Abstract
Using an in vivo arterio-venous loop-containing tissue-engineering chamber, we have created a variety of vascularized tissue blocks, including functional myocardium. The viability of the transplanted cells is limited by the rate of neovascularization in the chamber. A Nox2-containing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is thought to have a critical role in ischaemic angiogenesis. In this study we investigated whether NADPH oxidase is involved in the neovascularization process in the tissue-engineering chamber. New blood vessels originating from the venous and the arterial ends of the loop could be identified after 3 days, and the vessel density (by lectin staining) peaked after 7 days and was maintained for at least 14 days. This was accompanied by granulation tissue formation and concomitant increase in the mRNA level of Nox4 NADPH oxidase. Although the total level of Nox2 mRNA in the chamber tissue decreased from day 3 to day 7, immunohistochemistry identified a strong expression of Nox2 in the endothelial cells of the new vessels. In human microvascular endothelial cells, the NADPH oxidase inhibitor apocynin reduced NADPH oxidase activity and inhibited the angiogenic responses in vitro. Local treatment with the NADPH oxidase inhibitors apocynin or gp91ds-tat peptide significantly suppressed the vessel growth in the chamber. In conclusion, NADPH oxidase-dependent redox signalling is important for neovascularization in this novel tissue-engineering chamber in vivo, and boosting this signalling might be a new approach to extending vascularization and tissue growth.
Keywords: angiogenesis, NADPH oxidase, redox signalling, tissue engineering
Introduction
Growing new tissues by tissue engineering (TE) relies upon spontaneous invasion of capillaries from the surrounding vascular bed into the tissue construct. In contrast, we have focused on building tissue grafts around an artificial circulation circuit produced by embedding an arterio-venous loop (AVL) in a closed polycarbonate chamber filled with isolated tissues or cells [1]. Using this novel in vivo TE chamber, vascularized soft tissues including fat, muscle and connective tissues have been created [2–5]. In addition, a pancreatic organoid has been created by seeding adult mouse islets into the chamber, and the engineered islet graft effectively reduced blood glucose in type 1 diabetic mice [6]. Further, using neonatal rat cardiomyocytes in the rat chamber system, 3-dimensional strips of myocardial tissue have been created, which displayed contractions with intrinsic rhythms and could be paced, as well as producing positive chronotropic and inotropic responses in vitro to stretch, calcium and β-adrenoceptor agonists [7].
Survival of the transplanted cells and the resulting tissue grafts in the TE chamber largely depends on the establishment of a functional vasculature capable of delivering sufficient oxygen and nutrients. Vascularization of the chamber largely depends on spontaneous angiogenesis originating from the venous and arterial ends of the parent AVL [8]. Fibrin exudes from the AVL and inflammatory cells rapidly infiltrate the matrix after loop construction, and this inflammatory component may be the intrinsic stimulus for the angiogenic process inside the chamber [8]. Despite this vigorous vascularization, cells still become hypoxic [9], and this hypoxia may further augment angiogenesis. Clearly, promoting neovascularization inside the chamber is critical for preventing hypoxia and hence facilitating growth of the constructed tissue graft in the chamber.
Angiogenesis is a complex, tightly regulated process that occurs through degradation of extracellular matrix, and the migration, proliferation and morphogenesis of endothelial cells, involving many growth factors, cytokines and multiple signalling pathways [10]. Vascular endothelial growth factor (VEGF) is one of the most important angiogenic growth factors involved in the angiogenic response both in vitro and in vivo. Recently it has been found that VEGF stimulates generation of reactive oxygen species (ROS) in endothelial cells, and ROS are involved in mediating the angiogenic activities of endothelial cells [11–13]. Indeed, endogenously generated ROS are known to serve as second messengers, activating multiple intracellular signalling pathways that have key roles in vascular cell biology [13]. This notion is underlined by the observations that angiotensin II, a major stimulus for vascular ROS production, also plays an important role in angiogenesis [11, 12].
The reduced β-nicotinamide adenine dinucleotide phosphate (NADPH) oxidase has been identified in vascular tissues as a major source of ROS [14, 15]. Tojo et al. recently provided evidence that NADPH oxidase is directly involved in angiogenesis induced by ischemia in vivo. Using a hind limb ischaemia model, they showed that there was an increase in expression of the catalytic subunit Nox2 and generation of superoxide in the angiogenic area, and both the infiltrating inflammatory cells and resident vascular cells contributed to this process. Moreover, the angiogenic response was significantly impaired either by in vivo treatment with the antioxidant ebselen, or in Nox2-deficient mice [16], indicating that the Nox2 NADPH oxidase is a critical component in the signalling networks driving hypoxia-induced angiogenesis.
We have systemically characterized the angiogenic processes in the chamber [8], but little is known about the underlying cellular and molecular mechanisms. Based on the evidence of the involvement of NADPH oxidase in ischaemic angiogenesis, we set out to investigate whether NADPH oxidase is involved in the angiogenic process in the TE chamber.
Materials and methods
Animals and TE chamber construction
All procedures were carried out in compliance with the guidelines of the National Health and Medical Research Council (NHMRC) and approved by the institutional Animal Ethics Committee. Male Sprague Dawley rats each weighing 300–400g were anaesthetized with ketamine and xylazine (75 and 10 mg/kg respectively, i.p.), and the left and right femoral arteries and veins were exposed. A 15 mm-long segment of the right femoral vein was dissected out and anastomosed with the left femoral artery and vein. This produced an AVL consisting of the femoral artery, the vein graft and the femoral vein. The AVL was placed inside a polycarbonate chamber of about 0.55 ml in volume (inner diameter 13 mm, depth 4 mm), and the lid of the chamber was closed. The remnants of the severed superior epigastric vessels branching from the AVL were used to anchor the construct in the chamber. The chamber was then sutured to the groin musculature and the incision was closed. On day 3, 7, 10 or 14 after chamber implantation, the tissues formed in the chamber were collected for various analyses.
