Abstract
Background: The novel protein PTPIP51 (SwissProt accession code Q96SD6) is known to interact with two non-transmembrane protein-tyrosine phosphatases, PTP1B and TCPTP in vitro. Overexpression of the full-length protein induces apoptosis in HeLa and HEK293T cells (Lv et al. 2006). PTPIP51 shows a tissue-specific expression pattern and is associated with cellular differentiation and apoptosis in some mammalian tissues, especially in human follicular and interfollicular epidermis. PTPIP51 protein is expressed in all suprabasal layers of normal epidermis, whereas the basal layer contains PTPIP51 mRNA only but lacks the protein. Objectives: The expression of PTPIP51 was investigated in keratinocyte carcinomas, that is human basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) as well as Bowen's disease (BD) and keratoacanthomas (KAs) on a transcriptional (mRNA) and translational (immunohistochemical) level. Methods: Formalin-fixed, paraffin-embedded sections of BCCs, SCCs, KAs and BD, respectively, were analysed by RT-PCR, as well as immunohistochemistry and subsequent fluorescence microscopy. PTPIP51-positive cells of the tumour and the surrounding stroma were identified on the basis of specific morphological features by means of H & E staining. To obtain further information about a putative function of PTPIP51, a possible association of PTPIP51 with apoptotic cells, as well as an assumed negative correlation with proliferating cells was investigated by means of an in-situ TUNEL assay and Ki67/MIB-1 antigen staining, respectively. Co-immunostainings with PTPIP51 were performed for the following antigens: TCPTP, PTP1B and β-catenin. Results: PTPIP51-expression was detected in BCCs and SCCs of the skin, as well as in KAs and BD. Both types of keratinocyte carcinoma revealed a specific localization pattern of PTPIP51 in malignant keratinocytes. Whereas PTPIP51 -positive cells of BCC were found to form two cluster types with a different subcellular localization of the protein, i.e. cytoplasmic and nuclear or predominantly membranous, investigation of SCC revealed a meshwork-like appearance of PTPIP51-positive malignant keratinocytes, created by a mainly membranous localization. BD and KA resembled the findings of PTPIP51-expression in SCC. Furthermore, we observed a partial co-localization of PTP1B and PTPIP51 in BCC. SCC and BCC showed a co-expression and partial co-localization of PTPIP51 with β-catenin. Some PTPIP51-positive cells were found to undergo apoptosis. PTPIP51 was also expressed in cells comprising the surrounding stromal microenvironment. This was particularly noticed for endothelial cells lining peritumoural vessels as well as for infiltrating cells of both, the innate and the adaptive immune system. Conclusions: The results showed a distinct mainly membranous expression pattern of PTPIP51 in BCCs and SCCs. Since PTPIP51 was also detected in the peritumoural tissue, the protein may play a crucial role in keratinocyte tumour development.
Keywords: PTPIP51, PTP1B, β-catenin, keratinocyte carcinoma, non-melanoma skin cancer, basal cell carcinoma, squamous cell carcinoma
Introduction
PTPIP51 was originally detected by a yeast two-hybrid system as an interacting partner of two non-transmembrane protein-tyrosine phosphatases, PTP1B and TcPTP [1]. As reviewed by Ostman and colleagues [2] PTP1B plays a dual role in the tumourigenesis of different human cancers, either inhibiting or stimulating cancer-associated signalling processes [3].
A study performed by Stenzinger et al.[4] revealed that PTPIP51 is expressed in highly differentiated tissue, particularly in follicular and interfollicular epidermis, both requiring a careful balance between proliferation, differentiation and apoptosis. PTPIP51 protein is expressed in all suprabasal epidermal layers but not in the basal layer harbouring stem cells and proliferative cell units. It was also shown that PTPIP51 protein is expressed in HaCaT cells, partly genetically and partly phenotypically resembling ker-atinocytes in the early stages of squamous cell carcinogenesis [5, 6]. PTPIP51-expression in these cells was influenced by retinoic acid and 1,25(OH2)D3[7].
Therefore, we hypothesized that PTPIP51 may play a role in differentiation and apoptosis of cells. Independent observations by Lv and colleagues [8] as well as Roger and coworkers [9] seem to corroborate preliminary results obtained from the studies mentioned above.
Due to the specific expression pattern of PTPIP51 in mammalian epidermis and stimulated by the study of Lv and colleagues [8] who demonstrated the expression of PTPIP51 mRNA in several kinds of human cancers, investigation of the PTPIP51-expression profile in non-melanoma skin cancer (keratinocyte carcinoma, KC) is of great interest. This is further underscored by the fact that they are the most common tumours in humans with a worldwide increasing incidence [10]. Hence, KCs, namely, basal cell carcinomas (BCCs) as well as squamous cell carcinomas (SCCs) and their precancerous lesions were investigated for their PTPIP51 expression-pattern by immunohistochemistry and on atranscriptional level by RT-PCR. Keratoacanthoma (KA), an abortive malignancy that rarely progresses into an invasive squamous cell carcinoma, was also investigated for PTPIP51-expression [11, 12].
To obtain further information about the function of PTPIP51, co-immunostaining with a β-catenin-detecting antibody was performed, β-catenin serves as a gene-transcription factor and controls proliferation, differentiation, as well as cell–cell interaction [13, 14]. Hence, it is believed to play a central role in the development of SCCs. Moreover, the cadherin–catenin complex at epithelial adherens junctions is reported to be dephosphorylated by PTP1B [15, 16], an interacting partner of PTPIP51. With respect to those findings, co-immunostainings with PTPIP51 and the non-transmembrane PTPs, TCPTP and PTP1B, were carried out. An in-situ TUNEL assay as well as Ki-67 antigen staining was performed to check for PTPIP51 expression in apoptotic and proliferative tumour cells, respectively.
Material and methods
Tissue and section preparations
The study was performed with 22 formalin-fixed, paraffin-embedded samples of human keratinocyte carcinomas (10 BCCs and 6 SCCs), KAs (3) and BD (3), retrieved from the files of the Department of Dermatology and Andrology, Justus-Liebig-University, Giessen.
All BCC samples were reviewed according to the WHO-classification. SCCs were not graded according to Broders [17] since this system has some shortcomings concerning the clinical application of histologic results [18]. However, the tumour thickness, a valuable predictor of the metastaticrisk, was assessed [19]. The number of female cases was 14 (70%) and of male cases 6 (30%), respectively.
