Abstract
Background
Disability progression in multiple sclerosis (MS) remains incompletely understood. Unlike lesional measures, CNS atrophy has a strong correlation with disability. TMEV infection in SJL mice is an established model of progressive MS. We utilized in vivo MRI to quantify brain and spinal cord atrophy in this model and analyzed the temporal relationship between atrophy and disability.
Methods
Infected and control mice were followed for twelve months. Disability was assessed periodically using rotarod assay. Volumetric MRI datasets were acquired at 7 Tesla. Ventricular volume and C4-5 spinal cord cross-sectional area measurements were performed using Analyze 10.
Results
At three months, brain atrophy reached statistical significance (p=0.005). In contrast, disability did not differ until four months post infection (p=0.0005). Cord atrophy reached significance by nine months (p=0.009). By twelve months, brain atrophy resulted in 111.8% increased ventricular volume (p=0.00003), while spinal cord cross-sectional area was 25.6% reduced (p=0.001) among cases.
Conclusions
Our results suggest that significant brain atrophy precedes and predicts the development of disability, while spinal cord atrophy occurs late and correlates with severe disability. The observed temporal relationship establishes a framework for mechanisms of disability progression and enables further investigations of their underlying substrate.
Keywords: Multiple sclerosis, Atrophy, Disability, Magnetic Resonance Imaging
INTRODUCTION
Multiple sclerosis is a multifocal disease of the central nervous system. Histopathology is characterized by inflammatory demyelination, reactive gliosis, and axonal damage.1 MRI detected white matter lesions correspond to areas of demyelination and have been part of the MS diagnostic criteria for the past decade. However, lesion based metrics generally correlate poorly with clinical disability.2, 3 Axonal damage occurs early in disease and loss of axons, dendrites and neurons correlates well with clinical disability. Cortical and central atrophy have both been reported,4, 5 but pathologic mechanisms and relationship to inflammatory lesions remains unclear.
Theiler's Murine Encephalomyelitis Virus (TMEV) infection of mice is an established model of multiple sclerosis.6, 7 Intracerebral injection of TMEV induces a biphasic disease in susceptible strains of mice. Acute infection comprises a meningo-encephalomyelitis stage in all strains with subsequent full recovery. In susceptible strains, this is followed by a late stage of progressive demyelinating disease. TMEV infected SJL mice develop slowly progressive demyelination and disability over 9 to 12 months. Similar to experimental autoimmune encephalomyelitis (EAE), mostly spinal cord lesions develop. Despite the paucity of brain lesions, previous work from our laboratory demonstrated progressive cerebral atrophy in this model that is strongly correlated with disability.8 The study presented here further characterizes the distribution of CNS atrophy and the timing of development in relation to disability progression.
METHODS
TMEV model of MS
Progressive demyelinating disease was induced by intracerebral injection of TMEV into 6- to 8-week old mice. Animals were anesthetized with 1.5% inhalational isoflurane and then injected with 106 plaque-forming units of the Daniel’s strain of TMEV. Control animals underwent intracerebral injection of an equivalent volume of culture medium (DMEM; Mediatech, Manassas, VA). All experiments were approved by the Institutional Animal Care and Use Committee.
Animals and Imaging
Eight TMEV infected SJL mice and 4 controls were followed with MRI until mortality or 12 months post infection. Volume acquisition T1 and T2 weighted sequences were obtained using a Bruker Biospec (7 Tesla; Billerica, MA) horizontal bore small animal MRI system. Inhalational anesthesia (1.5% isoflurane) was used for the imaging procedure and vital parameters were monitored continuously via SA Instruments gating and monitoring system (SAI Inc, Stony Brook, NY). There was no animal loss during imaging. Four mice were lost due to the natural course of this model after the 6 month time point, and one control mouse.
Image analysis was performed using Analyze 10.0 (Mayo Clinic BIR). Total brain and ventricular volume measurements were performed using the coregistration, slice extraction, and semi-automated 3D ROI tools. Spinal cord area measurements were performed using the slice extraction and semi-automated 2D ROI tools. We used lateral ventricle volumes for determination of brain atrophy and cross-sectional area at the C4-5 interspace for determination of spinal cord atrophy. The datasets were analyzed independently by two investigators with excellent intra- and inter-rater agreement.