Ink perfusion of vasculature
Twelve millilitre of India ink was mixed with 6 ml of fish gelatin and 0.6 ml of phenol, and diluted with water to a final volume of 60 ml. During tissue harvesting, the femoral artery was cannulated, and perfused with heparin-saline to flush the blood from the AVL. The ink was then infused into the femoral artery with a syringe until the chamber became black. The tissue was removed and dehydrated by soaking with serial ethanol solutions of 70% (overnight), 2 × 90% (3 hrs each) and 100% (overnight). Finally the tissue was cleared with methylsalicylate and cut into 10–12 sections and preserved in glycerol. The tissue sections were photographed with a digital camera attached to a dissecting microscope.
Blood vessel density measurement
Tissues fixed with 4% paraformaldehyde were embedded in paraffin and sectioned in 6 pm thickness using a microtome (Reichert-Jung, Austria) and mounted on polylysine-coated slides. General tissue structure was studied by eosin-haematoxylin (H&E) staining. Blood vessels were labelled with biotiny-lated Griffonia Simplicifolia lectin (Vector Laboratories, 30 min. at room temperature) diluted in antibody diluent (Dako), and visualized with peroxidase-conjugated streptavidin (Dako) developed with 3’3-diaminobenzidine (DAB) chromogen (Dako). The vessel density was assessed with the computer-assisted stereological toolbox (CAST) system (Olympus). Each tissue block was evenly divided into five slices, from which a representative microtome section was obtained from each slice. Using an eyepiece with a grid, the blood vessel density (% of area) was estimated by the number of points that fell on blood vessels divided by the total number of points counted. The vessel density values of the five sections from each single tissue were then averaged.
Quantitative real-time PCR
Total RNA was extracted from freshly collected tissues using the TRI Reagent (Ambion) according to the manufacturer's instructions. The RNA was reverse-transcribed into cDNA using the TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Real time PCR was carried out with 50 ng sample cDNA in a total volume of 25 μl of a reaction mixture containing 12.5 μl of the TaqMan Universal PCR Master Mix (Applied Biosystems), 1.25 μl of the pre-designed rat specific primer-probe sets for Nox2 or Nox4 (Assays-on-Demand, Applied Biosystems). The PCR reaction (40 cycles) was performed on an ABI Prism 7000 system with the following settings: 2 min. at 50°C, 10 min. at 95°C and 40 cycles of 95°C for 30 sec. and 60°C for 1 min.
Immunohistochemistry
Paraffin sections were routinely dewaxed and re-hydrated. Antigen retrieval was performed by boiling the tissue section in sodium citrate buffer (pH 6.0) in a microwave for 10 min. The sections were then washed with phosphate-buffered saline (PBS), incubated with 3% H2O2 to inactivate the endogenous peroxidases, and blocked with 10% normal horse serum at room temperature for 1 hr. The primary antibody diluted in the antibody diluent (Dako) was applied overnight at 4°C. Biotinylated secondary antibodies were used in conjunction with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) to detect the bound primary antibodies, and the slides were developed with the AEC or DAB chromogen kits (both from Dako). The sections were counterstained with haematoxylin. Negative controls were performed by using normal IgG instead of the primary antibody.
In situ superoxide production
Frozen sections of 10 μm thickness were incubated with 10 μM dihydroethidium (DHE, Invitrogen) diluted in PBS at 37°C for 30 min. DHE can be oxidized by superoxide to generate the fluorescent product ethidium. After incubation, the slides were washed in PBS and mounted with the fluorescent mounting media (Dako). The fluorescence was visualized with a fluorescent microscope (Axioskop2, Zeiss) with a 590 nm filter. The specificity of the DHE fluorescence was confirmed by treating the sections with the superoxide dismutase mimetic Mn(lll)TMPyP (100 μg/ml in PBS), which was applied 30 min. before DHE and was present in the DHE solution thereafter.
In vitro experiments
Human microvascular endothelial cells (HMECs, a gift from Professor Philip Hogg, University of New South Wales, Sydney, Australia), derived from human skin microvasculature, were cultured in an endothelial cell culture media (EGM-2 BulletKit, Cambrex Corporation) containing 5% foetal calf serum (FCS), in a CO2/O2 incubator at 37°C. Lucigenin-enhanced chemiluminescence was used to measure NADPH oxidase activity as described previously [17]. Tube formation and wound healing assays were used to assess the angiogenic property of the cells in vitro. For the tube formation assay, cells were plated on Matrigel (BD Biosciences, San Jose, CA, USA) at a density of 5 × 104 cells per well in a 24-well plate. The capillary-like tube structures were examined using an invert microscope (Olympus CK40), and the tube formation was quantified by the average of total tube length from at least two randomly selected 100x fields using Axiovision software. For the wound-healing assay, cells were cultured in 6-well plates to confluency. A wound was made by scratching a line across the monolayer with a pipette tip. The width of the cell-free gap was measured continuously. The wound healing response was measured by the percentage reduction of the width of the scratch.
Drug treatment in vivo
To clarify the role of NADPH oxidase in angiogenesis, we applied the NADPH oxidase inhibitor apocynin in the AVL chamber. Stock solution of apocynin (1 M) dissolved in DMSO (dimethylsulfoxide) was mixed with Matrigel (BD Bioscience) to a final concentration of 10 mM. The mixed gel was stored on ice and 500 μl of the gel was pipetted into the chamber at the time of operation. Matrigel mixed with DMSO (1% of final concentration) was used as control. The chamber tissues were harvested 7 and 14 days after operation for vessel density analysis. Moreover, a specific peptidyl NADPH oxidase inhibitor gp91ds-tat [18] and the scrambled control peptide (all from Auspep, Australia) were also used (100 μM of final concentration in the Matrigel).