For immunohistochemistry, paraffin-sections of 4–5 μm thickness were cut, dried, deparaffinized in xylene and re-hydrated in graded alcohol. Prior to the staining procedure, antigen retrieval using microwave oven heating (2 × 5min., 10 mM, 800 W) in standard sodium citrate buffer (pH 6.0) was carried out for all antibodies used in this study.
Immunohistochemistry
Immunohistochemical staining was performed according to standard protocols.
The primary polyclonal antibody to PTPIP51 was used in 1:250 dilutions for immunocytochemistry and visualized by Alexa fluor 555 secondary antibody. Primary monoclonal antimouse antibodies used for double staining were as follows: MIB-1/Ki-67 (Dako, Cat ab28025), β-Catenin (MBL, Cat k0102–3) and PTP1B (Calbiochem, Cat PH02). The reaction was visualized by using Alexa fluor 488 secondary antibody. Nuclei were displayed by DAPI.
The in situ cell death detection kit ApoTag (Chemicon International, S7110) was used according to the instructions of the manufacturer.
H&E counterstaining
After obtaining photographs from immunostained samples, the coverslip of each slide was removed and the sections were subsequently stained with haematoxylin and eosin. Light microscopy was used to take H&E stained pictures of the corresponding immunohistochemical section.
PTPIP51 antibody production
The cDNA sequence encoding amino acids 131–470 were inserted into the BamHI and Hindlll sites of the plasmid pQE30 and expressed as His6-tagged protein in the protease deficient E. coli expression strain AD202 (ara D139DE(argF-lac)169 ompT1000:kan flh D5301 fruA25 relA1 rps150(strR) rbsR22 deoC1). The protein was purified to electrophoretic homogeneity by chromatography on a Ni-agarose column [1]. Immunization of rabbits was performed with 0.5 mg of the purified protein in 0.5 ml RIBI adjuvant followed by booster injections with 0.5 and 0.3 mg on day 14 and 21, respectively. The antiserum was collected on day 28. Monospecific antibodies were prepared following the method described by Olmsted [20]. Briefly, 2 mg of purified antigen were blotted on nitrocellulose after SDS electrophoresis. The protein band was marked with Ponceau solution and was cut out. After blocking the membrane strip with 1% low-fat milk powder in phosphate buffered saline, the membrane was incubated with the antiserum for 1 hr followed by extensive washing with Tris-ethylenediaminetetraacetic acid (EDTA)-buffered saline. The antibodies were eluted with 0.2 M glycine (pH 2.0) for 2 min., followed by immediate neutralization with 1 M triethanolamine. The assessment of the antibody specificity was described in more detail in Stenzinger et al. (2005) [4].
RNA extraction
RNA extraction from cryomaterial was performed using the RNA extraction kit RNeasy MINI (Qiagen, Hilden, Germany).
RNA extraction from paraffin material was performed as follows: Eight paraffin sections were collected in a reaction tube and deparaffinized in 500 μl xylene for 10 min. at 53°C. After centrifugation, pellet was re-suspended in 200 μl 1 M guanidine thiocyanate, 0.5% sarcosyl, 0.72%β-mercaptoethanol, 20 mM Tris-HCl (pH 7.5). After adding proteinase K to a final concentration of 0.5 μg/μl, samples were digested for 20 hrs at 58°C. Subsequently, 20 μl 2 M sodium acetate, 220 μl phenol (pH 4.3) and 60 μl chloroform/isoamylalcohol (24/1) were added. Samples were vortexed and centrifuged for 15 min. at 4°C. The aqueous layer was collected, 1 μl glycogen (10 mg/ml) added, and precipitated with 200 μl isopropanol. Samples were frozen for one hour at −20°C and centrifuged for 15 min. at 12,000 × g. Pellets were washed with 75% ethanol, air dried and re-suspended in 10 μl RNase-free water.
First strand synthesis
First-strand synthesis was performed using omniscript (cryomaterial) and sensiscript (paraffin material), according to the manufacturers protocol (Qiagen, Hilden, Germany).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
RT-PCR was performed on an iCycler using SYBR Green Supermix (BioRad, Munich, Germany) to visualize the respective amplicons [21]. Per sample, 2 μl (cryomaterial) and 4 μl (paraffin material) cDNA were used for amplification of PTPIP51. Cycling conditions were 95°C for 3 min., followed by 40 cycles of 95°C for 30 sec., 55°C for 30 sec. and 72°C for 30 sec. The following primers were employed (MWG, Ebersberg, Germany): 5′gcaggtggtgctatcaggtc3′ as forward primer and 5′agctccagggccaacttcatc3′ as reverse primer resulting in a 232 bp amplification product.
PCR products were visualized by agarose gel electrophoresis. While amplification of a 90 bp β-actin product served as positive control [22], negative controls included samples lacking reverse transcriptase.
Results
This study demonstrates the expression of PTPIP51 mRNA and protein in the two major types of keratinocyte carcinomas (BCC and SCC), as well as in KAs and BD.
Immunohistochemical staining of PTPIP51 was assessed with respect to both, the intracellular localization (membranous, cytoplasmic and nuclear) and the staining intensity in tumour tissue and the surrounding stromal microenvironment of each entity. Figure 1 summarizes the obtained results.
Fig 1.
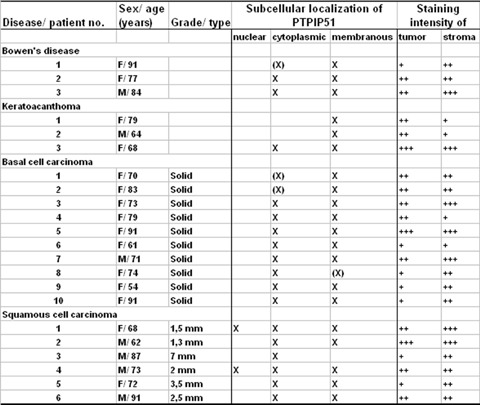
Summary of PTPIP51 expression in keratinocyte carcinomas and precancerous lesions. Staining intensity was assessed as strong (+++), moderate (++), weak (+), not reactive (−) and the staining pattern was divided into three groups: Nuclear, cytoplasmic and membranous and was marked by an X or (X). X indicates that this particular PTPIP51 staining pattern was observed for the majority of cells (>75%) and X in brackets (X) means a staining pattern observed for the minority of cells (<25%). Basal cell carcinoma (BCCs) were graded according to the WHO classification, for squamous cell carcinoma (SCCs), the tumour thickness is given in mm.
