor of nitric oxide synthase, and 10 µM ODQ, an inhibitor of soluble guanylyl cyclase; (n=4).
Acute increases in maximal rates of respiration can arise from effects at different steps of metabolism. We therefore probed for specificity of the effect of EPI on particular catabolic pathways relevant to myocardial protection [28]. A potential effect of EPI on glucose transport at the plasma membrane was first evaluated. EPI (1µM) failed to increase the rate of acidification of the extracellular medium when mitochondrial metabolism was blocked with oligomycin and rotenone, suggesting a lack of change of the rate of lactate production under conditions that stimulate glycolysis (Fig. 6C). Thus, EPI may be modulating mitochondrial respiration at a point distal to glucose uptake or glycolytic pyruvate production. The potential effect of EPI on oxidation of endogenous fatty acids was tested by inclusion of etomoxir, an inhibitor of carnitine palmitoyltransferase 1 [29]. Etomoxir (40 µM) prevented EPI’s stimulatory effect on cell respiration (ns; Fig. 6D). EPI’s effects on the oxidation of pyruvate (potentially derived from the incubation media or through glycolysis) were probed with the addition of α-cyano-4-hydroxycinnamic acid (CIN) an inhibitor of pyruvate uptake with some selectivity toward mitochondrial pyruvate transport [30]. CIN (400 µM) significantly inhibited the ability of EPI (1 µM) to enhance maximal rates of respiration (Fig. 6E).
Because we were unable to observe a stimulatory effect of EPI on rates of respiration of isolated rat heart mitochondria when the compound was added directly to the assay medium, EPI may be activating a cytoplasmic signaling cascade that converges on mitochondria. In fact, EPI stimulates the production of nitric oxide (NO) and downstream pathways in endothelial cells [9]. The possibility that EPI can modulate the NO-soluble guanylate cyclase (sGC) pathway as a means of regulating mitochondrial respiratory capacity in cardiomyocytes was assessed by measuring maximal rates of respiration in NRVM in the presence of 200 µM N-Nitro-L-arginine methyl ester (L-NAME), an inhibitor of NO synthase or 10 µM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-1-one (ODQ), an inhibitor of sGC. Pharmacologic inhibition of the generation of NO or cGMP production prevented EPI’s ability to enhance maximal rates of respiration in the cardiomyocytes (ns; Fig. 6F).
