Abstract
Key points
This study explores the state dependence of the hypercapnic ventilatory reflex (HCVR). We simulated an instantaneous increase or decrease of central chemoreceptor activity by activating or inhibiting the retrotrapezoid nucleus (RTN) by optogenetics in conscious rats.
During quiet wake or non-REM sleep, hypercapnia increased both breathing frequency (fR) and tidal volume (VT) whereas, in REM sleep, hypercapnia increased VT exclusively.
Optogenetic inhibition of RTN reduced VT in all sleep–wake states, but reduced fR only during quiet wake and non-REM sleep. RTN stimulation always increased VT but raised fR only in quiet wake and non-REM sleep.
Phasic RTN stimulation produced active expiration and reduced early expiratory airflow (i.e. increased upper airway resistance) only during wake.
We conclude that the HCVR is highly state-dependent. The HCVR is reduced during REM sleep because fR is no longer under chemoreceptor control and thus could explain why central sleep apnoea is less frequent in REM sleep.
Abstract
Breathing has different characteristics during quiet wake, non-REM or REM sleep, including variable dependence on 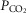 . We investigated whether the retrotrapezoid nucleus (RTN), a proton-sensitive structure that mediates a large portion of the hypercapnic ventilatory reflex, regulates breathing differently during sleep vs. wake. Electroencephalogram, neck electromyogram, blood pressure, respiratory frequency (fR) and tidal volume (VT) were recorded in 28 conscious adult male Sprague–Dawley rats. Optogenetic stimulation of RTN with channelrhodopsin-2, or inhibition with archaerhodopsin, simulated an instantaneous increase or decrease of central chemoreceptor activity. Both opsins were delivered with PRSX8-promoter-containing lentiviral vectors. RTN and catecholaminergic neurons were transduced. During quiet wake or non-REM sleep, hypercapnia (3 or 6%
. We investigated whether the retrotrapezoid nucleus (RTN), a proton-sensitive structure that mediates a large portion of the hypercapnic ventilatory reflex, regulates breathing differently during sleep vs. wake. Electroencephalogram, neck electromyogram, blood pressure, respiratory frequency (fR) and tidal volume (VT) were recorded in 28 conscious adult male Sprague–Dawley rats. Optogenetic stimulation of RTN with channelrhodopsin-2, or inhibition with archaerhodopsin, simulated an instantaneous increase or decrease of central chemoreceptor activity. Both opsins were delivered with PRSX8-promoter-containing lentiviral vectors. RTN and catecholaminergic neurons were transduced. During quiet wake or non-REM sleep, hypercapnia (3 or 6% 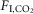 ) increased both fR and VT whereas, in REM sleep, hypercapnia increased VT exclusively. RTN inhibition always reduced VT but reduced fR only during quiet wake and non-REM sleep. RTN stimulation always increased VT but raised fR only in quiet wake and non-REM sleep. Blood pressure was unaffected by either stimulation or inhibition. Except in REM sleep, phasic RTN stimulation entrained and shortened the breathing cycle by selectively shortening the post-inspiratory phase. Phasic stimulation also produced active expiration and reduced early expiratory airflow but only during wake. VT is always regulated by RTN and CO2 but fR is regulated by CO2 and RTN only when the brainstem pattern generator is in autorhythmic mode (anaesthesia, non-REM sleep, quiet wake). The reduced contribution of RTN to breathing during REM sleep could explain why certain central apnoeas are less frequent during this sleep stage.
) increased both fR and VT whereas, in REM sleep, hypercapnia increased VT exclusively. RTN inhibition always reduced VT but reduced fR only during quiet wake and non-REM sleep. RTN stimulation always increased VT but raised fR only in quiet wake and non-REM sleep. Blood pressure was unaffected by either stimulation or inhibition. Except in REM sleep, phasic RTN stimulation entrained and shortened the breathing cycle by selectively shortening the post-inspiratory phase. Phasic stimulation also produced active expiration and reduced early expiratory airflow but only during wake. VT is always regulated by RTN and CO2 but fR is regulated by CO2 and RTN only when the brainstem pattern generator is in autorhythmic mode (anaesthesia, non-REM sleep, quiet wake). The reduced contribution of RTN to breathing during REM sleep could explain why certain central apnoeas are less frequent during this sleep stage.
Introduction
The retrotrapezoid nucleus (RTN) is a small cluster of lower brainstem neurons that are activated by hypercapnia and regulate several aspects of breathing, including inspiratory amplitude, breathing frequency and active expiration (Marina et al. 2010; Guyenet, 2014; Basting et al. 2015). The high responsiveness of RTN neurons to CO2 in vivo is attributed to several mechanisms: an intrinsic sensitivity to protons, paracrine effects from surrounding pH/CO2-sensitive astrocytes and inputs from other chemosensory neurons and the carotid bodies (Guyenet et al. 2005, 2010; Gestreau et al. 2010; Gourine et al. 2010; Huckstepp & Dale, 2011; Ramanantsoa et al. 2011; Wang et al. 2013). RTN neurons can therefore be viewed as central respiratory chemoreceptors (CRCs) that also function as chemoreflex integrator. These neurons mediate a large portion of the hypercapnic ventilatory reflex (HCVR) (Gestreau et al. 2010; Guyenet et al. 2010; Ramanantsoa et al. 2011; Wang et al. 2013).
The chemoreflex control of breathing is state-dependent. For example, although the respiratory chemoreflexes still operate during REM sleep, the breathing frequency (fR) is typically unaffected by hypoxia or hypercapnia (Coote, 1982; Berthon-Jones & Sullivan, 1984; Horner et al. 2002; Lovering et al. 2003; Nakamura et al. 2007). In addition, in conscious cats ventilated to apnoea during non-REM sleep, diaphragmatic EMG re-emerges during REM sleep (Orem et al. 2005).
Periodic breathing occurs during sleep under hypobaric hypoxia, in patients with congestive heart failure, or after chronic opiate treatment (Berssenbrugge et al. 1983; Burgess, 1997; Farney et al. 2003; Weil, 2004). The apnoeas have been tentatively attributed to recurrent episodes of CNS hypocarbia that silence CRCs (Dempsey et al. 2012; Marcus et al. 2014). This interpretation agrees with the finding that respiratory alkalosis silences RTN in conscious rats (Basting et al. 2015). In adult humans or cats, periodic breathing occurs only during non-REM sleep (Berssenbrugge et al. 1983; Farney et al. 2003; Lovering et al. 2012).
Congenital central hypoventilation syndrome (CCHS) is caused by Phox2b mutations (Amiel et al. 2003; Weese-Mayer et al. 2010). This disease is characterized by the loss of breathing automaticity during sleep and a greatly reduced HCVR (Amiel et al. 2003; Weese-Mayer et al. 2010). In mice, Phox2b mutations abort RTN development, which probably causes most of the HCVR deficit (Ramanantsoa et al. 2011). In patients with CCHS, breathing is much better maintained during REM than non-REM sleep (Fleming et al. 1980). In short, reduced CRC activity resulting from carotid body hyperactivity (hypoxia, congestive heart failure) (Marcus et al. 2014) or RTN lesion (putative cause of CCHS) appears to cause central sleep apnoea selectively during non-REM sleep.
The purpose of this study was to examine the state dependence of the HCVR. We simulated an instantaneous increase or decrease of CRC activity by activating or inhibiting the RTN optogenetically (Abbott et al. 2009; Basting et al. 2015). We then measured the resulting effects on breathing during periods of quiet wake, non-REM or REM sleep.
Methods
Animals
Experiments were performed on male Sprague–Dawley rats (n = 28; 400–550 g; Taconic, Germantown, NY, USA). All procedures conformed to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Virginia Animal Care and Use Committee. Animals were housed under standard 12 h light/dark cycle with ad libitum access to food and water.
Lentiviral constructs and vector preparation
This study used lentiviral vectors (LVV) that express their transgene under the control of the Phox2b-responsive artificial promoter PRSx8 (Hwang et al. 2001). When injected into the rostral ventrolateral medulla or ventrolateral pons (noradrenergic ‘A5’ area) as described in this study, these LVVs transduce Phox2b-expressing neurons that are virtually exclusively catecholaminergic (C1 and A5 neurons) and RTN neurons (Abbott et al. 2009). One of the LVVs (PRSx8-ChR2-mCherry) encoded the photoactivatable cation channel channelrhodopsin-2 (ChR2, H134R) fused to mCherry (Abbott et al. 2009) and the other the photoactivatable proton pump archaerhodopsin (ArchT3.0) fused to eYFP (PRSx8-Arch-eYFP) (Han et al. 2011; Mattis et al. 2012; Basting et al. 2015). The vectors were produced by the University of North Carolina virus core and diluted to a final titre of 3.0 × 108 viral particles per ml with sterile phosphate-buffered saline. We verified that the above viral dilution transduced Phox2b-expressing neurons selectively (Fig.9).
Figure 9. Location and phenotype of ChR2- or Arch-transduced neurons.
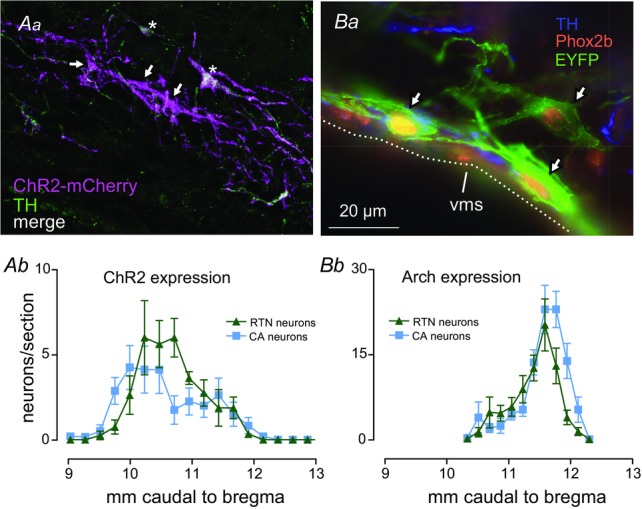
Aa, cluster of ChR2-transduced neurons within the rostral RTN (transverse plane; approximate location: 10.2 mm caudal to bregma). Three of five neurons were not TH-immunoreactive (arrows) and were presumptive RTN central respiratory chemoreceptors, the other two (stars) were TH-immunoreactive (A5 noradrenergic neurons). Ab, rostrocaudal distribution of ChR2-transduced RTN and catecholaminergic (CA) neurons (n = 8). Neurons were transduced on the left side of the brain only. Ba, three Arch-transduced putative RTN central respiratory chemoreceptor neurons (arrows) located next to the VMS (transverse section at ∼11.4 mm caudal to Bregma; green: Arch-EYFP; blue: TH; red: Phox2b). Bb, rostrocaudal distribution of Arch-EYFP-transduced neurons (n = 8; RTN neurons = TH−/Phox2b+/EYFP; CA neurons: TH+/ Phox2b+/EYFP+). Neurons were transduced bilaterally. Distance from bregma according to a standard rat atlas (Paxinos & Watson, 2005) (sections aligned using caudal end of facial motor nucleus as level 11.6 mm caudal to bregma). Calibration bar in Ba equals 100 μm (Aa) or 20 μm (Ba). CA, catecholamine; RTN, retrotrapezoid nucleus; TH, tyrosine hydroxylase; VMS, ventral medullary surface.
