Abstract
Adhesion of cancer cells to endothelium is considered an essential step in metastasis. However, we have shown in a previous study that when rat colon cancer cells are administered to the vena portae, they get stuck mechanically in liver sinusoids. Then, endothelial cells retract rapidly and cancer cells bind to hepatocytes. We investigated the molecular nature of these interactions between colon cancer cells and hepatocytes. Cancer cells in coculture with hepatocytes became rapidly activated with distinct morphological changes. Cancer cells formed long cytoplasmic protrusions towards hepatocytes in their close vicinity and these protrusions attached to microvilli of hepatocytes. Then, adhering membrane areas were formed by both cell types. Integrin subunits αv, α6 and β1 but not αL, β2, β3 and CD44 and CD44v6 were expressed on the cancer cells. In conclusion, colon cancer cells show an active behaviour to bind to hepatocytes, likely involving the integrin subunits av, a6 and B1, indicating that early events in colon cancer metastasis in liver are distinctly different than assumed thus far.
Keywords: colon cancer, metastasis, liver, hepatocyte, adhesion, integrin, CD44
Introduction
Colorectal cancer patients mainly die of metastatic disease rather than the primary tumour. Key events in metastasis are increased proteolytic activity, increased cell motility and altered expression of adhesion molecules [1–5]. Cell adhesion molecules can be classified into five major groups [3, 6]: integrins, selectins, cadherins, members of the immunoglobulin superfamily and other molecules. They all have been suggested to be involved in cancer progression and metastasis. Two types of adhesion molecules, the integrins and the CD44 hyaluronic acid receptors, are of particular importance with respect to the development of colon cancer and metastasis [7–13]. It is a generally accepted concept that adhesion of cancer cells in the capillary bed of a distant organ is based on interactions between the endothelium and cancer cells [1, 14–17].
The contrasting concept is that of mechanical entrapment of cancer cells in the first capillary bed that the cells encounter [18–21]. We have evidence that both concepts are at least partly correct because we have previously shown that colon cancer cells (CC531s) administered to the portal vein of rats bind to hepatocytes and not to sinusoidal endothelial cells [18]. It was observed that the cancer cells are arrested abruptly in sinusoids, endothelial cells retract locally within 30 min and specific molecular bridges are then formed between cancer cells and hepatocytes [18]. In the present study, we investigated these molecular interactions between these cell types in vitro and in vivo and focused on the involvement of integrins and CD44 variants.
Materials and methods
Animals
For all experiments, male syngeneic WAG-Rij rats of 200–220 g (Broekman, Someren, The Netherlands) were used, kept under constant environmental conditions with food and water ad libitum. Animal care was performed in accordance with the guidelines of the University of Amsterdam.
CC531s cancer cell line, culture and cytospins
An established colon carcinoma cell line, CC531s was cultured at 37°C as monolayers in RPMI-1640 Dutch Modification without L-glutamine (Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) foetal calf serum, 2 mM glutamine, 100 IU penicillin/ml and 100 mg streptomycin/ml (all from Invitrogen). Cells were washed with phosphate-buffered saline (PBS) and after detachment with the use of trypsin (0.05% w/v; Invitrogen) and ethylenediaminetetraacetic acid (EDTA) (0.02% w/v) in PBS and centrifu-gation (250 g, room temp, 5 min), single cell suspensions were obtained with a viability of at least 95%[22].
To investigate effects of trypsinization on surface molecules of the cells, cytospins of cancer cells were made by centrifugation of 250 μl cell suspension onto clean glass slides with a Hettich 1502 centrifuge (Hettich Zentrifugen, Tüv, Germany) at 400 g. Short-term in vitro cell cultures of 1, 2 and 4 hrs were made by culturing cancer cells on sterile clean glass slides. Long-term cancer cell cultures were made on clean round glass slides for up to 3 days. After gentle washing with PBS, cells were air-dried for 1 hr and stored at −20°C.
Cancer cells cultured on glass slides for 3 days were incubated in the presence of isolated hepatocytes [23]. After 1 hr, non-adhering cells were removed by washing with PBS. Then, attached cells were prepared for electron microscopy. Freshly isolated hepatocytes do not adhere to glass slides. Therefore, we could not perform the experiments the other way around as would be more closely resembling the in vivo situation.
Induction of tumours in rat liver
To induce tumours in livers of rats, the animals were anaesthetized by intraperitoneal injection of a mixture of 1 ml Hypnorm, 1 ml Midazolam and 2 ml water, 0.27 ml per 100 g body weight) and after a small midline incision, single cell suspensions of 2.5 × 106 CC531 s-eGFP cells in 0.5 ml PBS were injected into the portal vein. The animals were sacrificed at 4 hrs, 1 day, 2 days, 3 days or 3 weeks after injection of the cancer cells. The livers were removed immediately, and tumour-containing liver blocks were dissected and snap-frozen in liquid nitrogen for storage at -80°C until further use [18, 22].