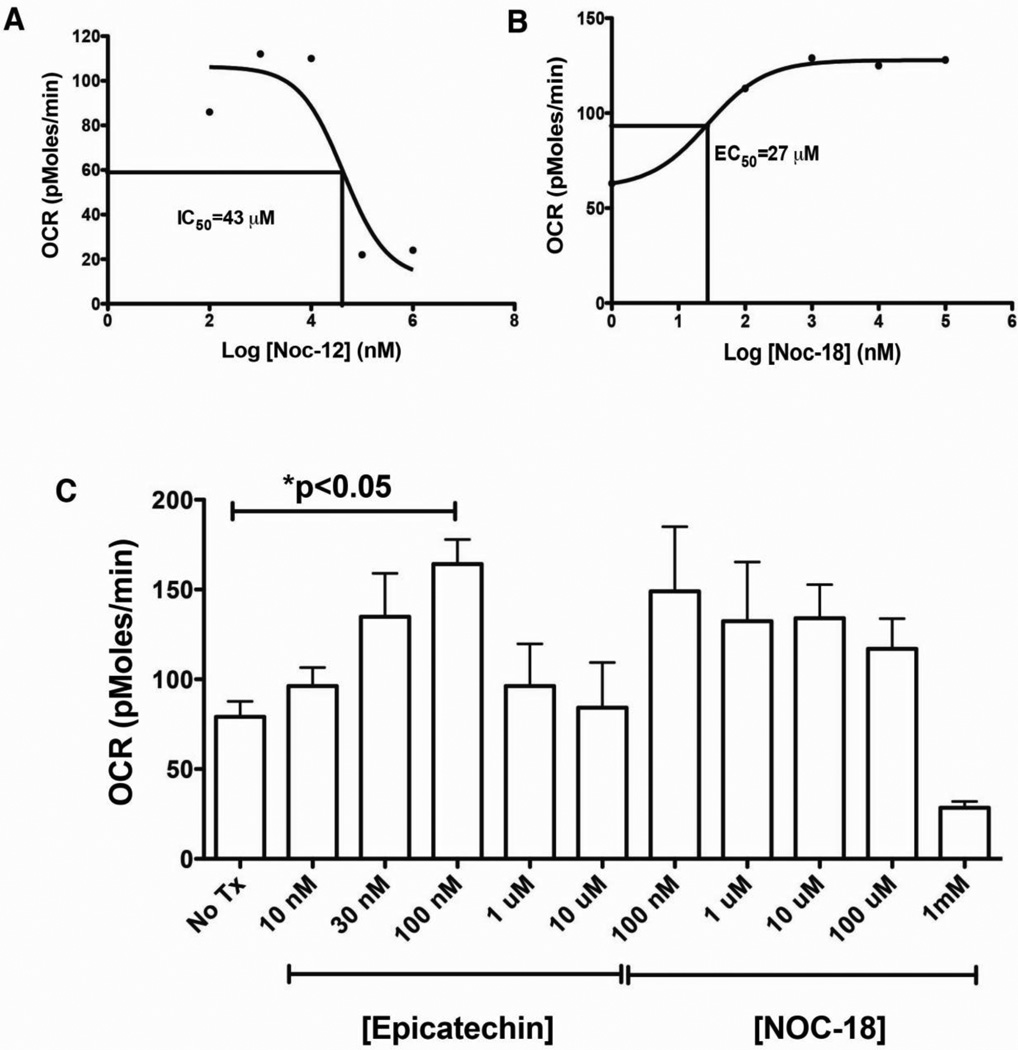
The effects of NO production on mitochondrial respiration have generally been described as inhibitory [31]. Given our observation of NO-dependent stimulation of respiration by EPI, we tested whether the effect of NO on intact cell respiration in C2C12 myoblasts could be mimicked by an NO-donor. Consistent with previous observations by others, NOC-12 induced respiratory inhibition with a half-maximal effect at 43 µM (Fig. 7A). In contrast, low concentrations of NOC-18 (DETA-NONOate) that donates NO at a lower rate than NOC-12, stimulated the maximal rate of C2C12 respiration (K0.5=27 µM) (Fig. 7B). In direct comparison of the effects of EPI and NOC-18 on C2C12 respiration, the maximal stimulatory effect of EPI and NOC-18 were similar (Fig. 7C).
Figure 7.
Effects of nitric oxide donors on C2C12 myoblast respiration with 10 mM glucose, 10 mM pyruvate and 2 mM glutaMax in unbuffered DMEM. Dose response curve of NOC-12 (A) and NOC-18 (B) on maximal rates of respiration in C2C12 myoblasts. (C) Comparison of the stimulatory effects of EPI and NOC-18; (n=4).
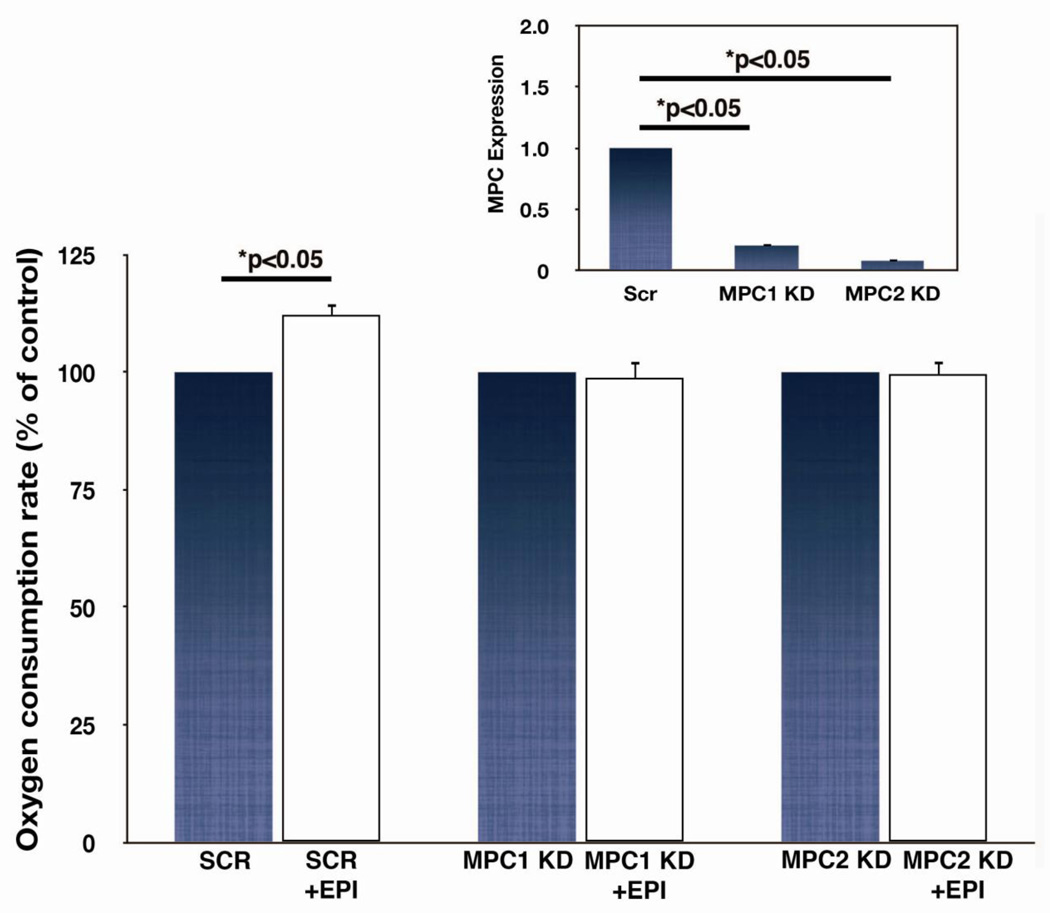
The essential components of the mitochondrial pyruvate transporter were recently identified as MPC1 and MPC2 [32,33]. We further tested involvement of the mitochondrial pyruvate transporter in the respiratory response to EPI by repressing expression of either MPC1 or MPC2 with lentiviral-mediated shRNA sequences in C2C12 myoblasts (Fig. 8). Knockdown of either paralog of the transporter prevented respiratory stimulation by EPI, suggesting involvement of mitochondrial pyruvate transport in this response.
Figure 8. Effects of MPC1 or MPC2 expression on epicatechin (EPI) stimulated C2C12 myoblast respiration.
MPC1 or MPC2 expression, measured by RT PCR was significantly repressed using lentiviral shRNAs in C2C12 myoblasts (inset). Rates of maximal uncoupler-stimulated respiration in intact myoblasts incubated in unbuffered DMEM containing pyruvate was measured in control, MPC1, or MPC2 knockdown cells (n=6).
Discussion
