Abstract
BACKGROUND
Endovascular stenting is an effective treatment for patients with clinically significant cerebral venous sinus stenosis. Traditionally, stenting is indicated in patients with elevated intravenous pressures on conventional venography; however, noninvasive monitoring is more desirable. Quantitative magnetic resonance angiography is an imaging modality that allows blood flow assessment noninvasively. Established in the arterial system, applications to the venous sinuses have been limited to date.
OBJECTIVE
In this study, we examined quantitative magnetic resonance venography (qMRV) flow in patients before and after venous stenting and correlated these results with intravenous pressure measurements and clinical outcomes.
METHODS
Five patients with intracranial hypertension (IH) secondary to venous sinus stenosis underwent cerebral venous stenting between 2009 and 2013 at a single institution. Preoperatively venous sinus flow was determined using qMRV, and intravenous pressure measured during venography. After stenting, intravenous pressure, qMRV flow, and clinical outcomes were assessed and compared.
RESULTS
A mean prestenotic intravenous pressure of 45.2 mmHg was recorded before stenting which decreased to 27.4mmHg afterwards (Wilcoxon signed rank test P=.04). Total jugular outflow on qMRV increased by 260.2 ml/min. Analysis of the change in intravenous pressure and qMRV flow identified a linear relationship (Pearson's correlation r= .926). All patients displayed clinical improvement, including vision.
CONCLUSION
Venous outflow by qMRV increases after endovascular stenting and correlates with significantly improved intravenous pressures. These findings establish qMRV as a useful adjunct to measure venous flow after stenting, and as a potential tool in the selection and postoperative surveillance of the cerebral venous sinus stenosis patient.
Keywords: Cerebral Flow, Cerebral Venous Sinus Stenosis, Intracranial Hypertension, Pseudotumor Cerebri, Quantitative MRV, Stenting, Venography
INTRODUCTION
Cerebral venous sinus stenosis is a significant factor in the pathogenesis of intracranial hypertension (IH).1, 2. Stenosis of the sinuses, in particular the distal transverse sinus, is associated with idiopathic intracranial hypertension (IIH), a syndrome characterized by signs and symptoms of elevated intracranial pressure (ICP) in the absence of cerebrospinal fluid or imaging abnormalities.3 Previous studies have identified venous sinus stenosis, either radiographically4, 5 or by intravenous pressure measurement,6, 7 in over 90% of IIH patients and fewer than 10% of controls.4-7
Endovascular stenting is a relatively new and effective treatment for patients with IH associated with venous sinus stenosis. 8 In treatment of IH, some authors assert that morphologically focal stenoses with large pressure gradients are most amenable to stenting, while diffuse stenoses without large pressure drop-offs are better treated with CSF diversion.9 It is recommended that all IH patients be evaluated with direct retrograde cerebral venography with manometry to make this distinction. This includes contrast venography and intravenous pressure measurement via femoral venous catheter, and is the current gold standard for characterizing venous sinus pathology.
Non-invasive studies, including magnetic resonance venography (MRV), have had mixed results in evaluating IH-related venous sinus stenosis.2, 9-12 Time-of-flight (TOF) MRV is limited by artifactual signal loss at sites of turbulent, in-plane flow including the distal transverse sinus, and is neither sensitive nor specific at diagnosing IH-related venous stenosis.2, 11 Gadolinium-enhanced (Gd) MRV can provide better anatomic imaging in the native sinus, but is susceptible to stent-related artifact and consequently can be unhelpful in assessing stent patency postoperatively.10 Unlike venography, neither TOF- nor Gd-MRV provide the intravenous pressure measurements necessary for hemodynamic characterization of venous stenosis. Quantitative MRA (qMRA) is a novel imaging modality that non-invasively measures local cerebral blood flow. In the arterial system, qMRA has been validated in a number of applications including risk stratification of vertebrobasilar insufficiency,11 peri-procedural evaluation of arterial stenting,12 and post-operative confirmation of stent patency.10, 13 Venous applications of qMRA (qMRV), however, have been less studied. With the exception of a case report featuring one of our patients14, our paper is the first to use qMRV to assess venous sinus stenosis and stenting.