Serial sections (8 μm thick) of liver specimens containing colon cancer tumours were cut with a motor-driven cryostat with rotary retracting microtome (Bright, Huntingdon, UK) at a cabinet temperature of -24°C. Sections were collected on clean glass slides at room temperature, and stored at -20°C until use.
Electron microscopy
CC531s cells cultured on glass slides in the presence of hepatocytes were fixed with 4% (v/v) paraformaldehyde and 1% (v/v) glutaraldehyde in 100 mM cacodylate buffer, pH 7.4, for 2 hrs at 4°C. After fixation, cells were rinsed with 100 mM cacodylate buffer, pH 7.4, for 40 min and post-fixed with 1% 0s04 (Merck, Darmstadt, Germany) in 100 mM cacodylate buffer, pH 7.4, for 1 hr at 4°C. Afterwards, samples were thoroughly rinsed with bidistilled water, dehydrated and embedded in epoxy resin LX-112 (Ladd,
Burlington, VT) according to standard procedures. Ultrathin sections (80 nm thick) were cut on an Ultracut E ultramicrotome (Leica Microsystems, Wetzlar, Germany) perpendicular to the glass slides. The ultrastructure was studied with an EM 420 transmission electron microscope (Philips, Eindhoven, The Netherlands). Areas of adhesion between colon cancer cells and hepatocytes were investigated with electron tomography. Electron tomographic reconstructions were made from 200 nm-thick sections, using two perpendicular tilt series (+557–55°, increment 5°) [24. 25].
Western blotting
For Western blotting, CC531 s cells were scraped to avoid the use of trypsin and were homogenized. Homogenates of 3-weeks-old liver tumours after being dissected from surrounding liver tissue were used as well. The samples were sonicated for 3 × 5 sec. at 14 A, in a concentration of 0.25 g per ml Eekhout buffer (1 M NaCI, 0.01% (v/v) Triton X-100 and 1 μM ZnCl2 in 10 mM sodium cacodylate buffer, pH 6.0), and stirred overnight at 4°C. After brief centrifugation at 10,000 g, 1 part of 3x concentrated Laemli loading buffer (30% (v/v) glycerol, 6% (w/v) sodium dodecyl sulphate, 0.3% (v/v) brome phenol blue, 10 mM dithiothreitol in 150 mM Tris/HCI, pH 6.8 was added to two parts supernatant. The samples were heated for 30 min. at 56°C or for 5 min. at 100°C and electrophoresis was performed on 10% SDS-polyacrylamide gel. Proteins were blotted onto nitrocellulose membranes (Schleicher & Schuel, Dassel, Germany) and the blots were stained immunohistochemically for various adhesion molecules (Table 1) according to standard procedures. Non-specific binding was blocked with 5% protifar in PBS-Tween for 1 hr. Incubations with primary antibodies were performed in the presence of 2.5% protifar in PBS-Tween. Control incubations were performed in the absence of primary antibodies.
Table 1.
Primary and secondary antibodies used for immunostaining and Western blotting
| Primary antibodies | |||
|---|---|---|---|
| Antigen | Isotype | Dilution | Origin |
| αL(CD11a) | Mouse | 1:50 IH | Instruchemie, Hilversum, The Netherlands |
| αv(CD51) | Arm. hamster | 1:250 WB | Pharmingen, San Diego, CA |
| α6; C28 (CD49f) | Arm. hamster | 1:10 IH 1:60 WB | Kind gift Dr. Wijnands, NKI Amsterdam, The Netherlands |
| β1 (CD29) | Arm. hamster | 1:200 IH 1:1000 WB | Pharmingen |
| β2(CD18) | Mouse | 1:50 IH | Instruchemie |
| β3(CD61) | 1:50 IH | Pharmingen | |
| CD44 | Mouse | 1:1000 IH 1:4000 WB | Pharmingen |
| CD44v6;1.1ASML | Mouse | 1:300 IH 1:800 WB | Kind gift Dr. Sleeman, Institut für Toxikologie und Genetik, Karlsruhe, Germany |
| Ulex Europaeus agglutinin 1 (UEA-1) | Lectin | 1:100 IH | Dakopatts, Glostrup, Denmark |
| UEA-HRP | Lectin | 1:70 IH | Dakopatts |
| Secondary antibodies | |||
|---|---|---|---|
| Antibody | Label | Dilution | Origin |
| Rabbit-anti-mouse IgG | PO | 1:200 IH 1:1000 WB | Dakopatts |
| Goat-anti-Arm. hamster IgG | PO | 1:120 IH 1:500 WB | Jackson, Baltimore, MA |
| Goat-anti-mouse IgG | FITC | 1:100 IH | Jackson |
| Goat-anti-Arm. hamster IgG | FITC | 1:100 IH | Jackson |
| Swine-anti-rabbit IgG | TRITC | 1:40 IH | Dakopatts |
| Rabbit-anti-mouse | TRITC | 1:200 IH | Dakopatts |
| Rabbit-anti-UEA-1 | PO | 1:60 IH | Dakopatts |
Abbreviations: Arm. hamster, Armenian hamster;
UEA-1, Ulex europaeus agglutinin-1;
FITC, fluorescein isothiocyanate;
TRITC, tetramethyl rhodamine isothiocyanate;
IH, immunohistochemistry and cytochemistry;
WB, Western blotting;
PO, peroxidase.
