Abstract
Rather different from their initial image as passive supportive cells of the CNS, the astrocytes are now considered as active partners at synapses, able to release a set of gliotransmitter-like substances to modulate synaptic communication within neuronal networks. Whereas glutamate and ATP were first regarded as main determinants of gliotransmission, growing evidence indicates now that the amino acid D-serine is another important player in the neuronal-glial dialogue. Through the regulation of glutamatergic neurotransmission through both N-methyl-D-aspartate (NMDA-R) and non-NMDA-R, D-serine is helping in modelling the appropriate connections in the developing brain and influencing the functional plasticity within neuronal networks throughout lifespan. The understanding of D-serine signalling, which has increased linearly in the last few years, gives new insights into the critical role of impaired neuronal-glial communication in the diseased brain, and offers new opportunities for developing relevant strategies to treat cognitive deficits associated to brain disorders.
Keywords: astrocytes, neuronal-glial communication, D-amino acid, glutamate, N-methyl-D-aspartate receptors, synaptic plasticity, aging, Alzheimer's disease, schizophrenia
Introduction
D-serine in the brain: regional expression and synaptic turnover
D-serine regulation of synaptic transmission and plasticity
D-serine signalling in normal aging and Alzheimer's disease
D-serine signalling and schizophrenia
Conclusions
Introduction
In the central nervous system (CNS), the astrocyte subtype of glial cells forms a continuous syncytium of highly fibrous cells with a dense core of processes interposed between neuronal elements, some of these cells also contacting local vasculature [1]. While providing for the physical and metabolic supports to neurones, the astrocytes also play a critical role as controllers of the extracellular space, regulating local pH and ion homeostasis and the reuptake of released neurotransmitters, functions that are essential for normal neuronal activity. Astrocytes were also seen as passive contributors to synaptic transmission, with very little electrical activity propagating through the glial syncitium, because of the abundant expression of K+ preventing cellular depolarization [2]. This electrical silence justified the experimental silence surrounding these cells until the mid 1980s [3, 4]. However in the last couple of decades, advances in quantitative calcium imaging techniques, combined with the development of confocal microscopy and elec-trophysiological patch-clamp recordings, have challenged this view of astrocytes as passive bystanders. Many results demonstrate now that astrocytes exhibit a specific form of cellular excitability based on intracellular calcium (Ca2+) elevations [5–7], which can be triggered by neuronal activity or by the exogenous application of a variety of neurotransmitters or neuropeptides (for review, see [8]).
These Ca2+ increases propagate as waves throughout the syncytium and promote the release of chemical molecules by astrocytes, which, in turn, are able to modulate synaptic transmission and to synchronize the activity of neuronal networks [9–11]. Because this intimate dialogue between astrocytes and neurones is critical for CNS integration [12], one could propose that abnormal signalling of this communication should be linked to neurological alterations and psychiatric disorders. Although this area of inquiry is still just emerging, increasing evidence indicates that changes in neuronal-glial cross-talk may indeed be causal factors in some diseases [13, 14].
This review aims to compile the available evidence to support the view that the amino acid D-serine is largely involved in the dialogue between astrocytes and neurones [15–17]. Further, it will discuss the most recent findings linking impaired D-serine signalling to cognitive deficits that occur in normal aging and neurological disorders including Alzheimer's disease and schizophrenia.
D-serine in the brain: regional expression and synaptic turnover
Although it took rather long to become established (see [8, 18] for reviews), the in situ existence of neurotransmitters such as glutamate in the brain tissue made acceptable the possibility that these neurotransmitters might also contribute to the neuronalglial communication-regulating functional integration in the brain. For D-serine, such a case was much less evident, since the free D-amino acids were initially considered as unnatural in tissues of higher species [19] and were thought to be active only in lower organisms, where they constitute a major element of cell walls of bacteria and control their rate of growth [20, 21]. The discovery of free D-aspartic acid in the brain of rodents and man, the localization of D-aspartate in specific neuronal populations and the characterization of uptake and release properties in primary cultures of astrocytes and cerebellar granule cells were successive indications for a functional role of D-amino acids in the brain of higher organisms [22–24]. The refinement of HPLC and in vivo micro-dialysis techniques, together with the use of stereoselective antibodies in immunohistochemical experiments now indicate that D-serine is predominantly confined to the brain, particularly concentrated in the cerebral cortex, hippocampus, striatum and amygdala in adult mammals [25–28]. In contrast, D-serine expression is developmentally regulated in the cerebellum, decreasing to trace levels after the first post-natal weeks [25–27]. An interesting view is that the regional distribution of D-serine complements that of another amino acid glycine, which is rather concentrated in the adult cerebellum, pons and spinal cord. Glycine, in addition to acting through strychnine-sensitive inhibitory receptors, is also known as an obligatory co-agonist required for the activation of the N-methyl-D-aspartate receptors (NMDA-R) [29, 30], the subtype of glutamate receptors critically involved in brain development and plasticity, learning and memory and cellular excitotoxicity. Because D-serine also concentrates in closed vicinity of these receptors in forebrain regions [28] and that D-[3H]serine selectively labels the glycine-binding site of NMDA-R, the amino acid was rapidly regarded as a putative endogenous ligand of the receptors in brain areas particularly involved in cognitive processes.
At cellular level, D-serine is predominantly enriched in grey matter within process-bearing glial cells that ensheath synapses, with the morphology of protoplasmic astrocytes [31, 32]. High concentrations of D-serine also occur in astrocytes surrounding local vasculature [28, 31, 32] while in the developing cerebellum, it is present in Bergmann glia, a specialized form of astrocytes involved in the migration, dendritogenesis, synaptogenesis and maturation of cerebellar neurones [33]. Initially, D-serine in the brain was considered to be confined to astrocytes but glutamatergic neurones of cerebral cortex, brainstem regions and olfactory bulbs, as well as microglial-like cells also contain the amino acid [32, 34–36]. Whether these respective sources contribute to modifying the free D-serine concentration at the synaptic cleft still remains equivocal, at least considering the striking numeric prevalence of astrocytes over neurones and given that a substantial release of D-serine from microglia cells requires pro-inflammatory stimuli [35, 37]. At subcellular levels, D-serine may be differentially compartmentalized depending on cellular types [34, 38]. In astrocytes and astrocytoma-derived C6 cells, D-serine is free in the cytoplasm but a large proportion also concentrates into vesicle-like compartments expressing the vesicle-associated protein 2 [VAMP2], a protein of the synaptic exocytotic machinery [38]. D-serine release is altered by concanamycin A, a vacuolar-type H+-ATPase inhibitor disrupting vesicular proton gradient, suggesting that some of the amino acid is stored in specific vesicular organelles that have yet to be identified [32, 38]. In contrast, treatment of neuronal preparations with bafilomycin A1, another potent blocker of the vesicular uptake of transmitters, is ineffective in modulating D-serine release [34]. In addition, vesicles isolated from cortical neurones that are filled up with conventional transmitters, do not incorporate D-serine, indicating that the neuronal pool of the amino acid is localized in a cytosolic non-vesicular compartment.
The issues of whether D-serine is synthesized, released and metabolized in situ in the brain are prerequisites in considering this amino acid as a signalling molecule (Fig. 1). Compelling evidence indicates that all of these criteria are achieved [15–17]. De novo synthesis of D-serine is promoted in the cerebral cortex by intraperitoneal injection of L-serine or by injection of L-[3H]-serine in rat brain [39, 40]. The synthesis is catalysed by the pyridoxal 5’-phosphate (PLP, vitamin B6)-dependent serine racemase (SR), a 37-kD protein that induces the direct racemization of L-serine to D-serine [41, 42]. The enzyme is highly selective for L-serine with few if any activity for L-alanine, L-threonine and L-aspartate. Serine racemase can also convert D-serine into L-serine, but at a much slower rate, indicating that under physiological conditions, the enzyme should predominantly synthesize D-serine [42]. The expression of SR mRNAs in human brain coincides with the high levels of endogenous D-serine and includes the forebrain and corpus callosum [41, 43–45]. In the brain, SR is expressed as a single transcript, consisting of four isoforms derived from the alternative use of various 5’ end exons [46]. Other, different splice forms occur in heart, skeletal muscle, kidney and liver tissues with at least three different sizes [43]. Initially confined to astrocytes [41, 42], SR both at RNA messenger and protein levels has been recently demonstrated also in neuronal cell bodies and processes in the forebrain and cerebellum [34, 36, 47]. The physiological relevance of this pool of neuronal SR still remains elusive and it was recently proposed that neurones predominantly synthesized D-serine whereas astrocytes preferentially accumulated the amino acid [48]. Another possibility is that neuronal SR could underlie the activation of cortical NMDA-R by D-serine through an autocrine or paracrine pathway [34].
