Abstract
The importin/exportin transport system provides the machinery involved in nucleocytoplasmic transport. Alterations of the levels of importins and exportins may play crucial roles in development, differentiation and transformation. Employing human leukaemia HL-60 cells, we and others have revealed the differentiation-associated changes in the protein and gene expression of these factors. The recent finding that a switch to the importin-α subtype triggers neural differentiation of embryonic stem cells underscores the importance of nucleocytoplasmic transport factors in cellular events. This review focuses on current research into the roles of importins and exportins in cell differentiation.
Keywords: importin·, exportin, differentiation, gene expression, nucleocytoplasmic transport
Introduction
Importins and exportins
NLS and NES
Cargo molecules
-
Importins and exportins In cellular differentiation
-
HL-60 cell differentiation
Importin expression
Exportèn expression
Monocyte differentiation
Terminal erythroìd differentiation
Neural differentiation
Cardiac differentiation
Keratìnocyte differentiation
Germ cell maturation
Muscle cell differentiation
-
Concluding remarks
Introduction
In eukaryotic cells, the nucleus is separated from the cytoplasm by a double-layered membrane, the nuclear envelope. Macromolecules such as RNA transcripts generated in the nucleus are exported to the ribosomes in the cytoplasm and proteins synthesized in the cytoplasm, such as histones, DNA and RNA polymerases and transcription factors, are imported into the nucleus. The importin/exportin transport system provides the machinery involved in nucleocytoplasmic transport of cargo molecules larger than ∼40 kD [1–12]. In this system, proteins that shuttle between the cytoplasm and the nucleus have generally a nuclear localization signal (NLS) sequence or a nuclear export signal (NES) sequence.
Importin-α recognizes to the classical NLS (cNLS) within a protein cargo and forms a ternary complex with importin-β1 to enter into the nucleus (Fig. 1). In another system, the cargo molecule with the NLS directly binds to importin-β and is transported into the nucleus. Exportin recognizes the NES in the cargo protein and the complex is exported from the nucleus by binding with the guanosine triphosphate (GTP)-bound form of the guanine nucleotide-binding protein Ran (RanGTP) (Fig. 1).
Fig 1.
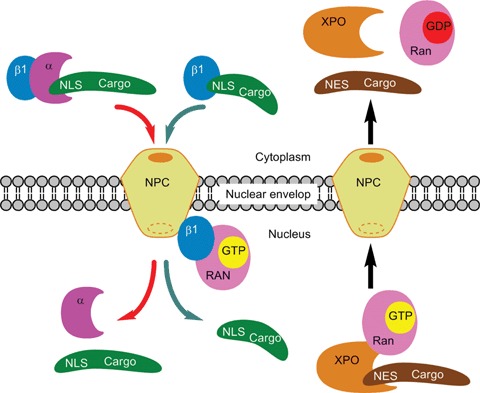
A basic model of importin/exportin-mediated nucleocytoplasmic transport of macromolecules. Importin-α (a) binds to the NLS within a protein cargo in the cytoplasm and forms a ternary complex with importin-β1 (β1) to enter into the nucleus. Some cargo molecules with the NLS can directly bind to importin-β1. In the nucleus, binding of RanGTP (the GTP-bound form of the small Ras family GTAse, Ran) to importin-β1 triggers the dissociation of the complex. For nuclear export, RanGTP stimulates binding of exportin (XPO) to an NES-containing cargo protein in the nucleus and the complex is exported to the cytoplasm, where hydrolysis of RanGTP to RanGDP results in complex disassembly.
Alterations in the expression of the components of the nuclear transport machinery would determine transport efficiency and plays crucial roles in development, differentiation and transformation. This review focuses on current research into the roles of importins and exportins in cellular differentiation.
Importins and exportins
The National Center for Biotechnology Information (NCBI) database shows that there are at least 18 importin and 6 exportin genes in human beings and 15 importin and 6 exportin genes in mice. Exportin-1 is frequently referred to as CRM1. Comparisons of these nucleocytoplasmic transport factors in different species have been hampered by the multiple names assigned. In this review, we use the terms for the human genes.
There have been a number of published comprehensive reports reviewing structural, functional, evolutional, mechanistic and regulational aspects of nucleocytoplasmic transport factors including importins and exportins [1–12]. Figure 1 illustrates a basic model for nuclear import/export pathways in which importins and exportins are involved.
In the importin-α-mediated nuclear import system, cytosolic importin-β1 forms a complex with importin-α, which binds to the cNLS contained in a cargo protein. After entering the nucleus through the nuclear pore complex (NPC), the ternary complex dissociates. The energy required for this dissociation is provided by GTP from RanGTP. Importin-α is recycled back to the cytoplasm in a complex with an importin-α re-exporter, cellular apoptosis susceptibility gene (CAS), in the presence of RanGTP [1, 9]. In some cases, importin-β1 recognizes the cNLS within a cargo in the cytoplasm without the importin-α adapter for entrance into the nucleus. Importin-β1 in the nucleus is recycled to the cytoplasm in a complex with RanGTP.
The nuclear export of proteins is mediated by exportins which bind to NES-containing cargo and RanGTP in the nucleus. The signal recognized by exportin-1 may be termed the classical NES. Dissociation of the ternary complex in the cytoplasm is promoted by Ran GTPase-activating protein to ensure the export of the cargo. Exportin-1 is known to be recycled into the nucleus by binding to an NPC component, Nup358 [6].
