Summary
Background
Seasonal variation has been reported in diagnosis of eosinophilic esophagitis (EoE), but results are not consistent across studies and there are no national-level data in the United States.
Aim
To determine if there is seasonal variation in diagnosis of esophageal eosinophilia and EoE in the U.S., while accounting for factors such as climate zone and geographic variation.
Methods
This was a cross-sectional study using a U.S. national pathology database. Patients with esophageal eosinophilia (≥15 eosinophils per high-power field) comprised the primary case definition and were compared to those with normal esophageal biopsies. We calculated the crude and adjusted odds of esophageal eosinophilia by season, as well as by day of the year. Sensitivity analyses were performed using more restrictive case definitions of EoE, and after stratification by climate zone.
Results
14,524 cases with esophageal eosinophilia and 90,459 normal controls were analyzed. The adjusted odds of esophageal eosinophilia were higher in the late spring and summer months, with the highest odds in July (aOR 1.13; 95%CI: 1.03–1.24). These findings persisted with increasing levels of esophageal eosinophilia, as well as across EoE case definitions. Seasonal variation was strongest in temperate and cold climates, and peak diagnosis varied by climate zone.
Conclusions
There is a mild but consistent seasonal variation in the diagnosis of esophageal eosinophilia and EoE, with cases more frequently diagnosed during summer months. These findings take into account climate and geographic differences, suggesting that aeroallergens may contribute to disease development or flare.
Keywords: Eosinophilic esophagitis, season, geography, climate
Introduction
Eosinophilic esophagitis (EoE) is a chronic esophageal disease characterized by symptoms of esophageal dysfunction and dense esophageal eosinophilia in the absence of other etiologies.1, 2 Both the incidence and prevalence of EoE have increased, particularly in the last decade.3–9 This rapid epidemiologic shift may be explained by environmental factors and studies have shown that EoE has been closely associated with food triggers, atopic disorders, and also environmental exposures.9–19 The pathogenesis is not completely understood, but is believed to be immune/allergen mediated.20 Evidence for this comes from animal models,21, 22 response to elimination diets and elemental formulas where all potential food antigens are removed,11–13 and cases that appear to be triggered by aeroallergens,16–18 including some environmental allergens that cross-react with certain food allergens.23, 24
Because of these associations, it has been hypothesized that there is seasonal variation in the diagnosis of EoE, with increased diagnosis during typical allergy seasons. Several reports have presented evidence of this association,5, 17, 25–29 but most of the studies were conducted at single centers with relatively small sample sizes. Additionally, some studies present conflicting results that suggest there is no association.6, 30, 31 Geographic and climate differences between study locations could explain these conflicting results,32, 33 but have not been accounted for in studies of seasonal variation.
The aim of the present study was to use a large, national pathology database to determine if there is seasonal variation in the detection and diagnosis of esophageal eosinophilia and EoE, while accounting for factors such as climate zone and geographic variation. We hypothesized we would observe seasonal variation in esophageal eosinophilia, but that this effect could be dependent on climate or geographic region.
Materials and Methods
Data sources and case definitions
We conducted a cross-sectional study of patients with esophageal biopsies examined between January 2009 and June 2012 by pathologists at Miraca Diagnostics, a specialized pathology laboratory serving outpatient endoscopy centers throughout the United States. Details of pathology protocols have been previously reported.32–35 In brief, samples from 43 states, DC, and Puerto Rico were processed centrally in one of three laboratories (Irving, Texas; Phoenix, Arizona; Boston, Massachusetts) with identical sectioning and staining procedures. Sub-specialty trained gastrointestinal pathologists applied standardized criteria for diagnoses. A central database collected biopsy reports, demographic information (patient age, sex, and zip code of residence), and indication for esophagogastroduodenoscopy, and the date when the procedure was performed.
Patients with esophageal eosinophilia were defined as those with ≥15 eosinophils per high power field (eos/hpf; 400× magnification with 22mm oculars; hpf area = 0.237mm2) on esophageal biopsy. These patients comprised our primary case group. Because of the standardized pathology coding, these subjects could be readily identified in the database, and the level of esophageal eosinophilia recorded. We excluded subjects with esophageal eosinophilia who had accompanying histologic findings of candidal or viral esophagitis. We also applied increasingly stringent criteria for the level of eosinophilia; patients were categorized by density of eosinophils, specifically ≥15 eos/hpf, ≥50 eos/hpf, and ≥100 eos/hpf.
To approximate patient disease status, we applied case definitions for eosinophilic esophagitis as previously described,32–35 using three increasingly stringent and specific definitions: 1) presence of ≥15 eos/hpf and documentation of dysphagia; 2) presence of ≥15 eos/hpf, documentation of dysphagia, and exclusion of patients with clinical data suggesting differential diagnoses (reflux/heartburn symptoms, reflux esophagitis, Barrett’s esophagus on biopsy, inflammatory bowel disease, and eosinophilic gastroenteritis); 3) presence of ≥15 eos/hpf, documentation of dysphagia, exclusion of the above differential diagnoses, and presence of eosinophilic microabscesses in the esophageal epithelium (defined as clusters of ≥4 contiguous eosinophils). For the control group, we selected patients with histologically normal esophageal biopsies. Specifically, in the squamous epithelium there was no evidence of inflammation of any type, mucosal disruption, infection, dysplasia, or neoplasia.
