Abstract
BACKGROUND
The effect of timing of initiation of concurrent radiation and chemotherapy after surgery on outcome of patients with glioblastoma (GBM) remains unclear.
OBJECTIVE
To further explore this issue, we analyzed 4 clinical trials for patients newly diagnosed with GBM receiving concurrent and adjuvant temozolomide.
METHODS
The cohort study included 198 adult patients with newly diagnosed supratentorial GBM who were enrolled from 2004 to 2010 in 4 clinical trials consisting of radiation plus temozolomide and an experimental agent. The interval to initiation of therapy was determined from the time of surgical resection. The partitioning deletion/substitution/addition algorithm was used to determine the cutoff points for timing of chemoradiation at which there was a significant difference in overall survival (OS) and progression-free survival (PFS).
RESULTS
The median wait time between surgery and initiation of concurrent chemoradiation was 29.5 days (range, 7–56 days). A short delay in chemoradiation administration (at 30–34 days) was predictive of prolonged OS (hazard ratio [HR]: 0.63, P = .03) and prolonged PFS (HR: 0.68, P = .06) compared with early initiation of concurrent chemoradiation (<30 days), after adjusting for protocol and baseline prognostic variables including extent of resection by multivariate analysis. A longer delay to chemoradiation beyond 34 days was not associated with improved OS or PFS compared with early initiation (HR: 0.94, P = .77 and HR: 0.91, P = .63, respectively).
CONCLUSION
A short delay in the start of concurrent chemoradiation is beyond the classic paradigm of 4 weeks post-resection and may be associated with prolonged OS and PFS.
Keywords: Chemotherapy, Concurrent chemoradiation, Delay, Glioblastoma, Radiation, Temozolomide, Timing
Despite advances in modern surgical and adjuvant therapies, glioblastoma (GBM) and high-grade gliomas remain challenging disease entities. The current treatment paradigm, as established by the European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada phase III trial, includes maximal safe surgical resection followed by external beam radiation therapy (RT) at 60 Gy with concurrent daily temozolomide (TMZ), followed by adjuvant TMZ.1 For recurrent disease, however, the optimal therapy remains unclear, and median overall survival (OS) from initial diagnosis remains <2 years.
With aggressive malignancies such as GBM, minimizing delay in initiation of cytotoxic therapies has been a widely held tenet in oncology. The effect of timing of radiotherapy on outcome has been evaluated in a variety of malignancies, such as breast,2,3 lung,4 and head and neck5,6 cancers, consistently showing higher recurrence rates and worse survival with delayed administration of adjuvant radiotherapy. However, in the context of GBM and high-grade gliomas, the relationship between delaying radiotherapy and outcome is less clear, with some studies demonstrating an association between delay in radiotherapy and poor survival,7,8 whereas no such impact on outcome was observed in other studies.9,10 A secondary analysis of 16 Radiation Therapy Oncology Group studies including with 2800 patients revealed a possible association with improved outcome with a moderate delay in radiotherapy initiation (>4 weeks).11 Yet, most of these studies were completed before radiotherapy with the establishment of concurrent TMZ as standard therapy for patients newly diagnosed with GBM, raising the question of whether these results remain relevant in the modern era of concurrent chemoradiation for GBM.
To explore the impact of timing of initiating radiotherapy with concurrent TMZ, we analyzed 4 clinical trials of patients with newly diagnosed GBM receiving concurrent and adjuvant TMZ with other agents(s) conducted at the University of California at San Francisco.
METHODS
Patient Population
The analysis included adult patients with newly diagnosed supratentorial GBM enrolled in 4 clinical trials consisting of radiation therapy (RT) plus TMZ and an experimental agent(s), including erlotinib,12 enzastaurin,13,14 and a combination of bevacizumab and erlotinib.15 These trials, all conducted at the University of California at San Francisco, accrued 202 adult patients from 2004 to 2010. The eligibility criteria and primary outcomes of these trials were published previously.12–15 The pretreatment, treatment, and survival data collected per the protocols were used, and the timing of initiation of therapy was defined as the time interval between definitive surgery and commencement of RT and concurrent TMZ, which was intended to be within 6 weeks per protocol eligibility.