NLS and NES
NLSs are nuclear targeting sequences which are recognized by importins. The best-characterized NLSs are cNLSs that have either one (monopartite) stretch such as PKKKRKV in SV40 large T antigen and EEKRKR in NF-κB p65 [5] or two (bipartite) stretches of basic amino acids. Some cNLSs are recognized directly by importin-β1 as exemplified by the sequence RKKRRQRRR in Hiv-1 Tat [13]. In the GenBank™ set of 5850 yeast proteins, 2639 (45%) proteins contain either a predicted monopartite or bipartite cNLS, suggesting the high prevalence of the classical nuclear import pathway [9].
Non-classical NLSs bind directly to the different importin-β homologues [3]. For example, the NLS with no cluster of basic amino acids in heterogeneous nuclear ribonucleoprotein A1 and other proteins is directly recognized by importin-β2/transportin-1/karyopherin-β2[14]. In addition, importin-independent nuclear entry systems are also known. These include viral protein R (Vpr) of immunodeficiency virus type 1 (HIV-1) and β-catenin, which can pass through the NPC by binding directly to NPC components [15, 16].
NESs recognized by exportins generally have short sequences with a cluster of hydrophobic amino acids such as RFLSLEPL and TPTDVRDVDI in cyclin D [5] and LQKKLEELEL in mitogen-activated protein kinase kinase [6]. Although exportin-1 has low affinity for regular NESs to achieve efficient release of export complexes from the NPC, there is another signal recognized by exportin-1 with high affinity [6]. One example is snurportin, which does not contain a canonical NES sequence but binds to exportin-1 through a larger domain [6].
Various types of intra- and inter-molecular masking of these transport signals regulate the efficiency of nucleocytoplasmic transport. Phosphorylation, changes in calcium concentrations and conformational changes for self-inhibition are representative events for such masking [5, 11].
Cargo molecules
Table 1 lists examples of macromolecules transported by nucleo-cytoplasmic transport factors. The importins and exportins selected here are those for which information on differentiation-associated changes in gene expression is available through our cDNA microarray analysis in human promyelocytic leukaemia HL-60 cells [17]. In many cases, an individual protein is carried by a specific importin or exportin, but some proteins are recognized by multiple isoforms as exemplified by NF-kB[18], c-Jun [19] and Oct3/4 [20] (Table 1).
Table 1.
Examples of cargo molecules transported by importins and exportins
| Human transport factor | NCBI official symbol | Cargo molecule | ||
|---|---|---|---|---|
| Importin-α1 | KPNA2 | Type 1 parathyroid hormone receptor [51] | IFN regulatory factor-1[39] | Oct3/4 [20] |
| lmportin-α3 | KPNA4 | NF-κβ p50/p65 [18] | RNA helicase A [52] | Oct3/4 [20] |
| Importin-α4 | KPNA3 | NF-κβ p50/p65 [18] | Bovine papillomavirus type1 E1 protein [53] | |
| Importin-α5 | KPNA1 | Stat3 [54] | Ebola virus VP24 [55] | Oct3/4 [20] |
| lmportin-βι | KPNB1 | Splicing factor PRPF31 [56] | Sex-determining factor SRY [57] | |
| Importin-β2 | TNP01 | HPV16 E6 oncoprotein [58] | HPV L1 major capsid proteins [59] | |
| Importin-β3 | RANBP5 | c-Jun [19] | Influenza A viral ribonucleoprotein [60] | NFAT [61] |
| lmportin-7 | IP07 | c-Jun [19] | Zinc finger protein EZI [62] | Histone H1 [63] |
| lmportin-8 | IP08 | Signal recognition particle protein 19 [64] | ||
| lmportin-9 | IP09 | c-Jun [19] | Protein phosphatase 2A [65] | |
| lmportin-11 | IP011 | Ribosomal protein L12 [66] | Ubiquitin-conjugating enzyme UbcM2 [67] | |
| lmportin-13 | IP013 | NF-YB/NF-YC heterodimer [68] | c-Jun [19] | Myopodin [69] |
| Transportin-2 | TNP02 | mRNA [70] | HuR [71] | hnRNP A1 [72] |
| Exportin-1 | XP01 | Cyclin D1 [73] | p53 [5] | Survivin [48] |
| Exportin-5 | XP05 | Double-stranded RNA binding protein Staufen2 [74] | Pre-miRNAs [75] | |
| Exportin-6 | XP06 | Profilin-actin complexes [76] | Actin [32] | |
| Exportin-7 | XP07 | IFI-α1 mRNA [77] | p50RhoGAP [78] | |
| Exportin-t | XPOT | Mature tRNAs [79] | tRNA-attached ribozymes [80] | |
Importins and exportins in cellular differentiation
HL-60 cell differentiation
Importin expression
HL-60 cells can be induced to differentiate into monocyte/macrophage-like and neutrophil-/granulocyte like cells in response to external stimuli such as 1α,25-dihydroxyvitamin D3, 12-O-tetradecanoyl-phorbol-13-acetate (TPA), all-trans-retinoic acid (ATRA) and dimethylsulfoxide [21, 22].