In this paper we use qMRV to examine flow rates in patients with IH undergoing stenting for cerebral venous sinus stenosis confirmed by venography and manometry. We hypothesize that after stenting, decreases in intravenous pressure will be matched by a corresponding increase in qMRV venous outflow and clinical improvement.
METHODS
Five consecutive patients with symptomatic IH were treated at our institution between June 2009 and November 2013, each presenting with either transverse or sigmoid sinus stenosis. Clinical examinations, angiography, and MR imaging were all retrospectively reviewed. Approval for the study was obtained by the institutional review board (IRB) at our institution. Prior to venous stenting, all patients underwent clinical history, a detailed physical examination including assessment of visual acuity by a neuro-ophthalmologist, and digital subtraction angiography to identify the location and severity of cerebral venous sinus stenosis. Appropriate surgical candidates exhibited headaches and visual symptoms refractory to medical management, elevated intracranial pressure improved by lumbar puncture, and venous sinus stenosis with elevated intravenous pressures on venogram performed at an earlier date. Qualifying patients first underwent baseline qMRV to measure venous flow within 1 week prior to stenting. Venography and intravenous pressure measurements were obtained before and after cerebral venous sinus stenting. Repeat qMRV was then obtained within 1 week after stenting. Following discharge, all patients received neurosurgical and neuro-ophthalmologic followup, including repeat visual acuity examination at 6 weeks.
Venography and Venous Stenting
All procedures were performed under general anesthesia. Patients had both the right and left groins prepared and the right common femoral artery and left common femoral veins were accessed. A 6-vessel angiogram was obtained through the arterial system with three dimensional venogram reconstructed from the venous phase. Patients were then placed on systemic heparin and a shuttle guide and microcatheter connected to manometer was used to measure the intravenous pressure at multiple points in the venous circulation, including the superior sagittal sinus, transverse sinus, sigmoid sinus, ipsilateral and contralateral jugular veins, and the stenotic segment of the venous system. The pressure distal to the stenotic segment was subtracted from the pressure proximal to it to calculate the trans-stenosis pressure gradient. Patients next underwent balloon angioplasty and stent deployment across the stenotic segment. Venous pressures were measured again at the same locations after angioplasty and stenting.
Quantitative MRV
MR imaging was performed within 1 week prior and after venous stenting. The study was performed on a 1.5 Tesla or 3 Tesla MRI scanner (GE Healthcare, Milwaukee, WI). 1.5T studies consisted of 3-D time of flight MR angiography and 2-D coronal time of flight MR venography with maximum intensity projection reconstructions of the large intracranial arteries and dural venous sinuses, respectively. 3T studies used 3-D time of flight MR angiography and venography. Quantitative flow measurements were made through the large intracranial arteries and dural venous sinuses using cardiac gated phase contrast MR angiography and venography with specialized software (Non-invasive Optimal Vessel Analysis; NOVA, VasSol Inc, Chicago, IL). The flow was measured along standard points of the arterial and venous sinus system and across the stenotic segment.
For this study, venous flow was reviewed at four locations: the superior sagittal sinus proximal to the stenotic segment, the region of stenosis, the jugular vein ipsilateral and downstream from the region of stenosis, and the contralateral jugular vein. The flow of the two jugular veins was added together to calculate the total jugular outflow. Flow measurements were made at the same location of each venous segment in preoperative and postoperative imaging.
Statistical Analysis
Single-tailed Wilcoxon signed rank tests were performed to compare intravenous pressures and qMRV flow before and after treatment. Statistically significant values were identified with a P<.05. The relationship between changes in the intravenous pressure and qMRV flow before and after treatment were assessed using Pearson’s correlation coefficient (r). All statistics were performed using SPSS (IBM Corporation, Armonk, NY).