Fig 1.
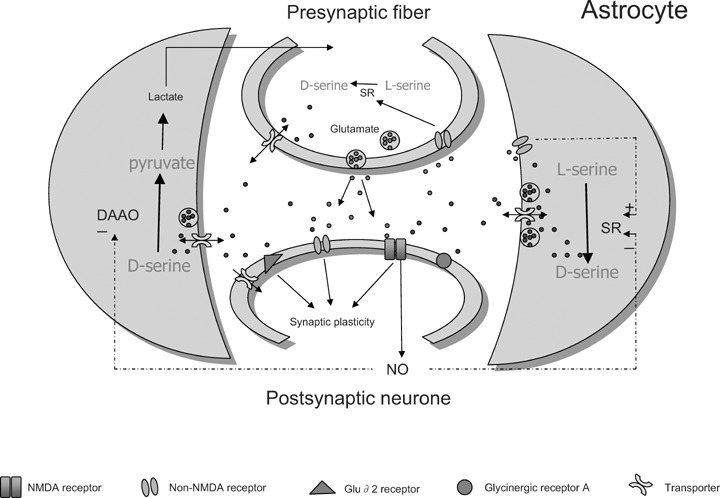
Schematic representation of mechanisms involving D-serine in neuronal-glial communication at synaptic cleft. Glutamate released upon depolarization of nerve terminals activates non-NMDA receptors present not only on post-synaptic neurones but also on membranes of astrocytes and presynaptic fibres. The synthesis of D-serine by serine racemase (SR) in glial cells and possibly in neurones is then promoted and D-serine is consequently released either by from a vesicular pool by exocytosis or from a cytosolic pool by transporters to act on post-synaptic NMDA receptors, in concert with glutamate. It may also depress fast glutamatergic transmission by directly binding on AMPA subtype of non-NMDA receptors or indirectly by acting on inhibitory glycinergic receptors. It also activates GluRδ2 receptors homologous to ionotropic glutamate receptors but not gated by glutamate. Through these different bindings, D-serine is able to promote and regulate synaptic plasticity within neuronal networks. Clearance of D-serine depends on transporters present on membranes of astrocytes and neurones. D-amino acid oxidase (DAAO) degrades D-serine into pyruvate, which is metabolized into lactate and given back to neurones. Activation of NMDA receptors induces the formation of nitric oxide, which diffuses to neighbouring astrocytes inhibiting SR activity and promoting DAAO activation. This mechanism allows neurones to regulate D-serine availability at synapses to avoid excessive activation of NMDA receptors.
Serine racemase activity is modulated by a variety of cofactors, likely to be present in the vicinity of the enzyme (reviewed in [49]). At physiological conditions, ATP and Mg2+ are very potent activators of SR, increasing 5- to 10-fold the rate of racemization [50]. Other divalent cations such as manganese or calcium, the latter acting through a direct binding to the enzyme, are also necessary for complete enzyme activity and D-serine synthesis may be altered by manipulating intracellular calcium concentration [51].
The activity of SR is also up- or down-regulated by a number of amino acids: D-serine activates, in a dose-dependent manner [52] whereas glycine and some compounds related to L-aspartic acid such as L-aspartate and L-homocysteic acid (but not L-glutamate), are potent inhibitors of the enzyme [53]. Nitric oxide decreases the catalytic activity of SR [52, 54], a mechanism that illustrates yet another aspect of the neuronal-glial communication: the inhibitory regulation by nitric oxide of SR activity represents thus a possible feedback mechanism that prevents excessive NMDA-R activation by reducing the availability of D-serine.
Emerging evidence indicates that the synthesis of D-serine is not only modulated by soluble cofactors but also depends on several physical protein-protein interactions. The transfection of C6 glioma cells or primay glial cultures with adenovirus containing the multi-PDZ domain glutamate receptor interacting protein (GRIP), which is usually linked to the GluR2/3 subunits of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) subtypes of glutamatergic receptors, increases the levels of D-ser-ine by twofold [55]. The interaction between SR and GRIP occurs in vivo since these proteins coimmunoprecipitate from brain extracts, and this association is required to trigger neuronal migration of granule cells within the developing cerebellum [55]. Although the PDZ6 domain of GRIP selectively interacts with the C-terminal portion of SR, short constructs restricted to this domain are unable to induce SR activation in COS7 cells and larger constructs involving PDZ4-5-6-7 and GAP2 domains are necessary, indicating that GRIP acts on SR by inducing a large confor-mational changes [56]. Another scaffolding protein for glutamate receptors, PICK1, also binds SR through its single PDZ domain [57]. Although the physiological relevance of this interaction has yet to be established, it may help for SR activation by promoting the phosphorylation of the enzyme by protein kinase C, known to interact tightly with PICK1 [58]. Indeed, it was recently reported that levels of D-serine is selectively decreased in the forebrain of PICK1 knockout mice whilst the levels of SR protein remains unchanged [59]. Not only the activation, but also the degradation of SR is dependent on protein-protein interactions. Serine race-mase is degraded by the ubiquitin-proteasome system, which mediates selective catabolization of many short-lived proteins [60]. The use of yeast two-hybrid technique recently demonstrated, both in vitro and in vivo, that the degradation of SR by the ubiquitin-proteasome system is prevented by its binding with the Golgin subfamily A member 3 (Golga3), a protein implicated in Golgi structure and membrane traffic. The protein interaction, which involves the coileD-coil domain of Golga3, decreases ubiquitylation and degradation rate and significantly enhances SR half-life, and constitutes an alternative way for regulating D-serine levels in the brain [61].
While the biosynthetic pathways for D-serine are now well characterized, the mechanisms regulating the storage, release and reuptake of the amino acid at synaptic cleft still remain to be clarified. The basic observation is that the activation of AMPA and metabotropic subtypes of glutamate receptors in vitro induces the release of D-serine [31, 38, 55]. However, these effects are less convincing in in vivo experiments [62, 63]. The AMPA-evoked D-serine release, both in glioma C6 cells and primary astrocytes, involves the SR-GRIP interaction and requires an increase in intracellular calcium concentration [38, 55]. Tetanus neurotoxin, which selectively cleaves the soluble N-ethyl-maleimide-sensitive attachment receptor (SNAREs), potently reduces the release of D-serine induced by glutamate receptor activation [38]. Since SNAREs are a class of proteins involved in the fusion of secretory vesicles to the plasma membrane, this indicates that the release of the amino acid results from an exocytotic event similar to that reported for the release of glutamate and ATP from astrocytes [64–66]. The physiological relevance of this calcium-dependent release of D-serine from a vesicular pool is still questioned, particularly when considering that basal extracellular levels of D-serine are not significantly affected in vivo by altering the extracellular calcium concentrations [26].
The alternative pathway is that of D-serine being released from the cytoplasmic compartment through a calcium-independent mechanism, such as the activation of a membrane channel driven by a chemical gradient [67] or through some carrier-mediated transport systems. One type of such transporter, expressed on astrocytes, is similar to the amino acid transporter ASCT2 since it is Na+-dependent and shows a low specificity for D-serine. The efflux of the amino acid by this transporter is coupled to an influx of L-serine within the astrocyte that may serve for de novo synthesis of D-serine by SR [68]. Other carrier-mediated transport systems, with higher specificity for the amino acid, are present at synaptic cleft. They include the Na+-independent alanine-serine-cysteine transporter 1 (Asc-1) primarily located on neuronal terminals [69–71] and the (Na+)-dependent, alanine-independent transporter [72, 73], which both control the re-uptake of D-serine, thus contributing to maintain a relatively low concentration of the amino acid in the extracellular fluid.
Searching for the pathways responsible for the degradation of D-serine has led to the effective rediscovery of D-amino acid oxidase (DAAO), a flavoprotein first isolated and characterized in the mid 1930s [74] but then forgotten since for a long time the free D-amino acids were not considered as natural components in the mammalian tissues. Several lines of evidence indicate currently that DAAO contributes to the degradation of D-serine in the CNS: its regional expression correlates inversely with the levels of endogenous amino acid in both developing and mature brain [31, 75–77] and its genetic deletion substantially increases D-serine levels in the hindbrain and cerebellum [78, 79] where the enzyme is highly expressed and confined in astrocytes [31, 75–77]. Interestingly, the activity of DAAO is enhanced by nitric oxide, indicating that the effect of NMDA-R to reduce the availability of D-serine occurs not only through the down-regulation of SR but also through an up-regulation of the degradation pathway [80].