The protein expression of importins-α1 (Table 2) and -α4 is greatly repressed in differentiated HL-60 cells, while that of importin-α7 is weakly down-regulated [23]. The protein expression of importin-α3 is down-regulated upon differentiation towards macrophage-like cells in contrast to the stable expression in cells differentiating into granulocyte-like cells [23] (Table 2).
Table 2.
Selected studies on differentiation-associated changes in gene/protein expression of importins and exportins
| Cells | HL-60 | HL-60 | HL-60 | HL-60 | ESC‡ | ESC | Monocytes |
|---|---|---|---|---|---|---|---|
| Cell fate | Macrophages | Granulocytes | Macrophages | Granulocytes | Cardiomyocytes | Neural cells | Macrophages |
| Method | Q-PCR | Q-PCR | WB§ | WB | Q-PCR | WB | Q-PCR/WB |
| Importin-α1 | Down | Down | Down | Down | Down | Up | |
| lmportin-α3 | Down | NC¶ | Down | NC | Up | Up | |
| Importin-α4 | Up | Up | NC | NC | Down | Up | Up |
| lmportin-βι | Down | Down | Down | ||||
| Transportin-2 | Down | Down | Up | ||||
| Exportin-1 | Down | Down | Down | ||||
| Exportin-5 | Down | Down | |||||
| Exportin-6 | NC | Down | |||||
| Exportin-7 | Down | Down | Down | Down | |||
| Exportin-t | Down | Down | |||||
| Ref. | [25] | [25] | [23] | [23] | [38] | [20] | [33] |
Embryonic stem cells.
Western blotting.
No change.
Consistent with the protein expression, the gene expression of most importins is down-regulated upon differentiation as examined by a cDNA microarray analysis [17] and a Q-PCR [24, 25] (Table 2). The result of Q-PCR indicates that the gene expression of importin-α5 is up-regulated upon differentiation towards macrophage-like cells [25] (Table 2).
The changes in the gene expression of importin-α3 are also compatible with those in the protein expression associated with the difference in differentiation of HL-60 cells. Thus, importin-α3 appears to have a very important role in directing cell lineages, monocyte/macrophages versus neutrophil/granulocytes [23].
ATRA induces a reduction in the gene expression of importin-a1 in HL-60 cells upon granulocytic differentiation (Table 2) with a transient up-regulation [25]. Similar observation has been made for cultured rat aortic smooth muscle cells [26].
The down-regulation of the gene expression of proteins related to nucleocytoplasmic transport may explain the differentiation-associated suppression of the growth of HL-60 cells [27, 28]. Another example of the involvement of importins in cell growth is the finding that RNAi-based down-regulation of the gene expression of importins-α3, -α5, -α7 and -β1 strongly inhibits the proliferation of HeLa cells [29].
The down-regulated expression of importin-α1 accompanied by the up-regulated expression of importin-a5 is seen in HL-60 cells both during TPA-mediated differentiation into macrophage-like cells and ATRA-mediated differentiation into granulocyte-like cells [25] (Table 2). The observation is in line with a recent finding that this switching triggers neural differentiation of mouse embryonic stem (ES) cells [20] (Table 2). The possibility that this switching is a hallmark of cell differentiation should be studied further.
Exportin expression
While the gene expression of five exportins (exportins-1, -5, -6, -7 and -t) is down-regulated in HL-60 cells differentiating towards granulocyte-like cells, the level of exportin-6 is maintained in HL-60 cells differentiating into macrophage-like cells [17, 24, 25] (Table 2). The difference in the expression of exportin-6 in addition to importin-α3 may be related to the differentiation of HL-60 cells into different lineages [25].
The down-regulation of exportins may be involved in the differentiation-associated inhibition of cell growth [27, 28]. Leptomycin B, an inhibitor of exportin-1, is known to prevent proliferation and cause cell cycle arrest at both G1 and G2 in rat 3Y1 fibroblasts [30]. Ratjadones inhibiting nuclear export by blocking exportin-1 also inhibit the growth of several types of eukaryotic cells [31].
Microinjected β-actin is accumulated in the nucleus of Xenopus oocytes unless exportin-6 is coinjected [32]. Thus, exportin-6 specifically mediates the nuclear export of β-actin and actin isoforms, and its expression is developmentally regulated in embryo-genesis. The nuclear accumulation of actin has been observed in cells treated with dimethylsulfoxide, which is an inducer of the differentiation of HL-60 cells toward granulocyte-like cells [32], and it is worth examining its possible relationship with the down-regulated gene expression of exportin-6 as observed in HL-60 cells differentiating into granulocyte-like cells with ATRA [25] (Table 2).
Monocyte differentiation
Macrophages induced to differentiate by macrophage colony-stimulating factor express higher levels of proteins and mRNAs for importins-α1, -α3 and -α5 than undifferentiated monocytes from human peripheral blood [33] (Table 2). Since HIV-1 Vpr is able to use these importins for nuclear entry, the observation provides an explanation of why monocytes are refractory to HIV-1-based vector transduction unlike mature macrophages [34]. The interaction between Vpr and importins may be a potential target for an antiviral agent by inhibiting nuclear entry.
Terminal erythroid differentiation
Terminal erythroid differentiation is the process by which immature precursor cells become erythrocytes in mammals. Exportin-7 appears to be very important to this event, since its gene expression is time-dependently up-regulated by erythropoietin treatment in erythroblasts isolated from the spleens of mice infected with an anaemia-inducing strain of the Friend leukaemia virus [35]. Its precise role, however, is not clear at present.