RT-PCR analysis revealed the presence of PTPIP51 mRNA in all investigated tissue samples including healthy epidermis (Fig. 2A and B). β-actin amplification served as an internal positive control [23, 24].
Fig 2.
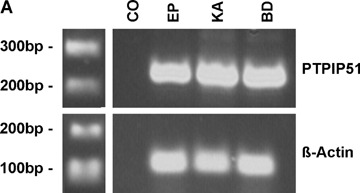
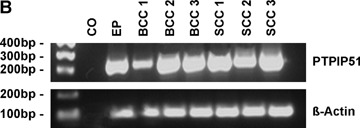
Expression of PTPIP51 in healthy epidermis, SCC, BCC, BD and KA as detected by RT-PCR RT-PCR was performed using primers specific to PTPIP51 as given in the Material and methods section, β-actin was amplified as an internal positive control and probes lacking reverse transcriptase served as negative controls (CO). (A) Samples of healthy epidermis (EP), keratoacanthoma (KA) and Bowen's disease (BD). The white bar between the marker and the lanes indicates that the additional lanes depicted by this photograph were cut out since they were probed with tissue samples unrelevant for the present study. (B) Samples of healthy epidermis (EP), basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For BCCs and SCCs, RT-PCR was performed with tumour tissue of three different patients (BCC1–3 andSCC1–3).
Immunohistochemical detection of PTPIP51 in BCCs
PTPIP51 -positive malignant keratinocytes predominantly showed a clustered formation, surrounded by keratinocyte fields lacking PTPIP51 protein. Single PTPIP51 -expressing keratinocytes were only rarely observed.
These cell-clusters revealed a combined cytoplasmic and nuclear staining of PTPIP51, this was especially observed for tumour nests situated beneath or dripping down from the epidermis. A second form of clustering was observed for keratinocytes of tumour nests located in the dermis preponderantly showing a strong membranous and faint cytoplasmic PTPIP51 -staining (Fig. 3A-C). Neighbouring cells sometimes revealed a strong PTPIP51 -positive rim at the opposing part of the cellular membrane (Fig. 3B and C). This finding was often found to be associated with immune cell infiltrates, comprising granulocytes and lymphocytes (Fig. 4).
Fig 3.
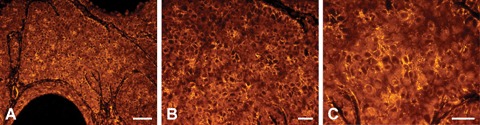
PTPIP51-immunostaining of a BCC (A) Overview. PTPIP51–positive cells in the malignant tissue lie in clusters showing a punctiform membranous localization of the protein. In malignant keratinocytes adjacent to the peritumoural stroma, PTPIP51 is localized at the basolateral segment of the cellular membrane. (B) Magnification of the same section (C) High-power view. Bar: A: 50 μm, B, C: 20 μm.
Fig 4.
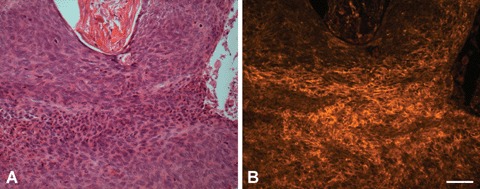
H+E staining and PTPIP51-immunostaining of inflammatory cells infiltrating a BCC (A) H+E staining. Note the eosinophils migrating through the malignant tissue (B) PTPIP51-immunostaining of the same section showing a membranous and attenuated cytoplasmic localization of PTPIP51 in eosinophils and adjacent tumour cells. Bar: 50 μm.
In some cases, however, the membranous distribution of PTPIP51 was located at the basolateral part of keratinocytes adjacent to the stromal tissue (Fig. 3A and B).
Immunohistochemical detection of PTPIP51 in SCCs
In contrast to BCCs, immunostaining of SCCs revealed a punctiform, mainly membranous localization of PTPIP51, thereby creating a meshwork-like appearance of adjacent keratinocytes (Fig. 5A-F). Other SCC samples, however, showed a focal faint cytoplasmic or somewhat stronger nuclear reaction (Fig. 5B). It was noticed that the intensity of the membranous staining of PTPIP51 was attenuated in keratinocytes adjacent to the surrounding stroma and in those encompassing intraepidermal and extraepidermal keratinization (Fig. 5B)
Fig 5.
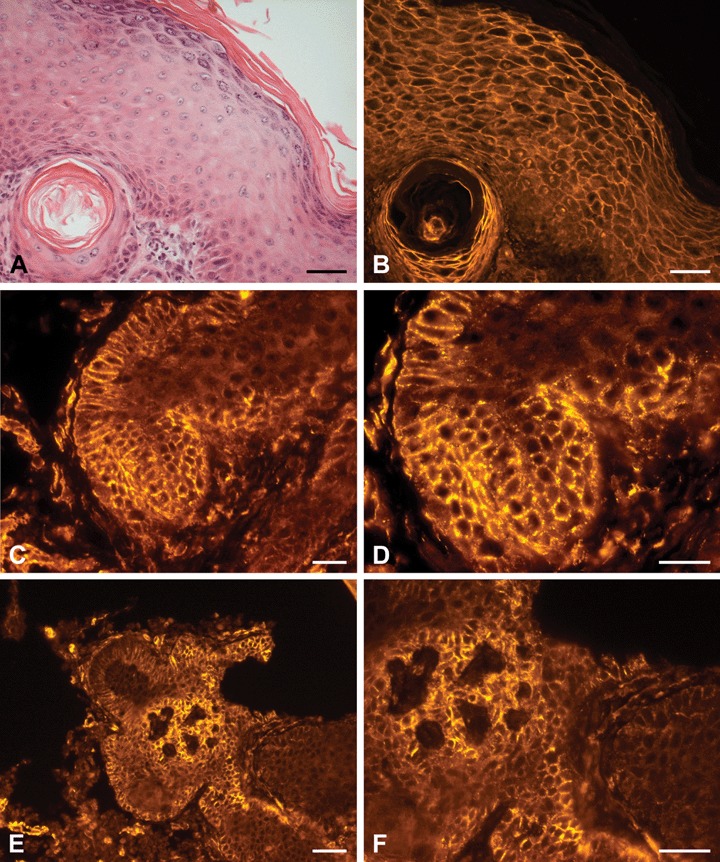
H+E staining and PTPIP51-immunostaining of aSCC (A) H+E staining of epidermis next to SCC tissue with a horn pearl beneath. (B) PTPIP51-immunostaining of the same section displays a strong membranous and faint nuclear staining of keratinocytes. Cells lining the horn pearl presented a more intense signal. (C) Membranous PTPIP51-immunostaining of a SCC (D) High-power view of the same section. PTPIP51 staining reveals a punctiform localization-pattern. (E) Membranous PTPIP51 -immunostaining of a SCC. Some keratinocytes show an attenuated staining and some even lack PTPIP51 (F) Magnification of the same section. Bar: A, B: 50 μm, C–F: 20 μm.