Although substantial immunoreactivity for DAAO has recently been found in human prefrontal cortex [44], its enzymatic activity is virtually undetectable in the telecephalon [81], suggesting the possibility of alternative pathways for the breakdown of the amino acid. The exact nature of these alternative pathways remains elusive, but it might involve an amazing property of SR which is able, both in vivo and in vitro, to catalyse the degradation of D-serine and L-serine, in an ATP-dependent manner, to pyruvate and ammonia viaan elimination of water [50, 82–84]. Thus, this property of SR, by slowing the rate of D-serine synthesis and by promoting the degradation of the amino acid and its precursor into pyruvate, appears as a potent putative mechanism to limit tissue levels of D-serine. Accordingly, activation of mutant SR with selective impairment of the eliminase activity significantly increases the levels of the amino acid in primary astrocytes cultures and HEK 293 cells [85]. On the other hand, the possibility of SR to enhance pyruvate levels may represent an important mechanism providing part of the energy required during periods of increased synaptic activity, because it creates extra substrate for mitochondrial oxida-tive phosphorylation and lactate production.
D-serine regulation of synaptic transmission and plasticity
The labelling of the glycine-binding site of NMDA-R by D-[3H]serine [86] and the capacity of D-serine to be at least as efficient as glycine in stimulating purified recombinant NMDA-R [87] support the view that the amino acid is a full agonist for this subtype of glutamatergic receptors, and even the de facto endogenous co-agonist in the brain. The use of enzymatic degradation to reduce D-serine from regions rich in the amino acid, for example, the hippocampus, brought the first convincing evidence to demonstrate that D-serine was indeed the endogenous ligand required for NMDA-R activation in these regions (see [15–17, 49]). Pre-treat-ment with DAAO potently reduced the spontaneous or agonist-evoked NMDA-R-mediated currents in hippocampal neuronal cell cultures [88] as well as the NMDA-R-mediated excitatory post-synaptic currents (EPSCs) electrically evoked at CA3-CA1 synapses of hippocampal slices [89]. In the same way, NMDA-R activation was impaired by DAAO in the hypothalamic supraoptic nucleus (SON), an area enriched in D-serine and where SR is highly expressed by astrocytes, whereas a pre-treatment with glycine oxidase to deplete the levels of glycine had no effect [90]. The NMDA-R-mediated EPSCs were altered in cultures of hippocampal neurones when the astrocytes were reduced or absent, resulting in a dramatic reduction in the concentration of D-serine in the supernatant; adding exogenous D-serine to these cultures restored a robust activation of the glutamatergic receptor [89]. These findings strongly argue for a critical role of the endogenous, astrocyte-derived, D-serine in regulating NMDA-R activation in regions enriched in the amino acid. However, some contribution from the glycine-dependent regulation cannot be totally ruled out since the inhibition of glycine transporter type 1, which normally removes glycine from the synaptic cleft, increased the amplitude of the NMDA-R component of the glutamatergic EPSC at hippocampal and cortical synapses [91–93]. An important issue is that D-ser-ine-mediated activation of NMDA-R was larger in pyramidal cells than in neighbouring GABAergic interneurones within CA1 hip-pocampal subfield [94], probably reflecting assemblies of diverse NMDA-R subunits that differentially respond to the agonist [87].
These findings indicate that beyond its primary role in activating NMDA-R, D-serine is also a dynamic partner involved in the regulation of excitation–inhibition balance within neuronal networks.
Endogenous D-serine also underlies NMDA-R activation outside the CNS, thus participating in the encoding of sensory inputs. Mutant mice with genetic deletion of DAAO display larger NMDA-R-mediated EPSCs at spinal cord dorsal horn neurons compared to wilD-type controls [95]. It is worth noticing that a similar increase occurs at CA3-CA1 hippocampal synapses in these animals [96], indicating that DAAO activity significantly contributes to limit D-serine levels in the forebrain, at least in the hippocampus, despite the fact that the expression of the enzyme is very low in this territory [28, 75–77]. Also, D-serine and SR are present in Müller cells and astrocytes, the two major glial cell types in the retina [97]. A pre-treatment with DAAO or D-serine deaminase, another D-serine degrading enzyme, reduces NMDA-R evoked currents as well as the NMDAr component of light-evoked synaptic responses in retinal ganglion cells [97, 98], which further extends the view of D-serine as a gliotransmitter necessary required for neuronal encoding of presynaptic inputs.
NMDA-R are a class of glutamate receptors involved in inducing lasting changes in synaptic strength between neurones, a form of functional plasticity proposed as to underlie cellular memory within neuronal networks. Forms of both long-term potentiation (LTP) or depression (LTD) of synaptic transmission may be driven by NMDA-R activation depending on the amplitude and kinetics of calcium influx flowing through the receptor, and which alters the phosphorylation/dephosphorylation rate and the density of AMPA/kainate subtype of glutamate receptors at active synapses [99, 100]. The critical role of astrocyte-derived D-serine on NMDA-R suggests that the capacity of functional plasticity in the brain might be closely related to the synaptic availability of the amino acid. Indeed, ‘pure’ cultures of hippocampal neurones grown in the absence of astrocytes are unable to express LTP and this defect of synaptic plasticity may be partially rescued by supplementing the external medium with exogenous D-serine [89]. Moreover, a pre-treatment of neuronal-glial co-cultures or hippocampal slices with DAAO also abolishes LTP expression [89, 101]. In the hypothalamic SON, a reversible decrease in astrocytic enshealthing of neurones occurs during lactation that down-regulates the occupancy of glycine-binding sites of NMDA-R by D-serine at synaptic cleft [90]. Consequently, the expression of NMDA-R-mediated synaptic plasticity is shifted toward higher activity values, a shift which is proposed to emphasize the activation of specific bursting firing neurones within SON such as those involved in suckling [90]. Thus, synaptic levels of D-serine appears as a prominent component of the neuronal-glial dialogue necessary for the brain to challenge changes in physiological or hormonal environment.
Although D-serine was initially thought to act specifically on NMDA-R, it may also affect the activation of AMPA/kainate receptors that underlies the fast glutamatergic transmission in most parts of the brain. In acutely isolated hippocampal neurones, D-ser-ine, but not L-serine, reduces kainate-induced currents, acting as a weak competitive antagonist of the receptor [102]. In hippocampal slice preparations, the amino acid, as well as the partial agonist
D-cycloserine, decreases AMPA-R-mediated synaptic responses, but indirectly through the activation of strychnine-sensitive inhibitory glycinergic receptors [103–105]. Whether these inhibitory effects induced by exogenous application of substantial doses of the amino acid are of any physiologically relevance may be questioned, considering that fast glutamatergic transmission is not affected in cerebral tissues depleted in D-serine by a pre-treatment with DAAO [89, 90, 101]. However, it is important to bear in that a single intraperitoneal administration of D-serine in rodents can induce a massive and prolonged increase in levels of the amino acid throughout the brain [106].
Recent evidence indicates that D-serine also binds to the glutamate-like receptor 82 (GluRδ2), a class of receptors homologous to ionotropic glutamate receptors but which are not gated by glutamate [107]. GluRδ2 receptors, which are localized on dendritic spines of Purkinje cells, are highly expressed during the first post-natal weeks [108], at the same period when Bergmann glial cells of cerebellar cortex are particularly enriched in D-serine, due to the weak expression of DAAO [25–27]. The ability of D-serine to affect the activation of GluR82 receptors [109] and the fact that the genetic deletion and naturally occurring mutations of GluR82 genes cause abnormal function of the parallel fibre-to-Purkinje cell and climbing fibre-to-Purkinje cell synapses [108] are further arguments in considering the binding of D-serine to GluR82 receptors as important factors during the synaptogenesis in the developing cerebellum. This mechanism is in addition to the property of D-serine released from Bergmann cells to promote, through the activation of NMDA-R, the migration of granule cells from the external to the internal granular layer in the cerebellum of neonatal mice [55]. Together, these experimental observations raise the possibility that D-serine-mediated neuronal-glial communication has a crucial role in the establishment of appropriate connections in the developing brain.