Neural differentiation
The expression of importin-α subtypes is strictly regulated during the neural differentiation of mouse ES cells [20]. The level of importin-α1 protein is high in undifferentiated ES cells, whereas the levels of importins-α3 and -α5 are low and undetectable, respectively (Table 2). The RNAi-based knockdown of importin-α1, the overexpression of importin-α5 or a combination thereof leads to neural differentiation. The transcription factors Oct3/4, SOX2 and Brn2 which play important roles in neural differentiation contain a single cNLS (Oct3/4 and Brn2) or two cNLSs (SOX2), and importin-α1 is involved in the nuclear transport of Oct3/4, which has a critical role in the maintenance of an undifferentiated ES-cell state. A decrease in importin-a1/Oct3/4 concomitant with the up-regulation of importin-α5, which is involved in the nuclear transport of SOX and Brn2 appears to lead to neural differentiation. Thus, the coordinated regulation of importin subtypes and their transcription factors appears to have a key role in cell-fate determination.
Surprisingly, transgenic Imp-α5−/− mice do not exhibit any obvious morphological or behavioural abnormalities [36]. Since the expression of importin-α4 is markedly increased in the brains of these knockout mice, a compensative mechanism may cover the lack of an importin subtype in mammals. Supporting this notion, an in vitro transport assay has shown that both importin-α5 and -α4 can import Brn2, although with differences in efficiency [37].
Cardiac differentiation
In cardiomyocytes differentiated from mouse ES cells, the gene expression of nuclear transport factors including importins, exportins, transportins, nucleoporins and Ran-related factors is globally down-regulated with a few exceptions as compared to ES cells [38]. In contrast to that during the neural differentiation of ES cells, the expression of importin-α5 is down-regulated (Table 2), suggesting that the difference may be related to cell fate. The up-regulated gene expression of transportin-2 and Ran-binding protein 6 is noticeable and may be related to the nuclear entry of cardiac transcription factors such as Mef2C, Nkx2.5 and Gata4.
Keratinocyte differentiation
Normal human epidermal keratinocytes (NHEKs) express the genes for importins-α1, -α3, -α4 and -α5, but not importin-a6 [39]. Stimulation with interferon (IFN)–γ, a modulator of epidermal proliferation and differentiation, up-regulates the protein expression of importin-α1 after 24 hrs, but down-regulates it by 48 hrs in NHEKs, corresponding to the mRNA expression. IFN-γ does not affect the gene expression of other importins. Since IFN-γ induces the expression of marker genes of keratinocyte differentiation, an increased nuclear entry of importin-α1-mediated signals at an early stage of IFN-γ treatment may facilitate the differentiation.
These observations may be related to the finding that importin-α1 is involved in the nuclear transport of IFN regulatory factor-1, a mediator of epidermal differentiation induced by IFN-γ. Overexpression and RNAi-based knockdown experiments have identified 54 genes modulated putatively by importin-α1 in NHEKs, including the genes for involucrin, keratin-1 and -10 [39]. However, overexpression of importin-α1 appears to induce no morphological changes as seen in differentiated keratinocytes, suggesting that importin-α1 by itself may not be sufficient to induce the full differentiation.
Germ cell maturation
Proteomic profiling of differentially expressed proteins in germinal vesicles and metaphase II arrested mouse oocytes has identified 12 proteins including importin-α1 that migrated differently on electrophoresis in a two-dimensional gel [40]. Thus, post-translational modification appears to take place during the maturation of oocytes.
In spermatogenesis, the mRNA expression of individual importin-α isoforms is differentially regulated [41]. Importin-α5 is expressed in differentiated spermatogonia through to the round spermatids in the adult mouse testis, suggesting its importance in mitotic and meiotic germ cells. The expression of importin-α1 is very limited, as its mRNA is present in spermatocytes but absent once the spermatids begins to elongate. Importin-α4 is expressed specifically in the mitotic germ cell populations, and importin-α3 in pachytene spermatocytes. Thus, mammalian spermatogenesis appears to be a model useful for further examination of the roles and regulation of nucleocytoplasmic transport factors in cellular differentiation and development, and information derived therefrom may have relevance to reproductive medicine.
The cellular and subcellular distribution of importins in spermatogenesis has been demonstrated comprehensively [42–44].
Muscle cell differentiation
The mouse muscle myoblast cell line C2C12 provides an excellent model for studying myogenesis in vitro and cell differentiation.
The RNA-binding protein HuR is critically involved in the formation of muscle fibres through its association with MyoD and myogenin mRNAs and is transported into the nucleus through a transportin-2-mediated pathway [45]. Transportin-2 is expressed in undifferentiated and differentiated C2C12 cells and transportin 1 appears to be expressed weakly only in mature myotubes. The involvement of transportin-2 in muscle cell differentiation has been demonstrated by an experiment in which RNAi-mediated depletion of transportin-2 expression lead to the expression of the myogenic transcription factors MyoD and myogenin. The disruption of the association between HuR and transportin-2 appears to be an important event leading to muscle cell differentiation.