RESULTS
Five patients underwent cerebral venous sinus stenting between 2009 and 2013 (Table). 3 patients were men, 2 were women. The mean age was 51 years (range 46-66y). 3 patients had dural arteriovenous fistulas at the time of stenting which were treated at a later date. Pressure and flow measurements were obtained after stenting but prior to additional treatments (Figure 1).
Table 1.
Intravenous Pressure, Flow, and Visual Acuity Before and After Cerebral Venous Sinus Stenting
| Patient | Location Sinus Stenosis |
Prestenotic Pressure (mmHg) |
Pressure Gradient (mmHg) |
Total Jugular Outflow (ml/min) |
Visual Acuity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After Stenting |
Baseline | After Stenting |
Baseline | After Stenting |
Baseline | After Stenting |
||
| 46/F | L Transverse - Sigmoid Junction |
31 | 34 | 11 | 12 | 707 | 491 | 20/40 L 20/30 R |
20/30 L 20/30 R |
| 66/M | R Transverse | 55 | 16 | 40 | −1 | 495 | 1234 | LP L 20/80 R |
20/400 L 20/50 R |
| 52/F | R Transverse | 49 | 20 | 28 | −1 | 273 | 963 | 20/30 L 20/50 R |
20/25 L 20/25 R |
| 61/M | L Transverse | 47 | 37 | 23 | 5 | 395 | 580 | 20/80 L 20/25 R |
20/50 L 20/20 R |
| 46/M | R Sigmoid | 44 | 30 | 24 | 9 | 341 | 244 | LPL 20/50 R |
LPL 20/40 R |
Pressure gradient = Intravenous pressure proximal to area of stenosis – pressure distal to area stenosis LP = Light Perception Only
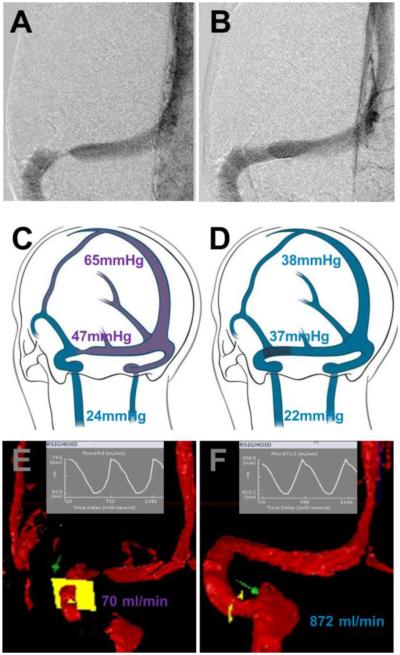
Figure 1.
Venography, Manometry, and Quantitative Flow before (left) and after (right) stenting of the left transverse sinus. A- Digital subtraction venography of the left transverse sinus showing severe stenosis. B- Digital subtraction venography after angioplasty and stenting, showing resolution of the transverse sinus stenosis. C- Illustrative map demonstrating elevated intravenous pressures at the superior sagittal sinus and stenotic segment of the sinus before stenting. D- Map demonstrating decreased intravenous pressures of the superior sagittal sinus and stenotic segment of the sinus after stenting. Ipsilateral internal jugular pressures were similar. E- qMRV flow map across the sigmoid sinus indicating poor flow secondary to transverse sinus stenosis. F- qMRV flow map demonstrating a significant increase of flow after stenting.
Intravenous Pressures
Preoperatively, the mean intravenous pressure immediately proximal to the region of stenosis was 45.2 mmHg (range 31-55 mmHg) and distal to the region of stenosis 20mmHg (15-24 mmHg), for a mean pressure gradient of 25.2mmHg (11-40mmHg). After stenting 4 of 5 patients displayed improvement; the mean pre-stenotic pressure decreased significantly to 27.4mmHg (16-34 mmHg; Wilcoxon signed rank test Z=−1.753, P=.04) (Figure 2). The mean pressure gradient also decreased by the same level of significance to 4.8mmHg (−1-12mmHg; Wilcoxon signed rank test Z=−1.753, P=.04).