Data analysis
Results are presented as mean ± standard error of the mean (SEM). Unpaired t-test or one-way analysis of the variance (anova) followed by post hoc Newman-Keuls t-test was used for statistical analysis and a value of P<0.05 was regarded as statistically significant.
Results
Angiogenic response in the chamber
The newly constructed AVL exhibited hyperpermeability with prominent fibrin exudation and leukocyte emigration that could be readily observed at 3 days (Fig. 1). Thereafter, this fibrin deposit was gradually organized with concomitant leukocyte infiltration, angiogenesis and fibrosis, and by 14 days the majority of the initial fibrin deposit was replaced by granulation tissue. The angiogenic response can be seen at 3 days with only occasional new capillaries composed of a single or two endothelial cells that could be identified by positive lectin staining proximal to the parent AVL. By day 7, the blood vessel density increased dramatically as compared to that at day 3 (Fig. 1). This change temporally and spatially accompanied the process of cellularization of fibrin matrix. At this stage, most of the new vessels only had a single layer of endothelial cells in the vessel wall (Fig. 1). Perfusion of the vascular bed of the chamber tissue showed that the angiogenic process was more active from the venous side as compared to the arterial side, with almost no new vessels sprouting from the vein graft (Fig. 1). The quantitative data of the new vessel density in the chamber tissue over the time course are shown in Figure 2.
Fig 1.
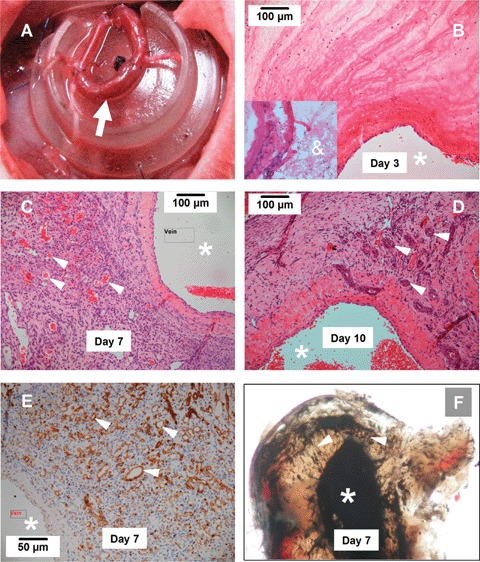
Angiogenic response in tissue-engineering (TE) chamber (A). Arrow indicates the arterio-venous loop constructed by a vein graft anastomosed to the femoral artery and vein. (B-D) Histology of the tissue formed in the TE chamber 3, 7 and 10 days after chamber implantation (H&E staining). The lumen of the vein side of the parent loop was indicated by *. The new blood vessels are indicated by arrow heads. Note that vessels with a multi-layered wall composed of mural cells can be observed in 10-day specimen. The insert in B shows that 1 day after construction, the chamber contained only a fibrin mesh (&) and a few infiltrating cells. (E) New vessels defined by Griffonia Simplicifolia lectin staining (brown, arrow heads) at 7 days. (F) Ink perfusion of the AV loop at 7 days demonstrates new vessels (arrow heads) sprouting form the parent vessel lumen (femoral vein). At each time point, 5–6 animals were analysed.
Fig 2.
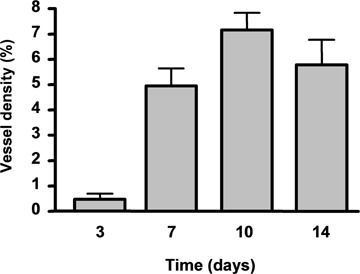
Density (% area) of new vessels in the chamber tissue 3,7,10 and 14 days after chamber implantation. Data are mean ± standard error of the mean (SEM), n = 3–4.
From day 10 to 14, the overall vessel density remained similar to that of day 7 (Fig. 1). Histological examination revealed that, as compared to day 7, many vessels at these later stages had a more distinct, thicker vessel wall, which has recruited multi-layered mural cells (Fig. 1). This indicates that vessel maturation occurred approximately 1 week after the AVL construction.
Nox NADPH oxidase expression
We measured the mRNA levels of the two predominant isoforms of the Nox subunit of NADPH oxidase expressed in endothelial cells, namely Nox2 and Nox4, in the chamber tissue. As shown in Figure 3, there was a time-dependent trend of increase in the Nox4 mRNA level, which was significantly greater at day 14 as compared to day 3. Interestingly, in contrast to the increasing trend of Nox4, Nox2 mRNA expression was significantly lower at days 7 to 14 as compared to day 3 (Fig. 3).
Fig 3.
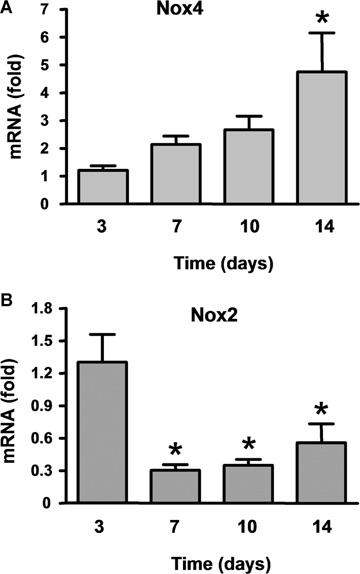
Time course of the expression levels of mRNA of the NADPH oxidase Nox4 and Nox2 in the chamber tissue. Data (mean ± S.E.M.) are expressed as fold of the mean level of day 3. *P < 0.05 versus day 3, one-way analysis of the variance (anova) followed by Newman-Keuls t-test, n = 6–7.