Statistical Analysis
OS and progression-free survival (PFS), measured from study registration to death/progression or last follow-up, were estimated using the Kaplan-Meier method. Analysis by the partitioning deletion/substitution/addition algorithm (partDSA) was used to determine the cutoff points for the timing of chemoradiation initiation at which there was a difference in OS and PFS.16,17 The log-rank test was used for comparison of survival between groups. The Cox proportional hazards model was used to assess the effect of timing of concurrent chemoradiation (in days) on outcomes, while adjusting for variables: treatment protocol, age, Karnofsky Performance Score (KPS), and extent of resection. Pretreatment clinical characteristics between groups were compared using the χ2 test (KPS, treatment regimen, extent of resection) and the Student t test (age).
RESULTS
Participants
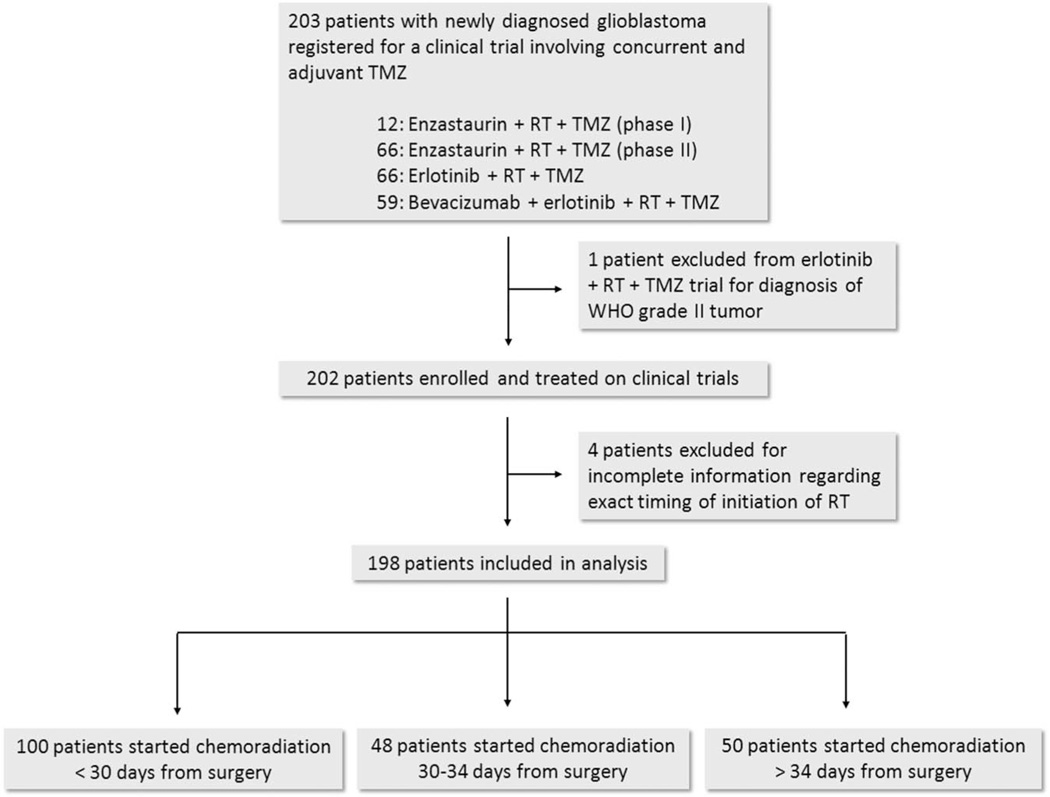
Of the 202 patients enrolled across the clinical trials, 198 patients were included in the current analysis (Figure 1). Four patients were excluded due to the inability to determine the exact timing of treatment initiation. The details of the trial studies selected for analysis are shown in Table 1.
FIGURE 1.
Flow diagram of patient inclusion. RT, radiotherapy; TMZ, temozolomide; WHO, World Health Organization.
TABLE 1.