Concluding remarks
Significant progress has been achieved in our understanding of the structure and function of nucleocytoplasmic transport factors including importins and exportins. Yet, information on which specific transport factors are expressed in which tissues and cells is still limited. Such information will be crucial to investigations aiming at human therapeutic applications. In addition, further studies should be done to see whether the results obtained from in vitro culture models will hold true in vivo as well.
Many trials are in progress as exemplified by the coupling of NLS peptides to DNA for gene therapy [11] and disruption of the interaction of NF-κB with importins-α1 and -α5 by NLS peptides [46]. Importin-α1 may have prognostic value in cancer [47], and inhibitors selectively targeting the survivin–exportin-1 interaction may be of therapeutic relevance [48].
Recently, the genes for transcription factors Oct4 and SOX2 have been identified as the minimum requirement for the repro-gramming of human somatic cells to pluripotency [49]. Oct4 can be transported into the nucleus in a complex with either importin-α1/-β1, importin-α3/-β1 or importin-α5/-β1 [20]. SOX2 may be imported with either of importin-α3/importin-β1, importin-a5/-p1 or importin-β1 [20]. The importin-β1 gene is one of the genes downstream of Oct4 [50]. Thus, nucleocytoplasmic transport factors are a potential target also in the field of regenerative medicine.
References
- 1.Koepp DM, Silver PA. Nucleocytoplasmic transport and cell proliferation. Biochim Biophys Acta. 1998;1377:39–47. doi: 10.1016/s0304-419x(97)00036-x. [DOI] [PubMed] [Google Scholar]
- 2.Moroianu J. Distinct nuclear import and export pathways mediated by members of the karyopherin beta family. J Cell Biochem. 1998;70:231–9. [PubMed] [Google Scholar]
- 3.Chook YM, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol. 2001;11:703–15. doi: 10.1016/s0959-440x(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 4.Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–14. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Poon IK, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–86. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 6.Kutay U, Güttinger S. Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 2005;15:121–4. doi: 10.1016/j.tcb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Hogarth C, Itman C, Jans DA, Loveland KL. Regulated nucleocytoplasmic transport in spermatogenesis: a driver of cellular differentiation? Bioessays. 2005;27:1011–25. doi: 10.1002/bies.20289. [DOI] [PubMed] [Google Scholar]
- 8.Conti E, Müller CW, Stewart M. Karyopherin flexibility in nucleocytoplas-mic transport. Curr Opin Struct Biol. 2006;16:237–44. doi: 10.1016/j.sbi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J Biol Chem. 2007;282:5101–5. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem. 2007;76:647–71. doi: 10.1146/annurev.biochem.76.052705.161529. [DOI] [PubMed] [Google Scholar]
- 11.Pouton CW, Wagstaff KM, Roth DM, Moseley GW, Jans DA. Targeted delivery to the nucleus. Adv Drug Deliv Rev. 2007;59:698–717. doi: 10.1016/j.addr.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–6. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 13.Truant R, Cullen BR. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol Cell Biol. 1999;19:1210–7. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee BJ, Cansizoglu AE, Süel KE, Louis TH, Zhang Z, Chook YM. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell. 2006;126:543–58. doi: 10.1016/j.cell.2006.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins Y, McEntee M, Weis K, Greene WC. Characterization of HIV-1 vpr nuclear import: analysis of signals and pathways. J Cell Biol. 1998;143:875–85. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoya F, Imamoto N, Tachibana T, Yoneda Y. Beta-catenin can be transported into the nucleus in a Ran-unassisted manner. Mol Biol Cell. 1999;10:1119–31. doi: 10.1091/mbc.10.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T, Tazoe H, Taguchi K, Koyama Y, Ichikawa H, Hayakawa S, Munakata H, Isemura M. DNA microarray analysis of changes in gene expression induced by 1,25-dihydroxyvitamin D3 in human promyelocytic leukemia HL-60 cells. Biomed Res. 2006;27:99–109. doi: 10.2220/biomedres.27.99. [DOI] [PubMed] [Google Scholar]
- 18.Fagerlund R, Kinnunen L, Köhler M, Julkunen I, Melén K. NF-{kappa}B is transported into the nucleus by importin {alpha}3 and importin {alpha}4. J Biol Chem. 2005;280:15942–51. doi: 10.1074/jbc.M500814200. [DOI] [PubMed] [Google Scholar]
- 19.Waldmann I, Wälde S, Kehlenbach RH. Nuclear import of c-Jun is mediated by multiple transport receptors. J Biol Chem. 2007;282:27685–92. doi: 10.1074/jbc.M703301200. [DOI] [PubMed] [Google Scholar]