Injections of virus and instrumentation
We used two different approaches for targeting Arch or ChR2 to RTN neurons: Arch was delivered bilaterally to transduce RTN neurons en masse in both rostral and caudal regions and the optical fibres were targeted to the caudal RTN to illuminate the region with the highest density of RTN neurons (Takakura et al. 2008). By contrast, we restricted ChR2 expression to the rostral portion of the RTN on the left side. This was done to photoactivate RTN neurons without involving the C1 neurons whose photoactivation produces arousal and boost breathing via glutamatergic mechanisms (Abbott et al. 2013b, 2014; Burke et al. 2014).
For LVV injection, the rats were anaesthetized with a mixture of ketamine (75 mg kg–1), xylazine (5 mg kg–1) and acepromazine (1 mg kg–1) given intraperitoneally (i.p.). The correct plane of anaesthesia was assessed by the absence of the corneal and hind-paw withdrawal reflexes. Additional anaesthetic was administered as necessary during surgery (25% of the original dose, i.p. or intramuscular). Body temperature was kept close to 37°C with a servo-controlled heating pad and a blanket. Rats received postoperative ampicillin (125 mg kg–1, i.p.), and ketoprofen [3–5 mg kg–1, subcutaneous (s.c.)] for two consecutive days. All surgical procedures were performed under aseptic conditions. The hair over the skull, neck and cheek were removed and skin disinfected. An incision over the mandible was made to expose the facial nerve for antidromic activation of facial motor neurons. The rat was then placed prone on a stereotaxic apparatus (bite bar set at −3.5 mm for flat skull; David Kopf Instruments Tujunga, CA, USA). A 1.5 mm diameter hole was drilled into the occipital plate on the left side (ChR2) or both sides (Arch) caudal to the parieto-occipital suture. Viral solutions were loaded into a 1.2 mm internal diameter glass pipette broken to a 25 μm tip (external diameter). To target the rostral RTN region with ChR2, the pipette was inserted at a 12–14 deg angle pointing towards the front of the animal and injections of 100–140 nl were made at two sites separated 200 μm (maximum 280 nl). These sites were located 100–200 μm below the rostral end of the facial motor nucleus whose lower edge was identified in each rat by mapping antidromic-evoked potentials elicited by stimulating the facial nerve (Brown & Guyenet, 1985). The Arch-LVV was injected bilaterally into four sites separated by 200 μm (total volume 400–600 nl side–1), extending rostrally from the caudal end of the facial motor nucleus.
We then implanted electrodes for recording the electroencephalogram (EEG), neck electromyogram (EMG) and optical fibre–ferrule assemblies for light stimulation (Sparta et al. 2012). For EEG recordings, stainless steel jeweller screws (Plastics One, Roanoke, VA, USA) were implanted extradurally (0.5–1 mm anterior, 1 mm lateral to bregma and 3 mm posterior, 2–2.5 mm lateral to bregma above the contralateral hemisphere). Two additional screws were implanted for structural support of the head stage and for grounding. Teflon-coated braided stainless steel wire (A-M Systems, Sequim, WA, USA) was stripped at the tip and wrapped around the implanted screws. Two additional wires were stripped at the tips and implanted in the superficial muscles of the neck for EMG recordings of postural activity. All wires were crimped to amphenol pins (A-M Systems) and inserted into a plastic headstage (Plastics One). Implantable optical fibres (230 μm, numerical aperture 0.39; Thorlabs, Newton, NJ, USA) were constructed using published methods (Sparta et al. 2012) and stereotaxically directed into the ventrolateral medulla 0.5–0.8 mm dorsal to the LVV injection sites. The head stage and optical fibre–ferrule assemblies were secured to the skull using a two-part epoxy (Loctite, Henkel, Düsseldorf, Germany). Incisions were then closed in two layers (muscle and skin) with absorbable sutures and vet bond adhesive. Rats recovered for a minimum of 4 weeks before experiments were conducted. A subset of animals was then implanted with radiotelemetry probes (PA-C10; Datasciences International, Saint Paul, MN, USA) to record blood pressure (BP) from the descending aorta via the right femoral artery. Animals were left to recover for at least another week before physiological experiments began.
Physiological experiments in freely behaving rats
Rats were first habituated to the testing environment, which consisted of a Buxco unrestrained plethysmography chamber modified to allow tethered EEG/EMG recordings and optical stimulation. This equipment was visually isolated and placed in low ambient noise conditions. On the day of the experiment, rats were lightly anaesthetized with isoflurane (induction with 5%, maintenance with 2% in pure oxygen for < 1 min) to permit cleaning of hardware and connections to the ferrule and EEG/EMG recording assembly. A 200 μm thick multimode optical fibre was terminated with a ferrule and mated to the implanted ferrule with a zirconia sleeve. Optical matching gel (Fibre Instrument Sales, Oriskany, NY, USA) was applied at the ferrule junction to minimize light loss. A minimum of 1 h was allowed for recovery from anaesthesia and the emergence of stable sleep patterns. Recordings were made between 12.00 and 18.00 h, over multiple days, with a minimum of 3 days’ rest between tests. The ventilatory response to RTN photoinhibition or activation was assessed using barometric, unrestrained whole body plethysmography (EMKA Technologies, Falls Church, VA, USA). The plethysmography chamber was continuously flushed with 1.5 l min–1 of 21% O2 balanced with N2. Three or 6% CO2 was added when required, leaving the oxygen percentage constant. Photoinhibition of RTN under hyperoxia (65% O2 in N2) was also performed, as previously described (Basting et al. 2015). The gas composition was regulated by computer-driven mass flow controllers for O2, N2 and CO2 (Alicat Scientific, Inc., Tucson, AZ, USA). Temperature and humidity within the plethysmography chamber were kept constant.
Optogenetics
Photoinhibition of Arch-expressing RTN neurons was achieved with a green laser (532 nm; Shanghai Laser and Optics Century, Shanghai, China). Photostimulation of ChR2-expressing RTN neurons was performed using a blue laser (473 nm; CrystaLaser, Reno, NV, USA). The lasers were controlled by TTL pulses from a Grass model S88 stimulator (AstroMed Inc., West Warwick, RI, USA). Continuous green light (∼5 mW) was applied bilaterally using a splitter through 200 μm thick multimode optical fibre (Thorlabs) in 10 s episodes of continuous illumination. This method effectively silences chemosensitive RTN neurons under anaesthesia (see fig.2 in Basting et al. 2015). ChR2-transduced neurons were excited with pulses of blue light (5 ms, ∼9 mW) delivered at 20 Hz for 20 s (Kanbar et al. 2010; Abbott et al. 2013a; Burke et al. 2014) or intermittently to entrain the breathing cycle (four 5 ms long pulses, 50 ms interval delivered at a frequency greater than resting fR, see Abbott et al. 2011).
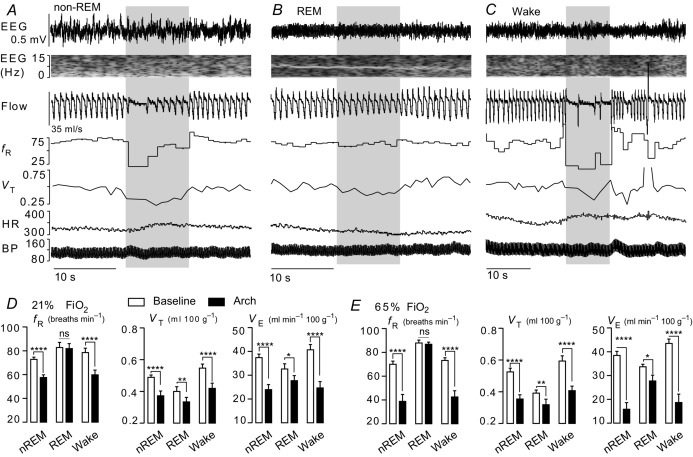
Figure 2. State dependence of the breathing changes evoked by retrotrapezoid nucleus inhibition.
A–C, hypoventilation caused by bilateral Arch activation (10 s) in one rat at 21% 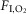 in non-REM sleep (A), REM sleep (B) and quiet wake (C). Arch activation decreased VT in all three states, decreased fR during non-REM sleep and quiet wake but did not change fR during REM sleep. Arch activation did not change BP, HR or sleep state. D, ventilation parameters at rest and during Arch activation in normoxia (21%
in non-REM sleep (A), REM sleep (B) and quiet wake (C). Arch activation decreased VT in all three states, decreased fR during non-REM sleep and quiet wake but did not change fR during REM sleep. Arch activation did not change BP, HR or sleep state. D, ventilation parameters at rest and during Arch activation in normoxia (21% 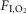 ; n = 8). E, ventilation parameters at rest and during Arch activation in hyperoxia (65%
; n = 8). E, ventilation parameters at rest and during Arch activation in hyperoxia (65% 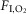 ; n = 8). The effects of Arch activation on ventilation were amplified in hyperoxia but qualitatively the same as in normoxia. In REM sleep, Arch activation had no effect on fR. Arch activation reduced VT under all conditions. Refer to online data supplement for video examples of Arch activation in hyperoxia. Significance (baseline vs. Arch stimulation; two-way repeated measures ANOVA with Bonferroni's correction for multiple comparisons): *P < 0.05, **P < 0.01, *** P < 0.005, ****P < 0.001. BP, blood pressure; HR, heart rate; nREM, non-REM.