Study Details
| Phase | Treatment | Reference |
|---|---|---|
| I | Enzastaurin (250–500 mg/d, concurrent and adjuvant) | Butowski et al13 |
| Radiation (60 Gy) | ||
| Concurrent and adjuvant temozolomide | ||
| II | Enzastaurin (250 mg/d, concurrent and adjuvant) | Butowski et al14 |
| Radiation (60 Gy) | ||
| Concurrent and adjuvant temozolomide | ||
| II | Erlotinib (100 mg/d concurrent, 150 mg/d adjuvant) | Prados et al12 |
| Radiation (60 Gy) | ||
| Concurrent and adjuvant temozolomide | ||
| II | Erlotinib (150 mg/d concurrent, 200 mg/d adjuvant) | Clarke et al15 |
| Bevacizumab (10 mg/kg every 2 wk, 4 wk post-surgery) | ||
| Radiation (60 Gy) | ||
| Concurrent and adjuvant temozolomide |
Descriptive Data
The pretreatment clinical characteristics of the patients, as a function of the treatment initiation interval, are listed in Table 2. The median time between surgery and initiation of concurrent RT + TMZ was 29.5 days (range, 7–56 days). The relationship between timing intervals of RT + TMZ initiation and pre-treatment clinical characteristics were assessed. Patients who were given RT + TMZ earlier were more likely to have undergone a biopsy than more extensive surgery (P = .006), and patients who were given RT + TMZ with a short delay were more likely to be younger (P = .02).
TABLE 2.
Pretreatment Characteristicsa
| Interval From Surgery to Start of Concurrent Chemoradiation |
P Value |
|||
|---|---|---|---|---|
| <30 days, n = 100 |
30–35 days, n = 48 |
>35 days, n=50 |
||
| Age, y, median | 56.4 | 51.3 | 57.8 | .02 |
| Age range, y | 27.3–80.0 | 22.6–72.9 | 21.3–74.3 | |
| KPS, % | ||||
| 60 | 3 (3) | 1 (2) | 1 (2) | .61 |
| 70 | 3 (3) | 2 (4) | 0 (0) | |
| 80 | 18 (18) | 9 (19) | 8 (16) | |
| 90 | 73 (73) | 33 (69) | 39 (78) | |
| 100 | 3 (3) | 3 (6) | 2 (4) | |
| Treatment, % | .08 | |||
| Erlotinib + TMZ | 37 (37) | 14 (29) | 14 (28) | |
| Enzastaurin + TMZ | 37 (37) | 11 (23) | 20 (40) | |
| Erlotinib + Bev + TMZ | 26 (26) | 23 (48) | 16 (32) | |
| Extent of resection (%) | .006 | |||
| Biopsy | 26 (26) | 2 (4) | 5 (10) | |
| STR | 40 (40) | 26 (54) | 29 (58) | |
| GTR | 33 (3) | 19 (40) | 15 (30) | |
| Extent of resection information not available | 1 (1) | 1 (2) | 1 (2) | |
KPS, Karnofsky Performance Scale; TMZ, temozolomide; Bev, bevacizumab; STR, subtotal resection; GTR, gross total resection.