We present a number of unique findings on the cardioprotective effects of the flavanol EPI, extending previous observations in two significant ways: 1) EPI was administered in a manner relevant to potential clinical use following cardiac ischemia. A single IV dose of EPI given 15 min before reperfusion decreased infarct size up to 3 wk after injury and preserved anterior wall thickness (i.e. limits adverse remodeling). More importantly, a second dose of EPI further reduced infarct size. The cardioprotective effects of EPI occurred independently of changes in hemodynamics. 2) We provide the novel mechanistic finding that EPI, working through NOS/sGC pathway stimulates mitochondrial pyruvate transport or oxidation. We found that inhibition of NO synthase, sGC, or knockdown of molecular components of the mitochondrial pyruvate carrier blocked the stimulatory effect of EPI on maximal respiration in cell models.
EPI’s ability to produce NO in endothelial cells has been documented by us, and treatment of endothelial cells with EPI leads to enhanced NO production that peaks at approximately 10 min [9]. So, there is precedence for EPI-induced NO production in another cell type. In turn, many investigations have reported that the administration of NO donors prevents IR injury to the heart [31]. Recent exciting findings suggest that complex I of the electron transport chain is the target of this form of NO-mediated cardioprotection. The levels of NO or NO metabolites reached in these studies are sufficient to induce respiratory inhibition that induces a slow, protective return of mitochondrial metabolism during early reperfusion [31,34]. In our studies, it does not appear that EPI-induced NO production reaches concentrations sufficient to induce respiratory inhibition, as we observe only respiratory stimulation in our models. Further, we made the unique observation that EPI-induced respiratory stimulation can be mimicked by acute treatment of cells with NOC-18, a relatively slow NO donor, and can be blocked by either an inhibitor of the mitochondrial pyruvate carrier (cyanocinnamate) or by shRNA-mediated down regulation of pyruvate carrier (MPC1 or MPC2) expression. This link between EPI, the NO/sGC pathway, and mitochondrial pyruvate metabolism has not previously been described.