Disability Assessment
Disability was monitored by periodic rotarod assay. This assay determines the time the animals are able to walk on a constantly accelerating rotating rod, and is a commonly used measure of disability in mice, capturing motor, sensory and coordination/balance-related components.8, 9 Two attempts were given to each animal at each time point, and the better score (the score suggestive of less disability) was used at each time point for data analysis. To minimize effects of motor learning, animals were trained on the rotarod for one month prior to the experiment.
Statistical Analysis
Intergroup differences were analyzed using Student's t-test and correlations were analyzed using Pearson Product Moment Correlation. All analysis was performed in SigmaPlot 11 (Systat software, Chicago, IL).
RESULTS
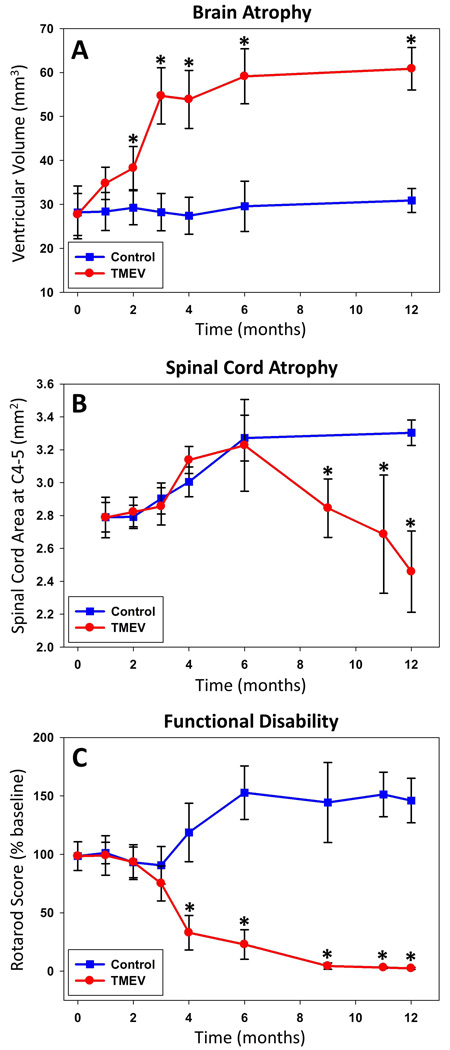
Significant brain atrophy was present by two months post infection (figure 1). Lateral ventricular volume was increased 32.4% by then (p=0.005) and plateaued at 111.8% by six months post infection (p=0.00003)(figure 2). Significant spinal cord atrophy was present by nine months post infection (figure 1). Spinal cord cross-sectional area was decreased 13.9% (p=0.009) by then with continued decline to 25.6% at twelve months post infection (p=0.001)(figure 2). Significant disability was present by four months post infection (72% lower, p=0.0005) with continued worsening through twelve months (figure 2).