Outcome Data and Main Results
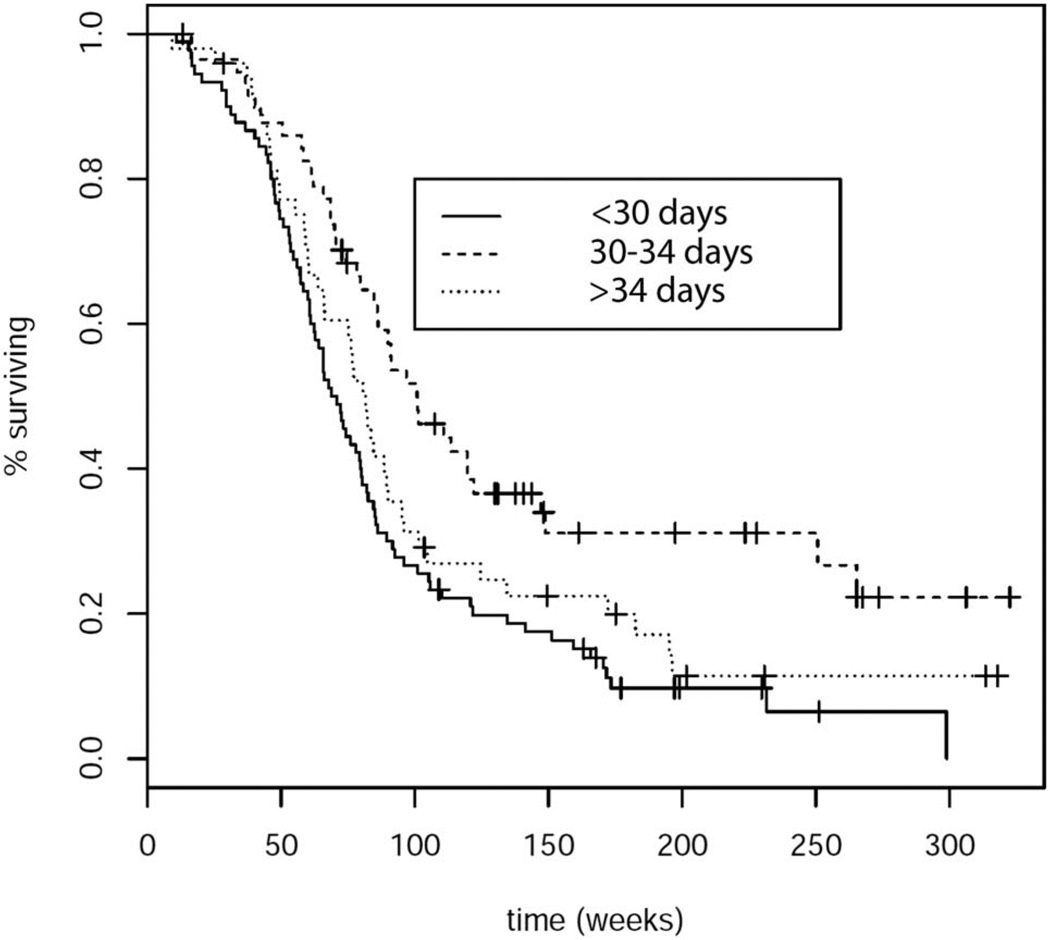
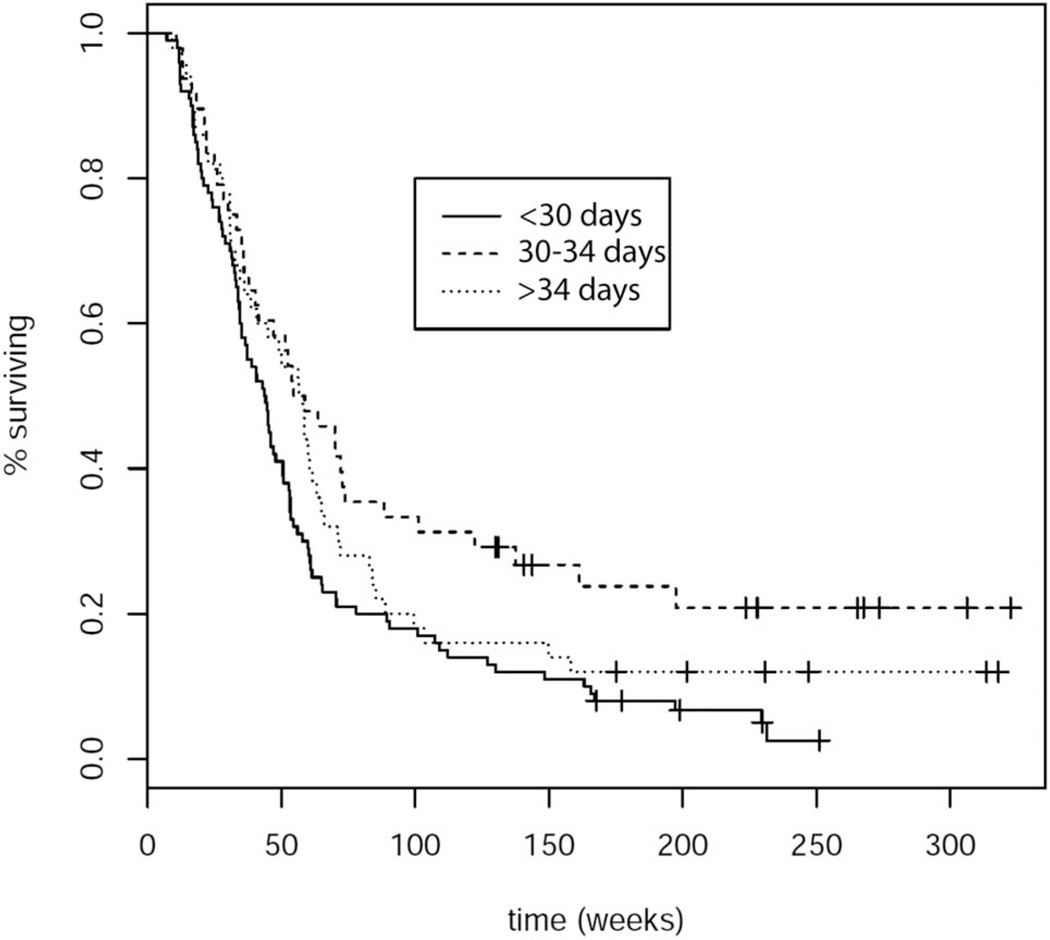
Analysis by partDSA revealed 2 time points of initiating concurrent chemoradiation at which there were differences in OS and PFS: between 29 and 30 days and between 34 and 35 days. A short delay in starting concurrent RT + TMZ (at days 30–34 post-surgery) was associated with prolonged OS (log-rank, P = .002, hazard ratio [HR]: 0.47, P < .001) (Figure 2) as well as prolonged PFS (log-rank, P = .004, HR: 0.59, P = .006) (Figure 3), compared with early initiation of concurrent chemoradiation therapy (<30 days). A longer delay of RT + TMZ initiation past 34 days was not associated with improved OS or PFS compared with early initiation within 30 days after surgery (HR: 0.76 P = .14; HR: 0.78, P = .15, respectively).
FIGURE 2.
Overall survival based on time interval to initiation of concurrent chemoradiation; log-rank test: P = .002.
FIGURE 3.
Progression-free survival based on time interval to initiation of concurrent chemoradiation; log-rank test: P = .004.
Multivariate analysis of variables including time intervals for chemoradiation, age, KPS, extent of resection, and treatment regimen revealed that time interval to chemoradiation (30–34 days) remained a statistically significant factor with respect to OS (HR: 0.63, P = .03), with trends toward significance for PFS (HR: 0.68, P = .06) (Table 3).