- 20.Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, Asally M, Kamachi Y, Kondoh H, Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2007;9:72–9. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- 21.Murao S, Gemmell MA, Callaham MF, Anderson NL, Huberman E. Control of macrophage cell differentiation in human promyelocytic HL-60 leukemia cells by 1,25-dihydroxyvitamin D3 and phorbol-12-myristate-13-acetate. Cancer Res. 1983;43:4989–96. [PubMed] [Google Scholar]
- 22.Miyaura C, Abe E, Suda T, Kuroki T. Alternative differentiation of human promyelocytic leukemia cells (HL-60) induced selectively by retinoic acid and 1 alpha,25-dihydroxyvitamin D3. Cancer Res. 1985;45:4244–8. [PubMed] [Google Scholar]
- 23.Köhler M, Fiebeler A, Hartwig M, Thiel S, Prehn S, Kettritz R, Luft FC, Hartmann E. Differential expression of classical nuclear transport factors during cellular proliferation and differentiation. Cell Physiol Biochem. 2002;12:335–44. doi: 10.1159/000067903. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Koyama Y, Hayakawa S, Munakata H, Isemura M. 1,25-Dihydroxyvitamin D3 suppresses exportin expression in human promyelocytic leukemia HL-60 cells. Biomed Res. 2006;27:89–92. doi: 10.2220/biomedres.27.89. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Ishigami Y, Okada N, Kaneko A, Fukutomi R, Isemura M. Differentiation-associated alteration in gene expression of importins and exportins in human leukemia HL-60 cells. Biomed Res. 2008;29:141–5. doi: 10.2220/biomedres.29.141. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Maltby KM, Miano JM. A novel retinoid-response gene set in vascular smooth muscle cells. Biochem Biophys Res Commun. 2001;281:475–82. doi: 10.1006/bbrc.2001.4362. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Koyama Y, Ichikawa H, Tsushima K, Abe K, Hayakawa S, Kuruto-Niwa R, Nozawa R, Isemura M. 1,25-Dihydroxyvitamin D3 suppresses gene expression of eukaryotic translation initiation factor 2 in human promyelocytic leukemia HL-60 cells. Cell Struct Funct. 2005;30:1–6. doi: 10.1247/csf.30.1. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, Shen Q, Mao WG, Li AP, Ye J, Liu QZ, Zou CP, Zhou JW. JWA, a novel signaling molecule, involved in all-trans retinoic acid induced differentiation of HL-60 cells. J Biomed Sci. 2006;13:357–71. doi: 10.1007/s11373-005-9068-0. [DOI] [PubMed] [Google Scholar]
- 29.Quensel C, Friedrich B, Sommer T, Hartmann E, Kohler M. In vivo analysis of importin alpha proteins reveals cellular proliferation inhibition and substrate specificity. Mol Cell Biol. 2004;24:10246–55. doi: 10.1128/MCB.24.23.10246-10255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida M, Nishikawa M, Nishi K, Abe K, Horinouchi S, Beppu T. Effects of lepto-mycin B on the cell cycle of fibroblasts and fission yeast cells. Exp Cell Res. 1990;187:150–6. doi: 10.1016/0014-4827(90)90129-x. [DOI] [PubMed] [Google Scholar]
- 31.Köster M, Lykke-Andersen S, Elnakady YA, Gerth K, Washausen P, Höfle G, Sasse F, Kjems J, Hauser H. Ratjadones inhibit nuclear export by blocking CRM1/exportin 1. Exp Cell Res. 2003;286:321–31. doi: 10.1016/s0014-4827(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 32.Bohnsack MT, Stüven T, Kuhn C, Cordes VC, Görlich D. A selective block of nuclear actin export stabilizes the giant nuclei of Xenopus oocytes. Nat Cell Biol. 2006;8:257–63. doi: 10.1038/ncb1357. [DOI] [PubMed] [Google Scholar]
- 33.Nitahara-Kasahara Y, Kamata M, Yamamoto T, Zhang X, Miyamoto Y, Muneta K, Iijima S, Yoneda Y, Tsunetsugu-Yokota Y, Aida Y. Novel nuclear import of Vpr promoted by importin alpha is crucial for human immunodeficiency virus type 1 replication in macrophages. J Virol. 2007;81:5284–93. doi: 10.1128/JVI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neil S, Martin F, Ikeda Y, Collins M. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J Virol. 2001;75:5448–56. doi: 10.1128/JVI.75.12.5448-5456.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koury S, Yarlagadda S, Moskalik-Liermo K, Popli N, Kim N, Apolito C, Peterson A, Zhang X, Zu P, Tamburlin J, Bofinger D. Differential gene expression during terminal erythroid differentiation. Genomics. 2007;90:574–82. doi: 10.1016/j.ygeno.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shmidt T, Hampich F, Ridders M, Schultrich S, Hans VH, Tenner K, Vilianovich L, Qadri F, Alenina N, Hartmann E, Köhler M, Bader M. Normal brain development in importin-alpha5 deficient-mice. Nat Cell Biol. 2007;9:1337–8. doi: 10.1038/ncb1207-1337. [DOI] [PubMed] [Google Scholar]
- 37.Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, Asally M, Kamachi Y, Kondoh H, Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2007;9:72–9. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Terzic C, Faustino RS, Boorsma BJ, Arrell DK, Niederländer NJ, Behfar A, Terzic A. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat Clin Pract Cardiovasc Med. 2007;4:68–76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- 39.Umegaki N, Tamai K, Nakano H, Moritsugu R, Yamazaki T, Hanada K, Katayama I, Kaneda Y. Differential regulation of karyopherin alpha 2 expression by TGF-beta1 and IFN-gamma in normal human epidermal keratinocytes: evident contribution of KPNA2 for nuclear translo-cation of IRF-1. J Invest Dermatol. 2007;127:1456–64. doi: 10.1038/sj.jid.5700716. [DOI] [PubMed] [Google Scholar]