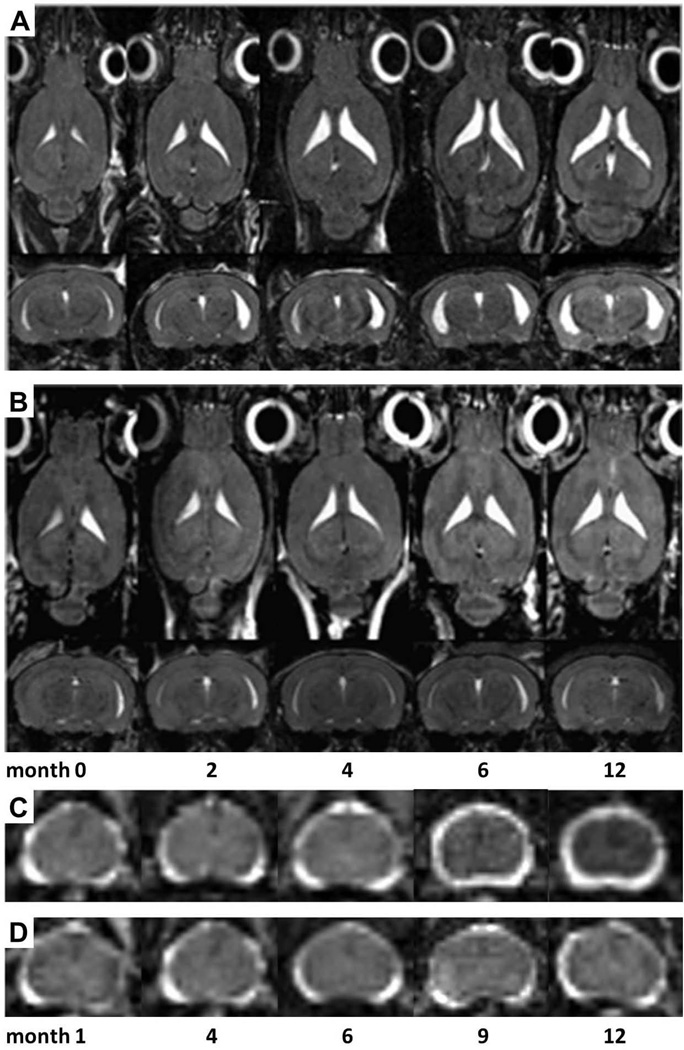
Figure 1. MRI assessed CNS atrophy.
Lateral ventricular volume increased from disease onset and peaked by six months post infection in TMEV infected SJL mice (A), but remained stable in controls (B). Spinal cord atrophy worsened from six months through twelve months post infection (C), but remained stable in controls (D).
Figure 2. Disability and CNS atrophy.
Brain and spinal cord atrophy were assessed by ventricular volume and spinal cord cross-sectional area, respectively. Functional disability was assessed by rotarod assay. Comparing TMEV infected SJL mice to controls, brain atrophy increased from disease onset and peaked by six months post infection (A). Spinal cord atrophy worsened from six months through twelve months post infection (B). Functional disability was apparent by four months post infection with continued worsening through twelve months (C).
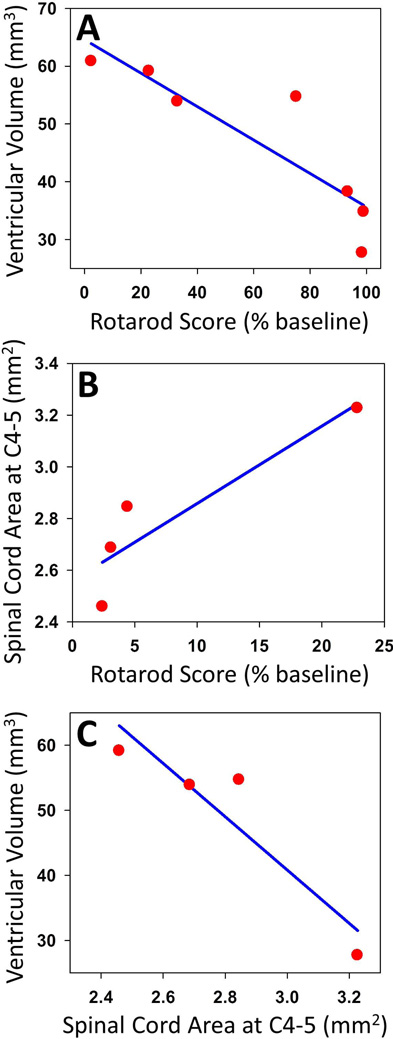
Brain atrophy showed a significant and strong negative correlation with functional disability (r=−0.88, p=0.01) throughout the observation period (figure 3). Spinal cord atrophy showed a strong correlation with functional disability trending toward significance (r=0.91, p=0.09) from months six through twelve (figure 3). Brain atrophy from zero through six months showed a strong correlation with spinal cord atrophy from six through twelve months (figure 3), also trending toward significance (r=−0.92, p=0.07).
Figure 3. Correlation.