Investigation of PTPIP51-expression in BD and keratoacanthoma resembled these results.
Surrounding stromal microenvironment of BCCs and SCCs
Healthy dermis hosts cells and structures, such as fibrocytes, glands as well as capillaries, microvessels and vessels, which are frequently but not always positive for PTPIP51 [4]. However, investigation of the stromal tumour microenviroment revealed a striking difference. There was a sheer abundance of PTPIP51-positive immune cells and endothelial cells expressing PTPIP51.
These cells differed also in their staining intensity compared to normal tissue.
A particularly strong reaction with the PTPIP51 antibody was observed in cells of the immune system infiltrating the peritumoural tissue (Fig. 6). The same staining intensity was also noticed for immune cells infiltrating the tumour tissue itself (Fig. 4).
Fig 6.
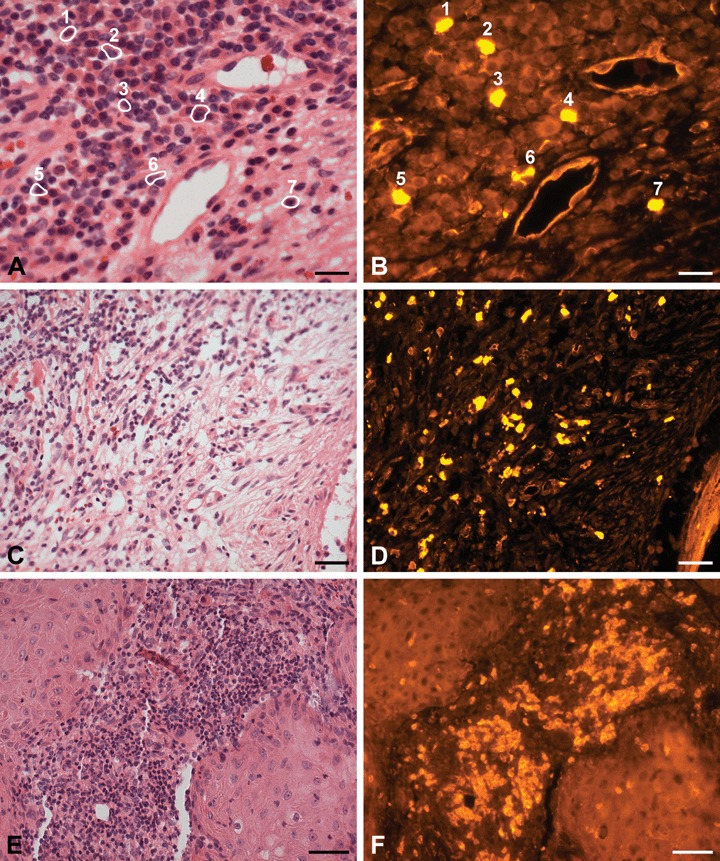
H+E staining and PTPIP51-immunostaining of peritumoural tissue of SCC (E, F) and BCC (A, B, C, D) (A) H+E staining of two microvessels embedded in surrounding stroma of BCC. The latter is infiltrated by lymphocytes and cells (numbered and marked by circlets) and (B) Some immune cells (1–4: neutrophils and eosinophils, 5–7: lymphocytes) and the endothelial cells express PTPIP51. (C) Overview. H+E staining of BCC stroma infiltrated by lymphocytes and some granulocytes (D) PTPIP51 is expressed in some of these immune cells. (E) H+E staining of an inflammatory infiltrate mainly comprising granulocytes and lymphocytes located in the stroma of a SCC. (F) PTPIP51 is expressed in many but not all of these immune cells. Bar: A–D: 20 μm, E and F: 50 μm.
Immune cells of both, the myeloid and lymphoid lineages expressed PTPIP51.
Figure 6(A and B) shows PTPIP51-positive granulocytes infiltrating the stroma of a BCC. Lymphocytes migrating through the peritumoural stroma were also positive to the PTPIP51 antibody in both stroma of BCCs and stroma of SCCs (Fig. 6C–F).
Endothelial cells lining venous and arterial microvessels and capillaries, being located nearby tumour nests, were also PTPIP51-positive (Fig. 7). Lumina packed with granulocytes and lymphocytes that showed a strong PTPIP51 labelling were detected quite frequently (Fig. 8). Through a comparison of different tumour samples and different regions within one sample, it was noticed, however, that some cells of the same immune cell type, that is neutrophils, expressed PTPIP51 and others did not. This observation is exemplified by Figure 8C–F.
Fig 7.
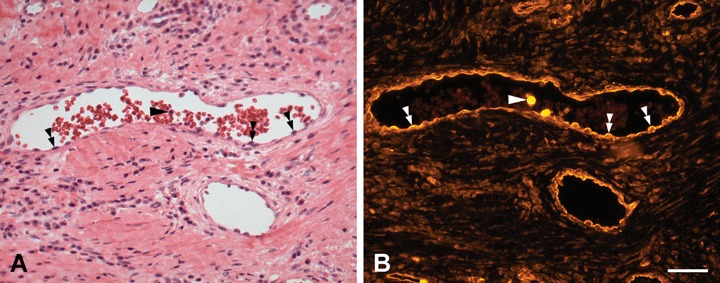
H+E staining and PTPIP51-immunostaining of microvessels in the peritumoural stroma of a BCC (A) H+E staining (B) Immunostaining of the same section. PTPIP51 is expressed in endothelial cells (double arrowheads) and two neutrophils in the vessel lumen (arrow). Both immune cells show an intense signal of the PTPIP51-antibody. Bar: 50 μm.