Immunohistochemistry and immunocytochemistry
Sections of livers containing tumours and colon cancer cells after culture were air-dried for at least 1 hr before fixation in acetone or 4% paraformaldehyde in PBS for 10 min at room temperature. After acetone fixation, sections were air-dried for 10 min before incubation with primary antibodies (Table 1). All incubations were performed in PBS, containing 0.2% bovine serum albumin and 1% normal rat serum to block non-specific binding, in a moist dark chamber for 60 min at room temperature.
Visualization of the antibodies bound to liver sections and cancer cells was performed either by coloured final reaction product to produce permanent preparations or by fluorescence for confocal microscopy.
Peroxidase-labelled secondary antibodies (Table 1) were visualized using 3-amino-9-ethylcarbazole (AEC) as peroxidase substrate (20 mg AEC in 5 ml dimethylformamide and 95 ml acetate buffer, pH 4.9, containing 0.01% hydrogen peroxide). The peroxidase reaction was performed for 10 min. at room temperature. Sections were rinsed in water, counter-stained with haematoxylin and mounted in glycerin-gelatin. The result was evaluated using standard light microscopy.
Fluorescence-labelled secondary antibodies (Table 1) were visualized with a Leica DM IRBE confocal laser scanning microscope SP2-A0BS or with a Leica DMRA wide-field fluorescense microscope (Leica). Nuclei were counterstained with 4,6'-diamidino-2-phenylindole (DAPI; 1 μg/ml; Sigma).
Results
Electron microscopy
Colon cancer cells cultured on glass slides were flat with few pseudopodia (Fig. 1A). The morphology of colon cancer cells hardly changed when hepatocytes were not in the direct vicinity. When hepatocytes were approaching cancer cells, cytoplasmic protrusions of cancer cells in the direction of hepatocytes were formed rapidly (within an hour) and cancer cells became more bulky (Fig. 1B). Contacts between cancer cells and hepatocytes started as contacts between protrusions of cancer cells and microvilli of hepatocytes (Fig. 1C) and resulted in large areas of parallel running plasma membranes (Fig. 1D).
Fig 1.
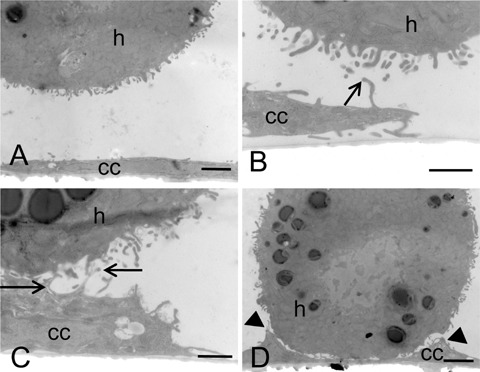
Electron micrographs of colon cancer cells and hepatocytes in coculture for 1 hr. CC531s cells were cultured on glass for 3 days and suspensions of hepatocytes were added. When hepatocytes were not in the vicinity, cancer cells (cc) were flattened with few small protrusions only and hepatocytes (h) were rounded (A). When hepatocytes were in close vicinity of cancer cells, cancer cells rapidly became bulky and formed protrusions in the direction of the hepatocytes (B). Contact between cancer cells and hepatocytes was established between cancer cell protrusions and microvilli of hepatocytes (arrows; B, C) and then stretches of parallel-running membranes were made (arrow heads; D). Bars = 2 pm (A, C) or 1 μmΊ (B, D).
Electron tomography revealed electron dense molecular contacts between cancer cells and hepatocytes (Fig. 2) similar to the contacts that have been observed previously in vivo[18]. These molecular contacts were present between the ridges of parallel running membranes (Fig. 1D), but not between pseudopodia of cancer cells and microvilli of hepatocytes (Fig. 1C).
Fig 2.

Electron micrograph (A) and electron tomographic reconstruction (B) of the intercellular space between a cultured colon cancer cell and a suspended hepatocyte after 1 hr coculture showing electron-dense molecular contacts. The reconstructed area (0.45 μm x 0.28 μm) is indicated. Thickness of the ultrathin section was 120 nm. Bar = 1 μm.
Western blotting of liver metastases and cultured colon cancer cells
Western blotting showed that cancer cells that were scraped after culture and 3-weeks-old tumours expressed integrin subunits av and β1, and CD44 and its splice variant CD44v6 (Fig. 3). Blotting of a6 was not possible with the antibody available, whereas blotting of αl, β2 and β3 was not performed because immunohistochemistry did not reveal any positivity on CC531s cells. Control incubations in the absence of primary antibodies were always negative.
Fig 3.