We performed immunohistochemistry to identify the protein expression of the Nox enzymes. On day 3, a high level of Nox2 expression could be identified in the endothelial cells of the parent AVL, and in a sub-population of infiltrating leukocytes.
Immunohistochemistry using an anti-macrophage/monocyte antibody (clone ED-1 on sections from the same specimen demonstrated that those Nox2 positive infiltrating cells in the fibrin mesh were also positive for ED-1, indicating that these cells are mononuclear cells or macrophages. It was also noted, however, that not all ED-1 positive cells expressed Nox2. Most of the small mononuclear cells that formed large clusters were found to be ED-1 positive but negative for Nox2 (data not shown). Taken together, we suggest that Nox2 expression is up-regulated in the infiltrating monocytes after they have differentiated into macrophages. On day 7, we found a highly concentrated expression of Nox2 in the endothelial cells of the new vessels, which were ED-1 negative (Fig. 4). A number of mononuclear cells scattered in the tissue were Nox2 positive, whereas these cells were probably ED-1 positive also (Fig. 4). In the day-10 specimen, Nox2 was detected in both endothelial cells and mural cells in the multi-layered wall of new vessels (Fig. 4). Interestingly, in some occasions we found that in the 7-day specimen, ED-1 positive cells could be identified adjacent to the new vessels, and some cells seemed to incorporate into the wall of the new vessels (Figure 4). This phenomenon could not be observed at 10 days.
Fig 4.
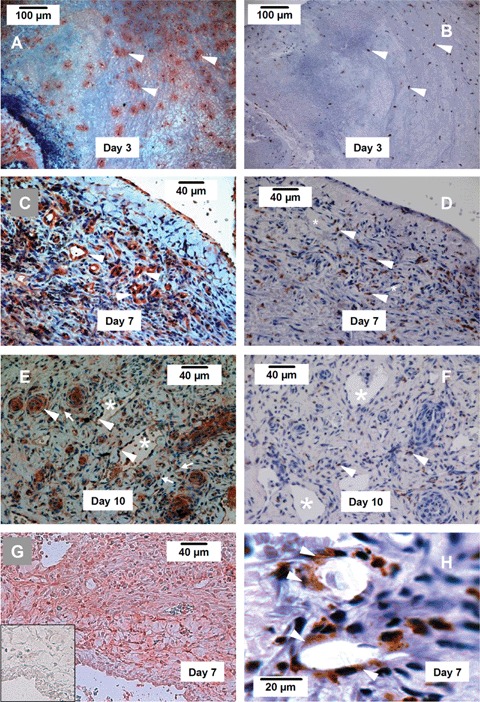
Immunohistochemistry showing the expression of Nox2, Nox4 and the mono-cytes/macrophage marker ED-1. (A, C, E) Nox2 expression (red) in new blood vessels (arrow heads) and infiltrating leukocytes (arrows) in the chamber tissue at 3, 7 and 10 days. (B, D, F) Expression of ED-1 (brown) in infiltrating leukocytes (arrow heads) in the chamber tissue at 3, 7 and 10 days. (G) Expression of Nox4 (red colour) in the chamber tissue at 7 days. The insert shows a negative control section, in which non-specific IgG was used instead of the primary antibody. (H) We have observed that at day 7, some ED-1 + cells appeared to be incorporated in the new vessel (arrow heads). Such ED-1 + cells could not be observed in the vessels at day 10. Endothelial cells of the new vessel were ED-1 negative. * indicates the lumen of new blood vessels, n = 4 at each time point.
We also attempted to localize Nox4 expression using the 7-day specimen, but the Nox4 antibody could not detect the antigen in paraffin sections. When Nox4 immunohistochemistry was performed in frozen sections, the specificity of the immunoreactivity was confirmed by negative control sections in which the primary antibody was replaced by equal concentration of non-specific IgG from the same species (Fig. 4G insert). In contrast to the highly localized expression of Nox2, Nox4 was expressed in most cells including the endothelium, mural cells and fibroblast-like spindle cells (Fig. 4). In comparison to the Nox2 staining, the endothelial cells did not express more Nox4 than other cell types.
Localization of superoxide production
At 3 days many DHF-labelled cells were detected in the fibrin matrix. The density of the fluorescent cells was higher at 7 days (mean fluorescence intensities being 16.3 ± 1.7 versus 25.6 ± 2.6 at 3 and 7 days respectively, P<0.05, t-test, n = 4–5) (Fig. 5). Comparison of the DHE fluorescent images with those of H&E-stained slides from the same specimen revealed that the granulation tissue, which was dominated by spindle shaped cells (presumably fibroblasts), endothelium and rounded mononuclear cells, exhibited intense DHE fluorescence. It was obvious that these areas were highly vascularized as demonstrated by lectin staining (Fig. 5). Some of the fluorescent cells formed structures that resembled new blood vessels (Fig. 5). However, given the poor resolution of tissue structure in the DHE fluorescent slides, it is difficult to precisely match the pattern of cellular superoxide with the NADPH oxidase expression.
Fig 5.