; n = 8). The effects of Arch activation on ventilation were amplified in hyperoxia but qualitatively the same as in normoxia. In REM sleep, Arch activation had no effect on fR. Arch activation reduced VT under all conditions. Refer to online data supplement for video examples of Arch activation in hyperoxia. Significance (baseline vs. Arch stimulation; two-way repeated measures ANOVA with Bonferroni's correction for multiple comparisons): *P < 0.05, **P < 0.01, *** P < 0.005, ****P < 0.001. BP, blood pressure; HR, heart rate; nREM, non-REM.
Data acquisition and analysis
Physiological signals were acquired and processed using Spike v7.03 software (CED Cambridge, UK). EEG and EMG were amplified and band pass filtered (EEG: 0.1–100 Hz, ×1000. EMG: 300–3000 Hz, ×1000) and acquired at a sampling frequency of 1 kHz. The signal generated by the differential pressure transducer connected to the plethysmography chamber was amplified and band pass filtered (0.1–15 Hz, ×100) and acquired at a sampling frequency of 1 kHz. The signal from the radiotelemetry probe was acquired at a sampling frequency of 0.2 kHz. Mean arterial pressure and heart rate (HR) were extracted from pulsatile BP recordings from the descending aorta based on values calibrated before implantation of the telemetry probe. Periods of non-REM sleep, REM sleep and quiet wake were classified based on EEG, EMG activity and the patterns of cardiovascular and breathing activity. During non-REM sleep, EEG spectra were dominated by delta activity (0.5–4 Hz), with little or no EMG activity and a stable breathing pattern, BP and HR. REM sleep was characterized by stable theta oscillations (6–8 Hz), neck muscle atonia, an irregular breathing pattern and bradycardia with BP fluctuations. Quiet wake was characterized by a reduction in total power with EMG tone and elevated HR. A minimum of six photoactivation trials were conducted in each rat in each state. Average breathing values were extracted from trials in non-REM sleep, REM sleep and quiet wake that were not contaminated by body movements, as indicated by EMG activity. Baseline values for all parameters were calculated from the 20 s before the photoactivation period. Respiratory frequency (fR, breaths per min) and tidal volume (VT, area under the curve during the inspiratory period calibrated to waveforms generated by injecting 5 ml of dry air from a syringe during the experiment, expressed in ml per 100 g body weight (BW)) were calculated using Spike software v7.3 (CED). These values were used to calculate minute ventilation (VE = fR × VT, expressed as ml 100 g BW min–1).
All data sets were tested for normality using the Shapiro–Wilk test, then differences within and between groups were determined using one- or two-way repeated measures (RM) ANOVA with Bonferroni multiple comparisons. Linear regression, Student's t test and non-parametric Kruskal–Wallis tests were also performed as required. All values are expressed as means ± SEM and significance indicated (one symbol, P < 0.05; two symbols, P < 0.01; three symbols, P < 0.005, four symbols, P < 0.0001).
Histology
Animals were deeply anaesthetized with sodium pentobarbital and perfused transcardially with 4% paraformaldehyde, brains removed and processed as described previously (Abbott et al. 2009; Burke et al. 2014). Immunohistochemistry with antibodies against tyrosine hydroxylase (sheep anti-TH, 1:2000; Millipore, Billerica, MA, USA), EYFP (chicken anti-GFP, 1:1000; AVES Labs, Tigard, OR, USA) or mCherry (rabbit anti-dsRed, 1:500, Clontech no. 632496; Clontech Laboratories, Mountain View, CA, USA) and Phox2b (rabbit anti-Phox2b, 1:8000, a gift from J.F. Brunet, Ecole Normale Superieure, Paris, France) were performed as previously described (Abbott et al. 2009; Burke et al. 2014). Cell mapping, counting and photography were done using the Neurolucida system (MicroBrightfield, Inc., Colchester, VT, USA) with a Zeiss Axioskop microscope with computer driven stage and Zeiss MRc camera. Cell counts were taken from a 1 in 6 series of 40 μm sections and only profiles containing a nucleus were counted.
Results
State dependence of the hypercapnic ventilatory reflex
Exposure to 3%  did not detectably perturb natural sleep in any of the rats (n = 10). Most rats (seven of 10) were also able to cycle through periods of non-REM and REM sleep in the presence of 6%
did not detectably perturb natural sleep in any of the rats (n = 10). Most rats (seven of 10) were also able to cycle through periods of non-REM and REM sleep in the presence of 6%  (Fig.1).
(Fig.1).
Figure 1. State dependence of the hypercapnic ventilatory reflex in rat.
A, breathing and cardiovascular function at rest. Shown from top to bottom: EEG power spectrum (0–15 Hz), EEG (raw signal), air flow signal (plethysmography, inspiration downward), fR (breaths min–1), VT (ml 100 g–1), HR (beats min–1) and BP (telemetric recording, mmHg). During non-REM sleep, EEG power was concentrated within the delta range (0.5–4 Hz), REM sleep was identified by a strong concentration of power in the 6–8 Hz range (theta) and quiet wake by a large reduction in total power (desynchronized state). Breathing during REM sleep was faster, more labile and shallower than during non-REM sleep. Bradycardia and BP fluctuations also commonly occurred in REM sleep, the result of co-activation of cardiac vagal and sympathetic vasomotor outflows by central command with impaired baroreflex function. B, breathing and cardiovascular function in hypercapnia (6% 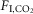 ; same rat as in A). VT and fR were markedly increased in non-REM sleep or wake. Transition from non-REM sleep into REM sleep caused an abrupt reduction in breathing rate that was reversed at the transition to wake (hatched horizontal lines denote the ∼50 breaths min–1 reduction in fR during REM sleep in this example). No differences in BP and HR regulation were observed under hypercapnia. C–E, fR, VT and VE at 0%, 3% and 6%
; same rat as in A). VT and fR were markedly increased in non-REM sleep or wake. Transition from non-REM sleep into REM sleep caused an abrupt reduction in breathing rate that was reversed at the transition to wake (hatched horizontal lines denote the ∼50 breaths min–1 reduction in fR during REM sleep in this example). No differences in BP and HR regulation were observed under hypercapnia. C–E, fR, VT and VE at 0%, 3% and 6% 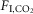 measured across sleep–wake states (n = 7). *Gain (slope of linear regressions) significantly different during REM vs. both quiet wake (P = 0.0001) and REM sleep (P = 0.004; see Results section for detailed statistics). BP, blood pressure; HR, heart rate; nREM, non-REM.
measured across sleep–wake states (n = 7). *Gain (slope of linear regressions) significantly different during REM vs. both quiet wake (P = 0.0001) and REM sleep (P = 0.004; see Results section for detailed statistics). BP, blood pressure; HR, heart rate; nREM, non-REM.
During REM sleep, breathing was either regular or very labile (Figs1, 4 and 6 and Supplemental Videos 3–4). The average fR measured in REM sleep was higher than during non-REM sleep and quiet wake (n = 10; REM sleep: 93 ± 3 breaths min–1; non-REM sleep: 74 ± 3; quiet wake: 77 ± 4; REM vs. non-REM, P = 0.0001; REM vs. wake, P = 0.0003; non-REM vs. wake, P = 0.2). VT (ml breath 100 g–1) was lower in REM sleep than non-REM sleep and quiet wake (n = 10; REM sleep: 0.32 ± 0.02; non-REM sleep: 0.41 ± 0.01; quiet wake: 0.44 ± 0.03; REM vs. non-REM, P = 0.003; REM vs. quiet wake, P = 0.002; non-REM vs. wake, P = 0.6). Minute ventilation (VE = fR × VT; ml min 100 g–1) was virtually identical across the three states (n = 10; REM sleep: 30 ± 2; non-REM sleep: 30 ± 1; quiet wake: 34 ± 2; one-way RM ANOVA: F2,14 = 2.969, P = 0.1).
Figure 4. Retrotrapezoid nucleus inhibition during REM sleep in hyperoxia.
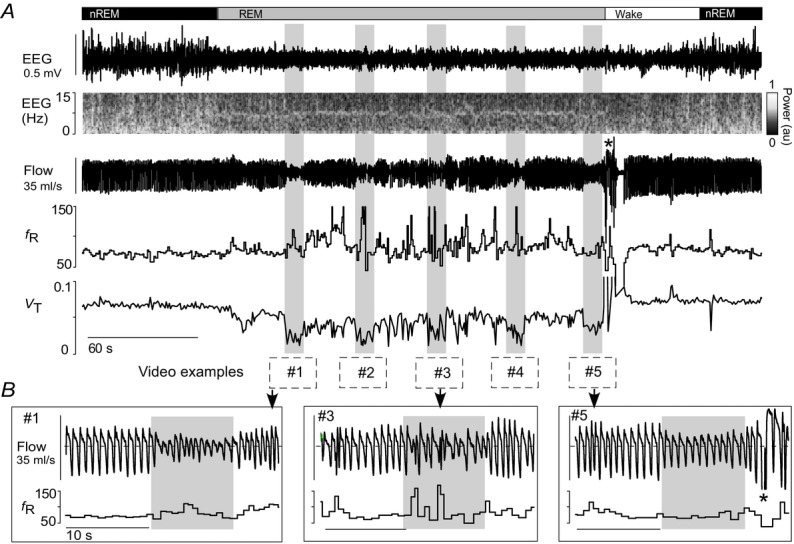
A, bilateral Arch activation (10 s; grey rectangles) in one rat at 65% 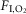 in REM sleep decreased VT in all trials, but did not reduce fR (same animal as Fig.3). Traces correspond to the five examples in the Supplemental videos 3–4. B, enlarged traces of trials 1, 3 and 5. Animal awoke from REM sleep 3 s after the fifth trial (asterisk) then returned to non-REM sleep. nREM, non-REM.
in REM sleep decreased VT in all trials, but did not reduce fR (same animal as Fig.3). Traces correspond to the five examples in the Supplemental videos 3–4. B, enlarged traces of trials 1, 3 and 5. Animal awoke from REM sleep 3 s after the fifth trial (asterisk) then returned to non-REM sleep. nREM, non-REM.