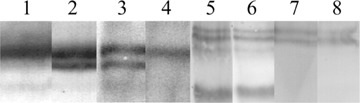
Western blots of homogenates of 3-weeks-old tumours (lanes 1, 3, 5 and 7) and cultured cancer cells (lanes 2, 4, 6 and 8) for the integrin subunits αv (1 and 2), β1 (3 and 4), CD44 (5 and 6) and CD44v6 (7 and 8). In culture, cancer cells express an av isoform which is lost during tumour progression, whereas in tumours a β1 isoform is expressed which is absent in cultured cells.
The anti-αν antibody revealed a band of 150 kD in both cultured cancer cells and tumours. A second band with a lower molecular weight was present in homogenates of cultured cells but not of tumours indicating expression of an αν-isoform by cancer cells in culture that is lost during tumour progression. Western blots stained with anti-βΐ antibodies revealed a 130 kD band both in cultured cancer cells and tumours. Tumour homogenates showed an extra β1 band that was absent in cultured cells. This was likely expressed by stromal cells. Staining of CD44 revealed in both cultured cells and tumours an 85 kD band indicating the CD44s form, and two isoforms of approximately 180 kD. These two 180 kD bands and not the 85 kD band were stained with the anti-CD44v6 antibody indicating that 180 kD isoforms contained the v6 domain (Fig. 3).
Immunohistochemistry of colon cancer tumours in liver
At 3 weeks after intraportal administration of CC531s cells, moderately-differentiated colon cancer tumours of 2–5 mm in diameter were present in the livers. Cancer cells were arranged in acinar structures surrounded by stromal cells (Fig. 4). The α6 and β1 integrin subunits as well as CD44 and CD44v6 were abundantly present in these tumours. The subunits al_, β2 and β3 could not be detected on cancer cells. The αL and β2 (which are the α and β chain of lymphocyte function-associated antigen-1 (LFA-1)) antibodies stained leukocytes (data not shown). Sections of rat spleen were used as positive control for specificity of the β3 antibody, and revealed intense staining of lymphocytes (data not shown). Immunolocaliza-tion of αv did not succeed with the antibodies available.
Fig 4.
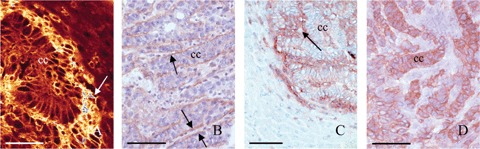
Localization of B1 (A; bar = 40 μmη),α6 (B; bar = 60 μmη) integrin subunits and CD44 (C; bar = 150 μπι) and its splice variant CD44v6 (D; bar = 60 μπι) in cryostat sections of rat liver containing 3-weeks-old tumours. The α1 integrin subunit is localized at the plasma membrane of cancer cells (cc) and is abundantly present in tumour stroma (arrow) (A). Localization of the a6 integrin subunit is restricted to the basal side of cancer cells in acini (arrows) (B). CD44 is localized at the plasma membrane of cancer cells (cc) and in tumour stroma (arrow) (C), whereas CD44v6 is restricted to cancer cells (D).
CD44 adhesion molecule showed similar staining patterns as the β1 integrin subunit (Figs. 4A, C and 5F) in stroma and peri-cellularly on cancer cells. Staining of CD44v6 was restricted to cancer cells showing a pericellular localization pattern (Figs. 4D and 51). Expression of α6 was restricted to the basal side of cancer cells (Fig. 5C). Tumours at 4 hrs to 3 days after cancer cell inoculation showed similar staining patterns.
Fig 5.
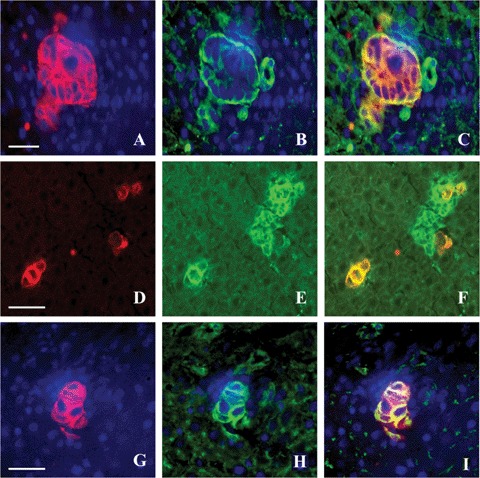
Double staining of colon cancer cells using Ulex Europaeus agglutinin (UEA) staining (A; bar = 30μm, D; bar = 50 μm and G; bar = 30 μm) and α6 (B), CD44 (E) and CD44v6 (H) staining and their respective overlays (C, F and I) in 3-days-old tumours in rat livers. Yellow represents colocalization of green and red. Cancer cells express a6 at the basal side (C), CD44 is expressed by cancer cells and stromal cells in tumours (F), and CD44v6 is exclusively expressed by cancer cells (I).