Figure 2.
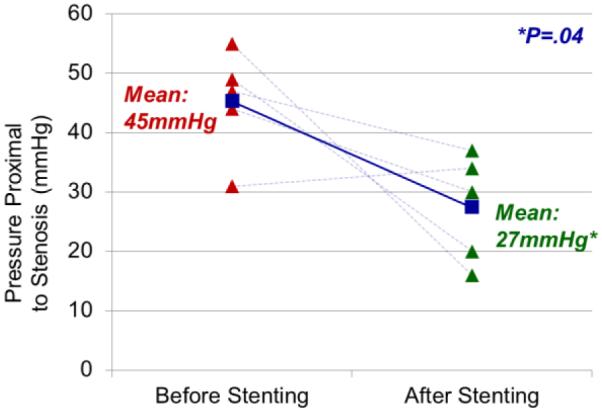
Intravenous pressure measurements before and after stenting. Intravenous pressure was measured by manometry proximal to the region of stenosis in all patients, with a mean of 45mmHg. After venous sinus stenting, pressures were measured again with a marked drop at the same location to a mean of 27 mmHg (Wilcoxon signed rank test Z=−1.753, P=.04).
qMRV Flow
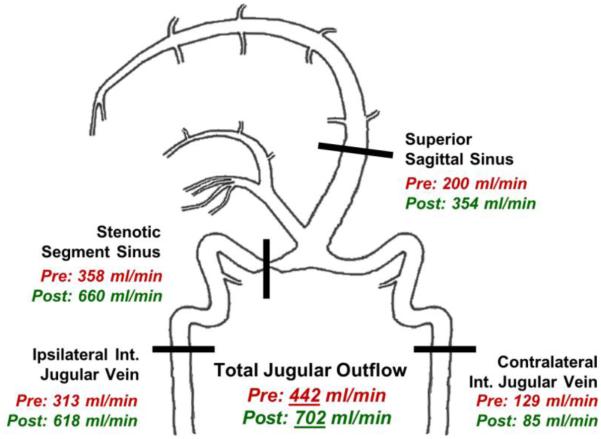
Venous flow was measured preoperatively by qMRV at four locations: the superior sagittal sinus proximal to the stenotic segment, the region of stenosis, the jugular vein ipsilateral and downstream from the region of stenosis, and the contralateral jugular vein. Preoperatively, a mean flow of 200ml/min was measured in the superior sagittal sinus, 358ml/min at the region of stenosis, 313ml/min at the ipsilateral jugular vein, and 129ml/min at the contralateral jugular vein (Figure 3). The total jugular outflow, measured as the sum of the two jugular veins, was 442ml/min. After stenting, the qMRV was repeated and mean flows increased to 354ml/min, 660ml/min, and 618ml/min in the superior sagittal sinus, at the region of stenosis, and the ipsilateral jugular vein, respectively. Although flow in the contralateral jugular vein decreased after stenting to 85ml/min, the total jugular outflow increased to 702ml/min, but was found only to trend toward significance (Wilcoxon signed rank test Z=−1.363, P=.087; Figure 3).
Figure 3.
Intracranial flow map before and after venous sinus stenting. There was an increase in flow, as measured by qMRV, of the superior sagittal sinus, stenotic segment of the sinus, and ipsilateral jugular vein after stenting. While total jugular outflow increased, the contralateral internal jugular vein exhibited a moderate drop after stenting.
Pressure-Flow Relationship
Intravenous pressures and flow on qMRV were plotted against each other to identify if there was a relationship. A strong, linear relationship between the improvement in intravenous pressure proximal to the region of stenosis (mean = 17.8mmHg) and increase in total jugular outflow (mean = 260.2 ml/min) was observed (Figure 4; Pearson’s correlation r=.926). A similar linear relationship was identified between the improvement of the intravenous pressure gradient (mean = 20.4mmHg) and the increase in total jugular outflow (Pearson’s correlation r=.934).
Figure 4.