D-serine signalling in normal aging and Alzheimer's disease
Even today, the exact nature of cellular and molecular substrates underlying learning and memory still remains an opened issue for the neurobiologist. However, this question has always been with us since the constant increase of the age of the population, driven by progress in the prevention and the treatment of chronic diseases and the improvements in hygiene and nutrition, has been shown to correlate with a higher incidence of cognitive defects, notably of learning and memory. The current hypothesis views memory formation as determined by the capacity of brain neuronal networks to express synaptic plasticity [100]. As a corollary to this postulate, a deficit in memory abilities should be linked to a parallel impairment of LTP and/or LTD induction at synapses. Normal aging is indeed a case in point, as demonstrated by numerous behavioural and electrophysiological studies performed mainly on rodents (see [109] for a review). The alteration of LTP expression is unambiguously related to a deficit of NMDA-R activation [110, 111] and is rescued in aged rats and senescence-accelerated mice by exogenous D-serine [101, 112]. These findings raise the intriguing possibility that changes in D-serine signalling in the age-related could be contributors to the dysfunctions of memory processing (Fig. 2). Impaired activation of NMDA-R by D-serine indeed occurs in aged tissues; it does not involve either a lower density of receptor or an altered affinity of D-serine-binding sites [113, 114] but a reduced level of the endogenous co-agonist [32, 101, 103]. To what extent the individual molecular determinants involved in synaptic storage of D-serine is affected by aging, has yet to be definitively established. A weaker expression of SR both at transcriptional and post-transcriptional levels indicates that impaired synthesis significantly contributes to the age-related decrease in D-serine levels [101]. It is possible that the degradation of the amino acid might not be affected since DAAO expression is unchanged [101], although the elimination of D-serine by the SR-dependent elimination-activated pathway may be affected. The ability of D-serine to prevent the age-related deficit of synaptic plasticity would make this amino acid an attractive tool in reducing memory dysfunctions in the aged. It is worth noticing that defects of spatial memory in aged rats [115, 116] and the disruption of the associative hippocampus-dependent eye-blink conditioning in old rabbits [117] are rescued by the partial agonist D-cycloserine, which also alleviates the age-related deficit of NMDA-R-mediated synaptic plasticity by acting on the glycine-binding site [118]. Unfortunately, the possibility of long-term treatments with large doses of D-cycloserine as well as with D-serine itself to rescue cognitive deficits has been discarded because they cause central side effects or induce DAAO-depenD-ent necrosis of the terminal portions of the proximal renal tubules respectively [119–121]. Alternately, the fact that SR activity is selectively affected in aged tissues opens new perspectives in the search of relevant strategies for reducing memory impairment associated with aging.
Fig 2.
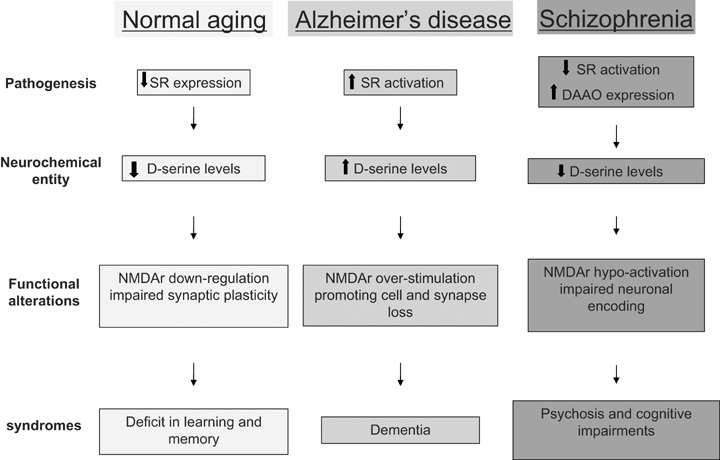
Schematic model outlying the contribution of abnormal D-serine signalling in normal aging, Alzheimer's disease and schizophrenia. Although cognitive impairments are prominent features in all cases, they may be correlated to either down- or up-regulation of D-serine signalling, indicating that the neuronal-glial dialogue must be strictly control to avoid pathological situations.
One striking feature of aging is that the degree of cognitive impairment can greatly vary amongst individuals, from a mild deficit to a severe dementia in the case of Alzheimer's disease (AD). Among the multiple aspects constituting the pathophysiology of AD, the excitotoxicity-induced cell and synapse loss driven by excessive exposure to glutamate and overstimulation of NMDA-R is now considered as a potent mechanism [122]. Indeed, the uncompetitive low-affinity NMDA-R blocker memantine, which enters the receptor-associated ion channel preferentially when it is excessively opened, not only protects against neurodegeneration but also improves cognitive deficits in AD patients and slows down the progression of the disease [123, 124]. Activation of microglial cells, as part of a chronic inflammatory response, is the prominent component of AD that drives neurototoxicity through the release of excitotoxins including glutamate [125, 126]. The amyloid β-peptide (Aβ) and the secreted form of β-amyloid precursor protein, two soluble factors involved in the pathogenesis of AD, not only promote glutamate release from microglia but, at the same time, stimulate SR expression and D-serine release from these glial cells [35, 37]. The transcriptional induction of SR by Aβ, which fits with the increase in messenger RNAs for SR in the brain of AD subjects [35], is promoted by the binding of inducible proto-oncogenes c-fos and JunB to the activator protein-1 sequence present on the first intron of SR gene [127]. Aβ also promotes SR activity post-transcriptionally through an increase in intracellular levels of calcium which up-regulates the activity of the enzyme [51]. On the other hand, SR-independent pathway may also underlie the Aβ-mediated increase in D-serine synthesis since the peptide activates L-phosphoserine phosphatase, an enzyme converting the endogenous isomer L-phosphoserine into L- and D-serine [128]. Rather curiously the issue of whether the levels of free D-serine are enhanced in the brains of AD is still a matter of debate in the literature (see [49]), probably because the levels of the amino acid are determined mostly at late stages of the disease, when clinical symptoms are obvious, whereas the neuro-toxic levels of D-serine are expected to occur in early neuroinflam-matory stages of the pathology.
Given the current state of researches, elevated D-serine levels may be considered as a pro-death signal in AD that promotes, in conjunction with glutamate, the neurotoxicity exhibited by inflammatory processes (Fig. 2). Accordingly, it is worth noticing that conditioned media from Aβ-treated microglia, containing elevated levels of D-serine, evoke a dramatic overload of calcium in neurones [35] while enzymatic degradation of the amino acid by DAAO or D-serine deaminase protects against cell death elicited by NMDA in vitro[129, 130]. These findings therefore raise an unexpected ‘dark side’ of neuronal-glial communication, through an exaggerated synaptic level of D-serine, as causative of cognitive deficits associated with AD since synapse loss is the strongest anatomical correlate of the degree of clinical impairment in the pathology [131].
D-serine signalling and schizophrenia
Schizophrenia is a severely disabling brain disorder with symptomatic onset in early adulthood, affecting approximately 1% of the population worldwide. The dopaminergic theory of treatment in schizophrenia has initially attracted a lot of attention but drugs that block dopamine D2 receptors appear most effective in treating the psychosis and have poor effects on the negative symptoms (alogia, apathy, associability, etc.) and cognitive impairments [132].