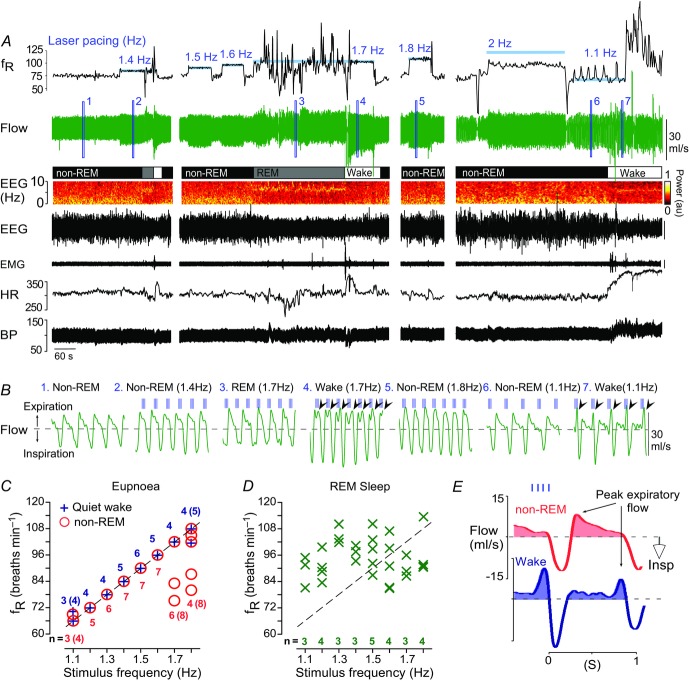
Figure 6. Breathing entrainment by RTN stimulation is state-dependent.
A, example of breathing entrainment elicited by photostimulation of RTN (trains of 4 × 3 ms light pulses at 20 Hz; stimulation at blue bars in fR trace). Faithful entrainment (fR = train frequency) in the range of 66–108 breaths min–1 (1.1–1.8 Hz) was obtained during non-REM sleep and wake but entrainment failed at 2 Hz. During REM sleep, no entrainment was produced. B, expanded plethysmography flow signals (light pulses delivered at vertical blue lines above flow traces; excerpts 1–7 taken from where indicated in the flow trace of A). During entrainment, the light pulses ‘settled’ spontaneously during late to mid expiration. Note that during non-REM sleep peak expiratory flow at rest (no. 1) or during entrainment (nos 2, 5–6) occurs in early expiration. However, in wake, RTN stimulation produced a switch in peak expiratory flow to the late expiratory phase (arrowheads in excerpts 4 and 7), indicative of active expiration (Abbott et al. 2011; Pagliardini et al. 2011). C, group data from 11 animals showing the relationship between stimulus train frequency and fR during non-REM sleep and wake states. Breathing was faithfully entrained between 1.2–1.6 Hz in all animals (single symbol for all animals); incomplete or unsuccessful entrainment outside this range is indicated by the individual symbols. Adjacent numbers are the number of animals that were successfully entrained at a given stimulation frequency (bracketed numbers indicate total rat number, including unsuccessful trials). Not every frequency was tested in every animal in each sleep–wake state; hence, the varying number of determinations for each tested frequency. D, group data during REM sleep (same rats as in C). There was no relationship between the stimulus train frequency and fR. E, laser light-triggered waveform averages of the respiratory flow signal during entrainment at 1.1 Hz (light pulses in sky blue) in non-REM sleep (red) and wake (deep blue) with the shaded regions denoting the expiratory flow. In this example, RTN stimulation switched peak expiratory flow from early expiration in non-REM sleep to late expiration (active expiration) in wake. BP, blood pressure; HR, heart rate; Insp, inspiration; RTN, retrotrapezoid nucleus.
Exposure to 3 and 6% 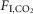 during non-REM and quiet wake increased fR, VT and VE linearly (Fig.1C–E). During REM sleep, VT also increased linearly with
during non-REM and quiet wake increased fR, VT and VE linearly (Fig.1C–E). During REM sleep, VT also increased linearly with 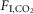 but fR was invariant. The slopes of the linear regression between VT and
but fR was invariant. The slopes of the linear regression between VT and 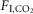 (ΔVT/Δ
(ΔVT/Δ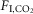 in ml 100 g–1/1%
in ml 100 g–1/1% 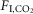 ) were not state-dependent (Fig.1D; n = 7; REM sleep: 0.04 ± 0.004; non-REM sleep: 0.05 ± 0.009; wake: 0.06 ± 0.009; One-way RM ANOVA: F2,10 = 3.537, P = 0.07). However, during REM sleep, fR was unaffected by CO2 (n = 7; 0%
) were not state-dependent (Fig.1D; n = 7; REM sleep: 0.04 ± 0.004; non-REM sleep: 0.05 ± 0.009; wake: 0.06 ± 0.009; One-way RM ANOVA: F2,10 = 3.537, P = 0.07). However, during REM sleep, fR was unaffected by CO2 (n = 7; 0% 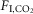 : 94 ± 3 bpm; 3%
: 94 ± 3 bpm; 3% 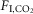 : 94 ± 4; 6%
: 94 ± 4; 6% 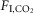 : 96 ± 4; one-way RM ANOVA: F2,12 = 0.1934, P = 0.8). Accordingly, the slopes of the linear regressions between fR and
: 96 ± 4; one-way RM ANOVA: F2,12 = 0.1934, P = 0.8). Accordingly, the slopes of the linear regressions between fR and 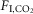 (ΔfR/Δ
(ΔfR/Δ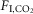 in breaths min–1/1%
in breaths min–1/1% 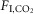 ) were markedly different (Fig.1C; n = 7; REM sleep: 0.3 ± 0.5; non-REM sleep: 8.9 ± 0.7; quiet wake: 9.3 ± 1.2; REM vs. non-REM, P < 0.0001; REM vs. quiet wake, P = 0.001; non-REM sleep vs. quiet wake, P > 0.9). Finally, the slopes of the regressions between VE and
) were markedly different (Fig.1C; n = 7; REM sleep: 0.3 ± 0.5; non-REM sleep: 8.9 ± 0.7; quiet wake: 9.3 ± 1.2; REM vs. non-REM, P < 0.0001; REM vs. quiet wake, P = 0.001; non-REM sleep vs. quiet wake, P > 0.9). Finally, the slopes of the regressions between VE and 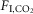 (ΔVE/Δ
(ΔVE/Δ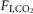 in ml min 100 g/1%
in ml min 100 g/1% 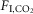 ) were also different during REM sleep vs. the other states (Fig.1E; n = 7; REM sleep: 4.2 ± 0.6; non-REM sleep: 9.7 ± 1.2; wake: 12.3 ± 0.6; REM vs. non-REM, P < 0.004; REM vs. wake, P < 0.0001; non-REM vs. wake, P = 0.05).
) were also different during REM sleep vs. the other states (Fig.1E; n = 7; REM sleep: 4.2 ± 0.6; non-REM sleep: 9.7 ± 1.2; wake: 12.3 ± 0.6; REM vs. non-REM, P < 0.004; REM vs. wake, P < 0.0001; non-REM vs. wake, P = 0.05).
In summary, in rats, fR is no longer under chemoreceptor control during REM sleep but the tidal volume component of the HCVR has approximately the same gain during all states.
Optogenetic inhibition of retrotrapezoid nucleus neurons in REM sleep reduces VT selectively
Arch was photoactivated in 10 s episodes during established periods of non-REM sleep, REM sleep or quiet wake. During non-REM sleep and quiet wake, Arch-mediated neuronal inhibition reduced both fR and VT whereas during REM sleep Arch activation reduced VT but did not change fR (Fig.2A–D) (n = 8; fR (baseline vs. Arch activation): non-REM sleep, P < 0.0001, REM sleep, P > 0.9, wake, P < 0.0001; VT (baseline vs. Arch activation): non-REM sleep, P < 0.0001; REM sleep, P = 0.001, wake, P < 0.0001; VE (baseline vs. Arch activation): non-REM, P < 0.0001; REM, P = 0.006; wake, P < 0.0001).
We also performed these experiments under hyperoxia (65% 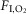 ; same eight rats), which silences the carotid bodies (Dejours, 1962; Gonzalez et al. 1994). As found previously (Basting et al. 2015), during non-REM sleep and quiet wake, Arch activation produced a much larger reduction of fR and VT than under normoxia (group data in Fig.2E, representative example in Fig.3 with corresponding Supplemental Videos 1–2). However, during REM sleep, again, Arch activation reduced VT but had no effect on fR (group data in Fig.2E, representative example in Fig.4 and corresponding Videos 3–4) [n = 8; fR (baseline vs. Arch activation): non-REM, P < 0.0001; REM, P > 0.9; wake, P < 0.0001. VT (baseline vs. Arch activation): non-REM, P = 0.0001; REM, P = 0.009; wake, P = 0.0001. VE (baseline vs. Arch activation): non-REM, P < 0.0001; REM, P = 0.04; wake, P < 0.0001].
; same eight rats), which silences the carotid bodies (Dejours, 1962; Gonzalez et al. 1994). As found previously (Basting et al. 2015), during non-REM sleep and quiet wake, Arch activation produced a much larger reduction of fR and VT than under normoxia (group data in Fig.2E, representative example in Fig.3 with corresponding Supplemental Videos 1–2). However, during REM sleep, again, Arch activation reduced VT but had no effect on fR (group data in Fig.2E, representative example in Fig.4 and corresponding Videos 3–4) [n = 8; fR (baseline vs. Arch activation): non-REM, P < 0.0001; REM, P > 0.9; wake, P < 0.0001. VT (baseline vs. Arch activation): non-REM, P = 0.0001; REM, P = 0.009; wake, P = 0.0001. VE (baseline vs. Arch activation): non-REM, P < 0.0001; REM, P = 0.04; wake, P < 0.0001].
Figure 3. Retrotrapezoid nucleus inhibition during non-REM sleep in hyperoxia.