Pearson Product Moment Correlation was used to assess dependence. Brain atrophy showed a significant and strong negative correlation with functional disability throughout the observation period (A). Spinal cord atrophy showed a strong correlation with functional disability from months six through twelve (B). Brain atrophy from zero through six months showed a strong correlation with spinal cord atrophy from six through twelve months (C).
DISCUSSION
We previously reported brain atrophy in correlation with neurologic function in this murine model of multiple sclerosis.8 In the study presented here, we demonstrate both brain and spinal cord atrophy in this model. The progressive atrophy in these two CNS regions, however, is temporally separated. Similarly, atrophy in these regions strongly correlates with disability, but over separate observation periods of the study. Brain atrophy preceded, and spinal cord atrophy followed, functionally detectable disability. As such, this study more completely delineates the presence and timing of CNS atrophy in this model, providing additional insight and avenues of investigation into pathologic mechanisms.
We utilized increasing ventricular volume as a measure of worsening brain atrophy. Although hydrocephalus is an alternate pathology that could cause increased ventricular volume in the absence of brain atrophy, hydrocephalus has been reported only in a neurovirulent experimentally modified TMEV strain and not in the wild-type Daniel’s strain used here.10 Moreover, hydrocephalus from the altered virus occurs within one week of infection, in contradistinction to the time course of ventricular enlargement reported here. As such, increasing ventricular volume should reflect worsening brain atrophy in this study. Importantly, the magnitude of ventricular enlargement observed in this model cannot be accounted for by white matter atrophy alone, given the relative volumes of cerebral white and gray matter, suggesting both cortical and subcortical atrophy contribute. This is analogous to MRI measured cortical atrophy in experimental autoimmune encephalomyelitis, another murine model of MS.11
Acute TMEV infection can lead to early neuronal loss in resistant strains of mice,12, 13 but this has not been significant in susceptible strains of mice.14 Moreover, the subacute time course of brain atrophy observed here should not be related to viral cytopathy, as both resistant and susceptible strains of mice clear virus from brain within two weeks of infection.15 Since lesions in the TMEV model primarily occur within the spinal cord and few are seen within the brain, central atrophy is also not likely related directly to lesion-associated mechanisms. Deep gray matter T2 hypointensity has been observed, which correlates with disability in the TMEV model16 and with gray matter atrophy in MS,17 but cortical lesions have not been reported in the TMEV model. Central atrophy, therefore, is most likely related to changes within the normal appearing white and gray matter (NAW+GM). Although no gross inflammatory infiltrate is seen in NAW+GM, lymphocyte numbers are increased compared to normal brain, with CD8+ cells being the most numerous.18 Cerebral T1 hypointensities, which reflect axonal and neuronal loss in MS,19 are seen in the TMEV model and are due to the activity of CD8+ cells,20, 21 suggesting that these lymphocytes directly contribute to axonal and neuronal damage.
We utilized spinal cord cross sectional area at the C4-5 interspace as a measure of spinal cord atrophy, which was significant only at the chronic phase of disease. This time course is suggestive of delayed axonal loss, but early decline in spinal cord cross sectional area may have been masked by edema related to inflammatory lesions. However, delayed atrophy would correlate well with pathologic studies showing axon loss with a similar time course affecting the lower cervical and mid thoracic cord,22, 23 which argues against a masking effect of edema. Since TMEV is known to persist within the spinal cord of susceptible mouse strains, viral cytopathy is an etiologic consideration for atrophy, but persistence is predominantly within macrophages.24 Whether spinal cord atrophy is secondary to or independent from brain atrophy is not clear. Since axon loss is predominantly seen within the ventral and lateral funiculi, areas where inflammatory demyelinating lesions are found, mechanisms associated with inflammatory cells and chronic loss of myelin have been proposed to underlie spinal cord atrophy.22 In contrast, early axonal injury within the spinal cord white matter has been reported in the absence of inflammatory cells and preceding demyelination.25 Since demyelination occurs in the same areas where early axonal injury is seen, apoptotic and Wallerian degeneration mechanisms triggered by cerebral TMEV infection have been proposed to underlie spinal cord atrophy,26 with inflammatory demyelinating lesions arising secondarily27 and further contributing to axonal injury and loss. The mechanisms of axonal injury mediated by inflammation are not entirely clear, but may also be due to the activity of CD8+ cells within the spinal cord NAWM.28 Further analysis of the mechanism of axonal injury mediated by inflammation needs to be performed and will be the subject of future studies.