Fig 8.
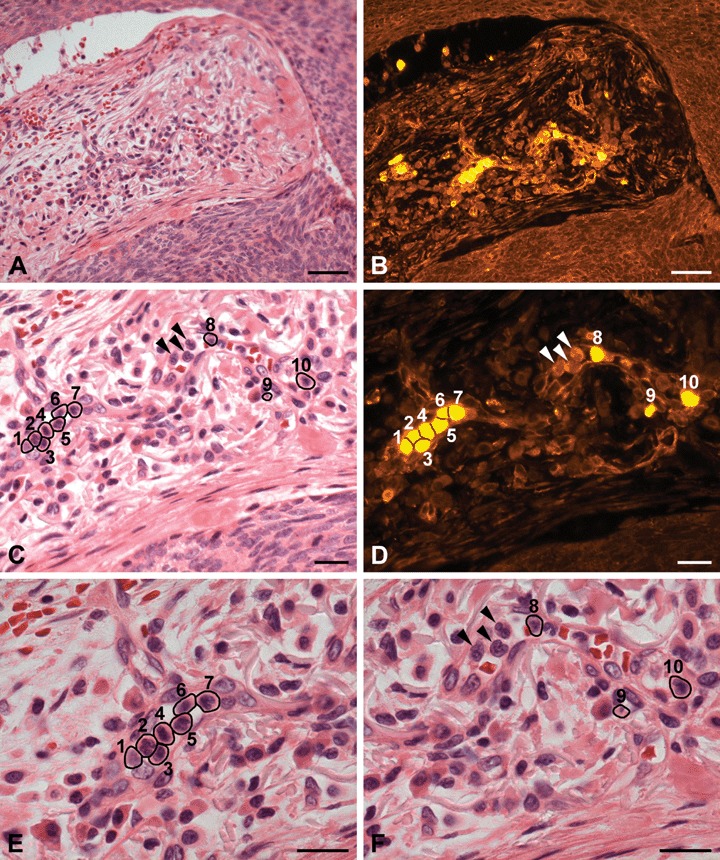
H+E staining and PTPIP51-immunostaining of a venous microvessel in the peritumoural tissue of a BCC. (A) H+E staining. Overview. (B) PTPIP51-immunostaining of the same section (C) H+E staining. Higher magnification of the same section. Multiple immune cells are located in the lumen of a capillary. The cells are numbered and marked by circlets. (D) PTPIP51-immunostaining of the same section. Not all immune cells (marked by arrowheads) are PTPIP51-positive. (E) and (F) provide a high-power view of (C) to identify PTPIP51-positive cells: 1–7: eosinophils and neutrophils; 8–10: neutrophils. As indicated by arrowheads, not all immune cells are PTPIP51-positive. Bar: A, B: 50 μm, C–F: 20 μm.
By comparing BCC and SCC stroma, we did not find a difference concerning the staining pattern or intensity of PTPIP51-positive immune cells and endothelial cells of microvessels and capillaries.
Doublestaining experiments of PTPIP51
-
(i) Ki67 and PTPIP51
MIB-1/Ki67 staining of BCC and SCC revealed that PTPIP51-positive malignant keratinocytes were not proliferating (data not shown). The same observation held true for PTPIP51 expressing immune cells infiltrating the stroma.
-
(ii) TUNEL-assay and PTPIP51
Co-immunostaining experiments of BCCs and SCCs with the PTPIP51 antibody and in situ TUNEL-labelling demonstrated only some PTPIP51-positive malignant cells executing apoptotic cell death. This finding is exemplified by Figure 9.
-
(iii) PTP1B and PTPIP51
An immunodetectable amount of the other PTPIP51 -interacting partner, that is TCPTP was neither noticed for SCCs nor for BCCs.
-
(iv) β-catenin and PTPIP51
Co-immunostaining of β-catenin and PTPIP51 in SCC showed a partial co-localization, which was mainly restricted to the membrane of malignant keratinocytes (Fig. 12A–C). In a few cells, a co-localization could also be detected in the nucleus or cytoplasm. These findings are in accordance with double-staining experiments performed with HaCaT cells (Fig. 12D–F). A co-expression and partial co-localization of PTPIP51 and β-catenin could also be detected in BCC.
Fig 9.
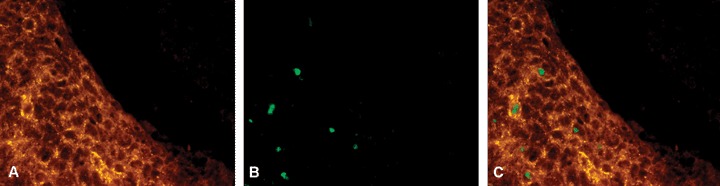
Detection of PTPIP51 and apoptosis in BCC tumour cells (A) PTPIP51 (B) in-situ TUNEL assay (C) Overlay. Not all PTPIP51-expressing cells are apoptotic. Bar: 20 μm.
Fig 12.
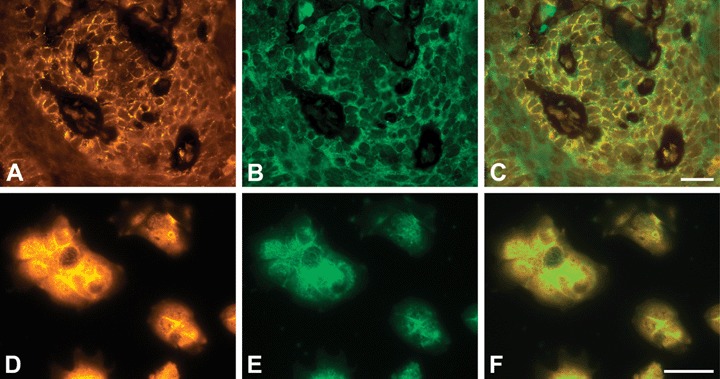
Co-immunostaining of PTPIP51 and β-catenin in a human SCC (A–C) and HaCaT cells (D–F) (A) PTPIP51, (B) β-catenin-immunostaining of the same section, (C) Overlay. Co-localization is indicated by yellow colour. (D) PTPIP51. Note the predominant membranous localization. (E) β-catenin (F) Overlay. Co-localization is indicated by yellow colour. Bar: 20 μm.