Polyphenols have been investigated for their potential to protect organs from ischemic injury. Previous studies have focused on the cardioprotective properties of EPI when given as pretreatment [5,6], and include observations of protection of mitochondria [10]. We previously demonstrated that 10 d of EPI pretreatment at 1mg/kg/day (by gavage) decreased infarct size 48 h and 3 wk after IR, and reduced inflammation, reactive oxygen species (ROS) generation and matrix metalloproteinase activity accompanied this protective effect [6]. Strikingly similar degrees of cardioprotection were observed using permanent coronary occlusion [5]. Because these previous studies utilized a pretreatment scheme in which time-dependent protective mechanisms may be in play, i.e. stimulation of angiogenesis [35], we wished to examine EPI’s therapeutic potential using a more clinically relevant paradigm to investigate the acute mechanisms of protection offered by EPI. In preliminary studies, animals subjected to IR were administered IV EPI from 1 – 50 mg/kg (15 min prior to reperfusion) resulting in a dose-dependent cardioprotective effect with no apparent adverse hemodynamic effects. Based on the data gathered, we utilized a low dose that had the efficacy of reducing infarct size at 48 h for the current study. Multiple dosing schemes have rarely been systematically examined and are of clinical relevance. Results with administration of a single dose yielded a moderate reduction in infarct size that was substantially reinforced by a second dose 12 hours later. Results demonstrate that IV EPI is not only decreasing reperfusion damage, but also decreasing the number of myocardial cells dying for ischemia or extending the protection against reperfusion damage. While the mechanism is unknown, this is a striking effect for a natural product. Thus, EPI, an apparently safe and well-tolerated compound [39], could conceivably be given in multiple doses to reinforce cardioprotection.
Molecular and cellular events underlying IR injury are complex and involve loss of ATP and ionic homeostasis promoting lactic acidosis, Ca2+ overload, and the generation of reactive nitrogen and oxygen species, all of which lead to mitochondrial dysfunction. Our study demonstrates EPI’s ability to maintain mitochondrial respiration after IR correlating with increased NADH, a decrease in mitochondrial Ca2+ overload and preservation of tissue ATP levels. EPI also preserved mitochondrial density. By preserving the capacity for energy production, EPI may prevent the loss of pyridine nucleotides that can be lost from mitochondria as a result of the mitochondrial permeability transition and reactive oxygen-stimulated poly(ADP-ribose) polymerase activity [40]. While other studies have demonstrated the ability of EPI to induce mitochondrial biogenesis and therefore increase mitochondrial density [7,8], this is likely not the case after only 1 h of treatment in the protocol of this study. Thus, it appears that EPI is acutely preventing loss of mitochondrial structure/function by a mechanism independent of biogenesis but clearly related to cellular energetics.
We utilized cultured cells to discover a novel mechanism of action of EPI to stimulate maximal mitochondrial respiratory function, which would be expected to preserve tissue function under energetically, stressed conditions. Our results suggest that EPI does not enhance glycolytic lactate production. However, when mitochondrial pyruvate uptake was inhibited, EPI stimulation of maximal rates of respiration was blocked. The carnitine palmitoyltransferase 1 inhibitor etomoxir increased maximal respiratory rates and in its presence, no further increases were observed with EPI. This observation suggests that when fatty acid oxidation is inhibited, a shift toward use of alternative substrate such as glucose or pyruvate may have occurred. Cardiac metabolism undergoes significant changes with pathological stresses [37]. Although fatty acid oxidation appears to be a primary source of energy during reperfusion of the ischemic heart, genetic and pharmacologic studies have demonstrated that optimal cardiac function during this period depends on the ability of mitochondria to oxidize both fatty acids and glucose [28,37]. Interestingly, agents designed to stimulate mitochondrial glucose metabolism such as the pyruvate dehydrogenase activator dichloroacetate, or compounds that partially inhibit mitochondrial beta oxidation including trimetazidine and ranolazine (although specificity of their effects have been questioned) have shown some cardioprotective benefits [28]. Our findings on the mechanism of action of EPI are consistent with this concept i.e. agents that stimulate mitochondrial glucose and pyruvate metabolism can provide cardioprotection. It should be noted that administration of pyruvate has been shown to be cardioprotective in a number of pre-clinical and clinical settings, but suffers from risks associated with administering the sodium salt of the anion as well as issues with conversion to a non-metabolizable dimer in aqueous solution [38].