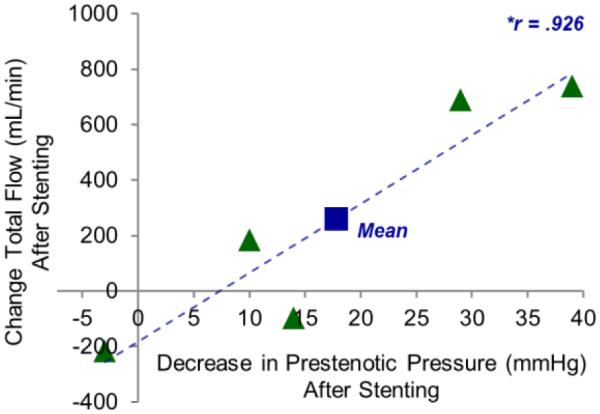
The relationship between total flow and intravenous pressures. The change in the total flow (sum of both jugular veins) and decrease of the intravenous pressure proximal to the region of stenosis after stenting for each patient were compared. A strong, linear relationship (Pearson’s correlation r=.926) was observed.
Visual Acuity
Visual acuity was measured by ophthalmologic examination prior to venous stenting and afterwards (Table). Pre-stenting acuity was measured by an ophthalmologist within 2 weeks of surgery; post-stenting acuity was reviewed from examinations at 6 weeks. Visual improvement was seen in at least one eye in each patient.
DISCUSSION
Cerebral Venous Sinus Stenosis and Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension (IIH), or pseudotumor cerebri, is a syndrome characterized by progressive headaches, vision loss, and other signs and symptoms of elevated ICP in the absence of CSF anomalies or abnormal imaging findings.3 Symptoms follow a relapsing and remitting course, and frequently recur despite maximal medical therapy in 60% of patients. 15Although episodes are self-limited, visual deficits are often progressive, with up to 57% of patients experiencing permanent visual deficits ranging from field cuts to complete blindness at 3 years.16 Traditional treatment for IIH includes medical management with acetazolamide and then CSF diversion in patients with refractory symptoms.17 CSF diversion is obtained through ventriculo- or lumbar-peritoneal shunts, but is complicated by a 2-year failure rate of 20-50%, with at least one revision needed in the lifetime of the majority of shunted patients.12
Stenosis of the cerebral venous sinuses is a relatively recent discovery observed in as many as 90% patients with IIH.4-7 The proposed pathophysiology of IIH arises from obstructed venous outflow at the stenotic segment, resulting in venous sinus hypertension that impedes CSF reabsorption across the arachnoid villi.9 The increased pressure from CSF compresses both the brain parenchyma and the compliant venous sinus system, causing the signs and symptoms of IIH and further obstructing venous outflow. The result is a positive feedback cycle of elevated intracranial pressures, worsening CSF reabsorption, and worsening sinus stenosis.18
Is venous sinus stenosis the cause or result of elevated intracranial pressure? An early paper by King et al. demonstrated that the steep pressure gradients measured in the distal transverse sinus of IIH patients were reversible, in nearly all cases, by CSF drainage.6The authors concluded that these patient’s sinus stenosis was not the cause but rather the result of intracranial hypertension, secondary to extraluminal compression of the sinus walls. This conclusion was further supported by the morphologic finding of bilateral smooth narrowing of the sinuses on venography.7 A later, contradicting study by Owler et al documented significant improvement with venous sinus stenting alone in patients with significant stenosis.9 Notably, these authors stratified sinus stenosis based on morphology and steepness of pressure gradient. Using these criteria, they proposed dividing IIH into two etiologic categories: “venogenic” IIH resulting from identifiable primary sinus pathology, and “non-venogenic” with compression secondary to elevated intracranial pressure alone or with normal venous sinuses.9
The distinctions of sinus stenosis morphology, pressure gradient, and etiology have important implications for management. Venous stenting is unlikely to be effective in the nonvenogenic IIH patient, as deployed stents cannot cover the entirety of the sinus system and extraluminal compressive forces will likely remain after stenting.19 These nonvenogenic patients exhibit generalized, extraluminal compression that is reversible with CSF removal and likely better treated with CSF diversion. The venogenic IIH patient with focal stenosis, however, stands to benefit greatly from endovascular stenting.9
The Pressure-Flow Relationship