TABLE 3.
Multivariate Cox Proportional Hazards Model for Overall Survival and Progression-Free Survivala
| Variable | Progression-Free Survival | Overall Survival | P Value | |||
|---|---|---|---|---|---|---|
| HR | 95% Confidence Interval | P Value | HR | 95% Confidence Interval | ||
| Age | 1.02 | 1.00–1.03 | .02 | 1.03 | 1.02–1.05 | <.001 |
| KPS | 3.45 | 1.36–8.73 | .01 | 3.64 | 1.55–8.55 | .003 |
| Treatment | ||||||
| Erlotinib + Bev + TMZ | ref | — | ref | — | ||
| Enzastaurin + TMZ | 1.75 | 1.20–2.56 | .004 | 1.34 | 0.92 | .13 |
| Erlotinib + TMZ | 1.81 | 1.27–2.67 | .003 | 1.17 | 0.79 | .44 |
| Extent of resection | ||||||
| STR and GTR | ref | — | ref | — | ||
| Biopsy | 2.16 | 1.42–3.28 | <.001 | 2.93 | 1.93–4.45 | <.001 |
| Time Interval to RT 1 TMZ (d) | ||||||
| <30 | ref | — | ref | — | ||
| 30–34 | 0.68 | 0.45–1.02 | .06 | 0.63 | 0.42–0.95 | .03 |
| >34 | 0.91 | 0.63–1.32 | .63 | 0.94 | 0.64–1.39 | .77 |
HR, hazard ratio; KPS, Karnofsky Performance Scale; Bev, bevacizumab; TMZ, temozolomide; ref, reference; STR, subtotal resection; GTR, gross total resection; RT, radiation therapy.