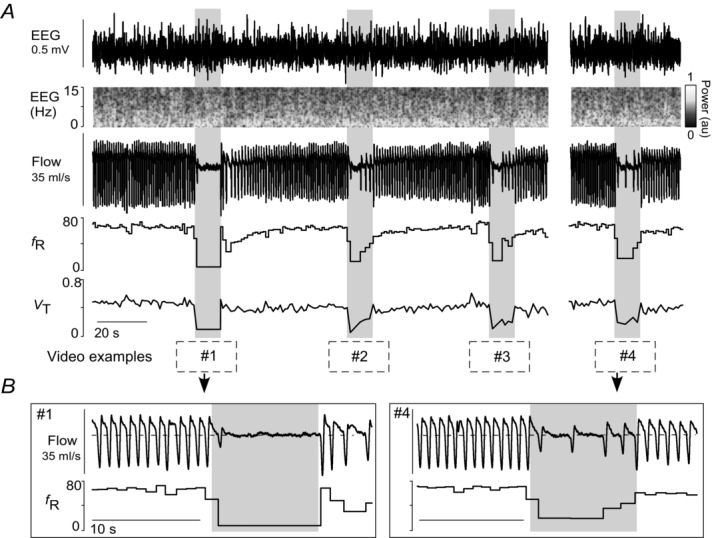
A, profound hypoventilation caused by bilateral Arch activation (10 s; grey rectangles) in one rat at 65% 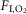 in non-REM sleep. Despite this sudden hypoventilation, the animal does not arouse from non-REM sleep (EEG delta wave activity: 0.5–4 Hz). Traces correspond to the four examples shown in Supplemental videos 1–2. B, enlarged traces of trials 1 and 4.
in non-REM sleep. Despite this sudden hypoventilation, the animal does not arouse from non-REM sleep (EEG delta wave activity: 0.5–4 Hz). Traces correspond to the four examples shown in Supplemental videos 1–2. B, enlarged traces of trials 1 and 4.
BP and HR were unaffected by photoactivation with green light (Fig.2A–C, Table1).
Table 1.
Blood pressure and HR at rest or during Arch or ChR2 photostimulation
| State | Non-REM sleep | REM sleep | Quiet wake | |||
|---|---|---|---|---|---|---|
| Variable | Rest | Arch | Rest | Arch | Rest | Arch |
| n | 5 | 5 | 4 | 4 | 5 | 5 |
| MAP (mmHg) | 109 ± 4 | 108 ± 4 | 119 ± 2 | 117 ± 2 | 115 ± 6 | 113 ± 5 |
| HR (bpm) | 300 ± 9 | 300 ± 9 | 289 ± 6 | 287 ± 9 | 310 ± 8 | 311 ± 8 |
| Variable | Rest | ChR2 | Rest | ChR2 | Rest | ChR2 |
| n | 7 | 7 | 7 | 7 | 7 | 7 |
| MAP (mmHg) | 109 ± 3 | 106 ± 3 | 114 ± 4 | 113 ± 4 | 109 ± 4 | 108 ± 3 |
| HR (bpm) | 306 ± 7 | 315 ± 8 | 298 ± 6 | 304 ± 5 | 314 ± 12 | 313 ± 12 |
Abbreviations: HR, heart rate; MAP, mean arterial blood pressure.
Telemetric blood pressure and HR recordings in rats habituated to the plethysmography chamber during various states of vigilance. No change in resting MAP or HR was observed during photostimulation of Arch+ or ChR2+ rats. Statistics: two-way ANOVA.
Retrotrapezoid nucleus activation in REM sleep increases VT but not fR
ChR2-transduced neurons were photoactivated (5 ms pulses, 20 Hz, 20 s episodes) during non-REM sleep, REM sleep and quiet wake. ChR2-mediated stimulation increased both fR and VT during non-REM sleep and quiet wake (Fig.5A, C and D). In REM sleep, VT increased but fR did not change (Fig.5B and D) [n = 10; fR (baseline vs. ChR2 stimulation): non-REM, P < 0.0001; REM, P = 0.6; quiet wake, P < 0.0001; VT (baseline vs. ChR2-stimulation: non-REM, P < 0.0001; REM, P = 0.0003; quiet wake, P < 0.0002].
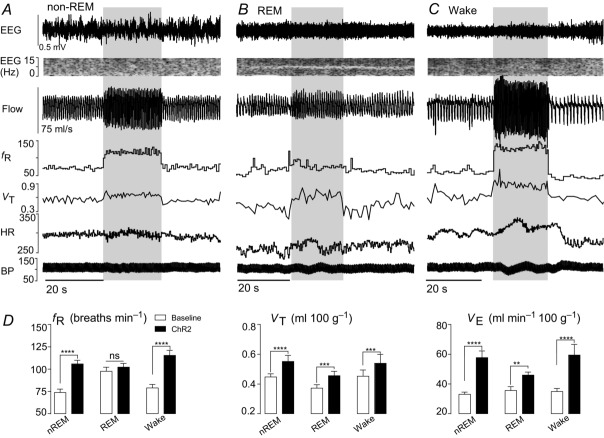
Figure 5. State dependence of the breathing changes evoked by rostral retrotrapezoid nucleus stimulation.
A–C, hyperventilation evoked by unilateral ChR2 activation (20 s, blue bar) in one rat at 21%  in non-REM sleep (A), REM sleep (B) and quiet wake (C). ChR2 activation increased VT in all three states, increased fR in non-REM and quiet wake states but had no effect on fR during REM sleep. ChR2 activation had little effect on BP and did not produce arousal from sleep. D, ventilation parameters at rest and during ChR2 activation in rats across sleep–wake states in normoxia (n = 10). Significance (baseline vs. Arch stimulation; two-way repeated measures ANOVA with Bonferroni's correction for multiple comparisons): **P < 0.01, ***P < 0.005, ****P < 0.001. BP, blood pressure; HR, heart rate; nREM, non-REM.
in non-REM sleep (A), REM sleep (B) and quiet wake (C). ChR2 activation increased VT in all three states, increased fR in non-REM and quiet wake states but had no effect on fR during REM sleep. ChR2 activation had little effect on BP and did not produce arousal from sleep. D, ventilation parameters at rest and during ChR2 activation in rats across sleep–wake states in normoxia (n = 10). Significance (baseline vs. Arch stimulation; two-way repeated measures ANOVA with Bonferroni's correction for multiple comparisons): **P < 0.01, ***P < 0.005, ****P < 0.001. BP, blood pressure; HR, heart rate; nREM, non-REM.
BP and HR were unchanged by the photostimulus (Fig.5A–C, Table1).
Phasic retrotrapezoid nucleus stimulation does not entrain breathing during REM sleep and does not produce active expiration during either form of sleep
As reported before (Abbott et al. 2011), short light trains (3–4 pulses per train, 20 Hz, 2–5 ms pulses) applied at frequencies above resting fR entrained the breathing cycle (range: 66–108 breaths min–1; Fig.6A). However, we found that pacing could be produced during non-REM sleep and quiet wake but never during REM sleep (Fig.6A–D).
In the absence of RTN stimulation, peak expiratory flow occurred in early expiration (E1) when the rats were awake or in non-REM sleep. This pattern persisted during phasic RTN photostimulation when the rats were in non-REM or REM sleep (Fig.6B and E). However, during quiet wake, the same RTN stimuli reduced E1 expiratory flow and produced a strong late expiratory flow (E2) signal indicative of active (abdominal) expiration (Fig.6B; black arrows). This pattern of breathing is better appreciated by examining stimulus-triggered averages of the plethysmography signal (Figs6E and 7A and B). In five of nine rats, a single large peak expiratory flow occurred during this late expiratory phase (Fig.7C). The remainder of the rats displayed a biphasic expiratory flow as per (Abbott et al. 2011). We divided the expiratory phase in two segments (E1 and E2) corresponding to each of the two peak expiratory flows and measured the expiratory airflow volume occurring in each phase at rest and during phasic RTN stimulation. In quiet wake, the volume of E1 expiratory airflow at rest was 0.38 ± 0.02 ml 100 g–1 and decreased with RTN stimuli to 0.25 ± 0.01 ml 100 g–1 (Fig.7D; n = 9; two-way ANOVA with Bonferroni's multiple comparisons: Wake (rest) vs. Wake (laser), P = 0.0005). Conversely, E2 expiratory airflow at rest was 0.14 ± 0.01 ml 100 g–1 and increased with RTN stimuli to 0.25 ± 0.02 ml 100 g–1 (P = 0.0001). There was no change in the relative E1 and E2 expiratory volume under resting conditions, nor with phasic RTN stimuli during non-REM sleep (Fig.7D); total expiratory airflow (volume of E1 + E2) did not differ between any groups (Fig.7D).
Figure 7. Wake state-dependent control of active expiration and upper airways resistance by the RTN.
A and B, event-triggered waveform averages of the plethysmography signal at rest (black trace) and during phasic RTN stimulation (colour trace) in one rat during non-REM sleep (A) or quiet wake (B). Arrows point to PEF. In non-REM sleep (A) PEF occurred in early expiration (E1) regardless of whether RTN was activated or not. In the wake state (B) PEF shifted from early to late expiration (E2; active expiration) during RTN stimulation and early expiratory flow (E1 phase) was markedly reduced, presumably due to increased upper airway resistance. C, relationship between peak expiratory flow and the respiratory cycle with RTN stimulation during non-REM and quiet wake states. n = 5. Mann–Whitney non-parametric test. *P = 0.02. D, expiratory airflow during early (E1; unfilled) and late (E2; grey fill) expiratory phases at rest or during phasic RTN stimuli in non-REM sleep or quiet wake. n = 9. Two-way ANOVA with Bonferroni's multiple comparison test. ***P < 0.0005. E, expiration; I, inspiration; PEF, peak expiratory airflow; RTN, retrotrapezoid nucleus.
In summary, phasic RTN stimulation at frequencies above resting fR entrained the breathing cycle. The trajectory of the expiratory flow was detectably altered only when the animals were awake. Under these circumstances, expiratory flow slowed during the early post-inspiratory phase and was strongly enhanced during the late expiratory phase.