Fig 10.
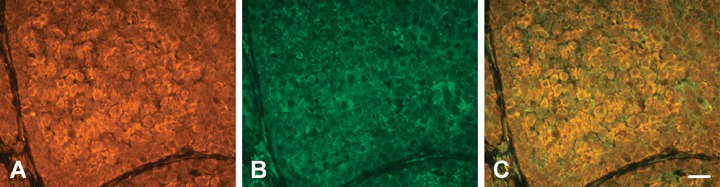
Co-immunostaining of PTPIP51 and PTP1B in a human BCC (A) PTPIP51, (B) PTP1B-immunostaining of the same section, (C) Overlay. Colocalization is indicated by yellow colour. Bar: 20 μm.
Fig 10.
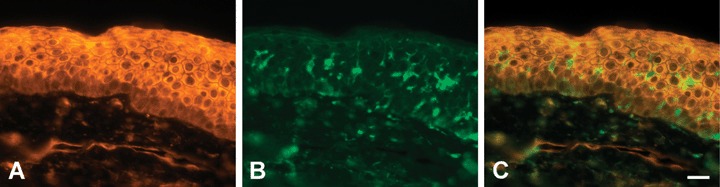
Co-immunostaining of PTPIP51 and PTP1B in healthy human epidermis (A) PTPIP51 (B) PTP1B-immunostaining of the same section. Note that only Langerhans cells express PTP1B. (C) Overlay. Bar: 20 μm.
Discussion
To our knowledge, this study demonstrates for the first time the mRNA expression and a distinct cellular localization of PTPIP51 protein in keratinocyte carcinomas, that is BCC and SCC. A specific expression pattern for PTPIP51 was also observed for pre-cancerous BD, as well as for KAs. Furthermore, PTPIP51 could be detected in specific cells and structures comprising the peritumoural stromal microenvironment.
Due to the steadily increasing incidence of non-melanoma skin cancer subsequently resulting in a higher therapeutic effort, these carcinomas are a major health burden greatly affecting public health care costs [25, 26].
Malignant tissue
Malignant keratinocytes showed a tumour-entity specific cellular localization of PTPIP51. However, not all malignant keratinocytes were found to be positive for PTPIP51.
Whereas, SCCs showed mainly a membranous localization creating a meshwork-like appearance of keratinocytes, immunostaining of BCCs revealed cell clusters of combined cytoplasmic and nuclear or strong membranous and faint cytoplasmic distribution of PTPIP51 within the cells.
If PTPIP51 und β-catenin are co-expressed in malignant cells of SCCs and BCCs, both proteins also co-localize, predominantly at the cellular membrane and in a few cases in the cytoplasm. Results of our immunohistochemical experiments with β-catenin are in accordance with observations made by El-Bahrawy and colleagues [27] as well as Brasanac and coworkers [28].
A similar staining pattern for PTPIP51 and β-catenin could also be observed in HaCaT cells resembling precancerous lesions of SCC by its genetic mutations. [5, 6]
A cytoplasmic pool of β-catenin serves as a nuclear gene-transcription factor that is integrated in the Wnt-pathway, thereby controlling proliferation and differentiation, as well as development of normal tissue. Alterations of this pathway play a role in carcino-genesis and non-melanoma skin cancer in particular [13, 14, 29]. Additionally, many BCCs have somatic mutations in the PTCH1 gene ultimately leading to a disturbed hedgehog-signalling pathway. It is speculated that these alterations may lead to increased Wnt-expression [30, 31]. A stable pool of β-catenin, however, is bound to the adherens junctions of epithelial cells, bridging the cadherin cell–cell adhesion molecules with the actin containing cytoskeleton. Dephosphorylation of tyrosine residues on β-catenin by an interacting partner of PTPIP51, PTP1B maintains cadherin–actin cytoskeletal linkage [15, 16]. Bearing in mind the partial co-expression and co-localization of β-catenin and PTPIP51 in SCCs, one can speculate that PTPIP51 plays a role in this bridging process.
v-Src-kinase is known to phosphorylate β-catenin in vitro, causing destabilization of this anchorage system [32, 33]. V-Src belongs to a Src-kinase superfamily, which is well known to play a major role in the regulation of cell growth, differentiation, survival, cell shape, migration and as a protooncogene in cancer development [34, 35]. Interestingly, biochemical experiments revealed that PTPIP51 is tyrosine-phosphorylated on Y176 by c-Src and v-Src kinase in vitro (Schreiner and Hofer, personal communication).
Lv and colleagues [8] reported an inherent N-terminal mitochondrial targeting sequence for PTPIP51 that is also responsible for induction of apoptosis in vitro. The in vivo immunohistochemical results presented in this study, however, revealed only a few PTPIP51-positive cells to execute apoptosis as identified by TUNEL-labelling. These findings may be explained by putative isoforms or splicing variants expressed in different tissues that lack this N-terminal sequence, which is mandatory for the execution of apoptosis [4, 8]. This hypothesis is supported by the fact that PTPIP51 was not found to be exclusively restricted to mitochondria but was present at the cellular membrane and to a lower extent in the cytosol of malignant keratinocytes [7, 8, 36]. The intracellular localization may also be explained by an in vivo interaction with PTP1B or Src-kinases.
The stem cell compartment of healthy epidermis and seminiferous epithelium harboring proliferative cell units lack PTPIP51 protein [4]. Ki 67 staining of keratinocyte carcinomas revealed that proliferative cells in both tumour and tumour stroma did not express an immunodetectable amount of PTPIP51.
Peritumoural stroma
PTPIP51 was also found to be expressed in cells comprising the peritumoural stroma. This microenvironment serving as a soil for the malignant seeds provides a connective-tissue framework and includes immune and inflammatory cells, as well as fat cells and blood vessels [37, 38]. Hence, interactions between the tumour and the surrounding stroma play an important role in epithelial tumourigenesis [39] and can largely determine the phenotype of the tumour [40, 41].
Angiogenesis
Tumour angiogenesis is a crucial process in tumourigenesis not only promoting tumour growth but also the progression from pre-malignant to malignant and invasive cancers [34, 42]. VEGF produced by malignant cells stimulates endothelial cell proliferation and angiogenesis. Malignant tissue may also induce the production of VEGF in their surrounding stroma [43]. Inhibition of VEGF activity resulted in reduced tumour angiogenesis and tumour growth. VEGF-receptor 2 blockade, in particular, induced a shift from a highly malignant to a pre-malignant, non-invasive cancer [44].