An important insight into the pathophysiology of schizophrenia came from the observation that administration of low doses of the uncompetitive NMDA-R antagonists phencyclidine (PCP) and ketamine reproduced both the negative and positive cardinal symptoms of schizophrenia [133]. Although a reduced glutamate availability in the brain of schizophrenic patients may account for some of the NMDA-R hypofunction, both experimental investigations and clinical trials support the view that impaired activation of D-serine-binding sites is also concerned (see [134] for a review). For example, the cognitive deficit induced in rats by PCP is reversed by a treatment with D-serine, while placebo-controlled trials showed a reduction in severity of simptomatology, as well as improvement in cognition on administration of D-serine, glycine or, to a lesser extent, D-cycloserine to schizophrenic individuals receiving concurrent antipsychotics agents (e.g. olanzapine and risperidone) [135]. Several studies tried to determine the molecular and cellular mechanisms that might link impaired NMDA-R activation in schizophrenia with the signalling of D-serine (Fig. 2). Changes in the density of NR1 subunit of the receptor, which bear the D-serine binding site, occur in the brain of schizophrenic patients, but these changes are very patchy, depending on several factors such as age and specific forebrain region concerned [133]. In contrast, compelling evidence shows that D-serine levels (but not those of L-serine and L-glutamate) are reduced in serum as well as cerebrospinal fluid of schizophrenic patients (reviewed in [136]), indicating that the production or breakdown of the amino acid are affected in the illness. The involvement of D-serine synthesis in the aetiology of schizophrenia is also suggested by the association of the disease with single nucleotide polymorphisms (SNPs) of the 5’ region of the SR gene, such as the IVS1a+-465C allele, which reduces the expression of the gene [137, 138]. However, no consensus has been reached yet regarding the changes in SR protein levels in the cerebral cortex of schizophrenic subjects – both increased and decreased expression have been reported (see [44]). The weaker deposit of D-serine in brains of schizophrenic patients may also result from changes in the degradation pathway. Indeed, not only SR but also DAAO is affected in schizophrenia [139]. In the search for SNPs associated with the disease, two overlapping genes G30 and G72 have been isolated from chromosome region 13q-q34 that is genetically linked to schizophrenia [140]. When the transcription of G30 gene does not result in a stable protein, G72 induces the formation of a polypeptide that specifically interacts with DAAO, promoting the activation of the enzyme [140]. G72 expression is enhanced in the prefrontal cortex of schizophrenia subjects and SNPs variations in the G72 gene region increase risk of cognitive impairment associated to the disease (see [139]). Thus, the association of both DAAO and G72 with schizophrenia [141] together with the increase in DAAO activity by a G72 protein product indicates the involvement of an enhanced D-serine degradation as a possible mechanism underlying NMDA-R hypofunction in the disease. Some have argued that such data are not the relevant in the psychiatric context since the cortical localization of schizophrenia did not fit well with the regional expression of DAAO, which was found to be restricted to the hindbrain regions and cerebellum, at least in rodent brains [31, 75–77]. However, recent evidence showed the unequivocal expression of DAAO in the human cerebral cortex [44, 136]. In addition, there are emerging views considering the cerebellum as an integral part of the circuitry affected in neuropsychiatric disorders, in general and in schizophrenia, in particular [142]. The cerebellar expression of DAAO is up-regulated in brains of schizophrenic subjects [44, 81] while protein levels of the enzyme are elevated in the hippocampus [136]. Interestingly, the hippocampal increase in DAAO expression correlates with duration of illness, not with age [136], and this is a strong support for the view that changes in DAAO activity are an aetiological mechanism giving rise to NMDA-R dysfunction in schizophrenia [133].
Conclusions
During the last decades, our knowledge regarding the neuronalglial communication as a potent cellular substrate involved in brain information processing has markedly increased [8, 10–12]. Through the release of active molecules such as the atypical amino acid D-serine, this communication has the potential to influence profoundly the functionality of neuronal networks by modulating neurotransmission in the short term and by helping to establish efficacy in the long term. New insights into a ‘yin and yang’ of D-serine signalling have profound implications for our understanding of the role of the neuronal-glial cross-talk in the healthy and diseased brain. When the amino acid is able to promote development and plasticity of the CNS, it may also cause neuronal death or loss of synaptic flexibility through either up- or down-regulation of its metabolic pathway. These findings are therefore clear indications that a strict control of the level of neuronal-glial dialogue is an important prerequisite in avoiding the development of various brain pathologies. Although the present review focuses on cognitive consequences of impaired D-serine signalling, there is no doubt that this view is rather restrictive. On one hand, it is now obvious that D-serine manages NMDA-R-dependent neurotransmission and up- or down-activation of this pathway is involved in a large range of neurological disorders including stroke, epilepsy and peripheral neuropathies. A contribution of D-serine in these pathologies is therefore likely and indeed is supported by a set of recent experimental data. Ischaemia-induced neuronal cell death simulated in vitro by exposing neurones to oxygen- and glucose-free conditions is prevented by a pre-treatment with DAAO [129] while administration of both D-serine alone or in combination with morphine potentiates antinociception through the stimulation of supraspinal NMDA receptor [143]. On the other hand, we can imagine that only a few pages of the catalogue of D-serine-dependent brain dysfunctions have been yet written since all cellular mechanisms regulated by the amino acid are far from being unravelled. This view is confirmed by the recent discovery that D-serine may also act through non-NMDA-R-dependent processes [101–103, 105]. Although there is no longer any doubt about considering D-serine signalling as main avenue in the process of neuronal-glial dialogue in brain and periphery, the details on how it affects the coding and processing in the CNS in normal and pathological conditions have yet to be clarified. However, in the light of the results discussed in this review, these details represents an exciting challenge for the neuroscientists in their search of new therapeutical approaches required by the desire to improve the scourge of various neurological disorders.
References
- 1.Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcircula-tion. Trends Neurosci. 2003;26:340–4. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci. 2001;21:7901–8. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocam-pal astrocytes in situ. J Neurophysiol. 1997;78:461–77. doi: 10.1152/jn.1997.78.1.461. [DOI] [PubMed] [Google Scholar]
- 4.Sontheimer H, Waxman SG. Expression of voltage-activated ion channels by astrocytes and oligodendrocytes in the hippocampal slice. J Neurophysiol. 1993;70:1863–73. doi: 10.1152/jn.1993.70.5.1863. [DOI] [PubMed] [Google Scholar]
- 5.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–3. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 6.Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–40. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 7.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–30. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–93. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 9.Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–7. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auld DS, Robitaille R. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron. 2003;40:389–400. doi: 10.1016/s0896-6273(03)00607-x. [DOI] [PubMed] [Google Scholar]
- 11.Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–42. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 12.Verkhratsky A, Toescu EC. Neuronal-glial networks as substrate for CNS integration. J Cell Mol Med. 2006;10:826–36. doi: 10.1111/j.1582-4934.2006.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 13.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 15.Miller RF. D-Serine as a glial modulator of nerve cells. Glia. 2004;47:275–83. doi: 10.1002/glia.20073. [DOI] [PubMed] [Google Scholar]
- 16.Mustafa AK, Kim PM, Snyder SH. D-Serine as a putative glial neurotransmitter. Neuron Glia Biol. 2004;1:275–81. doi: 10.1017/S1740925X05000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliet SH, Mothet JP. Molecular determinants of D-serine-mediated gliotransmis-sion: from release to function. Glia. 2006;54:726–37. doi: 10.1002/glia.20356. [DOI] [PubMed] [Google Scholar]