During retrotrapezoid nucleus-imposed pacing, selective reduction of the post-inspiratory phase accounts for the reduction of breathing cycle length
Phasic RTN stimulation entrained breathing equally during non-REM sleep and quiet wake (Fig.8A and B, see also Fig.6C). As previously observed, the light stimuli always settled in the mid- to late-expiratory phase (Potts et al. 2005; Abbott et al. 2011; Pagliardini et al. 2011). During non-REM sleep, pacing shortened the expiratory phase (Fig.8C; n = 8; Kruskal–Wallis H = 41.93, P < 0.0001) without changing the inspiratory duration (Fig.8C; Kruskal–Wallis H = 7.027, P = 0.4). During wake, the post-inspiratory phase (E1) could be clearly distinguished from the active expiratory phase (E2). Pacing shortened the E1 phase (Fig.8D; n = 7; Kruskal–Wallis H = 23.76, P = 0.001) without altering the E2 phase (Kruskal–Wallis H = 3.962, P = 0.8) nor inspiratory duration (Kruskal–Wallis H = 3.510, P = 0.8).
Figure 8. Phasic retrotrapezoid nucleus stimulation selectively shortens the post-inspiratory phase.
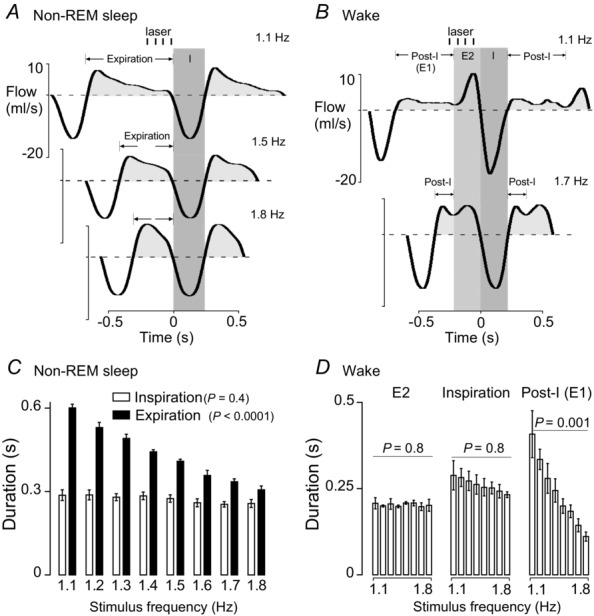
A and B, event-triggered waveform averages of the plethysmography signal during light-induced breathing entrainment in one rat during non-REM sleep (A) and during quiet wake (B). ChR2-transduced retrotrapezoid nucleus neurons were photostimulated with trains of 4 × 3 ms long light pulses (20 Hz; represented as vertical blue bars) delivered at various frequencies between 1.1 and 18 Hz. Active expiration (large flow signal immediately preceding inspiration) was observed only during quiet wake periods. During non-REM sleep we measured only the I and E phases. During quiet wake, the duration of the late expiratory flow peak was defined as the E2 phase and the post-inspiratory phase (E1) represents the balance of the expiratory duration. C and D, group data from eight animals. Effect of stimulation frequency on the various phases of the breathing cycle evaluated using unpaired, non-parametric one-way ANOVA (Kruskal–Wallis). E, expiration; I, inspiration.
Histology
The ChR2-transduced neurons (mCherry-positive, mCherry detection enhanced by immunohistochemistry) were located under the facial motor nucleus within the rostral half of RTN (Fig.9Aa and Ab)(Fig.9 (Takakura et al. 2008). The number of transduced RTN neurons (TH-negative) counted (1:6 series of coronal sections) was 32 ± 5 per rat (n = 8). These sections also contained 27 ± 7 catecholaminergic neurons judged to be A5 noradrenergic neurons rather than C1 adrenergic neurons based on location. RTN contains approximately 2000 neurons in rats (Takakura et al. 2008). On average, only 10% (range 5–18%) were therefore transduced.
The distribution of Arch-transduced (EYFP-immunoreactive) neurons was comparable (though bilateral) and centred on the caudal rather than rostral half of RTN (Fig.9Ba and Bb)(Fig.9. We identified 77 ± 15 Arch-transduced RTN neurons (EYFP+/TH−/Phox2b+) and 97 ± 10 catecholaminergic, probably C1, neurons in a one in six series of transverse sections (n = 8). Thus, 23% of RTN CRCs (range 20–42%) were detectably transduced with Arch (6 × 77/2000).
Green laser light control experiments
To control for possible non-selective effects of continuous application of green light, the light was delivered in 10 s episodes bilaterally to the RTN of 5 rats that had received injections of vector but in which transduced neurons were not detected postmortem (Arch− rats; n = 5). The location of the optical fibre tips was recorded for both Arch− (n = 5) and Arch+ rats (n = 8). The optic fibre tips were positioned in the same region of the ventrolateral medulla oblongata in both rat groups, mostly dorsal to the RTN neurons (Fig.10A). Light delivery during non-REM sleep or quiet wake had no effect on breathing in Arch− rats (Fig.10B and C).
Figure 10. Photostimulation of the RTN in control animals has no effect on breathing.
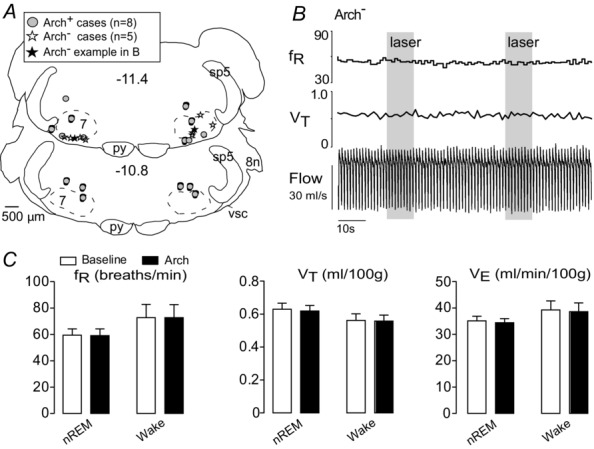
A, location of the bilateral optic fibre tips identified in control rats (Arch−; n = 5) and Arch-expressing rats (Arch+; n = 8) overlap in the ventrolateral medulla. These sites are plotted on two transverse brain sections closest to their location. The tip sites of example B are represented by the filled star. Stereotaxic coordinates (transverse planes posterior to bregma) correspond to the atlas of Paxinos & Watson (2005). B, bilateral illumination of the RTN region with green light (10 s, ∼5 mW) in an Arch− rat produced no change in breathing variables. C, group data from five Arch− animals show no effect of photostimulation of RTN with constant green light (10 s, ∼5 mW) on breathing variables in either non-REM sleep or quiet wake states (two-way repeated measures ANOVA). nREM, non-REM; RTN, retrotrapezoid nucleus
Discussion
We report two new findings. First, the respiratory drive contributed by RTN has the same state dependency as the HCVR. Specifically, like CO2, RTN regulates fR and VT during wake and non-REM sleep whereas, during REM sleep, neither RTN nor CO2 exert any control over fR. Second, RTN stimulation reduces post-inspiratory (E1) airflow and elicits active E2 expiration but this pattern is observed only during wake.
Loss of fR control contributes to reduced chemoreflex gain during REM sleep
As reported before (Fagenholz et al. 1976; Sullivan et al. 1979; Coote, 1982; Douglas et al. 1982a; Douglas et al. 1982b; Berthon-Jones & Sullivan, 1984; Smith et al. 1997; Horner et al. 2002), the HCVR was similar during non-REM sleep and quiet wake but significantly reduced during REM sleep. We also confirm that hypercapnia does not change fR during REM sleep (Berthon-Jones & Sullivan, 1984; Horner et al. 2002; Nakamura et al. 2007). By contrast, hypercapnia reportedly produces comparable increases in diaphragmatic EMG and VT across wake, non-REM and REM sleep states (Haxhiu et al. 1987; Horner et al. 2002; Nakamura et al. 2007). Consistent with these observations, a given increment of 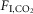 produced a similar VT increase during wake, non-REM sleep and REM sleep in our experiments.
produced a similar VT increase during wake, non-REM sleep and REM sleep in our experiments.
In short, the HCVR persists during REM sleep but with reduced gain because this reflex operates only via changes in VT. This characteristic seems present in all mammals, including humans. Its impact on the HCVR gain is presumably larger in species such as rodents in which fR stimulation contributes most to the reflex. As is the case during REM sleep, homeostatic control of 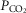 via changes in VT persists during voluntary breathing (Haouzi & Bell, 2009; Ohashi et al. 2013).
via changes in VT persists during voluntary breathing (Haouzi & Bell, 2009; Ohashi et al. 2013).
Inability of retrotrapezoid nucleus to regulate fR contributes to reduced chemoreflex during REM sleep
In rats, the breathing pattern in REM sleep alternates between periods during which fR is increased but remains fairly regular, or increased with periods of marked breathing lability. In neither phase could we modify fR by stimulating or inhibiting RTN. Thus the inability of RTN neurons to control fR underlies, partly at least, the loss of chemoreceptor control over fR during REM sleep. Our observations also rule out the hypothesis that RTN could be mediating the tachypnoea occurring during REM sleep (Fraigne & Orem, 2011).
Two possibilities could explain RTN's inability to regulate fR during REM sleep: (a) RTN could be silent or unexcitable, or (b) this nucleus could remain active and CO2 responsive but its effect on the rhythm generator could be gated out downstream. Although RTN neurons are mildly activated by serotonin, a transmitter whose release presumably decreases during REM sleep in this brain region as elsewhere (Veasey et al. 1995; Mulkey et al. 2007), the first interpretation (a) is the least plausible. First, optogenetic activation of RTN or hypercapnia still increased VT during REM sleep indicating that RTN does remain excitable. Even if distinct subtypes of RTN neurons regulated fR vs. VT it seems unlikely that only the former would become unresponsive to ChR2 activation during REM sleep. Second, optogenetic inhibition of RTN inhibited VT similarly during wake, non-REM and REM sleep indicating that these neurons remain active at rest during REM sleep. Accordingly, a more plausible explanation is that, during REM sleep, neither RTN nor CO2 controls fR because the respiratory rhythm generator is unresponsive to chemoreceptor input.