Immunocytochemistry of cultured colon cancer cells
CC531 s cells expressed α6 and β1 integrin subunits but not αL, β2 and β3 subunits (Table 2). CD44 and its CD44v6 splice variant were expressed only after longer periods of culture (Fig. 6). Apparently, trypsin treatment during cell harvest resulted in the proteolytic removal of the CD44 and CD44v6 epitope. Staining intensities were rather homogenous, with clusters of cells staining slightly more intense. Antigens were localized pericellularly, suggesting localization of the adhesion molecules at the plasma membrane (Fig. 7).
Table 2.
Immunohistochemical staining of integrin subunits and CD44 isoforms on rat CC531s colon cancer cells in cytospins and after various periods of culture
| Molecule | Cytospin | 1 hr | 2hrs | 4 hrs | 3 days |
|---|---|---|---|---|---|
| αL | - | - | - | - | - |
| α6 | + | + | + | + | +++ |
| β1 | + | + | ++ | ++ | +++ |
| β2 | - | - | - | - | - |
| β3 | - | - | - | - | - |
| CD44 | - | - | - | + | +++ |
| CD44v6 | - | - | - | ± | +++ |
-, No staining; ±, heterogeneous staining; + to +++, mild to abundant staining.
Fig 6.
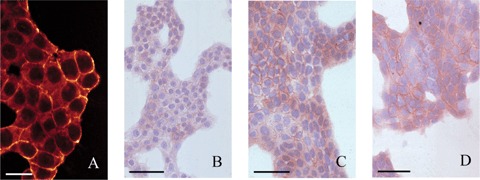
Colon cancer cells cultured for 3 days stained for B1 (A; bar = 20 μm), α6 (B; bar = 50 μm), CD44 (C; bar = 40 μm) and CD44v6 (D; bar = 40 μm). All antigens were localized at the plasma membrane of cancer cells.
Fig 7.
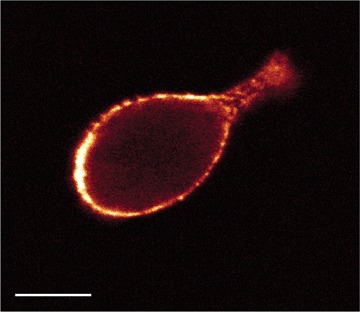
Optical section obtained with confocal microscopy of a cultured colon cancer cell stained for CD44. Localization of CD44 was restricted to the cell surface. Bar = 40 μηι.
Discussion
We have previously shown that colon cancer cell arrest in rat liver sinusoids is due to size restriction rather than adhesion to endothelium [18]. We did not find a single cancer cell in the per-fusate of livers collected from the hepatic vein indicating that entrapment in the sinuoids was absolute. This would not happen when arrest was due to selective adhesion to the sinusoidal endothelium [26, 27].
After cancer cell arrest, endothelial cells retract and cancer cells have direct molecular interactions with hepatocytes in vivo[18]. Retraction of endothelium occurs rapidly (within 30 min) after cancer cell arrest. Other studies have shown that cancer cells can disrupt the endothelial barrier in capillaries either by induction of apoptosis [26–31], or secretion of Vascular endothelial growth factor (VEGF) [32], reactive oxygen species [33, 34], arachidonic acid metabolites such as 12(S)-hydroxyeicosate-traenoic acid [35, 36] or the induction of inflammatory processes [37]. However, VEGF, reactive oxygen species and arachidonic acid metabolites are secreted by cancer cells after adhering to endothelial cells and apoptosis is not induced within 30 min [38]. Inflammatory responses and/or interactions between cancer cells and leukocytes or platelets [37] have never been observed either in our previous study [18, 38] or in the present study. Therefore, in our model endothelial retraction is likely due to mechanical stress caused by the cancer cells arrested in the sinusoids. Endothelial cells are very sensitive to mechanical stress [39].
The Electron Microscopical (EM) analysis of cocultures of colon cancer cells and hepatocytes in vitro shows an active behaviour of cancer cells rapidly induced by hepatocytes that are in close vicinity. This active behaviour ultimately results in large membrane areas of cell-cell contacts. These membrane areas are characterized by protein bridges that closely resembled the structures that have been observed between cancer cells and hepatocytes in vivo[18]. Adhesion molecules are likely involved. It has been hypothesized that colon carcinoma cells may use similar mechanisms that are involved in invasion and migration of leukocytes [2, 3]. In the present study, neither cultured cancer cells nor colon cancer tumours in rat liver at different stages of development expressed either subunits al_ or 32 of LFA-1. Therefore, it is unlikely that these cancer cells use ‘immune cell’ adhesion molecules including αLβ2, αdβ2, αmβ2 and αxβ2 for their interactions with hepatocytes. It cannot be excluded that very late antigen-4 (VLA-4, also known as integrin α4β1) was involved. VLA-4 has been shown recently to play an essential role in the formation of pre-metastatic niches by bone marrow progenitor cells [40].
Furthermore, 33 was not expressed on cultured cancer cells or in the tumours at any stage of development. This suggests that the integrins αvβ3 and αlbβ3 are not involved either. On the other hand, αν, α6, β1, CD44 and CD44v6 were expressed on cultured cancer cells and in metastases as shown by immunohistochem-istry and Western blotting. Involvement of α6, and/or CD44v6 in cancer cell adhesion has been reported previously [12, 13, 41–45].