The observed temporal relationship between brain atrophy, disability and spinal cord atrophy is informative. The demonstration that brain atrophy precedes disability onset suggests this is proximate to initiating events of the pathologic cascade. That this atrophy occurred in the absence of significant cerebral lesions implicates pathologic mechanisms separate from inflammatory demyelination and underscores the need to further elucidate pathology occurring in normal appearing white and gray matter. The fact that spinal cord atrophy follows disability onset may suggest this is a marker of, and less likely causative of, progressive functional decline. Spinal cord edema, however, may confound initial cross sectional area decline. Importantly, this temporal relationship establishes a framework for mechanisms of disability progression and informs further investigations of their underlying substrate. The array of genetically distinct mouse strains should facilitate further delineation of the pathologic mechanisms within the TMEV model, with MRI enabling in vivo assessment of CNS atrophy. Better understanding of the initiating events and subsequent pathologic cascade will provide insight into potential therapeutic interventions that could impact disability progression in MS.
ACKNOWLEDGEMENTS
This work was supported by NIH NINDS R01NS58698 to Istvan Pirko and R01NS60881 to Aaron J. Johnson.
Funding Source: This work was supported by NIH NINDS R01NS58698 to Istvan Pirko and R01NS60881 to Aaron J. Johnson. The sponsors had no involvement with study design; collection, analysis and interpretation of data; writing of the manuscript; or in the decision to submit for publication.
Footnotes
Disclosures: The authors have no financial conflicts of interest.
Contributor Information
M. Mateo Paz Soldán, Email: pazsoldan.mateo@mayo.edu.
Mekala R. Raman, Email: raman.mekala@mayo.edu.
Jeffrey D. Gamez, Email: gamez.jeffrey@mayo.edu.
Anne K. Lohrey, Email: aklohrey@gmail.com.
Yi Chen, Email: chey2@ucmail.uc.edu.
Istvan Pirko, Email: pirko@mayo.edu.
Aaron J. Johnson, Email: johnson.aaron2@mayo.edu.
References
- 1.Prineas JW. Pathology of multiple sclerosis. In: Cook SD, editor. Handbook of multiple sclerosis. 3rd. New York: Marcel Dekker; 2001. pp. 289–324. [Google Scholar]
- 2.Stadelmann C, Albert M, Wegner C, Bruck W. Cortical pathology in multiple sclerosis. Curr Opin Neurol. 2008;21:229–234. doi: 10.1097/01.wco.0000318863.65635.9a. [DOI] [PubMed] [Google Scholar]
- 3.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 4.De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60:1157–1162. doi: 10.1212/01.wnl.0000055926.69643.03. [DOI] [PubMed] [Google Scholar]
- 5.Kalkers NF, Ameziane N, Bot JC, Minneboo A, Polman CH, Barkhof F. Longitudinal brain volume measurement in multiple sclerosis: rate of brain atrophy is independent of the disease subtype. Arch Neurol. 2002;59:1572–1576. doi: 10.1001/archneur.59.10.1572. [DOI] [PubMed] [Google Scholar]
- 6.Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler's virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denic A, Johnson AJ, Bieber AJ, Warrington AE, Rodriguez M, Pirko I. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–29. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirko I, Johnson AJ, Chen Y, et al. Brain atrophy correlates with functional outcome in a murine model of multiple sclerosis. Neuroimage. 2011;54:802–806. doi: 10.1016/j.neuroimage.2010.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson AJ, Upshaw J, Pavelko KD, Rodriguez M, Pease LR. Preservation of motor function by inhibition of CD8+ virus peptide-specific T cells in Theiler's virus infection. FASEB J. 2001;15:2760–2762. doi: 10.1096/fj.01-0373fje. [DOI] [PubMed] [Google Scholar]