State-dependent control of active expiration by retrotrapezoid nucleus
Active expiration (Janczewski & Feldman, 2006; Feldman et al. 2013), can be elicited by activating RTN and surrounding catecholamine (CA) neurons in conscious rats (Abbott et al. 2011; and present results). In the in situ working heart–brainstem preparation in which lumbar expiratory activity persists at rest, this outflow is eliminated by inhibiting the same neuronal mix (Marina et al. 2010). The present study adds three novel elements. First, active expiration is elicited by stimulating the rostral portion of RTN, which contains hypercapnia-activated neurons but no C1 cells (Takakura et al. 2008). Thus, C1 cell stimulation is not required to produce active expiration. Second, RTN stimulation causes active expiration only when the rats are awake. Third, we suggest that upper airways resistance increases in parallel with active expiration, as denoted by the significant reduction in early expiratory (E1) airflow and the proportional increase in late expiratory (E2) airflow. In sum, we show that, along with active expiration, RTN stimulation produces a brief wake state-dependent facilitation of glottis closure immediately after inspiration to maintain expiratory lung volume, presumably for increased gas exchange. Thus, RTN stimulation appears capable of increasing alveolar ventilation via at least four mechanisms: increased inspiratory tidal volume; increased fR; brief retention of inspired air during the early expiratory phase; and active expiration. According to the present results, the latter two mechanisms operate only during the waking state. However, we do not exclude the possibility that active expiration could be triggered even during non-REM sleep by stimulating a larger fraction of RTN neurons than in the present study.
Components of the circuitry responsible for active expiration (expiratory rhythm generator) overlap anatomically with the caudal RTN (Pagliardini et al. 2011; Feldman et al. 2013; Tupal et al. 2014). As shown here, active expiration can be triggered by stimulating RTN neurons located rostral to this region (Pagliardini et al. 2011; Feldman et al. 2013). Thus RTN, as defined in this laboratory (Phox2b+/VGLUT2+/NK1R+ neurons located ventral to the facial motor nucleus) (Guyenet & Mulkey, 2010), can activate this oscillator but is probably not part of it. Consistent with this interpretation, RTN stimulation elicits active expiration only during wake whereas the same neurons increase fR and inspiratory amplitude equally during non-REM sleep and quiet wake; the same reasoning applies for the effects of RTN on post-inspiratory airflow. The fact that RTN can only facilitate active expiration or laryngeal adduction during wake signifies that, in the absence of exercise, the recruitment of these muscles for breathing requires both a high level of CRC activation and a heightened network excitability presumably conferred by wake-ON neuromodulators (e.g. serotonin, noradrenaline, orexin) (Doi & Ramirez, 2008).
RTN innervates all the respiratory pattern generator (RPG) regions that harbour excitatory pump premotor neurons (Ballantyne & Richter, 1986; Dobbins & Feldman, 1994; Yokota et al. 2007; Bochorishvili et al. 2012). The excitatory input from RTN plausibly increases VT by enhancing the discharges of these premotor cells. Phrenic motor neurons remain active regardless of the state of vigilance hence the relative state independence of the control of inspiratory amplitude by RTN and other chemoreceptors. By contrast, the activity of lumbar and other expiratory pump muscles is highly state-dependent, like that of other postural muscles or the musculature regulating upper airway resistance. This characteristic probably explains why RTN stimulates active expiration only during wake. Reductions of the activity of serotonin, noradrenaline and orexin neurons probably contribute to the reduced excitability of expiratory motor neurons or their cognate premotor inputs during sleep (Doi & Ramirez, 2008; Horner, 2009; Saper et al. 2010).
Expiratory airflow is regulated by upper airway resistance. The reduced E1 airflow with phasic RTN stimulation is probably a facilitation of glottic adduction by laryngeal constrictor (LC) muscles. The post-inspiratory activity of LC motor neurons could be enhanced by the polysynaptic drive via the Kolliker Fuse, a major target of RTN (Dutschmann & Herbert, 2006; Bochorishvili et al. 2012), and/or a network effect that augments phasic inspiratory inhibition of LC motor neurons and strengthens their post-inhibitory rebound (Sun et al. 2008; Bautista et al. 2010).
Retrotrapezoid nucleus stimulation selectively shortens the post-inspiratory phase
During quiet wake, phasic RTN stimulation increased fR strictly by shortening the post-inspiratory (E1) phase. In vitro, somatic afferent stimulation also produces tachypnoea in this manner (Potts et al. 2005). In this case, the tachypnoea is attributed to disinhibition of the rhythmogenic pre-inspiratory/inspiratory (preI/I) neurons of the pre-Bötzinger complex as follows (Potts et al. 2005; Smith et al. 2013): activation of inhibitory E2 Bötzinger neurons by somatic afferents would cause early termination of E1 inhibitory neurons and, consequently, earlier depolarization of the preI/I neurons (Potts et al. 2005). RTN neurons could produce tachypnoea via this mechanism because they are excitatory and innervate the Bötzinger region (Mulkey et al. 2004; Bochorishvili et al. 2012). However, RTN neurons also project heavily to the pre-Bötzinger complex, including to the NK1 receptor expressing neurons that are suspected to include the preI/I cells (Gray et al. 1999; Bochorishvili et al. 2012). An excitatory input from RTN to these cells should also theoretically be able to reduce the duration of their inhibition during E1, allowing their burst discharges to occur earlier and fR to rise.
Why is retrotrapezoid nucleus no longer controlling fR during REM sleep?
The transition from an autorhythmic homeostatically regulated state (anaesthesia, reduced preparations, non-REM sleep and quiet wake) to other forms of breathing (voluntary, emotional, REM sleep) is poorly understood. However, in general terms, the ‘commanded’ model, where inspiration is still triggered by the pontomedullary RPG, but its timing is tightly controlled by powerful inhibitory inputs from higher source(s) (Richter & Smith, 2014) seems the most plausible for several reasons. The pontomedullary RPG is clearly engaged during both REM sleep and volitional control (Lovering et al. 2006) and the integrity of the pre-Bötzinger circuitry is required for normal breathing during both REM and non-REM sleep (McKay & Feldman, 2008). Finally, given that RTN projections are propriobulbar (Bochorishvili et al. 2012), the homeostatic control of VT during REM sleep must occur via the pontomedullary RPG.
Post-inspiratory inhibition is probably far more critical to the voluntary control of breathing than to breathing automaticity during quiet wake and non-REM sleep (Guz, 1997; Richter & Smith, 2014). The irregular breathing pattern present during REM sleep may share this characteristic. A plausible reason why RTN no longer controls fR during REM sleep could be that the excitatory effect of RTN on the preI/I neurons is gated out during REM sleep by powerful inhibitory inputs from outside the pontomedullary RPG, which clamp the membrane potential of these neurons during the expiratory phase of the breathing cycle (Richter & Smith, 2014).
Physiological and pathophysiological relevance
The present observations could explain why many forms of central sleep apnoea, including CCHS occur predominantly during non-REM sleep (Berssenbrugge et al. 1983; Farney et al. 2003; Lovering et al. 2012). During REM sleep, VE depends more on fR, which rises while VT decreases. Because fR is no longer under chemoreceptor control during REM sleep, VE is less dependent on 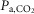 than during non-REM sleep. Therefore, recurrent cycles of hyperventilation and apnoeas presumably cannot be sustained during REM sleep because, as shown here in rats, CRCs such as RTN contribute much less to ventilation.
than during non-REM sleep. Therefore, recurrent cycles of hyperventilation and apnoeas presumably cannot be sustained during REM sleep because, as shown here in rats, CRCs such as RTN contribute much less to ventilation.
Experimental limitations
PRSx8-based LVVs transduce a mixed population of RTN and catecholaminergic neurons as described before (Abbott et al. 2009; Marina et al. 2010; Abbott et al. 2011). In the ChR2 experiments, most transduced neurons (54%) were TH-negative hence putative CRCs. These neurons were located within the rostral half of RTN, which contains A5 noradrenergic neurons but few C1 adrenergic cells (Takakura et al. 2008). Selective C1 cell stimulation raises BP massively in conscious rats (Burke et al. 2014). Here BP was unaffected. Thus few C1 neurons could have been directly or synaptically activated and, consequently, these neurons could not have produced the observed breathing stimulation. Some contribution of A5 neuron stimulation to the breathing effects observed presently cannot be excluded.
For the loss of function experiments (Arch experiments), we needed to target the caudal RTN to transduce more CRCs. This region contains numerous C1 cells and, consequently, 56% of the Arch-transduced neurons were TH-immunoreactive. Under anaesthesia, Arch activation effectively silenced RTN neurons (Basting et al. 2015). In anaesthetized rats, sympathetic tone is high, the C1 cells are very active and their inhibition produces profound hypotension consistent with their sympathoexcitatory function (Schreihofer & Guyenet, 1997; Burke et al. 2008; Marina et al. 2011; Guyenet et al. 2013). Here, in conscious rats, Arch photostimulation had no effect on BP. One explanation is that sympathetic tone is low in resting unstressed and normoxic animals and the transduced C1 cells have a correspondingly low resting discharge. Another possibility is that the Arch-transduced C1 cells were inadequately hyperpolarized by the light. In any event, the lack of BP change indicates that C1 cell inhibition was minor at best. Consequently, a change in the activity of the C1 cells is very unlikely to have contributed significantly to the observed respiratory changes.
Summary and conclusions
RTN regulates both fR and VT when the pontomedullary RPG is autorhythmic (anaesthesia, non-REM sleep, quiet wake) but RTN no longer controls fR during REM sleep. We speculate that during REM sleep, as during voluntary breathing, the expiratory phase of the breathing cycle is controlled by suprabulbar inputs that powerfully inhibit the pre-Bötzinger complex and shunt out the excitatory effect of RTN on the rhythmogenic core. A higher resting fR that is no longer subject to control by RTN could explain the absence of periodic breathing and central sleep apnoea during REM sleep in humans.
Additional information
Competing interests
None.
Author contributions
P.G.R.B., R.L.S., T.M.B. and P.G.G. designed the experiments. P.G.R.B., R.K. and T.M.B. performed and analysed the physiological experiments. W.M.H., K.E.V. and R.L.S. did the histology. P.G.R.B. and P.G.G. wrote the manuscript.
Funding
This work was supported by National Institutes of Health Grants HL28785 and HL74011 to P.G.G.