DISCUSSION
Key Results
Our current analysis appears to support the results found by Blumenthal et al11 that a modest wait time (4 weeks, ie, 28 days in their analysis and 30 days in ours) is associated with improved survival outcomes. However, due to the retrospective nature of our analysis, there is a significant potential for confounding: the treating physicians may have tendencies to rush more fragile-appearing patients into adjuvant therapy, thus patients with shorter waiting times would have included those patients with a greater number of poor prognostic factors, such as older age, worse KPS, or less than gross total resection achieved at the time of surgery. This phenomenon was found to be true to varying degrees in previous reports as well as in our current analysis, in which patients with the shortest delay (<30 days) tended to be older and were more likely to have a subtotal resection or biopsy. However, this factor alone does not reconcile the discrepancies in the literature because our results, as well as those of Blumenthal et al,11 Noel et al,10 Irwin et al,8 and Do et al,7 all remained significant even after adjusting for these possible confounding prognostic factors on multivariate analyses. However, there may be additional prognostic factors not captured in our controlled variables (eg, age, KPS) that may have influenced the treating clinician’s decision to start adjuvant radiotherapy earlier or later. For example, the differences in tumor regrowth before initiation of RT were not fully accounted for in our analysis because patients did not routinely undergo repeat scans immediately before initiating RT. Along these lines, the heterogeneity of prognostic factors not captured by the multivariate analyses among the different cohorts studied may also explain some of the discrepant results observed in the literature.
Interpretation
The impact of delay in adjuvant radiotherapy for GBM has been studied in a number of retrospective series. The largest series to date, by Blumenthal et al,11 analyzed more than 2800 patients enrolled in 16 Radiation Therapy Oncology Group trials and demonstrated not only a lack of any deleterious effects, but even improved survival times with delays up to 4 weeks after surgery. Noel et al9 and Lai et al10 found no association of worsened outcome and waiting times before initiating radiotherapy, even at 8 weeks. Two series, however, found that delays in receiving adjuvant radiotherapy decreased survival in patients.7,8 All previous studies discussed previously, with the exception of the study by Noel et al, were completed before the establishment of concurrent and adjuvant TMZ as standard therapy for newly diagnosed GBM. The heterogeneous nature of treatments for newly diagnosed GBM in the pre-TMZ era may explain some of the discrepant findings in the literature regarding the impact of timing of radiotherapy initiation. The current cohort all received radiotherapy with concurrent and adjuvant TMZ as established by the European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada phase III trial.1
An alternative explanation for our findings is the differential spectrum of wait times included in the cohorts studied. Blumenthal et al,11 suspected waiting more than 6 weeks was not beneficial, and indeed our results confirmed that a short delay, but not a longer delay, was associated with improved outcomes. Although we found no detrimental effects associated with waiting times beyond 34 days compared with short waiting times (<30 days), it remains possible that worse survival is seen when this delay is even longer, as was the case for the patients studied by Irwin et al.8
Additionally, when radiation is given too early after an initial insult of surgery, the effects may be detrimental. One mechanistic hypothesis states that hypoxia and edema of the surgical bed resulting from resection may diminish the radiosensitivity of the region.11 Shrinking of the tumor cavity can also be seen up to 4 weeks, which suggests the need for larger radiation field sizes and hence greater potential for radiation-induced injury when given earlier postoperatively.18 Rat model studies have demonstrated higher levels of brain injury with early initiation of radiation.19
Differences in statistical methods used may also contribute to the varying results described in the literature. Most studies dichotomized timing as a binary variable, using the median length of delay in the cohort as the cutoff threshold. Other studies modeled timing in categories using quartiles or percentiles, and 2 studies considered length of delay as a continuous variable in weeks. By using the partDSA method, our analysis was unique and allowed the data to dictate the cutoffs in timing to initiation of therapy. This is in contrast to previous analyses, including 1 reported by Blumenthal et al that used quartiles or percentiles as cutoff points. Use of partDSA likely allowed for a more sensitive analysis of the effect of waiting time on outcome, and using percentiles in the Noel et al10 analysis may have failed to detect a small signal that was potentially present. However, the favorable interval of 30 to 34 days after surgery is a narrow window, and there are obvious practical challenges in universally recommending that everyone initiate chemoradiation during this narrow range of time. In addition, although the 30- to 34-day window was the one that was identified as the best fit in our partDSA model, the true biological window may be larger. Thus, we caution against this possibility of overfitting in our model, and validation in an external cohort is recommended.