Interestingly, observations made by Tinsley and colleagues and other research groups suggest that activated neutrophils induce Src kinase-mediated tyrosine phosphorylation of endothelial β-catenin, thus leading to gap-formation and hyperpermeability of adjacent endothelial cells [45, 46]. Since endothelial cells of peritumoural capillaries and microvessels as well as a subset of neutrophils located therein were found to be positive for PTPIP51, the protein may play a role in the aforementioned processes.
Immune system
Immune cells in general play a complex and sometimes antagonistic role by promoting carcinogenesis on the one hand and keeping malignant tissue under immune surveillance on the other hand.
However, deletion of B- and T cells comprising the adaptive immune system results in attenuated pre-malignant progression and reduced skin carcinoma incidence, which is most likely caused by a halted chronic inflammation [47]. Innate immune cells promote angiogenesis and thus tumourigenesis of skin cancer by matrix metalloproteinase production. Furthermore, mast cells of the innate immune system seem to be crucial for keratinocytes to achieve hyperproliferative growth characteristics during neoplastic development [48, 49, 50]. PTPIP51-positive innate and adaptive immune cells invading malignant tissue, that is macrophages, eosinophils, neutrophils and lymphocytes may serve as important mediators nourishing chronic peri- and intratumoural inflammation. Since distinct cells of one immune cell type, that is neutrophils were found to express PTPIP51 and others were not, these findings may be interpreted as a selective up- and down-regulation of PTPIP51 in immune cells, putatively altering the functional state of these cells. Since both interacting partners of PTPIP51, TCPTP and PTP1B are involved in immune cell signalling and myeloid development [51], an ongoing study aims to investigate PTPIP51-expression in differentiating immune cells of both, the myeloid and the lymphoid lineages.
Acknowledgments
We thank Mrs. T. Papadakis (Institute of Anatomy and Cell Biology, Giessen: Germany) for helpful technical advices and Dr. HW. Hofer (Department of Biology, Konstanz, Germany) for critically reading the manuscript.
References
- 1.Porsche A. Allensbach UFO Publishers. 2001;414 Identifikation von Interaktion-spartnern der T-Zell Protein-Tyrosin-Phosphatase durch das Lex-A Two Hybrid System thesis (PhD) University of Konstanz.; Vol. [Google Scholar]
- 2.Östman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–20. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 3.Tonks NK, Muthuswamy SK. A Brake becomes an accelerator: PTP1B- A new therapeutic target for breast cancer. Cancer Cell. 2007;3:214–6. doi: 10.1016/j.ccr.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Stenzinger A, Kajosch T, Tag C, Porsche A, Welte I, Hofer HW, Steger K, Wimmer M. The novel protein PTPIP51 exhibits tissue- and cell-specific expression. Histochem Cell Biol. 2005;123:19–28. doi: 10.1007/s00418-004-0732-7. [DOI] [PubMed] [Google Scholar]
- 5.Boukamp P, Popp S, Altmeyer S, Hulsen A, Fasching C, Cremer T, Fusenig NE. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer. 1997;19:201–14. doi: 10.1002/(sici)1098-2264(199708)19:4<201::aid-gcc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Ashton KJ, Carless MA, Griffiths LR. Cytogenetic alterations in nonmelanoma skin cancer: a review. Genes Chromosomes Cancer. 2005;43:239–48. doi: 10.1002/gcc.20183. [DOI] [PubMed] [Google Scholar]
- 7.Stenzinger A, Schreiner D, Pfeiffer T, Tag C, Hofer HW, Wimmer M. Epidermal growth factor-, transforming growth fac-tor-β, retinoic acid- and 1,25-Dihydroxyvitamin D3-regulated expression of the novel protein PTPIP51 in ker-atinocytes. Cells Tissues Organs. 2006;184:76–87. doi: 10.1159/000098949. [DOI] [PubMed] [Google Scholar]
- 8.Lv BF, Yu CF, Chen YY, Guo JH, Song QS, Ma LD, Shi TP, Wang L. Protein tyrosine phosphatase interacting protein 51 (PTPIP51) is a novel mitochondria protein with an N-terminal mitochondrial targeting sequence and induces apoptosis. Apoptosis. 2006;11:1489–501. doi: 10.1007/s10495-006-8882-9. [DOI] [PubMed] [Google Scholar]
- 9.Roger J, Goureau O, Sahel JA, Gouillonneaux X. Use of suppression subtractive hybridization to identify genes regulated by ciliary neutrophic factor in postnatal retinal explants. Molecular Vision. 2007;13:206–19. [PMC free article] [PubMed] [Google Scholar]
- 10.Albert MR, Weinstock MA. Keratinocyte carcinoma. CA Cancer J Clin. 2003;53:292–302. doi: 10.3322/canjclin.53.5.292. [DOI] [PubMed] [Google Scholar]
- 11.Kwittken J. Dermatologic pseudobenignities. Mt Sinai JMed. 1980;47:34–7. [PubMed] [Google Scholar]
- 12.Schwartz RA. Keratoacanthoma: a clinicopathologic enigma. Dermatol Surg. 2004;30(2 Pt 2):326–33. doi: 10.1111/j.1524-4725.2004.30080.x. [DOI] [PubMed] [Google Scholar]
- 13.Doglioni C, Piccinin S, Dementis S, Cangi MG, Pecciarini L, Chiarelli C, Armellin M, Vukosavljevic T, Boiocchi M, Maestro R. Alterations of beta-catenin pathway in non-melanoma skin tumors: loss of alpha-ABC nuclear reactivity correlates with the presence of beta-catenin gene mutation. Am J Pathol. 2003;163:2277–87. doi: 10.1016/s0002-9440(10)63585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Balsamo J, Arregui C, Leung T, Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J Cell Biol. 1998;143:523–32. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu G, Arregui C, Lilien J, Balsamo J. PTP1B modulates the association of beta-catenin with N-cadherin through binding to an adjacent and partially overlapping target site. J Biol Chem. 2002;277:49989–97. doi: 10.1074/jbc.M206454200. [DOI] [PubMed] [Google Scholar]
- 17.Broders AC. The grading of carcinoma. Minn Med. 1925;8:726–30. [Google Scholar]