- 18.Perea G, Araque A. Glial calcium signaling and neuron-glia communication. Cell Calcium. 2005;38:375–82. doi: 10.1016/j.ceca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Corrigan JJ. D-amino acids in animals. Science. 1969;164:142–9. doi: 10.1126/science.164.3876.142. [DOI] [PubMed] [Google Scholar]
- 20.Smith JL, Higuchi K. Studies on the nutrition and physiology of Pasteurella pestis. V. Inhibition of growth by D-serine and its reversal by various compounds. J Bacteriol. 1960;79:539–43. doi: 10.1128/jb.79.4.539-543.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitney JG, Grula EA. A major attachment site for D-serine in the cell wall mucopep-tide of Micrococcus lysodeikticus. Biochim Biophys Acta. 1968;158:124–9. doi: 10.1016/0304-4165(68)90079-2. [DOI] [PubMed] [Google Scholar]
- 22.Drejer J, Larsson OM, Schousboe A. Characterization of uptake and release processes for D- and L-aspartate in primary cultures of astrocytes and cerebellar granule cells. Neurochem Res. 1983;8:231–43. doi: 10.1007/BF00963923. [DOI] [PubMed] [Google Scholar]
- 23.Dunlop DS, Neidle A, McHale D, Dunlop DM, Lajtha A. The presence of free D-aspartic acid in rodents and man. Biochem Biophys Res Commun. 1986;141:27–32. doi: 10.1016/s0006-291x(86)80329-1. [DOI] [PubMed] [Google Scholar]
- 24.Man EH, Fisher GH, Payan IL, Cadilla-Perezrios R, Garcia NM, Chemburkar R, Arends G, Frey WH., 2nd D-aspartate in human brain. J Neurochem. 1987;48:510–5. doi: 10.1111/j.1471-4159.1987.tb04122.x. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto A, Nishikawa T, Oka T, Takahashi K. Endogenous D-serine in rat brain: N-methyl-D-aspartate receptor-related distribution and aging. J Neurochem. 1993;60:783–6. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto A, Oka T, Nishikawa T. Extracellular concentration of endogenous free D-serine in the rat brain as revealed by in vivo microdialysis. Neuroscience. 1995;66:635–43. doi: 10.1016/0306-4522(94)00597-x. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto A, Oka T, Nishikawa T. Anatomical distribution and postnatal changes in endogenous free D-aspartate and D-serine in rat brain and periphery. Eur J Neurosci. 1995;7:1657–63. doi: 10.1111/j.1460-9568.1995.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 28.Schell MJ, Brady RO, Jr, Molliver ME, Snyder SH. D-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci. 1997;17:1604–15. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 30.Verdoorn TA, Kleckner NW, Dingledine R. Rat brain N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Science. 1987;238:1114–6. doi: 10.1126/science.2825347. [DOI] [PubMed] [Google Scholar]
- 31.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and gluta-mate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–52. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams SM, Diaz CM, Macnab LT, Sullivan RK, Pow DV. Immuno-cytochemical analysis of D-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia. 2006;53:401–11. doi: 10.1002/glia.20300. [DOI] [PubMed] [Google Scholar]
- 33.Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int. 2002;77:94–108. doi: 10.1046/j.0022-7722.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 34.Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281:14151–62. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- 35.Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW. Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J Neuroinflammation. 2004;1:2. doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasuda E, Ma N, Semba R. Immunohistochemical evidences for localization and production of D-serine in some neurons in the rat brain. Neurosci Lett. 2001;299:162–4. doi: 10.1016/s0304-3940(01)01502-6. [DOI] [PubMed] [Google Scholar]
- 37.Wu S, Basile AS, Barger SW. Induction of serine racemase expression and D-serine release from microglia by secreted amyloid precursor protein (sAPP) Curr Alzheimer Res. 2007;4:243–51. doi: 10.2174/156720507781077241. [DOI] [PubMed] [Google Scholar]
- 38.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci USA. 2005;102:5606–11. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunlop DS, Neidle A. The origin and turnover of D-serine in brain. Biochem Biophys Res Commun. 1997;235:26–30. doi: 10.1006/bbrc.1997.6724. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi K, Hayashi F, Nishikawa T. In vivo evidence for the link between L- and D-serine metabolism in rat cerebral cortex. J Neurochem. 1997;69:1286–90. doi: 10.1046/j.1471-4159.1997.69031286.x. [DOI] [PubMed] [Google Scholar]
- 41.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96:13409–14. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolosker H, Sheth KN, Takahashi M, Mothet J-P, Brady RO, Jr, Ferris CD, Snyder SH. Purification of serine race-mase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci USA. 1999;96:721–5. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Miranda J, Santoro A, Engelender S, Wolosker H. Human serine racemase: molecular cloning, genomic organization and functional analysis. Gene. 2000;256:183–8. doi: 10.1016/s0378-1119(00)00356-5. [DOI] [PubMed] [Google Scholar]
- 44.Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW. D-amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci. 2007;26:1657–69. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia M, Liu Y, Figueroa DJ, Chiu CS, Wei N, Lawlor AM, Lu P, Sur C, Koblan KS, Connoly TM. Characterization and localization of a human serine racemase. Mol Brain Res. 2004;125:96–104. doi: 10.1016/j.molbrainres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Yamada K, Ohnishi T, Hashimoto K, Ohba H, Iwayama-Shigeno Y, Toyoshima M, Okuno A, Takao H, Toyota T, Minabe Y, Nakamura K, Shimizu E, Itokawa M, Mori N, Iyo M, Yoshikawa T. Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol Psychiatry. 2005;57:1493–503. doi: 10.1016/j.biopsych.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Yoshikawa M, Takayasu N, Hashimoto A, Sato Y, Tamaki R, Tsukamoto H, Kobayashi H, Noda S. The serine racemase mRNA is predominantly expressed in rat brain neurons. Arch Histol Cytol. 2007;70:127–34. doi: 10.1679/aohc.70.127. [DOI] [PubMed] [Google Scholar]
- 48.Wolosker H. NMDA receptor regulation by D-serine: new findings and perspectives. Mol Neurobiol. 2007;36:152–64. doi: 10.1007/s12035-007-0038-6. [DOI] [PubMed] [Google Scholar]
- 49.Martineau M, Baux G, Mothet JP. D-ser-ine signalling in the brain: friend and foe. Trends Neurosci. 2006;29:481–91. doi: 10.1016/j.tins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 50.De Miranda J, Panizzutti R, Foltyn VN, Wolosker H. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proc Natl Acad Sci USA. 2002;99:14542–7. doi: 10.1073/pnas.222421299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook SP, Galve-Roperh I, Martinez del Pozo A, Rodriguez-Crespo I. Direct calcium binding results in activation of brain serine racemase. J Biol Chem. 2002;277:27782–92. doi: 10.1074/jbc.M111814200. [DOI] [PubMed] [Google Scholar]
- 52.Shoji K, Mariotto S, Ciampa AR, Suzuki H. Regulation of serine racemase activity by D-serine and nitric oxide in human glioblastoma cells. Neurosci Lett. 2006;392:75–8. doi: 10.1016/j.neulet.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 53.Dunlop DS, Neidle A. Regulation of serine racemase activity by amino acids. Mol Brain Res. 2005;133:208–14. doi: 10.1016/j.molbrainres.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 54.Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc Natl Acad Sci USA. 2007;104:2950–5. doi: 10.1073/pnas.0611620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci USA. 2005;102:2105–10. doi: 10.1073/pnas.0409723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baumgart F, Mancheno JM, Rodriguez-Crespo I. Insights into the activation of brain serine racemase by the multi-PDZ domain glutamate receptor interacting protein, divalent cations and ATP. FEBS J. 2007;274:4561–71. doi: 10.1111/j.1742-4658.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 57.Fujii K, Maeda K, Hikida T, Mustafa AK, Balkissoon R, Xia J, Yamada T, Ozeki Y, Kawahara R, Okawa M, Huganir RL, Ujike H, Snyder SH, Sawa A. Serine race-mase binds to PICK1: potential relevance to schizophrenia. Mol Psychiatry. 2006;11:150–7. doi: 10.1038/sj.mp.4001776. [DOI] [PubMed] [Google Scholar]
- 58.Staudinger J, Lu J, Olson EN. Specific interaction of the PDZ domain protein PICK1 with the COOH terminus of protein kinase C-alpha. J Biol Chem. 1997;272:32019–24. doi: 10.1074/jbc.272.51.32019. [DOI] [PubMed] [Google Scholar]
- 59.Hikida T, Mustafa AK, Maeda K, Fujii K, Barrow RK, Saleh M, Huganir RL, Snyder SH, Hashimoto K, Sawa A. Modulation of D-serine levels in brains of mice lacking PICK1. Biol Psychiatry. 2008;63:997–1000. doi: 10.1016/j.biopsych.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hershko A, Heller H. Occurrence of a polyubiquitin structure in ubiquitin-protein conjugates. Biochem Biophys Res Commun. 1985;128:1079–86. doi: 10.1016/0006-291x(85)91050-2. [DOI] [PubMed] [Google Scholar]