The results of this study demonstrate that qMRV flow measurements correlate closely with invasive measurements of intravenous pressure in patients following stenting for cerebral sinus stenosis. After sinus stenting, intravenous pressures proximal to the region of stenosis and the pressure gradient across the region of stenosis decreased significantly (Figure 2), with numbers similar to those seen in the literature.8 Quantitative flow measurements across the region of stenosis, the ipsilateral jugular vein, and total jugular outflow increased after stenting (Figure 3). These measurements approached, but did not achieve significance, which we attribute to our small sample size. Flow from the contralateral jugular vein decreased, predictably, after stenting, suggesting shunting to the side of lower resistance. In our discussion, we use total jugular outflow, rather than a single sinus, as our dependent variable as we feel this bilateral measurement more closely approximates venous outflow of the brain and its relationship to intravenous and intracranial pressures. Improvements in total jugular outflow were found to correlate with intravenous pressures and the pressure gradient (Figure 4), suggesting a strong, linear relationship. As we later discuss, this introduces qMRV flow as a potential surrogate for intravenous pressure measurements in the diagnosis and postoperative management of cerebral venous sinus stenosis.
One notable outlier was observed in our patient cohort. This patient had the lowest pressure proximal to the region of stenosis (31 mmHg), lowest trans-stenosis pressure gradient (11mmHg), and highest total jugular outflow (707ml/min) prior to stenting. After stenting, this patient had a modest increase in intravenous pressure and a decrease in total jugular outflow (−216ml/min). While this observation represents only a single patient, it suggests that this patient’s stenosis was not flow-limiting, and may not have been ideal for stenting. It is notable also that the patient’s symptoms have recurred on multiple occasions since stenting. Nonetheless, the changes in pressure and flow in this patient are congruent with the remainder of our cohort, supporting the linear relationship between the two variables even in an unexpected clinical outcome.
The Role of Quantitative MRV Imaging
Endovascular venous sinus stenting is emerging as a powerful treatment option for a subset of patients with IIH-related sinus stenosis.12 In a recent analysis by Puffer et al, of 143 IIH patients undergoing stenting, 88% displayed headache relief, 97% papilledema improvement, and 87% visual recovery at a mean of 22.3 months.8
Despite these promising results, however, a substantial minority of patients experience recurrence of IIH symptoms. Even in patients with significant clinical improvement, the likelihood of headache recurrence or visual symptoms in the IIH population is very high. This clinical reality, combined with the risk of in-stent restenosis, demands an accurate tool to assess long-term stent patency. At our institution, we traditionally have performed repeat venography for significant symptom recurrence and at regular post-stenting intervals. While complication rates are relatively low,16 repetitive venography remains invasive, inconvenient, and potentially hazardous. Since the results of this study, we have changed our practice to encompass qMRV as a noninvasive means of monitoring stent patency. If an IIH patient with an endovascular stent returns with recurrent symptoms but a stable ophthalmologic examination, we next repeat the qMRV study. If qMRV flows are stable from previous imaging, this suggests stable intravenous pressure and symptomatic treatment is appropriate. Only if clinical suspicion remains high do we repeat venography. As our results suggest a strong linear relationship between sinus flow and intravenous pressure, flow on qMRV in this regard can serve as a surrogate for venography pressure measurements. We feel that this option introduces a role for qMRV as a powerful surveillance tool in the management of the demanding IIH patient.
qMRV may also find potential as a screening tool to predict patients who may benefit from endovascular stenting. As described in earlier studies,9 patients with focal sinus stenosis and significant pressure gradients usually respond well to endovascular stents. As part of the screening process of IIH patients, qMRV may find utility; should significant flow restriction be detected, conventional venography and intravenous manometry may be pursued. Patients with confirmed sinus stenosis and a high pressure gradient would then make excellent candidates for stenting. We have begun to implement this strategy to our new IIH patients; it is our hope that we may detect more patients amenable to stenting and avoid the risks and long-term management of shunting.