Limitations
Main limitations of our analysis include its retrospective design and the lack of molecular marker data such as IDH mutation or MGMT methylation status. A more robust multivariate survival analysis would have included these markers for interactions; however, the clinical trials studied predated the recognition of the significance of such markers, and thus the tumors were not routinely tested for them.
It is also difficult to distinguish whether the effects seen on the timing of chemoradiation initiation were due to the timing of the radiotherapy or the timing of TMZ administration. Because the timing of concurrent TMZ varied with initiation of radiotherapy, the variation in initiation of TMZ may have had an impact on clinical outcomes as well. Mathematical modeling of tumor responses to RT and TMZ suggest that TMZ given concomitantly with RT synergistically enhances the radiosensitivity of GBM.20 Studies on administering TMZ before RT for patients with newly diagnosed GBM revealed inferior outcomes compared with standard administration of concomitant RT with TMZ, and it remains unclear how much impact the large delay (of up to 4 months) of RT initiation had on the ultimate patient outcome.21 These studies suggest a greater potential impact of adjuvant radiotherapy timing in GBM management.
Generalizability
To date, the current evidence regarding the impact of timing of radiotherapy or concurrent chemoradiation remains exclusively retrospective in nature. Thus, these studies, including the current analysis, are subject to the limitations of retrospective analyses, such as the possible presence of bias of clinicians opting to rush patients who are likely to do poorly into adjuvant therapy. However, as laid out by Blumenthal et al,11 a prospective, randomized trial to study such effects is challenging due to issues of ethics and clinical equipoise. Still, this study is the second large study to demonstrate a clear clinical benefit associated with a short delay in initiation adjuvant radiotherapy and the first to demonstrate improved outcomes associated with a short delay in concurrent chemoradiation with TMZ.
CONCLUSION
Although we caution against universal deliberate delay of concurrent chemoradiation, the results may have implications for clinical trials entry. It may be of interest to examine whether the timing of RT, in either the RT alone or RT plus TMZ arm, is of significance in the original European Organisation for Research and Treatment of Cancer study from which TMZ gained approval.