- 61.Dumin E, Bendikov I, Foltyn VN, Misumi Y, Ikehara Y, Kartvelishvily E, Wolosker H. Modulation of D-serine levels via ubiq-uitin-dependent proteasomal degradation of serine racemase. J Biol Chem. 2006;281:20291–302. doi: 10.1074/jbc.M601971200. [DOI] [PubMed] [Google Scholar]
- 62.Ciriacks CM, Bowser MT. Measuring the effect of glutamate receptor agonists on extracellular D-serine concentrations in the rat striatum using online microdialysis-capillary electrophoresis. Neurosci Lett. 2006;393:200–5. doi: 10.1016/j.neulet.2005.09.080. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto A, Kanda J, Oka T. Effects of N-methyl-D-aspartate, kainate or veratridine on extracellular concentrations of free D-serine and L-glutamate in rat striatum: an in vivo microdialysis study. Brain Res Bull. 2000;53:347–51. doi: 10.1016/s0361-9230(00)00357-9. [DOI] [PubMed] [Google Scholar]
- 64.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–20. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 65.Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J. Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem. 2007;282:24185–97. doi: 10.1074/jbc.M700452200. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–53. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 67.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–40. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 68.Ribeiro CS, Reis M, Panizzutti R, de Miranda J, Wolosker H. Glial transport of the neuromodulator D-serine. Brain Res. 2002;929:202–9. doi: 10.1016/s0006-8993(01)03390-x. [DOI] [PubMed] [Google Scholar]
- 69.Helboe L, Egebjerg J, Moller M, Thomsen C. Distribution and pharmacology of alanine-serine-cysteine transporter 1 (asc-1) in rodent brain. Eur J Neurosci. 2003;18:2227–38. doi: 10.1046/j.1460-9568.2003.02966.x. [DOI] [PubMed] [Google Scholar]
- 70.Matsuo H, Kanai Y, Tokunaga M, Nakata T, Chairoungdua A, Ishimine H, Tsukada S, Ooigawa H, Nawashiro H, Kobayashi Y, Fukuda J, Endou H. High affinity D- and L-serine transporter Asc-1: cloning and dendritic localization in the rat cerebral and cerebellar cortices. Neurosci Lett. 2004;358:123–6. doi: 10.1016/j.neulet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 71.Rutter AR, Fradley RL, Garrett EM, Chapman KL, Lawrence JM, Rosahl TW, Patel S. Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating D-serine reuptake in the mouse CNS. Eur J Neurosci. 2007;25:1757–66. doi: 10.1111/j.1460-9568.2007.05446.x. [DOI] [PubMed] [Google Scholar]
- 72.Javitt DC, Balla A, Sershen H. A novel alanine-insensitive D-serine transporter in rat brain synaptosomal membranes. Brain Res. 2002;941:146–9. doi: 10.1016/s0006-8993(02)02557-x. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto N, Tomita U, Umino A, Nishikawa T. Uptake of D-serine by synaptosomal P2 fraction isolated from rat brain. Synapse. 2001;42:84–6. doi: 10.1002/syn.1103. [DOI] [PubMed] [Google Scholar]
- 74.Krebs HA. Metabolism of amino-acids: deamination of amino-acids. Biochem J. 1935;29:1620–44. doi: 10.1042/bj0291620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horiike K, Tojo H, Arai R, Nozaki M, Maeda T. D-amino-acid oxidase is confined to the lower brain stem and cerebellum in rat brain: regional differentiation of astrocytes. Brain Res. 1994;652:297–303. doi: 10.1016/0006-8993(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 76.Moreno S, Nardacci R, Cimini A, Ceru MP. Immunocytochemical localization of D-amino acid oxidase in rat brain. J Neurocytol. 1999;28:169–85. doi: 10.1023/a:1007064504007. [DOI] [PubMed] [Google Scholar]
- 77.Urai Y, Jinnouchi O, Kwak KT, Suzue A, Nagahiro S, Fukui K. Gene expression of D-amino acid oxidase in cultured rat astro-cytes: regional and cell type specific expression. Neurosci Lett. 2002;324:101–4. doi: 10.1016/s0304-3940(02)00184-2. [DOI] [PubMed] [Google Scholar]
- 78.Hashimoto A, Nishikawa T, Konno R, Niwa A, Yasumura Y, Oka T, Takahashi K. Free D-serine, D-aspartate and D-alanine in central nervous system and serum in mutant mice lacking D-amino acid oxidase. Neurosci Lett. 1993;152:33–6. doi: 10.1016/0304-3940(93)90476-2. [DOI] [PubMed] [Google Scholar]
- 79.Morikawa A, Hamase K, Inoue T, Konno R, Niwa A, Zaitsu K. Determination of free D-aspartic acid, D-serine and D-alanine in the brain of mutant mice lacking D-amino acid oxidase activity. J Chromatogr B Biomed Sci Appl. 2001;757:119–25. doi: 10.1016/s0378-4347(01)00131-1. [DOI] [PubMed] [Google Scholar]
- 80.Shoji K, Mariotto S, Ciampa AR, Suzuki H. Mutual regulation between serine and nitric oxide metabolism in human glioblastoma cells. Neurosci Lett. 2006;394:163–7. doi: 10.1016/j.neulet.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 81.Hamase K, Konno R, Morikawa A, Zaitsu K. Sensitive determination of D-amino acids in mammals and the effect of D-amino-acid oxidase activity on their amounts. Biol Pharm Bull. 2005;28:1578–84. doi: 10.1248/bpb.28.1578. [DOI] [PubMed] [Google Scholar]
- 82.Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M, Li P, Toney MD, Kartvelishvily E, Wolosker H. Serine racemase modulates intracellular D-serine levels through an alpha,beta-elimination activity. J Biol Chem. 2005;280:1754–63. doi: 10.1074/jbc.M405726200. [DOI] [PubMed] [Google Scholar]
- 83.Strisovsky K, Jiraskova J, Barinka C, Majer P, Rojas C, Slusher BS, Konvalinka J. Mouse brain serine racemase catalyzes specific elimination of L-serine to pyruvate. FEBS Lett. 2003;535:44–8. doi: 10.1016/s0014-5793(02)03855-3. [DOI] [PubMed] [Google Scholar]
- 84.Strisovsky K, Jiraskova J, Mikulova A, Rulisek L, Konvalinka J. Dual substrate and reaction specificity in mouse serine racemase: identification of high-affinity dicarboxylate substrate and inhibitors and analysis of the beta-eliminase activity. Biochemistry. 2005;44:13091–100. doi: 10.1021/bi051201o. [DOI] [PubMed] [Google Scholar]
- 85.Neidle A, Dunlop DS. Allosteric regulation of mouse brain serine racemase. Neurochem Res. 2002;27:1719–24. doi: 10.1023/a:1021607715824. [DOI] [PubMed] [Google Scholar]
- 86.McDonald JW, Penney JB, Johnston MV, Young AB. Characterization and regional distribution of strychnine-insensitive [3H]glycine binding sites in rat brain by quantitative receptor autoradiography. Neuroscience. 1990;35:653–68. doi: 10.1016/0306-4522(90)90336-3. [DOI] [PubMed] [Google Scholar]
- 87.Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. Functional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem. 1995;65:454–8. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- 88.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97:4926–31. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci USA. 2003;100:15194–9. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–84. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 91.Chen L, Muhlhauser M, Yang CR. Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2003;89:691–703. doi: 10.1152/jn.00680.2002. [DOI] [PubMed] [Google Scholar]
- 92.Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, Schmidt CJ, Bergeron R. Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiol. 2004;557:489–500. doi: 10.1113/jphysiol.2004.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martina M, ME BT, Halman S, Tsai G, Tiberi M, Coyle JT, Bergeron R. Reduced glycine transporter type 1 expression leads to major changes in glutamatergic neuro-transmission of CA1 hippocampal neurones in mice. J Physiol. 2005;563:777–93. doi: 10.1113/jphysiol.2004.080655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martina M, Krasteniakov NV, Bergeron R. D-serine differently modulates NMDA receptor function in rat CA1 hippocampal pyramidal cells and interneurons. J Physiol. 2003;548:411–23. doi: 10.1113/jphysiol.2002.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wake K, Yamazaki H, Hanzawa S, Konno R, Sakio H, Niwa A, Hori Y. Exaggerated responses to chronic nociceptive stimuli and enhancement of N-methyl-D-aspartate receptor-mediated synaptic transmission in mutant mice lacking D-amino-acid oxidase. Neurosci Lett. 2001;297:25–8. doi: 10.1016/s0304-3940(00)01658-x. [DOI] [PubMed] [Google Scholar]
- 96.Maekawa M, Watanabe M, Yamaguchi S, Konno R, Hori Y. Spatial learning and long-term potentiation of mutant mice lacking D-amino-acid oxidase. Neurosci Res. 2005;53:34–8. doi: 10.1016/j.neures.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 97.Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA. 2003;100:6789–94. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gustafson EC, Stevens ER, Wolosker H, Miller RF. Endogenous D-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J Neurophysiol. 2007;98:122–30. doi: 10.1152/jn.00057.2006. [DOI] [PubMed] [Google Scholar]
- 99.Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–50. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 100.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 101.Mothet JP, Rouaud E, Sinet PM, Potier B, Jouvenceau A, Dutar P, Videau C, Epelbaum J, Billard JM. A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell. 2006;5:267–74. doi: 10.1111/j.1474-9726.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 102.Gong XQ, Zabek RL, Bai D. D-serine inhibits AMPA receptor-mediated current in rat hippocampal neurons. Can J Physiol Pharmacol. 2007;85:546–55. doi: 10.1139/y07-040. [DOI] [PubMed] [Google Scholar]