Overall, data were available for 292,621 unique patients with esophageal biopsies. Of these, a total of 106,990 met definitions for either case or control status. We further excluded participants living in Puerto Rico and restricted to patients with complete information for all key covariates (i.e. age, sex, and zip code of residency), resulting in a final study population 104,983 participants. This study was deemed exempt from ongoing review by the UNC Institutional Review Board.
Statistical analysis
We first described the distribution of demographic characteristics for patients with normal biopsies and those with esophageal eosinophilia (≥15 eos/hpf), our primary case group. We then compared the distribution of demographic and disease characteristics by the season and month in which the procedure was conducted. Specifically, we assessed for differences in age, sex, clinical symptoms (dysphagia, heartburn, chest pain, abdominal pain/dyspepsia, nausea/vomiting, or weight loss), and histological features (eosinophil counts and microabscesses). Seasons were defined as follows: winter (December-February); spring (March–May); summer (June–August); autumn (September–November). We used a chi-squared test to assess differences in proportions and ANOVA to assess for differences in means.
We assessed the relationship between the procedure date (as a proxy for the date of diagnosis of esophageal eosinophilia) and levels of esophageal eosinophilia or EoE case definitions in several ways. We first used logistic regression to estimate the relationship between month of procedure and each case definition. Specifically, we estimated the odds of disease in each month as compared to the odds in March. This was chosen as the reference category because the greatest number of controls had diagnostic procedures in that month. Next, to evaluate the relationship with a finer time gradient, we used generalized additive models (GAMs) to assess the log-odds of disease by the day of the year. GAMs are an extension of linear models which can accommodate binary outcomes and do not impose assumptions on the shape of the relationship between variables (i.e. that the relationship is linear).36 GAMs replace the traditional beta coefficient from a logistic regression model with functions (in this case a locally weighted regression smoothing function) in solving regression equations. Plotting these functions enabled us to explore the shape of the relationship between the date of diagnosis and esophageal eosinophilia. For GAMs, day of the year was coded as 1 through 365. All models adjusted for age (in 10 year increments) and sex (male, female) as potential confounders. Analyses were performed in SAS (version 9.2; SAS Institute Inc, Cary, NC) or R (version 2.12.02; Vienna Austria) using the GAM package.37
Secondary analyses
As previous assessments have demonstrated that climate may be related to eosinophilic esophagitis,32 we assessed whether associations were impacted by a patient’s climate zone of residence. To obtain residential climate information we geographically located each patient’s zip code (GIS ArcMap; version 9.3; ESRI Inc., Redlands, CA). Using the geographic location of the zip code, we then linked each participant to their Köppen-Geiger climate zone (as updated by Kottek et al. in 2006;38 spatial data available: http://koeppen-geiger.vu-wien.ac.at/present.htm). Climate zones were collapsed into arid, temperate, cold, and equatorial climates to ensure that there were sufficient numbers of participants in each climate type for analyses. We assessed for effect modification by climate zone by conducting stratified analyses for each zone.
Results
Patient characteristics
Of the 104,983 unique patients with esophageal biopsies over the study time frame who met inclusion criteria, 14,524 patients had esophageal eosinophilia and 90,459 were normal controls (Table 1). Patients with esophageal eosinophilia were generally younger (45.0 vs. 53.9, p < 0.001), more likely to be male (64.0% vs 34.9%, p < 0.001), and had a lower proportion of abdominal pain (25.3 vs. 40.4%, p < 0.001) compared to the normal population. There was a higher proportion of heartburn, nausea/vomiting, and chest pain in those with normal biopsies. A higher percentage of endoscopies were performed during the months of March through June as compared to the rest of the year, but the proportion of patients in each group did not vary according to month of endoscopy.
Table 1.