This study does have several limitations. Our patient sample included only five patients; although sufficient to provide a proof of concept, this sample size provided inadequate power to demonstrate significant changes in venous flow after stenting. The study follow-up was also varied, ranging from 12mo to 5y. In this time period, none of our patients demonstrated stent failure. While we believe that decreased flow on qMRV will detect restenosis or obstruction, this has not yet occurred in practice. Finally, our patients also served as their own controls, comparing qMRV flow before stenting to afterwards. While reliable, this limits the use of qMRV to patients initially imaged and then followed. Without a standardized “normal” value for venous sinus flow, it is difficult to determine if a new patient’s qMRV flow is abnormal. While studies of arterial disease have validated qMRA flow abnormalities by comparison to healthy controls and angiographic findings, 11, 13, 20, 21 previous studies using qMRV have only been in context of pathology, notably AVMs.12, 21 Further validation of venous flow measurements with healthy volunteers may help guide future management decisions.
CONCLUSION
This study is the first to demonstrate a strong, linear relationship between venous flow measured by qMRV and intravenous sinus pressures. Measurements were made in context of IH patients with clinically significant cerebral venous sinus stenosis improved after stenting. By demonstrating qMRV flow as a potential surrogate for venography and pressure measurements, these findings introduce a potentially valuable role of qMRV in the selection, treatment, and postoperative management of the otherwise challenging IH patient.
Acknowledgments
DISCLOSURE OF FUNDING: None
DISCLOSURES:
Darian R. Esfahani: None
Matthew Stevenson: None
Heather E Moss: Research to Prevent Blindness (unrestricted departmental grant), NIH K23 EY024345 and P30 EY01792
Sepideh Amin-Hanjani: NIH; Material Research Support GE Healthcare and VasSol, Inc Victor Aletich: Consultant for Cordis-Codman
Fady T. Charbel: Shareholder VasSol, Inc; Consultant for Transonic, Inc
Ali Alaraj: NIH; Consultant for Cordis-Codman
REFERENCES
- 1.Liebig T, Henkes H, Brew S, Miloslavski E, Kirsch M, Kuhne D. Reconstructive treatment of dural arteriovenous fistulas of the transverse and sigmoid sinus: transvenous angioplasty and stent deployment. Neuroradiology. 2005 Jul;47(7):543–551. doi: 10.1007/s00234-005-1377-5. [DOI] [PubMed] [Google Scholar]
- 2.Johnston I, Kollar C, Dunkley S, Assaad N, Parker G. Cranial venous outflow obstruction in the pseudotumour syndrome: incidence, nature and relevance. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2002 May;9(3):273–278. doi: 10.1054/jocn.2001.0986. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Subcommittee of the International Headache S The International Classification of Headache Disorders: 2nd edition. Cephalalgia : an international journal of headache. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 4.Kelly LP, Saindane AM, Bruce BB, et al. Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure? Clinical neurology and neurosurgery. 2013 Aug;115(8):1215–1219. doi: 10.1016/j.clineuro.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003 May 13;60(9):1418–1424. doi: 10.1212/01.wnl.0000066683.34093.e2. [DOI] [PubMed] [Google Scholar]
- 6.King JO, Mitchell PJ, Thomson KR, Tress BM. Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology. 1995 Dec;45(12):2224–2228. doi: 10.1212/wnl.45.12.2224. [DOI] [PubMed] [Google Scholar]
- 7.King JO, Mitchell PJ, Thomson KR, Tress BM. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology. 2002 Jan 8;58(1):26–30. doi: 10.1212/wnl.58.1.26. [DOI] [PubMed] [Google Scholar]