We evaluated expression of α6, β1, CD44 and CD44v6 on cancer cells as they were administered to rats, namely after trypsinization. It was found that α6 and β1 were still present at the plasma membrane after trypsinization, whereas CD44 and CD44v6 protein could not be detected. Staining of CD44 and CD44v6 reappeared weakly after 4 hrs of culture (Table 2). Apparently, the integrin subunits α6 and β1 were resistant to trypsinization and CD44 and CD44v6 were not. This implies that subunits a6 and 61 and not CD44 and CD44v6 can be involved in immediate interactions between hepatocytes and cancer cells after initial cancer cell arrest in the sinusoids. Enns etal.[14, 15] showed that α6β1, α6β4, but not ανβ3 integrins are crucial for cancer cell adhesion in liver sinuoids. Others did find a role for αvβ3 in liver metastasis [46] and 61 integrins in the binding of colon cancer cells to laminin [47]. It remains to be established whether αv, α6 and β1 integrin subunits are directly involved in the interactions between colon cancer cells and hepatocytes but integrins can participate in cell-cell interactions, for example, when members of the immunoglobulin superfamily or Extracellular Matrix molecules are attached to the hepatocytes such as heparins [48] or fibronectin [49]. As a consequence of binding of αv, α6 and β1-containing integrins, other processes involved in metastasis such as proteolysis and cell motility can be initiated [4, 5, 50, 51].
In conclusion, we have previously found evidence for colon cancer cell dissemination in liver in vivo due to size restriction in the sinusoids and subsequent adhesion between cancer cells and hepatocytes and not the endothelium. In the present study, we further elucidated these molecular interactions between cancer cells and hepatocytes in situ and in vitro. Our findings provide evidence that both concepts for cancer cell arrest in a capillary bed, mechanical entrapment due to size restriction and arrest due to adhesive interactions are correct and are not mutually exclusive.
Acknowledgments
This study was sponsored by the Dutch National Computing Facilities Foundation for the use of supercomputing facilities with financial support from the Netherlands Organization for Scientific Research (NWO). The careful preparation of the manuscript by Trees Pierik and the figures by Jan Peeterse are gratefully acknowledged.
References
- 1.Fidler J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2002;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 3.Haier J, Nicolson GL. Cell biology and clinical implications of adhesion molecules in colorectal diseases: colorectal cancers, infections and inflammatory bowel diseases. Clin Exp Metast. 2000;18:623–38. doi: 10.1023/a:1013114200750. [DOI] [PubMed] [Google Scholar]
- 4.Lah TT, Duran Alonso MB, Van Noorden CJF. Antiprotease therapy in cancer: hot or not? Expert Opin Biol Ther. 2006;6:257–79. doi: 10.1517/14712598.6.3.257. [DOI] [PubMed] [Google Scholar]
- 5.Deryugina El, Quigley JP. Matrix metallo-proteineases and tumor metastasis. Cancer Metast. Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 6.Hynes RO. Cell adhesion: old and new questions. Trends Cell Biol. 1999;9:M33–7. [PubMed] [Google Scholar]
- 7.Ohnishi Y, Fujii H, Murakami K, Sakamoto T, Tsukada K, Fujimaki M, Kojima M, Saiki I. A new pseudo-peptide analogue of the Arg-Gly-Asp (RGD) sequence inhibits liver metastasis of colon 26-L5 carcinoma cells. Cancer Lett. 1998;124:157–63. doi: 10.1016/s0304-3835(97)00473-4. [DOI] [PubMed] [Google Scholar]
- 8.Harada N, Mizoi T, Kinouchi M, Hoshi K, Ishii S, Shiiba K, Sasaki I, Matsuno S. Introduction of antisense CD44s cDNA down-regulates expression of overall CD44 isoforms and inhibits tumor growth and metastasis in highly metastatic colon carcinoma cells. IntJ Cancer. 2001;91:67–75. doi: 10.1002/1097-0215(20010101)91:1<67::aid-ijc1011>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Fujisaki T, Tanaka Y, Fujii K, Mine S, Saito K, Yamada S, Yamashita U, Irimura T, Eto S. CD44 stimulation induces inte-grin-mediated adhesion of colon cancer cell lines to endothelial cells by up-regulation of integrins and c-Met and activation of integrins. Cancer Res. 1999;59:4427–34. [PubMed] [Google Scholar]
- 10.Isozaki H, Ohyama T, Mabuchi H. Expression of cell adhesion molecule CD44 and sialyl Lewis A in gastric carcinoma and colorectal carcinoma in association with hepatic metastasis. Int J Oncol. 1998;13:935–42. doi: 10.3892/ijo.13.5.935. [DOI] [PubMed] [Google Scholar]