- 10.Tsunoda I, McCright IJ, Kuang LQ, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler's murine encephalomyelitis virus variant. J Neuropathol Exp Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 11.MacKenzie-Graham A, Rinek GA, Avedisian A, et al. Cortical atrophy in experimental autoimmune encephalomyelitis: in vivo imaging. Neuroimage. 2012;60:95–104. doi: 10.1016/j.neuroimage.2011.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buenz EJ, Sauer BM, Lafrance-Corey RG, et al. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am J Pathol. 2009;175:668–684. doi: 10.2353/ajpath.2009.081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe CL, Lafrance-Corey RG, Sundsbak RS, Lafrance SJ. Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain. J Neuroinflammation. 2012;9:50. doi: 10.1186/1742-2094-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsunoda I, Kurtz CI, Fujinami RS. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- 15.Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler's murine encephalomyelitis virus. Acta Neuropathol. 1996;91:595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- 16.Pirko I, Johnson AJ, Lohrey AK, Chen Y, Ying J. Deep gray matter T2 hypointensity correlates with disability in a murine model of MS. J Neurol Sci. 2009;282:34–38. doi: 10.1016/j.jns.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil M, Enzinger C, Langkammer C, et al. Quantitative assessment of brain iron by R(2)* relaxometry in patients with clinically isolated syndrome and relapsing-remitting multiple sclerosis. Mult Scler. 2009;15:1048–1054. doi: 10.1177/1352458509106609. [DOI] [PubMed] [Google Scholar]
- 18.Lassmann H, Ransohoff RM. The CD4-Th1 model for multiple sclerosis: a critical [correction of crucial] re-appraisal. Trends Immunol. 2004;25:132–137. doi: 10.1016/j.it.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Bitsch A, Kuhlmann T, Stadelmann C, Lassmann H, Lucchinetti C, Bruck W. A longitudinal MRI study of histopathologically defined hypointense multiple sclerosis lesions. Ann Neurol. 2001;49:793–796. doi: 10.1002/ana.1053. [DOI] [PubMed] [Google Scholar]
- 20.Pirko I, Nolan TK, Holland SK, Johnson AJ. Multiple sclerosis: pathogenesis and MR imaging features of T1 hypointensities in a [corrected] murine model. Radiology. 2008;246:790–795. doi: 10.1148/radiol.2463070338. [DOI] [PubMed] [Google Scholar]
- 21.McDole J, Suidan G, Boespflug E, et al. A translatable molecular approach to determining CD8 T-cell epitopes in TMEV infection. Hum Immunol. 2008;69:805–810. doi: 10.1016/j.humimm.2008.08.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGavern DB, Murray PD, Rodriguez M. Quantitation of spinal cord demyelination, remyelination, atrophy, and axonal loss in a model of progressive neurologic injury. J Neurosci Res. 1999;58:492–504. doi: 10.1002/(sici)1097-4547(19991115)58:4<492::aid-jnr3>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denic A, Bieber A, Warrington A, Mishra PK, Macura S, Rodriguez M. Brainstem 1H nuclear magnetic resonance (NMR) spectroscopy: marker of demyelination and repair in spinal cord. Ann Neurol. 2009;66:559–564. doi: 10.1002/ana.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton HL, Twaddle G, Jelachich ML. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsunoda I, Kuang LQ, Libbey JE, Fujinami RS. Axonal injury heralds virus-induced demyelination. Am J Pathol. 2003;162:1259–1269. doi: 10.1016/S0002-9440(10)63922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsunoda I. Axonal degeneration as a self-destructive defense mechanism against neurotropic virus infection. Future Virol. 2008;3:579–593. doi: 10.2217/17460794.3.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsunoda I, Tanaka T, Saijoh Y, Fujinami RS. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am J Pathol. 2007;171:1563–1575. doi: 10.2353/ajpath.2007.070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deb C, Lafrance-Corey RG, Schmalstieg WF, et al. CD8+ T cells cause disability and axon loss in a mouse model of multiple sclerosis. PLoS One. 2010;5:e12478. doi: 10.1371/journal.pone.0012478. [DOI] [PMC free article] [PubMed] [Google Scholar]