- 8.Puffer RC, Mustafa W, Lanzino G. Venous sinus stenting for idiopathic intracranial hypertension: a review of the literature. Journal of neurointerventional surgery. 2013 Sep 1;5(5):483–486. doi: 10.1136/neurintsurg-2012-010468. [DOI] [PubMed] [Google Scholar]
- 9.Owler BK, Parker G, Halmagyi GM, et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. Journal of neurosurgery. 2003 May;98(5):1045–1055. doi: 10.3171/jns.2003.98.5.1045. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakaran S, Wells KR, Jhaveri MD, Lopes DK. Hemodynamic changes following wingspan stent placement--a quantitative magnetic resonance angiography study. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2011 Apr;21(2):e109–113. doi: 10.1111/j.1552-6569.2009.00425.x. [DOI] [PubMed] [Google Scholar]
- 11.Amin-Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke; a journal of cerebral circulation. 2005 Jun;36(6):1140–1145. doi: 10.1161/01.STR.0000166195.63276.7c. [DOI] [PubMed] [Google Scholar]
- 12.Brisman JL. Wingspan stenting of symptomatic extracranial vertebral artery stenosis and perioperative evaluation using quantitative magnetic resonance angiography: report of two cases. Neurosurgical focus. 2008;24(2):E14. doi: 10.3171/FOC/2008/24/2/E14. [DOI] [PubMed] [Google Scholar]
- 13.Amin-Hanjani S, Alaraj A, Calderon-Arnulphi M, Aletich VA, Thulborn KR, Charbel FT. Detection of intracranial in-stent restenosis using quantitative magnetic resonance angiography. Stroke; a journal of cerebral circulation. 2010 Nov;41(11):2534–2538. doi: 10.1161/STROKEAHA.110.594739. [DOI] [PubMed] [Google Scholar]
- 14.MacIntosh PW, Jain S, Moss HE, Volpe NJ, Alaraj A. A school of red herring. Survey of ophthalmology. 2014 Apr 23; doi: 10.1016/j.survophthal.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skau M, Brennum J, Gjerris F, Jensen R. What is new about idiopathic intracranial hypertension? An updated review of mechanism and treatment. Cephalalgia : an international journal of headache. 2006 Apr;26(4):384–399. doi: 10.1111/j.1468-2982.2005.01055.x. [DOI] [PubMed] [Google Scholar]
- 16.Galgano MA, Deshaies EM. An update on the management of pseudotumor cerebri. Clinical neurology and neurosurgery. 2013 Mar;115(3):252–259. doi: 10.1016/j.clineuro.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Wall M, McDermott M, Kupersmith M. Idiopathic intracranial hypertension--reply. Jama. 2014 Sep 10;312(10):1060. doi: 10.1001/jama.2014.8903. [DOI] [PubMed] [Google Scholar]
- 18.Donnet A, Metellus P, Levrier O, et al. Endovascular treatment of idiopathic intracranial hypertension: clinical and radiologic outcome of 10 consecutive patients. Neurology. 2008 Feb 19;70(8):641–647. doi: 10.1212/01.wnl.0000299894.30700.d2. [DOI] [PubMed] [Google Scholar]
- 19.Rohr A, Dorner L, Stingele R, Buhl R, Alfke K, Jansen O. Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR. American journal of neuroradiology. 2007 Apr;28(4):656–659. [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer AM, Amin-Hanjani S, Alaraj A, Charbel FT. Quantitative magnetic resonance angiography in the evaluation of the subclavian steal syndrome: report of 5 patients. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2009 Jul;19(3):250–252. doi: 10.1111/j.1552-6569.2008.00297.x. [DOI] [PubMed] [Google Scholar]
- 21.Langer DJ, Song JK, Niimi Y, et al. Transarterial embolization of vein of Galen malformations: the use of magnetic resonance imaging noninvasive optimal vessel analysis to quantify shunt reduction. Report of two cases. Journal of neurosurgery. 2006 Jan;104(1 Suppl):41–45. doi: 10.3171/ped.2006.104.1.41. [DOI] [PubMed] [Google Scholar]