- 11.Reeder JA, Gotley DC, Walsh MD, Fawcett J, Antalis TM. Expression of anti-sense CD44 variant 6 inhibits colorectal tumor metastasis and tumor growth in a wound environment. Cancer Res. 1998;58:3719–26. [PubMed] [Google Scholar]
- 12.Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, Van Den Berg FM, Ponta H, Herrlich P, Pals ST. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993;53:4754–6. [PubMed] [Google Scholar]
- 13.Coppola D, Hyacinthe M, Fu L, Cantor AB, Karl R, Marcet J, Cooper DL, Nicosia SV, Cooper HS. CD44V6 expression in human colorectal carcinoma. Hum Pathol. 1998;29:627–35. doi: 10.1016/s0046-8177(98)80014-2. [DOI] [PubMed] [Google Scholar]
- 14.Enns A, Korb T, Schlueter K, Gassmann P, Spiegel HU, Senninger B, Mitjans F, Haier J. o^5-lntegrins mediate early steps of metastasis formation. Eur J Cancer. 2005;41:1065–72. doi: 10.1016/j.ejca.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Enns A, Gassmann P, Schlueter K, Korb T, Spiegel HU, Senninger B, Mitjans F, Haier J. Integrins can directly mediate metastatic tumor cell adhesion within the liver sinusoids. J Gastrointest Surg. 2004;8:1049–60. doi: 10.1016/j.gassur.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nature Med. 2000;6:100–2. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 17.Haier J, Korb T, Hotz B, Spiegel HU, Senninger N. An intracital model to monitor tumor cell adhesion within the hepatic microcirculation. J Gastrointest Surg. 2003;7:507–15. doi: 10.1016/S1091-255X(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 18.Mook ORF, Van Marie J, Vreeling-Sindelarova H, Jonges R, Frederiks WM, Van Noorden CJF. Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology. 2003;38:295–304. doi: 10.1053/jhep.2003.50297. [DOI] [PubMed] [Google Scholar]
- 19.Naumov GN, Wilson SM, MacDonald IC, Schmidt EE, Morris VL, Groom AC, Hoffman RM, Chambers AF. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J Cell Sci. 1999;112:1835–42. doi: 10.1242/jcs.112.12.1835. [DOI] [PubMed] [Google Scholar]
- 20.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 21.Wong CW, Song C, Grimes MM, Fu W, Dewhirst MW, Muschel RJ, Al-Mehdi AB. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol. 2002;161:749–53. doi: 10.1016/S0002-9440(10)64233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffini P, Smorenburg SM, Verbeek FJ, Van Noorden CJF. Three-dimensional reconstruction of colon carcinoma metastases in liver. JMicrosc. 1997;187:12–21. doi: 10.1046/j.1365-2818.1997.2140770.x. [DOI] [PubMed] [Google Scholar]
- 23.Groen AK, Sips HJ, Vervoorn RC, Tager JM. Intracellular compartmentation under control of alanine metabolism in rat liver parenchymal cells. Eur J Biochem. 1982;122:87–93. doi: 10.1111/j.1432-1033.1982.tb05851.x. [DOI] [PubMed] [Google Scholar]
- 24.Jonges R, De Moor E, Boon PNM, Van Marie J, Dietrich AJJ, Grimbergen CA. Three-point repositioning of axes: three-dimensional alignment procedure for electron microscope tomography using three markers. MicrRes Techn. 1996;33:516–26. doi: 10.1002/(SICI)1097-0029(19960415)33:6<516::AID-JEMT7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Jonges R, Boon PNM, Van Marie J, Dietrich AJJ, Grimbergen CA. CART: a controlled algebraic reconstruction technique for electron microscopic tomography of embedded, sectioned specimen. Ultramicroscopy. 1999;76:203–19. doi: 10.1016/s0304-3991(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 26.Schliiter K, Gassmann P, Enns A, Korb T, Hemping-Bovenkerk A, Holzen J, Haier J. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol. 2006;169:1064–73. doi: 10.2353/ajpath.2006.050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Im JH, Fu W, Wang H, Bhatia SK, Hammer DA, Kowalska MA, Muschel RJ. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64:8613–9. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 28.Braet F, Nagatsuma K, Saito M, Soon L, Wisse E, Matsuura T. The hepatic sinusoidal endothelial lining and colorectal liver metastases. Worls J Gastroenterol. 2007;13:821–5. doi: 10.3748/wjg.v13.i6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmers M, Vekemans K, Vermijlen D, Asosingh K, Kuppen P, Bouwens L, Wisse E, Braet F. Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer. 2004;112:793–802. doi: 10.1002/ijc.20481. [DOI] [PubMed] [Google Scholar]
- 30.Vekemans K, Braet F, Wisse E. CC531S-induced damage of the rat liver sinusoidal endothelial lining is mediated by the Fas/FasL pathway. Hepatology. 2003;38:1314. doi: 10.1053/jhep.2003.50460. [DOI] [PubMed] [Google Scholar]