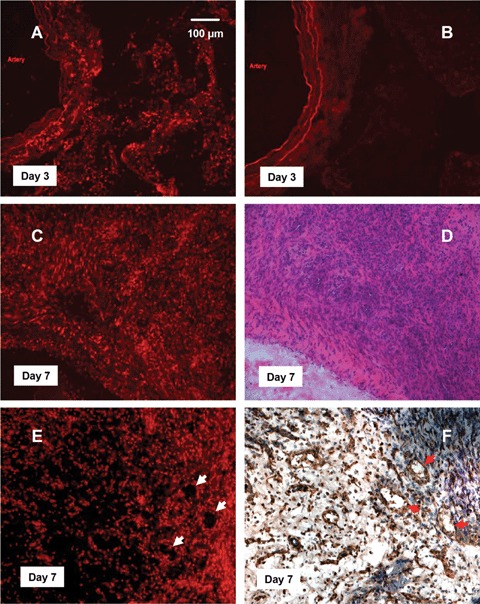
In situ superoxide production detected by dihydroethidium (DHE) fluorescence. (A, C) superoxide production in 3- and 7-day specimens respectively. (B) A 3-day sample pre-incubated with the superoxide dismutase mimetic Mn(lll)TMPyP, showing that the fluorescence was superoxide specific. Some autofluorescence was observed only in the elastic layers of the parent AV loop. (D) H&E staining of the 7-day sample, showing that intense DHE fluorescence was present in the highly cellularized granulation tissue with numerous new vessels. (E, F) Comparison of DHE fluorescence with lectin reactivity in consecutive sections demonstrated that some of the fluorescent cells formed ring structures that resembled the new blood vessels (arrows), n = 3 at each time point.
Apocynin inhibited NADPH oxidase activity and angiogenesis in vitro
In order to determine the role of NADPH oxidase in angiogenesis in vitro, we used the NADPH oxidase inhibitor apocynin in cultured HMECs. Treatment of HMECs with apocynin (2 and 10 mM for 1 hr) concentration-dependently reduced the NADPH oxidase activity (Fig. 6A). Next, we examined whether long-term inhibition of NADPH oxidase by apocynin affects the survival of endothelial cells. As shown in Figure 6B, the cell number was slightly reduced (P<0.05) by apocynin at 24 hrs, while at 72 hrs, the reduction in cell number was more obvious. This observation is consistent with our previous finding that inhibition of NADPH oxidase expression in HMECs induced an apoptotic response (caspase 3/7 activation). Then we assessed in vitro angiogenic responses in HMECs using tube formation and wound healing assays. Apocynin at both concentrations significantly inhibited the tube formation and wound healing responses (Figs 6C and D). In the following in vivo studies, we used 10 mM of apocynin for local treatment.
Fig 6.
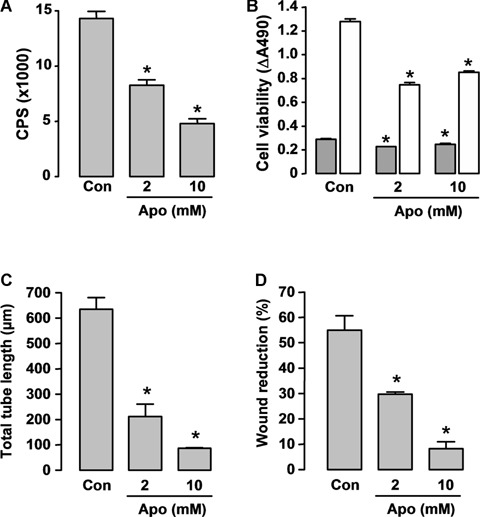
Effects of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor apocynin on (A) NADPH oxidase activity, (B) cell viability and (C, D) angiogenic responses in cultured Human microvascular endothelial cells (HMEC) cells. (A) Cells were detached by trypsinization and resuspended in Hank's balanced salt solution. Apocynin (Apo) was added 60 min before assay. Superoxide release was measured by lucigenin (5 μM)-enhanced chemiluminescence after stimulation with 100 μM NADPH in a topcount microplate reader (model 9912, Packard). The results are expressed as count per second (CPS) per 105 cells (n = 5). (C) Con, DMSO control. (B) Cell survival was assessed 24 (grey bars) and 72 hrs (hatched bars) after apocynin treatment with the CellTiter-96 AQueous One Solution kit, and the results are expressed as differences of absorbance at 490 nm between wells containing cells and buffer only (background) (n = 4). (C) In vitro angiogenesis was assessed by the tube formation assay (observed 6 hrs after cell plating on Matrigel, n = 3). Apocynin was added at the time of plating. (D) Wound-healing assay in HMECs observed 18 hrs after scratching (n = 3). Apocynin was added at the time of scratching. * P < 0.05 versus corresponding control (Con), one-way anova.
Apocynin suppressed vessel growth in the chamber
To determine the role of NADPH oxidase in neovascularization of the AVL chamber, we tested the effects of apocynin locally applied in the chamber. As shown in Figure 7A, treatment with apocynin remarkably retarded the vessel growth into the Matrigel surrounding the AV loop. The vessel density between the artery and vein stems was reduced by about 50% in the apocynin-treated chambers at 14 days (Fig. 7B). We also found that the apocynin-containing Matrigel did not prevent the infiltration of inflammatory cells prior to vessel development in unorganized areas (Fig. 7A). To further confirm the effects of NADPH oxidase inhibition on neovascularization, we tested the effects of a specific peptidyl inhibitor gp91ds-tat (100 μM). Treatment with gp91ds-tat for 14 days reduced the vessel density by 84.8% as compared to those treated with the scrambled control peptide (Fig. 7C).
Fig 7.