Study limitations
As a polycyclic phenol, EPI may has pleiotropic effects. In this study, potential effects on endoplasmic reticulum (ER) stress and ER Ca2+ transport have not been explored but can be in future studies. In addition, the study was performed using healthy rodents and this has been recognized as a limitation for the translatability of preclinical models to the clinic. Thus, our results would need to be verified using larger models and possibly those that have underlying health problems such as diabetes. The study also relied on the use of cell cultures using established systems (NRVM and myoblasts). However, these approaches allowed us to gain greater insight as to the possible mechanisms underlying EPI effects.
Conclusions
Our findings of NO-mediated stimulation of pyruvate-dependent respiration are novel and provocative. Additional studies are necessary to further identify upstream signaling events, for instance whether the pyruvate carrier is directly regulated by nitrosation or PKG-mediated phosphorylation. Regardless, these observations provide a unique perspective on EPI-mediated regulation of mitochondrial function. Our findings also support further consideration of EPI as a cardioprotective agent with therapeutic potential beyond consideration as a dietary supplement. Given its wide safety margin [39] and apparent additive effects with multiple dosing in reperfusion injury demonstrated here, the evaluation of direct administration of EPI warrants further studies.
Highlights.
Administration of EPI in a manner relevant to potential clinical use.
Single IV dose of EPI decreased infarct size and limited adverse remodeling.
Second dose of EPI further reduced infarct size and adverse remodeling.
EPI stimulates mitochondrial pyruvate transport through NOS/sGC pathway.
Acknowledgments
Funding Sources
The authors are grateful to Dr. Joan Heller Brown’s laboratory for provision of the neonatal rat ventricular myocytes (PO1 HL085577). This work was supported by NIH HL43617, AT4277, MD000220 to Dr. Villarreal, and DK92154 to Dr. Villarreal and Murphy and Conacyt Mexico # 129889 to Dr. Ceballos. Funding for Christine De La Fuente was provided from the National Institutes of Health Minority Access to Research Careers-Undergraduate Student Training for Academic Research Grant (NIH MARC U*STAR GM08228). Part of the work was performed at the National Center for Microscopy and Imaging Research funded by NIH P41GM103412-24.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none to disclose
References
- 1.Walters AM, Porter GA, Brookes PS. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res. 2012;111:1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 4.Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. Bmj. 2011;343:d4488. doi: 10.1136/bmj.d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki KG, Taub PR, Barraza-Hidalgo M, Rivas MM, Zambon AC, Ceballos G, et al. Effects of (−)-epicatechin on myocardial infarct size and left ventricular remodeling after permanent coronary occlusion. J Am Coll Cardiol. 2010;55:2869–2876. doi: 10.1016/j.jacc.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki KG, Romero-Perez D, Barraza-Hidalgo M, Cruz M, Rivas M, Cortez-Gomez B, et al. Short- and long-term effects of (−)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H761–H767. doi: 10.1152/ajpheart.00413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taub PR, Ramirez-Sanchez I, Ciaraldi TP, Perkins G, Murphy AN, Naviaux R, et al. Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: effects of epicatechin rich cocoa. Clin Transl Sci. 2012;5:43–47. doi: 10.1111/j.1752-8062.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, et al. (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol (Lond) 2011;589:4615–4631. doi: 10.1113/jphysiol.2011.209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (−)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55:1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prince PSM. A biochemical, electrocardiographic, electrophoretic, histopathological and in vitro study on the protective effects of (−)epicatechin in isoproterenol-induced myocardial infarcted rats. Eur J Pharmacol. 2011;671:95–101. doi: 10.1016/j.ejphar.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779–2811. doi: 10.1089/ars.2010.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sies H, Hollman PCH, Grune T, Stahl W, Biesalski HK, Williamson G. Protection by flavanol-rich foods against vascular dysfunction and oxidative damage: 27th Hohenheim Consensus Conference. Adv Nutr. 2012;3:217–221. doi: 10.3945/an.111.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 14.Tompkins AJ, Burwell LS, Digerness SB, Zaragoza C, Holman WL, Brookes PS. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta. 2006;1762:223–231. doi: 10.1016/j.bbadis.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths EJ. Mitochondria and heart disease. Adv Exp Med Biol. 2012;942:249–267. doi: 10.1007/978-94-007-2869-1_11. [DOI] [PubMed] [Google Scholar]