- 31.Vekemans K, Timmers M, Vermijlen D, De Zanger R, Wisse E, Braet F. CC531 s colon carcinoma cells induce apoptosis in rat hepatic endothelial cells by the Fas/FasL-mediated pathway. Liver Internat. 2003;23:283–93. doi: 10.1034/j.1600-0676.2003.00840.x. [DOI] [PubMed] [Google Scholar]
- 32.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–9. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190:310–29. doi: 10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH525>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Soares FA, Shaughnessy SG, MacLarkey WR, Orr FW. in vivo. Lab Invest. 1994;71:480–9. Quantification and morphologic demonstration of reactive oxygen species produced by Walker 256 tumor cells in vitro and during metastasis. [PubMed] [Google Scholar]
- 35.Honn KV, Tang DG, Grossi IM, Duniec ZM, Timar J, Renaud C, Leithauser M, Blair I, Johnson CR, Diglio CA, et al. Tumor cell-derived 12(S)-hydroxye-icosatetraenoic acid induces microvascular endothelial cell retraction. Cancer Res. 1994;54:565–74. [PubMed] [Google Scholar]
- 36.Honn KV, Tang DG, Grossi IM, Renaud C, Duniec ZM, Johnson CR, Diglio CA. Enhanced endothelial cell retraction mediated by 12(S)-HETE: a proposed mechanism for the role of platelets in tumor cell metastasis. Exp Cell Res. 1994;210:1–9. doi: 10.1006/excr.1994.1001. [DOI] [PubMed] [Google Scholar]
- 37.Weiss L, Orr FW, Honn KV. Interactions of cancer cells with the microvasculature during metastasis. FASEB J. 1988;2:12–21. doi: 10.1096/fasebj.2.1.3275560. [DOI] [PubMed] [Google Scholar]
- 38.Mook OR, Van Marie J, Vreeling-Sindelarova H, Jonges R, Frederiks WM, Van Noorden CJF. Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology. 2003;38:1315. doi: 10.1053/jhep.2003.50297. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi M, Ishida T, Traub O, Corson MA, Berk BC. Mechanotransduction in endothelial cells: temporal signaling events in response to shear stress. J Vase Res. 1997;34:212–9. doi: 10.1159/000159225. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonals DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1 -positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;55:901–6. [PubMed] [Google Scholar]
- 42.Wewer UM, Shaw LM, Albrechtsen R, Mercurio AM. The integrin alpha 6 beta 1 promotes the survival of metastatic human breast carcinoma cells in mice. Am J Pathol. 1997;151:1191–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Rabinovitz I, Nagle RB, Cress AE. Integrin alpha6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metast. 1995;13:481–91. doi: 10.1007/BF00118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okazaki K, Nakayama Y, Shibao K, Hirata K, Nagata N, Itoh H. Enhancement of metastatic activity of colon cancer as influenced by expression of cell surface antigens. J Surg Res. 1998;78:78–84. doi: 10.1006/jsre.1998.5298. [DOI] [PubMed] [Google Scholar]
- 45.Van Rossen MEE, Hofland LJ, Van den Tol MP, Van Koetsveld PM, Jeekel J, Marquet RL, Van Eijck CHJ. Effect of inflammatory cytokines and growth factors on tumour cell adhesion to the peritoneum. J Pathol. 2001;193:530–7. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH805>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 46.Kikkawa H, Kaihou M, Horaguchi N, Uchida T, Imafuku H, Takiguchi A, Yamazaki Y, Koike C, Kuruto R, Kakiuchi T, Tsukada H, Takada Y, Matsuura N, Oku N. Role of integrin αvβ3 in the early phase of liver metastasis: PET and IVM analyses. Clin Exp Metast. 2002;19:717–25. doi: 10.1023/a:1021356019563. [DOI] [PubMed] [Google Scholar]
- 47.Kitayama J, Nagawa H, Tsuno N, Osada T, Hatano K, Sunami E, Saito H, Muto T. Laminin mediates tethering and spreading of colon cancer cells in physiological shear flow. BrJ Cancer. 1999;80:1927–34. doi: 10.1038/sj.bjc.6690622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smorenburg SM, Van Noorden CJF. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol Rev. 2001;53:93–105. [PubMed] [Google Scholar]
- 49.Kemperman H, Wijnands YM, Roos E. αV integrins on HT-29 colon carcinoma cells: adhesion to fibronectin is mediated solely by small amounts of αVβ6, and αVβ5 is codistributed with actin fibers. Exp Cell Res. 1997;234:156–64. doi: 10.1006/excr.1997.3599. [DOI] [PubMed] [Google Scholar]
- 50.Mook ORF, Frederiks WM, Van Noorden CJF. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Ada. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann UB, Westphal JR, Waas ET, Becker JC, Ruiter DJ, Van Muijen GN. Coexpression of integrin alpha(v)beta3 and matrix metalloproteinase-2 (MMP-2) coincides with MMP-2 activation: correlation with melanoma progression. J Invest Dermatol. 2000;115:625–32. doi: 10.1046/j.1523-1747.2000.00114.x. [DOI] [PubMed] [Google Scholar]