- 103.Junjaud G, Rouaud E, Turpin F, Mothet JP, Billard JM. Age-related effects of the neuromodulator D-serine on neurotransmission and synaptic potentiation in the CA1 hippocampal area of the rat. J Neurochem. 2006;98:1159–66. doi: 10.1111/j.1471-4159.2006.03944.x. [DOI] [PubMed] [Google Scholar]
- 104.Krasteniakov NV, Martina M, Bergeron R. Subthreshold contribution of N-methyl-D-aspartate receptors to long-term potentia-tion induced by low-frequency pairing in rat hippocampal CA1 pyramidal cells. Neuroscience. 2004;126:83–94. doi: 10.1016/j.neuroscience.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 105.Rouaud E, Billard JM. D-cycloserine facilitates synaptic plasticity but impairs gluta-matergic neurotransmission in rat hippocampal slices. Br J Pharmacol. 2003;140:1051–6. doi: 10.1038/sj.bjp.0705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hashimoto A, Chiba S. Effect of systemic administration of D-serine on the levels of D- and L-serine in several brain areas and periphery of rat. Eur J Pharmacol. 2004;495:153–8. doi: 10.1016/j.ejphar.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 107.Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, Olsen L, Vestergaard B, Egebjerg J, Gajhede M, Traynelis SF, Kastrup JS. Ionotropic glutamate-like receptor delta2 binds D-serine and glycine. Proc Natl Acad Sci USA. 2007;104:14116–21. doi: 10.1073/pnas.0703718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–52. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 109.Billard JM. Ageing, hippocampal synaptic activity and magnesium. Magnes Res. 2006;19:199–215. [PubMed] [Google Scholar]
- 110.Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging. 1997;18:445–52. doi: 10.1016/s0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 111.Potier B, Poindessous-Jazat F, Dutar P, Billard JM. NMDA receptor activation in the aged rat hippocampus. Exp Gerontol. 2000;35:1185–99. doi: 10.1016/s0531-5565(00)00122-4. [DOI] [PubMed] [Google Scholar]
- 112.Yang S, Qiao H, Wen L, Zhou W, Zhang Y. D-serine enhances impaired long-term potentiation in CA1 subfield of hippocampal slices from aged senescence-accelerated mouse prone/8. Neurosci Lett. 2005;379:7–12. doi: 10.1016/j.neulet.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 113.Miyoshi R, Kito S, Doudou N, Nomoto T. Age-related changes of strychnine-insensitive glycine receptors in rat brain as studied by in vitro autoradiography. Synapse. 1990;6:338–43. doi: 10.1002/syn.890060405. [DOI] [PubMed] [Google Scholar]
- 114.Nagata Y, Uehara T, Kitamura Y, Nomura Y, Horiike K. D-serine content and D-[3H]serine binding in the brain regions of the senescence-accelerated mouse. Mech Ageing Dev. 1998;104:115–24. doi: 10.1016/s0047-6374(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 115.Aura J, Riekkinen P., Jr Pre-training blocks the improving effect of tetrahydroaminoacridine and D-cycloserine on spatial navigation performance in aged rats. Eur J Pharmacol. 2000;390:313–8. doi: 10.1016/s0014-2999(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 116.Baxter MG, Lanthorn TH, Frick KM, Golski S, Wan RQ, Olton DS. D-cycloser-ine, a novel cognitive enhancer, improves spatial memory in aged rats. Neurobiol Aging. 1994;15:207–13. doi: 10.1016/0197-4580(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 117.Thompson LT, Disterhoft JF. Age- and dose-dependent facilitation of associative eyeblink conditioning by D-cycloserine in rabbits. Behav Neurosci. 1997;111:1303–12. doi: 10.1037//0735-7044.111.6.1303. [DOI] [PubMed] [Google Scholar]
- 118.Billard JM, Rouaud E. Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. Eur J Neurosci. 2007;25:2260–8. doi: 10.1111/j.1460-9568.2007.05488.x. [DOI] [PubMed] [Google Scholar]
- 119.Krug AW, Volker K, Dantzler WH, Silbernagl S. Why is D-serine nephrotoxic and alpha-aminoisobutyric acid protective? Am J Physiol Renal Physiol. 2007;293:F382–90. doi: 10.1152/ajprenal.00441.2006. [DOI] [PubMed] [Google Scholar]
- 120.Lewis WC, Calden G, Thurston JR, Gilson WE. Psychiatric and neurological reactions to cycloserine in the treatment of tuberculosis. Dis Chest. 1957;32:172–82. doi: 10.1378/chest.32.2.172. [DOI] [PubMed] [Google Scholar]
- 121.Williams RE, Lock EA. D-serine-induced nephrotoxicity: possible interaction with tyrosine metabolism. Toxicology. 2004;201:231–8. doi: 10.1016/j.tox.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 122.Tannenberg RK, Scott HL, Westphalen RI, Dodd PR. The identification and characterization of excitotoxic nerve-endings in Alzheimer disease. Curr Alzheimer Res. 2004;1:11–25. doi: 10.2174/1567205043480591. [DOI] [PubMed] [Google Scholar]
- 123.Lipton SA. The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res. 2005;2:155–65. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- 124.Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system – too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 125.Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem. 2007;101:1205–13. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vesce S, Rossi D, Brambilla L, Volterra A. Glutamate release from astrocytes in physiological conditions and in neurode-generative disorders characterized by neuroinflammation. Int Rev Neurobiol. 2007;82:57–71. doi: 10.1016/S0074-7742(07)82003-4. [DOI] [PubMed] [Google Scholar]
- 127.Wu S, Barger SW. Induction of serine racemase by inflammatory stimuli is dependent on AP-1. Ann N Y Acad Sci. 2004;1035:133–46. doi: 10.1196/annals.1332.009. [DOI] [PubMed] [Google Scholar]
- 128.Heese K, Nagai Y, Sawada T. Induction of rat L-phosphoserine phosphatase by amyloiD-beta (1–42) is inhibited by interleukin-11. Neurosci Lett. 2000;288:37–40. doi: 10.1016/s0304-3940(00)01197-6. [DOI] [PubMed] [Google Scholar]
- 129.Katsuki H, Nonaka M, Shirakawa H, Kume T, Akaike A. Endogenous D-serine is involved in induction of neuronal death by N-methyl-D-aspartate and simulated ischemia in rat cerebrocortical slices. J Pharmacol Exp Ther. 2004;311:836–44. doi: 10.1124/jpet.104.070912. [DOI] [PubMed] [Google Scholar]
- 130.Shleper M, Kartvelishvily E, Wolosker H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005;25:9413–7. doi: 10.1523/JNEUROSCI.3190-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 132.Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9:329–36. doi: 10.1007/s11920-007-0041-7. [DOI] [PubMed] [Google Scholar]
- 133.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 134.Millan MJ. N-methyl-D-aspartate receptors as a target for improved antipsychotic agents: novel insights and clinical perspectives. Psychopharmacology. 2005;179:30–53. doi: 10.1007/s00213-005-2199-1. [DOI] [PubMed] [Google Scholar]
- 135.Coyle JT. Substance use disorders and Schizophrenia: a question of shared glutamatergic mechanisms. Neurotox Res. 2006;10:221–33. doi: 10.1007/BF03033359. [DOI] [PubMed] [Google Scholar]
- 136.Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 137.Goltsov AY, Loseva JG, Andreeva TV, Grigorenko AP, Abramova LI, Kaleda VG, Orlova VA, Moliaka YK, Rogaev El. Polymorphism in the 5’-promoter region of serine racemase gene in schizophrenia. Mol Psychiatry. 2006;11:325–6. doi: 10.1038/sj.mp.4001801. [DOI] [PubMed] [Google Scholar]
- 138.Morita Y, Ujike H, Tanaka Y, Otani K, Kishimoto M, Morio A, Kotaka T, Okahisa Y, Matsushita M, Morikawa A, Hamase K, Zaitsu K, Kuroda S. A genetic variant of the serine racemase gene is associated with schizophrenia. Biol Psychiatry. 2007;61:1200–3. doi: 10.1016/j.biopsych.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 139.Boks MP, Rietkerk T, van de Beek MH, Sommer IE, de Koning TJ, Kahn RS. Reviewing the role of the genes G72 and DAAO in glutamate neurotransmission in schizophrenia. Eur Neuropsychopharmacol. 2007;17:567–72. doi: 10.1016/j.euroneuro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 140.Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin-Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz-Fuertes R, Meguenni S, Aurich-Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA. 2002;99:13675–80. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shinkai T, De Luca V, Hwang R, Müller DJ, Lanktree M, Zai G, Shaikh S, Wong G, Sicard T, Potapova N, Trakalo J, King N, Matsumoto C, Hori H, Wong AH, Ohmori O, Macciardi F, Nakamura J, Kennedy JL. Association analyses of the DAOA/G30 and D-amino-acid oxidase genes in schizophrenia: further evidence for a role in schizophrenia. Neuromolecular Med. 2007;9:169–77. doi: 10.1007/BF02685890. [DOI] [PubMed] [Google Scholar]
- 142.Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–86. [PMC free article] [PubMed] [Google Scholar]
- 143.Yoshikawa M, Ito K, Maeda M, Akahori K, Takahashi S, Jin XL, Matsuda M, Suzuki T, Oka T, Kobayashi H, Hashimoto A. Activation of supraspinal NMDA receptors by both D-serine alone or in combination with morphine leads to the potentiation of antinociception in tailflick test of rats. Eur J Pharmacol. 2007;565:89–97. doi: 10.1016/j.ejphar.2007.02.042. [DOI] [PubMed] [Google Scholar]