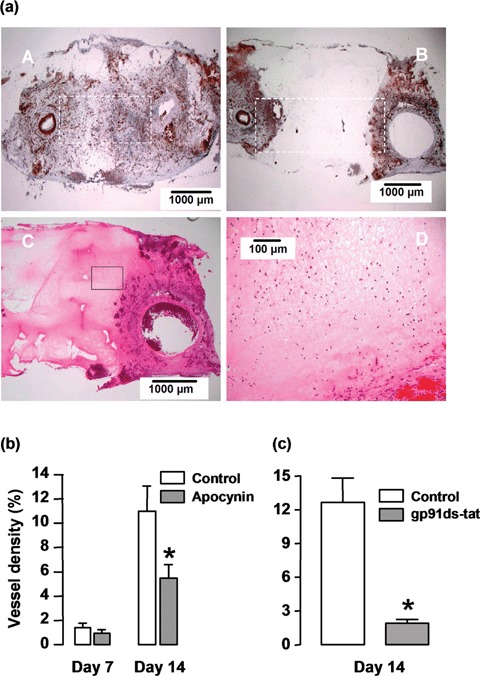
Effects of NADPH oxidase inhibitors apocynin and gp91ds-tat on angiogenesis in the chamber in vivo. (A, panels A, B) lectin staining of sections from the 14-day samples without (panel A) and with (panel B) apocynin (10 mM) treatment, (panel C) H&E staining showing that the apocynin-containing Matrigel (the pink matrix) did not prevent the infiltration of inflammatory cells prior to vessel development in unorganized areas, (panel D) higher magnification of the outlined area in panel C. (b) Quantitative data of the vessel density (% area) in control and apocynin-treated chamber tissues at 7 and 14 days. The vessel density was assessed in an area as outlined in panels A & B. * P<0.05 versus control, unpaired t-test, n = 5. (c) Effects of gp91ds-tat (100 μM) on neovascularization. A scrambled peptide was used as control. * P < 0.005 versus control, unpaired t-test, n = 5.
Discussion
Vascular NADPH oxidase and the ROS molecules produced by this enzyme have emerged as key regulators of angiogenesis [12]. In this study, we provide evidence that NADPH oxidase is intimately involved in the angiogenic process in vivo inside our tissue-engineering chambers. A major difference between our study and previous ones is that the angiogenic process in the chamber occurred in the absence of any obvious physiological or pathophysiological triggers of angiogenesis, such as tissue ischaemia or release of growth factors [11, 16, 19]. Rather, the new vessel growth sprouted spontaneously from a patent arterial-venous loop, occurring in a milieu of granulation tissue formation. This process was preceded by early fibrin deposition and subsequent inflammatory cell infiltration, which resembles the wound healing process in the skin. Interestingly, our finding is supported by a recent report that low concentrations of H2O2 accelerated, whereas catalase slowed, the angiogenesis and healing process of dermal wounds in vivo[20]. Taken together, we suggest that NADPH oxidase and subsequent redox signalling have important roles in promoting tissue regeneration by acting as key intermediates in angiogenesis.
The expression of Nox4 increased with the formation and maturation of new vessels during tissue growth in the TE chamber. A role of Nox4 in promoting angiogenesis is emerging. It was found that focal brain ischaemia resulted in prominent expression of Nox4 in the new capillaries, indicating an involvement of Nox4 in hypoxia-induced angiogenesis [21]. Recently, we demonstrated that blockade of Nox4 expression or function inhibited angiogenic responses in cultured endothelial cells [22]. On the other hand, the importance of Nox2 in angiogenesis has been documented under both in vitro and in vivo conditions [11, 16]. A limitation of the present study is that we could not distinguish the specific roles of Nox2 and Nox4 in neovascularization in the chamber. Given that both Nox2 and Nox4 are expressed and are involved in redox modulation in endothelial cells [11, 23], we speculate that both isoforms may be involved in promoting neovascularization in the chamber.
Immunohistochemistry experiments revealed that Nox4 is expressed in mesenchymal cells as well as in endothelial cells, indicating that Nox4 may be also involved in vessel maturation. This is supported by the observation that Nox4 expression continued to increase even after the vessel density has reached the plateau. In mature arteries, Nox4 is expressed in both vascular smooth muscle cells and fibroblasts [24, 25]. There is evidence that NADPH oxidase and ROS molecules are involved in regulating proliferation and phenotypic switch of mural cells [25–27]. Moreover, Nox4 expressed in mesenchymal cells may stimulate angiogenesis via paracrine effects by releasing cell permeable H2O2[28], which has potent pro-angiogenic actions [12]. It seems, however, paradoxical that Nox2 expression decreased at day 7 as compared to day 3, while there was a dramatic concomitant increase in the vessel density. Immunohistochemistry experiments revealed that at day 3, the majority of cells in the chamber appeared to be ED-1 positive, indicating that these cells are mono-cytes and differentiated macrophages. Nox2 was present in a population of these infiltrating leukocytes, with an especially strong expression in those cells migrated into the fibrin mesh, reflecting that there is an up-regulation of Nox2 during the course of maturation of macrophages [29]. At this stage, non-leukocytes were rare in the chamber. At day 7, we identified a high level of Nox2 expression localized to the endothelium of new vessels, which was accompanied by a relatively lower level of expression in the scattered ED-1+ cells. It was noted that many of the cells in the 7-day tissue were Nox2 null. Therefore, the decreased overall Nox2 expression over time is likely to be due to the recruitment and accumulation of a large proportion of Nox2 null cells. This spatial pattern of Nox2 expression is entirely in agreement with that reported in ischaemic muscle, where increased Nox2 protein was primarily present in infiltrating leukocytes at 3 days and in newly formed capillaries at 7 days after ischaemia [16].
We used a pharmacological inhibitor of NADPH oxidase, apocynin, to clarify the role of NADPH oxidase in mediating the angiogenic process in the chamber. Apocynin has been widely used as a tool to block NADPH oxidase-dependent ROS formation and been shown to effectively inhibit angiogenic responses both in vitro and in vivo[19, 30, 31]. Consistent with these results, we confirmed that treatment of human microvascular endothelial cells with apocynin significantly suppressed NADPH oxidase activity, inhibited angiogenic responses assessed by tube formation and wound healing assays, and compromised the cell survival. Finally, we showed that local treatment with apocynin significantly retarded vessel growth into the Matrigel, resulting in a 50% reduction of the mean vessel density in the chamber tissue at 14 days. This result was further confirmed by the specific NADPH oxidase inhibitor gp91ds-tat. The delayed angiogenesis following NADPH oxidase inhibition was unlikely to be due to altered inflammatory response prior to the new vessel development, since leukocyte infiltration in the Matrigel was obvious (Fig. 6A), similar to that occurring in the fibrin clot in chambers without Matrigel (Fig. 1). On the other hand, compromised cell viability is unlikely to have a major role in the reduced angiogenesis in apocynin-treated chambers, because our in vitro experiments showed that apocynin only marginally reduced the viability of HMEC cells. By comparing the data in Figure 2 and Figure 7B, it is clear that inclusion of Matrigel in the chamber markedly delayed the time course of vascularization. The reason is unknown, but may be because of restricted exudation of fibrin, which acts as a more optimal matrix for angiogenesis. The insignificant effect of apocynin at day 7 could be because of the low baseline values that rendered our morphological measurement method less sensitive.