- 16.Ha S-J, Kim W. Mechanism of Ischemia and Reperfusion Injury to the Heart: From the Viewpoint of Nitric Oxide and Mitochondria. Chonnam Medical Journal. 2010;46:129–139. [Google Scholar]
- 17.Morin D, Hauet T, Spedding M, Tillement J. Mitochondria as target for antiischemic drugs. Adv Drug Deliv Rev. 2001;49:151–174. doi: 10.1016/s0169-409x(01)00132-6. [DOI] [PubMed] [Google Scholar]
- 18.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 19.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushnareva YE, Wiley SE, Ward MW, Andreyev AY, Murphy AN. Excitotoxic injury to mitochondria isolated from cultured neurons. J Biol Chem. 2005;280:28894–28902. doi: 10.1074/jbc.M503090200. [DOI] [PubMed] [Google Scholar]
- 21.Wiley SE, Andreyev AY, Divakaruni AS, Karisch R, Perkins G, Wall EA, et al. Wolfram Syndrome protein, Miner1, regulates sulphydryl redox status, the unfolded protein response, and Ca2+ homeostasis. EMBO Mol Med. 2013;5:904–918. doi: 10.1002/emmm.201201429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol, Cell Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 25.de Jesús García-Rivas G, Guerrero-Hernández A, Guerrero-Serna G, Rodríguez-Zavala JS, Zazueta C. Inhibition of the mitochondrial calcium uniporter by the oxo-bridged dinuclear ruthenium amine complex (Ru360) prevents from irreversible injury in postischemic rat heart. Febs J. 2005;272:3477–3488. doi: 10.1111/j.1742-4658.2005.04771.x. [DOI] [PubMed] [Google Scholar]
- 26.Riess ML, Camara AKS, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol. 2002;283:H53–H60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- 27.Di Lisa F, Menabò R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 28.Lopaschuk GD, Folmes CDL, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101:335–347. doi: 10.1161/CIRCRESAHA.107.150417. [DOI] [PubMed] [Google Scholar]
- 29.Lagoa R, Graziani I, Lopez-Sanchez C, Garcia-Martinez V, Gutierrez-Merino C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim Biophys Acta. 2011;1807:1562–1572. doi: 10.1016/j.bbabio.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Halestrap AP, Denton RM. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974;138:313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 32.Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen Y-C, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzig S, Raemy E, Montessuit S, Veuthey J-L, Zamboni N, Westermann B, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 34.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez-Sanchez I, Nogueira L, Moreno A, Murphy A, Taub P, Perkins G, et al. Stimulatory effects of the flavanol (−)-epicatechin on cardiac angiogenesis: additive effects with exercise. J Cardiovasc Pharmacol. 2012;60:429–438. doi: 10.1097/FJC.0b013e318269ae0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 37.Kolwicz SC, Jr, Tian R. Metabolic Therapy at the Crossroad: How to Optimize Myocardial Substrate Utilization? Trends Cardiovasc Med. 2009;19:201–207. doi: 10.1016/j.tcm.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallet RT, Sun J, Knott EM, Sharma AB, Olivencia-Yurvati AH. Metabolic cardioprotection by pyruvate: recent progress. Exp Biol Med (Maywood) 2005;230:435–443. doi: 10.1177/153537020523000701. [DOI] [PubMed] [Google Scholar]
- 39.Richelle M, Tavazzi I, Enslen M, Offord EA. Plasma kinetics in man of epicatechin from black chocolate. Eur J Clin Nutr. 1999;53:22–26. doi: 10.1038/sj.ejcn.1600673. [DOI] [PubMed] [Google Scholar]
- 40.Schriewer JM, Peek CB, Bass J, Schumacker PT. ROS-mediated PARP activity undermines mitochondrial function after permeability transition pore opening during myocardial ischemia-reperfusion. J Am Heart Assoc. 2013;2(2):e000159. doi: 10.1161/JAHA.113.000159. [DOI] [PMC free article] [PubMed] [Google Scholar]