- 40.Vitale AM, Calvert ME, Mallavarapu M, Yurttas P, Perlin J, Herr J, Coonrod S. Proteomic profiling of murine oocyte maturation. Mol Reprod Dev. 2007;74:608–16. doi: 10.1002/mrd.20648. [DOI] [PubMed] [Google Scholar]
- 41.Hogarth CA, Calanni S, Jans DA, Loveland KL. Importin alpha mRNAs have distinct expression profiles during sper-matogenesis. Dev Dyn. 2006;235:253–62. doi: 10.1002/dvdy.20569. [DOI] [PubMed] [Google Scholar]
- 42.Hogarth C, Itman C, Jans DA, Loveland KL. Regulated nucleocytoplasmic transport in spermatogenesis: a driver of cellular differentiation. Bioessays. 2005;27:1011–25. doi: 10.1002/bies.20289. [DOI] [PubMed] [Google Scholar]
- 43.Loveland KL, Hogarth C, Mendis S, Efthymiadis A, Ly J, Itman C, Meachem S, Brown CW, Jans DA. Drivers of germ cell maturation. Ann N Y Acad Sci. 2005;1061:173–82. doi: 10.1196/annals.1336.018. [DOI] [PubMed] [Google Scholar]
- 44.Hogarth CA, Jans DA, Loveland KL. Subcellular distribution of importins correlates with germ cell maturation. Dev Dyn. 2007;236:2311–20. doi: 10.1002/dvdy.21238. [DOI] [PubMed] [Google Scholar]
- 45.van Der Giessen K, Gallouzi IE. Involvement of transportin 2-mediated HuR import in muscle cell differentiation. Mol Biol Cell. 2007;18:2619–29. doi: 10.1091/mbc.E07-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham MD, Cleaveland J, Nadler SG. An intracellular targeted NLS peptide inhibitor of karyopherin alpha: NF-kappa B interactions. Biochem Biophys Res Commun. 2003;300:403–7. doi: 10.1016/s0006-291x(02)02863-2. [DOI] [PubMed] [Google Scholar]
- 47.Dankof A, Fritzsche FR, Dahl E, Pahl S, Wild P, Dietel M, Hartmann A, Kristiansen G. KPNA2 protein expression in invasive breast carcinoma and matched peritumoral ductal carcinoma in situ. Virchows Arch. 2007;451:877–81. doi: 10.1007/s00428-007-0513-5. [DOI] [PubMed] [Google Scholar]
- 48.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 49.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 50.Du Z, Cong H, Yao Z. Identification of putative downstream genes of Oct-4 by suppression-subtractive hybridization. Biochem Biophys Res Commun. 2001;282:701–6. doi: 10.1006/bbrc.2001.4636. [DOI] [PubMed] [Google Scholar]
- 51.Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Type 1 parathyroid hormone receptor (PTH1R) nuclear trafficking: association of PTH1R with importin alpha1 and beta. Endocrinology. 2006;147:3326–32. doi: 10.1210/en.2005-1408. [DOI] [PubMed] [Google Scholar]
- 52.Aratani S, Oishi T, Fujita H, Nakazawa M, Fujii R, Imamoto N, Yoneda Y, Fukamizu A, Nakajima T. The nuclear import of RNA helicase A is mediated by importin-alpha3. Biochem Biophys Res Commun. 2006;340:125–33. doi: 10.1016/j.bbrc.2005.11.161. [DOI] [PubMed] [Google Scholar]
- 53.Bian XL, Rosas-Acosta G, Wu YC, Wilson VG. Nuclear import of bovine papillo-mavirus type 1 E1 protein is mediated by multiple alpha importins and is negatively regulated by phosphorylation near a nuclear localization signal. J Virol. 2007;81:2899–908. doi: 10.1128/JVI.01850-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma J, Cao X. Regulation of Stat3 nuclear import by importin alpha5 and importin alpha7 via two different functional sequence elements. Cell Signal. 2006;18:1117–26. doi: 10.1016/j.cellsig.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol. 2006;80:5156–67. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkie SE, Morris KJ, Bhattacharya SS, Warren MJ, Hunt DM. A study of the nuclear trafficking of the splicing factor protein PRPF31 linked to autosomal dominant retinitis pigmentosa (ADRP) Biochim Biophys Acta. 2006;1762:304–11. doi: 10.1016/j.bbadis.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Forwood JK, Kaur G, Jans DA. Nuclear import properties of the sex-determining factor SRY. Methods Mol Biol. 2007;390:83–98. doi: 10.1007/978-1-59745-466-7_6. [DOI] [PubMed] [Google Scholar]
- 58.Le Roux LG, Moroianu J. Nuclear entry of high-risk human papillomavirus type 16 E6 oncoprotein occurs via several pathways. J Virol. 2003;77:2330–7. doi: 10.1128/JVI.77.4.2330-2337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson LM, Rose RC, Moroianu J. The L1 major capsid protein of human papillo-mavirus type 11 interacts with Kap beta2 and Kap beta3 nuclear import receptors. Virology. 2003;306:162–9. doi: 10.1016/s0042-6822(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 60.Mayer D, Molawi K, Martínez-Sobrido L, Ghanem A, Thomas S, Baginsky S, Grossmann J, García-Sastre A, Schwemmle M. Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J Proteome Res. 2007;6:672–82. doi: 10.1021/pr060432u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, Goto H. Acetate inhibits NFAT activation in T cells via importin beta1 interference. Eur J Immunol. 2007;37:2309–16. doi: 10.1002/eji.200737180. [DOI] [PubMed] [Google Scholar]