We detected cellular superoxide generation using DHE fluorescence. Because of the poor preservation of tissue structure in frozen sections and the low resolution of the fluorescent image, it is difficult to precisely match the pattern of cellular superoxide with the NADPH oxidase expression. Nevertheless, we have observed that superoxide was mainly produced by the macrophages as detected in the fibrin matrix at 3 days, and later on by the granulation tissue which was highly vascularized. This is consistent with the findings in ischaemic muscle, where both macrophage and activated endothelial cells contributed to the increased ROS production, with the former component being prominent at day 3 and the later at day 7 [16]. On the other hand, our data indicate that other cells, such as fibroblasts and smooth muscle cells were also sources of ROS in the granulation tissue. Moreover, whether superoxide itself is directly involved in mediating the angiogenic signalling of angiogenesis is unknown, while evidence suggests that hydrogen peroxide might be a more promising candidate.
It should be noted that the new vessel formation in the TE chamber is somehow different from conventional angiogenesis in ischaemic tissues [32], which is a process of new vessel development from pre-existing capillaries or post-capillary venules. In contrast, all new vessels in the TE chamber are derived from large conduit vessels. Our study revealed that the angiogenic response in the chamber was preceded by a prominent inflammatory response. It is known that macrophages have important roles in regulating angiogenesis, and this is mainly achieved through paracrine actions by releasing proteases, growth factors, cytokines and autacoids, virtually impacting each phase of the angiogenic process including extracellular matrix degradation, endothelial cell migration and proliferation and vascular maturation [33]. In addition, myeloid lineage mononuclear cells have a high plasticity and under different conditions they exhibit the potential to differentiate toward different phenotypes [34–36]. Moreover, there appears to be a remarkable phenotypic overlap between monocytes and angiogenic progenitor cells [37]. Interestingly, we have identified some ED-1+ cells in the extreme proximity to the newly formed vessels in the 7-day specimen, and some cells appeared to be integrated in the vessel wall. This phenomenon could not be observed at 10 days. This finding complements the report by Jabs and co-workers showing that in granulation tissue, some peripheral blood mononuclear cells have acquired myofibroblast phenotype [38]. However, this phenomenon occurred only occasionally; it is unclear whether this indicates transdifferentiation of monocytes into mural cells and incorporation into new vessels. Moreover, we have not obtained convincing evidence that endothelial progenitors have a role during this process by using cell surface markers, such as CD133. These findings indicate that vasculogenesis is unlikely to have a major role in neovascularization of the chamber.
In summary, our study has identified an important role of NADPH oxidase-dependent redox signalling in promoting neovascularization in in vivo TE. This mechanism may represent a new target in promoting tissue growth in our proprietary TE chamber.
Acknowledgments
This work was supported by Project Grants from the National Health and Medical Research Council of Australia and Grants-in-Aid from the National Heart Foundation of Australia. G.J.D. is a Principal Research Fellow of the NHMRC. The authors thank Jason Palmer for technical assistance with the histology and immunohistochemistry; and Nancy Guo for performing the real time PCR assay.
References
- 1.Cassell OC. Hofer SO, Morrison WA, Knight KR. Vascularisation of tissue-engineered grafts: the regulation of angiogenesis in reconstructive surgery and in disease states. Br J Plast Surg. 2002;55:603–10. doi: 10.1054/bjps.2002.3950. [DOI] [PubMed] [Google Scholar]
- 2.Cassell OC, Morrison WA, Messina A, Penington AJ, Thompson EW, Stevens GW, Perera JM, Kleinman HK, Hurley JV, Romeo R, Knight KR. The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Ann N YAcad Sci. 2001;944:429–42. doi: 10.1111/j.1749-6632.2001.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 3.Hofer SO, Knight KM, Cooper-White JJ, O’Connor AJ, Perera JM, Romeo-Meeuw R, Penington AJ, Knight KR, Morrison WA, Messina A. Increasing the volume of vascularized tissue formation in engineered constructs: an experimental study in rats. Plast Reconstr Surg. 2003;111:1186–92. doi: 10.1097/01.PRS.0000046034.02158.EB. ; discussion 93–4. [DOI] [PubMed] [Google Scholar]
- 4.Messina A, Bortolotto SK, Cassell OC, Kelly J, Abberton KM, Morrison WA. Generation of a vascularized organoid using skeletal muscle as the inductive source. FASEB J. 2005;19:1570–2. doi: 10.1096/fj.04-3241fje. [DOI] [PubMed] [Google Scholar]
- 5.Mian R, Morrison WA, Hurley JV, Penington AJ, Romeo R, Tanaka Y, Knight KR. Formation of new tissue from an arte-riovenous loop in the absence of added extracellular matrix. Tissue Eng. 2000;6:595–603. doi: 10.1089/10763270050199541. [DOI] [PubMed] [Google Scholar]
- 6.Knight KR, Uda Y, Findlay MW, Brown DL, Cronin KJ, Jamieson E, Tai T, Keramidaris E, Penington AJ, Rophael J, Harrison LC, Morrison WA. Vascularized tissue-engineered chambers promote survival and function of transplanted islets and improve glycemic control. Faseb J. 2006;20:565–7. doi: 10.1096/fj.05-4879fje. [DOI] [PubMed] [Google Scholar]