Supporting Information
Videos S1
Videos S2
Videos S3
Videos S4
References
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE. Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Stornetta RL, Coates MB. Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci. 2011;31:16410–16422. doi: 10.1523/JNEUROSCI.3280-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Coates MB, Stornetta RL. Guyenet PG. Optogenetic stimulation of C1 and retrotrapezoid nucleus neurons causes sleep state-dependent cardiorespiratory stimulation and arousal in rats. Hypertension. 2013a;61:835–841. doi: 10.1161/HYPERTENSIONAHA.111.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Depuy SD, Nguyen T, Coates MB, Stornetta RL. Guyenet PG. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J Neurosci. 2013b;33:3164–3177. doi: 10.1523/JNEUROSCI.1046-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Holloway BB, Viar KE. Guyenet PG. Vesicular glutamate transporter 2 is required for the respiratory and parasympathetic activation produced by optogenetic stimulation of catecholaminergic neurons in the rostral ventrolateral medulla of mice in vivo. Eur J Neurosci. 2014;39:98–106. doi: 10.1111/ejn.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C. Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Ballantyne D. Richter DW. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basting TM, Burke PG, Kanbar R, Viar KE, Stornetta DS, Stornetta RL. Guyenet PG. Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosis. J Neurosci. 2015;35:527–543. doi: 10.1523/JNEUROSCI.2923-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista TG, Burke PG, Sun QJ, Berkowitz RG. Pilowsky PM. The generation of post-inspiratory activity in laryngeal motoneurons: a review. Adv Exp Med Biol. 2010;669:143–149. doi: 10.1007/978-1-4419-5692-7_29. [DOI] [PubMed] [Google Scholar]
- Berssenbrugge A, Dempsey J, Iber C, Skatrud J. Wilson P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J Physiol. 1983;343:507–524. doi: 10.1113/jphysiol.1983.sp014906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon-Jones M. Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol. 1984;57:59–67. doi: 10.1152/jappl.1984.57.1.59. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Stornetta RL, Coates MB. Guyenet PG. Pre-Botzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J Comp Neurol. 2012;520:1047–1061. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DL. Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res. 1985;56:359–369. doi: 10.1161/01.res.56.3.359. [DOI] [PubMed] [Google Scholar]
- Burgess KR. Central sleep apnoea and heart failure (Part I) Respirology. 1997;2:243–253. doi: 10.1111/j.1440-1843.1997.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Burke PG, Li Q, Costin ML, McMullan S, Pilowsky PM. Goodchild AK. Somatostatin 2A receptor-expressing presympathetic neurons in the rostral ventrolateral medulla maintain blood pressure. Hypertension. 2008;52:1127–1133. doi: 10.1161/HYPERTENSIONAHA.108.118224. [DOI] [PubMed] [Google Scholar]
- Burke PG, Abbott SB, Coates MB, Viar KE, Stornetta RL. Guyenet PG. Optogenetic stimulation of adrenergic C1 neurons causes sleep state-dependent cardiorespiratory stimulation and arousal with sighs in rats. Am J Respir Crit Care Med. 2014;190:1301–1310. doi: 10.1164/rccm.201407-1262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH. Respiratory and circulatory control during sleep. J Exp Biol. 1982;100:223–244. doi: 10.1242/jeb.100.1.223. [DOI] [PubMed] [Google Scholar]
- Dejours P. Chemoreflexes in breathing. Physiol Rev. 1962;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Smith CA, Blain GM, Xie A, Gong Y. Teodorescu M. Role of central/peripheral chemoreceptors and their interdependence in the pathophysiology of sleep apnea. Adv Exp Med Biol. 2012;758:343–349. doi: 10.1007/978-94-007-4584-1_46. [DOI] [PubMed] [Google Scholar]
- Dobbins EG. Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Doi A. Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas NJ, White DP, Weil JV, Pickett CK, Martin RJ, Hudgel DW. Zwillich CW. Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis. 1982a;125:286–289. doi: 10.1164/arrd.1982.125.3.286. [DOI] [PubMed] [Google Scholar]
- Douglas NJ, White DP, Weil JV, Pickett CK. Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis. 1982b;126:758–762. doi: 10.1164/arrd.1982.126.5.758. [DOI] [PubMed] [Google Scholar]
- Dutschmann M. Herbert H. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Fagenholz SA, O'Connell K. Shannon DC. Chemore-ceptor function and sleep state in apnea. Pediatrics. 1976;58:31–36. [PubMed] [Google Scholar]
- Farney RJ, Walker JM, Cloward TV. Rhondeau S. Sleep-disordered breathing associated with long-term opioid therapy. Chest. 2003;123:632–639. doi: 10.1378/chest.123.2.632. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming PJ, Cade D, Bryan MH. Bryan AC. Congenital central hypoventilation and sleep state. Pediatrics. 1980;66:425–428. [PubMed] [Google Scholar]
- Fraigne JJ. Orem JM. Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. Sleep. 2011;34:425–434. doi: 10.1093/sleep/34.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R. Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci USA. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A. Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K. Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM. Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the PreBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol. 2014;4:1511–1562. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. RespirPhysiol Neurobiol. 2010;173:244–255. doi: 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL. Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL. Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG. Abbott SB. C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–R204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz A. Brain, breathing and breathlessness. Respir Physiol. 1997;109:197–204. doi: 10.1016/s0034-5687(97)00050-9. [DOI] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R. Boyden ES. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi P. Bell HJ. Control of breathing and volitional respiratory rhythm in humans. J Appl Physiol. 2009;106:904–910. doi: 10.1152/japplphysiol.90675.2008. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, van Lunteren E, Mitra J. Cherniack NS. Comparison of the response of diaphragm and upper airway dilating muscle activity in sleeping cats. Respir Physiol. 1987;70:183–193. doi: 10.1016/0034-5687(87)90049-1. [DOI] [PubMed] [Google Scholar]
- Horner RL. Emerging principles and neural substrates underlying tonic sleep-state-dependent influences on respiratory motor activity. Philos Trans R Soc Lond B Biol Sci. 2009;364:2553–2564. doi: 10.1098/rstb.2009.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H. Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO2 in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT. Dale N. Redefining the components of central CO2 chemosensitivity – towards a better understanding of mechanism. J Physiol. 2011;589:5561–5579. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Carlezon WA, Jr, Isacson O. Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- Janczewski WA. Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbar R, Stornetta RL, Cash DR, Lewis SJ. Guyenet PG. Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am J Respir Crit Care Med. 2010;182:1184–1194. doi: 10.1164/rccm.201001-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering AT, Dunin-Barkowski WL, Vidruk EH. Orem JM. Ventilatory response of the cat to hypoxia in sleep and wakefulness. J Appl Physiol. 2003;95:545–554. doi: 10.1152/japplphysiol.01051.2002. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Fraigne JJ, Dunin-Barkowski WL, Vidruk EH. Orem JM. Medullary respiratory neural activity during hypoxia in NREM and REM sleep in the cat. J Neurophysiol. 2006;95:803–810. doi: 10.1152/jn.00615.2005. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Fraigne JJ, Dunin-Barkowski WL, Vidruk EH. Orem JM. Tonic and phasic drive to medullary respiratory neurons during periodic breathing. Respir Physiol Neurobiol. 2012;181:286–301. doi: 10.1016/j.resp.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus NJ, DR R, Schultz EP, Xia XH. Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2014;592:391–408. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF. Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Korsak A, Simms AE, Allen AM, Paton JF. Gourine AV. Control of sympathetic vasomotor tone by catecholaminergic C1 neurones of the rostral ventrolateral medulla oblongata. Cardiovasc Res. 2011;91:703–710. doi: 10.1093/cvr/cvr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O'Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, Yizhar O. Deisseroth K. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2012;9:159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC. Feldman JL. Unilateral ablation of pre-Botzinger complex disrupts breathing during sleep but not wakefulness. Am J Respir Crit Care Med. 2008;178:89–95. doi: 10.1164/rccm.200712-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA. Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA. Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y. Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Izumizaki M, Atsumi T. Homma I. CO2 homeostasis is maintained in conscious humans by regulation of tidal volume, but not of respiratory rhythm. Respir Physiol Neurobiol. 2013;186:155–163. doi: 10.1016/j.resp.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Orem JM, Lovering AT. Vidruk EH. Excitation of medullary respiratory neurons in REM sleep. Sleep. 2005;28:801–807. doi: 10.1093/sleep/28.7.801. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K. Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- Potts JT, Rybak IA. Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci. 2005;25:1965–1978. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J. Goridis C. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW. Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 2014;29:58–71. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J. Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer AM. Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Smith CA, Henderson KS, Xi L, Chow C-M, Eastwood PR. Dempsey JA. Neural-mechanical coupling of breathing in REM sleep. J Appl Physiol. 1997;83:1923–1932. doi: 10.1152/jappl.1997.83.6.1923. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Borgmann A, Rybak IA. Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van ZR. Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2012;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CE, Murphy E, Kozar LF. Phillipson EA. Ventilatory responses to CO2 and lung inflation in tonic versus phasic REM sleep. J Appl Physiol. 1979;47:1305–1310. doi: 10.1152/jappl.1979.47.6.1304. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Berkowitz RG. Pilowsky PM. GABA A mediated inhibition and post-inspiratory pattern of laryngeal constrictor motoneurons in rat. Respir Physiol Neurobiol. 2008;162:41–47. doi: 10.1016/j.resp.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM. Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S, Huang WH, Picardo MC, Ling GY, Del Negro CA, Zoghbi HY. Gray PA. Atoh1-dependent rhombic lip neurons are required for temporal delay between independent respiratory oscillators in embryonic mice. Elife. 2014;3:e02265. doi: 10.7554/eLife.02265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW. Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J. Bayliss DA. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci. 2013;33:16033–16044. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA. Trang H. An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med. 2010;181:626–644. doi: 10.1164/rccm.200807-1069ST. [DOI] [PubMed] [Google Scholar]
- Weil JV. Sleep at high altitude. High Alt Med Biol. 2004;5:180–189. doi: 10.1089/1527029041352162. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S. Yasui Y. Glutamatergic neurons in the Kolliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: a combined retrograde tracing and in situ hybridization study in the rat. Neurosci Res. 2007;59:341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos S1
Videos S2
Videos S3
Videos S4