- 62.Saijou E, Itoh T, Kim KW, Iemura S, Natsume T, Miyajima A. Nucleocytoplasmic shuttling of the zinc finger protein EZI Is mediated by importin-7-dependent nuclear import and CRM1-independent export mechanisms. J Biol Chem. 2007;282:32327–37. doi: 10.1074/jbc.M706793200. [DOI] [PubMed] [Google Scholar]
- 63.Jäkel S, Albig W, Kutay U, Bischoff FR, Schwamborn K, Doenecke D, Görlich D. The importin beta/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 1999;18:2411–23. doi: 10.1093/emboj/18.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dean KA, von Ahsen O, Görlich D, Fried HM. Signal recognition particle protein 19 is imported into the nucleus by importin 8 (RanBP8) and transportin. J Cell Sci. 2001;114:3479–85. doi: 10.1242/jcs.114.19.3479. [DOI] [PubMed] [Google Scholar]
- 65.Lubert EJ, Sarge KD. Interaction between protein phosphatase 2A and members of the importin beta superfamily. Biochem Biophys Res Commun. 2003;303:908–13. doi: 10.1016/s0006-291x(03)00434-0. [DOI] [PubMed] [Google Scholar]
- 66.Plafker SM, Macara IG. Ribosomal protein L12 uses a distinct nuclear import pathway mediated by importin 11. Mol Cell Biol. 2002;22:1266–75. doi: 10.1128/MCB.22.4.1266-1275.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sommer T, Jarosch E. Pardon me–no access without ubiquitin. Dev Cell. 2005;8:4–5. doi: 10.1016/j.devcel.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 68.Kahle J, Baake M, Doenecke D, Albig W. Subunits of the heterotrimeric transcription factor NF-Y are imported into the nucleus by distinct pathways involving importin beta and importin 13. Mol Cell Biol. 2005;25:5339–54. doi: 10.1128/MCB.25.13.5339-5354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang J, Ke G, You W, Peng Z, Lan J, Kalesse M, Tartakoff AM, Kaplan F, Tao T. Interaction between importin 13 and myopodin suggests a nuclear import pathway for myopodin. Mol Cell Biochem. 2008;307:93–100. doi: 10.1007/s11010-007-9588-1. [DOI] [PubMed] [Google Scholar]
- 70.Shamsher MK, Ploski J, Radu A. Karyopherin beta 2B participates in mRNA export from the nucleus. Proc Natl Acad Sci USA. 2002;99:14195–9. doi: 10.1073/pnas.212518199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Güttinger S, Mühlhäusser P, Koller-Eichhorn R, Brennecke J, Kutay U. Transportin2 functions as importin and mediates nuclear import of HuR. Proc Natl Acad Sci USA. 2004;101:2918–23. doi: 10.1073/pnas.0400342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rebane A, Aab A, Steitz JA. Transportins 1 and 2 are redundant nuclear import factors for hnRNP A1 and HuR. RNA. 2004;10:590–9. doi: 10.1261/rna.5224304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benzeno S, Diehl JA. C-terminal sequences direct cyclin D1-CRM1 binding. J Biol Chem. 2004;279:56061–6. doi: 10.1074/jbc.M411910200. [DOI] [PubMed] [Google Scholar]
- 74.Macchi P, Brownawell AM, Grunewald B, DesGroseillers L, Macara IG, Kiebler MA. The brain-specific double-stranded RNA-bind-ing protein Staufen2: nucleolar accumulation and isoform-specific exportin-5-dependent export. J Biol Chem. 2004;279:31440–4. doi: 10.1074/jbc.C400226200. [DOI] [PubMed] [Google Scholar]
- 75.Perron MP, Provost P. Protein interactions and complexes in human microRNA biogenesis and function. Front Biosci. 2008;13:2537–47. doi: 10.2741/2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stüven T, Hartmann E, Görlich D. Exportin 6: a novel nuclear export receptor that is specific for profilin· 79. actin complexes. EMBO J. 2003;22:5928–40. doi: 10.1093/emboj/cdg565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kimura T, Hashimoto I, Nagase T, Fujisawa J. CRM1-dependent, but not 80. ARE-mediated, nuclear export of IFN-alpha1 mRNA. J Cell Sci. 2004;117:2259–70. doi: 10.1242/jcs.01076. [DOI] [PubMed] [Google Scholar]
- 78.Mingot JM, Bohnsack MT, Jäkle U, Görlich D. Exportin 7 defines a novel general nuclear export pathway. EMBO J. 2004;23:3227–36. doi: 10.1038/sj.emboj.7600338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arts GJ, Kuersten S, Romby P, Ehresmann B, Mattaj IW. The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J. 1998;17:7430–41. doi: 10.1093/emboj/17.24.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuwabara T, Warashina M, Sano M, Tang H, Wong-Staal F, Munekata E, Taira K. Recognition of engineered tRNAs with an extended 3' end by exportin-t (Xpo-t) and transport of tRNA-attached ribozymes to the cytoplasm in somatic cells. Biomacromolecules. 2001;2:1229–42. doi: 10.1021/bm0101062. [DOI] [PubMed] [Google Scholar]