- 7.Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, McCombe D, Wright CE, Itescu S, Angus JA, Morrison WA. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–60. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 8.Lokmic Z. Stillaert F. Morrison WA. Thompson EW, Mitchell GM. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21:511–22. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- 9.Hofer SO, Mitchell GM, Penington AJ, Morrison WA, RomeoMeeuw R. Keramidaris E. Palmer J, Knight KR. The use of pimonidazole to characterise hypoxia in the internal environment of an in vivo tissue engineering chamber. Br J Plast Surg. 2005;58:1104–14. doi: 10.1016/j.bjps.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Simons M. Integrative signaling in angiogenesis. Mol Cell Biochem. 2004;264:99–102. doi: 10.1023/b:mcbi.0000044379.25823.03. [DOI] [PubMed] [Google Scholar]
- 11.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing I\IAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–7. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 12.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of I\IAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 13.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–66. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK, Sorescu D, Ushio-Fukai M. I\IAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 15.Jiang F, Drummond GR, Dusting GJ. Suppression of oxidative stress in the endothelium and vascular wall. Endothelium. 2004;11:79–88. doi: 10.1080/10623320490482600. [DOI] [PubMed] [Google Scholar]
- 16.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–55. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 17.Jiang F, Guo Y, Salvemini D, Dusting GJ. Superoxide dismutase mimetic M40403 improves endothelial function in apolipoprotein(E)-deficient mice. Br J Pharmacol. 2003;139:1127–34. doi: 10.1038/sj.bjp.0705354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular 0(2)(-) and systolic blood pressure in mice. Circ Res. 2001;89:408–14. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 19.Angermayr B, Fernandez M, Mejias M, Gracia-Sancho J, Garcia-Pagan JC, Bosch J. NAD(P)H oxidase modulates angiogenesis and the development of portosystemic collaterals and splanchnic hyperaemia in portal hypertensive rats. Gut. 2006;56:560–4. doi: 10.1136/gut.2005.088013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13:211–20. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase N0×4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–8. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of NOX4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vase Biol. 2007;27:2319–24. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 23.Ago T, Kitazono T, Ooboshi H, lyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, lida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–33. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 24.Chamseddine AH, Miller FJ., Jr Gp91phox contributes to NADPH oxidase activity in aortic fibroblasts but not smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H2284–9. doi: 10.1152/ajpheart.00459.2003. [DOI] [PubMed] [Google Scholar]
- 25.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-betal1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–73. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 26.Su B, Mitra S, Gregg H, Flavahan S, Chotani MA, Clark KR, Goldschmidt-Clermont PJ, Flavahan NA. Redox regulation of vascular smooth muscle cell differentiation. Circ Res. 2001;89:39–46. doi: 10.1161/hh1301.093615. [DOI] [PubMed] [Google Scholar]
- 27.Shen WL, Gao PJ, Che ZQ, Ji KD, Yin M, Yan C, Berk BC, Zhu DL. NAD(P)H oxidase-derived reactive oxygen species regulate angiotensin-ll induced adventitial fibroblast phenotypic differentiation. Biochem Biophys Res Commun. 2006;339:337–43. doi: 10.1016/j.bbrc.2005.10.207. [DOI] [PubMed] [Google Scholar]
- 28.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi H, Fujinawa T, Kuribayashi F, Nakanishi A, Imajoh-Ohmi S, Goto M, Kanegasaki S. Induction of essential components of the superoxide generating system in human monoblastic leukemia U937 cells. J Biochem. 1994;116:742–6. doi: 10.1093/oxfordjournals.jbchem.a124590. [DOI] [PubMed] [Google Scholar]
- 30.Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–6. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- 31.Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, Caldwell RB. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain RK. Molecular regulation of vessel maturation. NatMed. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 33.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 34.Ninomiya K, Takahashi A, Fujioka Y, Ishikawa Y, Yokoyama M. Transforming growth factor-beta signaling enhances transdifferentiation of macrophages into smooth muscle-like cells. Hypertens Res. 2006;29:269–76. doi: 10.1291/hypres.29.269. [DOI] [PubMed] [Google Scholar]
- 35.Sharifi BG, Zeng Z, Wang L, Song L, Chen H, Qin M, Sierra-Honigmann MR, Wachsmann-Hogiu S, Shah PK. Pleiotrophin induces transdifferentiation of monocytes into functional endothelial cells. Arterioscler Thromb Vase Biol. 2006;26:1273–80. doi: 10.1161/01.ATV.0000222017.05085.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuter U, Champion C, Kain HL. Transdifferentiation of human monocytes into fibroblast-like cells in vitro. Ger J Ophthalmol. 1995;4:182–7. [PubMed] [Google Scholar]
- 37.Loomans CJ, Wan H, de Crom R, van Haperen R, de Boer HC, Leenen PJ, Drexhage HA, Rabelink TJ, van Zonneveld AJ, Staal FJ. Angiogenic murine endothelial progenitor cells are derived from a myeloid bone marrow fraction and can be identified by endothelial NO synthase expression. Arterioscler Thromb Vase Biol. 2006;26:1760–7. doi: 10.1161/01.ATV.0000229243.49320.c9. [DOI] [PubMed] [Google Scholar]
- 38.Jabs A, Moncada GA, Nichols CE, Waller EK, Wilcox JN. Peripheral blood mononuclear cells acquire myofibroblast characteristics in granulation tissue. J Vase Res. 2005;42:174–80. doi: 10.1159/000084406. [DOI] [PubMed] [Google Scholar]