Acknowledgments
Disclosures
Dr Chang receives research support from Schering, Novartis, and Quest. Dr Han is supported by the Neurosurgery Research and Education Foundation Research Fellowship. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Abbreviations
- GBM
glioblastoma
- KPS
Karnofsky Performance Score
- OS
overall survival
- partDSA
partitioning deletion/substitution/addition algorithm
- PFS
progression-free survival
- RT
radiation therapy
- TMZ
temozolomide
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Froud PJ, Mates D, Jackson JS, et al. Effect of time interval between breast-conserving surgery and radiation therapy on ipsilateral breast recurrence. Int J Radiat Oncol Biol Phys. 2000;46(2):363–372. doi: 10.1016/s0360-3016(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 3.Vujovic O, Perera F, Dar AR, et al. Does delay in breast irradiation following conservative breast surgery in node-negative breast cancer patients have an impact on risk of recurrence? Int J Radiat Oncol Biol Phys. 1998;40(4):869–874. doi: 10.1016/s0360-3016(97)00922-x. [DOI] [PubMed] [Google Scholar]
- 4.Choi N, Baumann M, Flentjie M, et al. Predictive factors in radiotherapy for non-small cell lung cancer: present status. Lung Cancer. 2001;31(1):43–56. doi: 10.1016/s0169-5002(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 5.Ampil FL, Buechter KJ, Bairnsfather LE, Shockley WW. Timing and dosage of postoperative radiotherapy for squamous cell carcinoma of the upper aerodigestive tract. J Oral Maxillofac Surg. 1993;51(11):1194–1197. doi: 10.1016/s0278-2391(10)80287-3. [DOI] [PubMed] [Google Scholar]
- 6.Bastit L, Blot E, Debourdeau P, Menard J, Bastit P, Le Fur R. Influence of the delay of adjuvant postoperative radiation therapy on relapse and survival in oropharyngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2001;49(1):139–146. doi: 10.1016/s0360-3016(00)01376-6. [DOI] [PubMed] [Google Scholar]
- 7.Do V, Gebski V, Barton MB. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol. 2000;57(2):131–136. doi: 10.1016/s0167-8140(00)00257-7. [DOI] [PubMed] [Google Scholar]
- 8.Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol. 2007;85(3):339–343. doi: 10.1007/s11060-007-9426-z. [DOI] [PubMed] [Google Scholar]
- 9.Lai R, Hershman DL, Doan T, Neugut AI. The timing of cranial radiation in elderly patients with newly diagnosed glioblastoma multiforme. Neuro Oncol. 2010;12(2):190–198. doi: 10.1093/neuonc/nop004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noel G, Huchet A, Feuvret L, et al. Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol. 2012;109(1):167–175. doi: 10.1007/s11060-012-0883-7. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal DT, Won M, Mehta MP, et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27(5):733–739. doi: 10.1200/JCO.2008.18.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butowski N, Chang SM, Lamborn KR, et al. Enzastaurin plus temozolomide with radiation therapy in glioblastoma multiforme: a phase I study. Neuro Oncol. 2010;12(6):608–613. doi: 10.1093/neuonc/nop070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butowski N, Chang SM, Lamborn KR, et al. Phase II and pharmacogenomics study of enzastaurin plus temozolomide during and following radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma. Neuro Oncol. 2011;13(12):1331–1338. doi: 10.1093/neuonc/nor130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke JL, Molinaro AM, Phillips JJ, et al. A single-institution phase II trial of radiation, temozolomide, erlotinib, and bevacizumab for initial treatment of glioblastoma. Neuro Oncol. 2014;16(7):984–990. doi: 10.1093/neuonc/nou029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinaro AM, Lostritto K, van der Laan M. partDSA: deletion/substitution/addition algorithm for partitioning the covariate space in prediction. Bioinformatics. 2010;26(10):1357–1363. doi: 10.1093/bioinformatics/btq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lostritto K, Strawderman RL, Molinaro AM. A partitioning deletion/substitution/addition algorithm for creating survival risk groups. Biometrics. 2012;68(4):1146–1156. doi: 10.1111/j.1541-0420.2012.01756.x. [DOI] [PubMed] [Google Scholar]
- 18.Iuchi THK, Kodama T, Tohyama N, et al. Eur Soc Radiother Oncol. Barcelona, Spain: 2010. Brain deformation after surgery of glioblastoma should we take MRI for planning of irradiation. [Google Scholar]
- 19.Peker S, Abacioglu U, Sun I, Yuksel M, Pamir MN. Irradiation after surgically induced brain injury in the rat: timing in relation to severity of radiation damage. J Neurooncol. 2004;70(1):17–21. doi: 10.1023/b:neon.0000040820.78643.0a. [DOI] [PubMed] [Google Scholar]
- 20.Barazzuol L, Burnet NG, Jena R, Jones B, Jefferies SJ, Kirkby NF. A mathematical model of brain tumour response to radiotherapy and chemotherapy considering radiobiological aspects. J Theor Biol. 2010;262(3):553–565. doi: 10.1016/j.jtbi.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Chinot OL, Barrié M, Fuentes S, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol. 2007;25(12):1470–1475. doi: 10.1200/JCO.2006.07.4807. [DOI] [PubMed] [Google Scholar]