- 18.Heffner DK. Let's make grading of squa-mous cell carcinomas more meaningful to clinicians (via “Ed's Insight”) Ann Diagn Pathol. 2002;6:399–403. doi: 10.1053/adpa.2002.36652. [DOI] [PubMed] [Google Scholar]
- 19.Breuninger H, Black B, Rassner G. Microstaging of squamous cell carcinomas. Am J Clin Pathol. 1990;94:624–7. doi: 10.1093/ajcp/94.5.624. [DOI] [PubMed] [Google Scholar]
- 20.Olmsted JB. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981;256:11955–7. [PubMed] [Google Scholar]
- 21.Brehm R, RUttinger C, Fischer P, Gashaw I, Winterhager E, Kliesch S, Bohle RM, Steger K, Bergmann M. Transition from preinvasive carcinoma in situ to seminoma is accompanied by a reduction of connexin 43 expression in Sertoli cells and germ cells. Neoplasia. 2006;8:499–509. doi: 10.1593/neo.05847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biermann K, Heukamp LC, Steger K, Zhou H, Franke FE, Sonnack V, Brehm R, Berg J, Bastian PJ, Muller SC, Wang-Eckert L, Buettner R. Genome-wide expression profiling reveals new insights into pathogenesis and progression of testicular germ cell tumors. Cancer Genomics Proteomics. 2007;4:359–68. [PubMed] [Google Scholar]
- 23.de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, Swinkels DW, Span PN. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest. 2005;85:154–9. doi: 10.1038/labinvest.3700208. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Higgins PJ, Crawford DR. Control selection for RNA quantitation. Biotechniques. 2000;29:332–7. doi: 10.2144/00292rv02. [DOI] [PubMed] [Google Scholar]
- 25.Brooke RC. Basal cell carcinoma. Clin Med. 2005;5:551–4. doi: 10.7861/clinmedicine.5-6-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstock MA. Controversies in the public health approach to keratinocyte carcinomas. BrJ Dermatol. 2006;154(Suppl 1):3–4. doi: 10.1111/j.1365-2133.2006.07228.x. [DOI] [PubMed] [Google Scholar]
- 27.El-Bahrawy M, El-Masry N, Alison M, Poulsom R, Fallowfield M. Expression of beta-catenin in basal cell carcinoma. Br J Dermatol. 2003;148:964–70. doi: 10.1046/j.1365-2133.2003.05240.x. [DOI] [PubMed] [Google Scholar]
- 28.Brasanac D, Boricic I, Todorovic V, Tomanovic N, Radojevic S. Cyclin A and beta-catenin expression in actinic kerato-sis, Bowen's disease and invasive squamous cell carcinoma of the skin. Br J Dermatol. 2005;153:1166–75. doi: 10.1111/j.1365-2133.2005.06898.x. [DOI] [PubMed] [Google Scholar]
- 29.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 30.Saldanha G, Fletcher A, Slater DN. Basal cell carcinoma: a dermatopathological and molecular biological update. Br J Dermatol. 2003;148:195–202. doi: 10.1046/j.1365-2133.2003.05151.x. [DOI] [PubMed] [Google Scholar]
- 31.Tilli CM, Van Steensel MA, Krekels GA, Neumann HAM, Ramaekers FCS. Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol. 2005;152:1108–24. doi: 10.1111/j.1365-2133.2005.06587.x. [DOI] [PubMed] [Google Scholar]
- 32.Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–14. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–66. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–9. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 35.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 36.Stenzinger A, Schreiner D, Tag C, Wimmer M. Expression of the novel protein PTPIP51 in rat liver: an immunohisto-chemical study. Hlstochem Cell Biol. 2007;128:77–84. doi: 10.1007/s00418-007-0298-2. [DOI] [PubMed] [Google Scholar]
- 37.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 38.Mueller MM, Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation. 2002;70:486–97. doi: 10.1046/j.1432-0436.2002.700903.x. [DOI] [PubMed] [Google Scholar]
- 39.Mueller MM, Fusenig NE. Friends or foes – bipolar effects of the tumor stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 40.Radisky D, Hagios C, Bissell MJ. Tumors are unique organs defined by abnormal signaling and context. Semin Cancer Biol. 2001;11:87–95. doi: 10.1006/scbi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 41.Van Kempen LCL, Rhee JS, Dehne K, Lee J, Edwards DR, Coussens LM. Epithelial carcinogenesis: dynamic interplay between neoplastic cells and their microenvironment. Differentiation. 2002;70:610–23. doi: 10.1046/j.1432-0436.2002.700914.x. [DOI] [PubMed] [Google Scholar]
- 42.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:947–70. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 43.Fukumura D, Xavier R, Sugiura T, Chen Y, Park CE, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–25. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 44.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Malting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3:1222–7. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- 45.Tinsley JH, Ustinova EE, Xu W, Yuan SY. Src-dependent, neutrophil-mediated vascular hyperpermeability and beta-catenin modification. Am J Physiol Cell Physiol. 2002;283:1745–51. doi: 10.1152/ajpcell.00230.2002. [DOI] [PubMed] [Google Scholar]
- 46.Ali N, Yoshizumi M, Yano S, Sone S, Ohnishi H, Ishizawa K, Kanematsu Y, Tsuchiya K, Tamaki T. The novel Src kinase inhibitor M475271 inhibits VEGF-induced vascular endothelial-cadherin and beta-catenin phosphorylation but increases their association. J Pharmacol Sci. 2006;102:112–20. doi: 10.1254/jphs.fp0060357. [DOI] [PubMed] [Google Scholar]
- 47.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Coussens LM, Raymond WW, Bergers G, Laig-Websters M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–90. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Junankar SR, Eichten A, Kramer A, de Visser KE, Coussens LM. Analysis of immune cell infiltrates during squamous carcinoma development. J Investig Dermatol Symp Proc. 2006;11:36–43. doi: 10.1038/sj.jidsymp.5650024. Sep. [DOI] [PubMed] [Google Scholar]
- 51.Simoncic PD, McGlade CJ, Tremblay ML. PTP1B and TC-PTP: novel roles in immune-cell signaling. Can J Physiol Pharmacol. 2006;84:667–75. doi: 10.1139/y06-012. [DOI] [PubMed] [Google Scholar]