Demographic characteristics, clinical symptoms, and histological features of patients with esophageal biopsies
| Normal esophageal biopsies (n = 90459) | Esophageal eosinophiliaa (n = 14524) | p | |
|---|---|---|---|
| Demographic characteristic | |||
| Age (yrs) mean ± SD (IQR) | 53.86 ± 17.11 (43.57–66.25) | 44.96 ± 16.24 (33.48–55.78) | <0.001 |
| Male n (%) | 31526 (34.85) | 9298 (64.02) | <0.001 |
| Clinical symptoms/EGD indications – n (%) | |||
| Dysphagia/odynophagia | 23317 (25.78) | 3449 (23.75) | <0.001 |
| Heartburn | 45887 (50.75) | 5989 (41.24) | <0.001 |
| Chest pain | 5480 (6.06) | 485 (3.34) | <0.001 |
| Abdominal pain/dyspepsia | 36543 (40.40) | 3715 (25.28) | <0.001 |
| Nausea/vomiting | 7560 (8.36) | 843 (5.80) | <0.001 |
| Weight loss | 2774 (3.07) | 230 (1.58) | <0.001 |
| Suspected EoE | 18348 (20.28) | 6655 (45.8) | <0.001 |
| Histological features | |||
| Maximum eosinophil count, mean ± SD (IQR) | 0 ± 0.06 (0–0) | 37.02 ± 24.14 (20–50) | <0.001 |
| Eosinophil microabscesses n (%) | 0 (0.00) | 3449 (23.75) | <0.001 |
| Month of endoscopy and biopsy | |||
| January | 7947 (8.79) | 1308 (9.01) | 0.085 |
| February | 8332 (9.21) | 1346 (9.27) | |
| March | 9272 (10.25) | 1406 (9.68) | |
| April | 8843 (9.78) | 1358 (9.35) | |
| May | 8491 (9.39) | 1386 (9.54) | |
| June | 8518 (9.42) | 1447 (9.96) | |
| July | 6097 (6.74) | 1031 (7.1) | |
| August | 6845 (7.57) | 1129 (7.77) | |
| September | 6440 (7.12) | 981 (6.75) | |
| October | 6722 (7.43) | 1049 (7.22) | |
| November | 6535 (7.22) | 1036 (7.13) | |
| December | 6417 (7.09) | 1047 (7.21) | |
Patients with esophageal eosinophilia on esophageal biopsy with a maximum count of ≥15 eos/hpf
Seasonal variation in detection of esophageal eosinophilia and EoE
Examination of the cases of esophageal eosinophilia by season revealed no statistical differences in the age or proportion of male patients in each of the four seasons (Table 2). There were also no substantial differences between clinical symptoms or indication for EGD with season. Of note, the proportions of patients with individual symptoms were generally similar in each season.
Table 2.
Characteristics of cases of esophageal eosinophilia (≥15 eos/hpf) by season
| Winter (n = 3701) | Spring (n = 4150) | Summer (n = 3607) | Autumn (n = 3066) | p | |
|---|---|---|---|---|---|
| Characteristic | |||||
| Age (yrs) mean ± SD (IQR) | 45.55 ± 16.15 (34.16–56.50) | 44.89 ± 16.49 (33.43–55.89) | 44.10 ± 16.35 (32.31–54.80) | 45.35 ± 15.83 (34.59–55.97) | 0.0008 |
| Male n (%) | 2448 (66.14) | 2644 (63.71) | 2245 (62.45) | 1961 (63.96) | 0.006 |
| Clinical symptoms/EGD indications n (%) | |||||
| Dysphagia/odynophagia | 2026 (54.74) | 2182 (52.58) | 1901 (52.70) | 1689 (55.09) | 0.06 |
| Heartburn | 1490 (40.26) | 1755 (42.29) | 1476 (40.92) | 1268 (41.36) | 0.32 |
| Chest pain | 97 (2.62) | 154 (3.66) | 130 (3.60) | 106 (3.46) | 0.04 |
| Abdominal pain/dyspepsia | 901 (24.34) | 1076 (25.93) | 976 (27.06) | 762 (24.85) | 0.04 |
| Nausea/vomiting | 193 (5.21) | 250 (6.02) | 211 (5.85) | 189 (6.16) | 0.33 |
| Weight loss | 50 (1.35) | 67 (1.61) | 71 (1.97) | 42 (1.37) | 0.13 |
| Histological features | |||||
| Maximum eosinophil count, mean ± SD (IQR) | 36.17 ± 23.64 (20–48) | 37.45 ± 25.45 (20–50) | 37.44 ± 24.17 (20–50) | 36.96 ± 24.23 (20–50) | 0.07 |
| Eosinophil microabscesses | 867 (23.43) | 1017 (24.51) | 862 (23.90) | 703 (22.93) | 0.44 |
Using the month of March as a reference, the adjusted odds of esophageal eosinophilia were slightly higher in the late spring and summer months (Table 3). The highest odds of diagnosing esophageal eosinophilia occurred in the months of June and July. The mild variation by month was also observed in analyses with increasing levels of esophageal eosinophilia (Table 3). For example, in those with > 100 eosinophils/hpf on biopsy, the month of July had an adjusted odds ratio of 1.24 (CI: 0.88 – 1.73). Analyses using our constructed EoE case definitions also indicated a slight increase in odds of diagnosis in the spring and summer months (Table 4).
Table 3.
Association between month of diagnosis and increasing levels of esophageal eosinophilia
| Month | ≥15 eos/hpf (n=14524)c
|
≥50 eos/hpf (n=3824)c
|
≥100 eos/hpf (n=836)c
|
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | aORb (95% CI) | OR (95% CI) | aORb (95% CI) | OR (95% CI) | aORb (95% CI) | |
| January | 1.09 (1.00, 1.18) | 1.07 (0.98, 1.16) | 1.00 (0.86, 1.17) | 0.97 (0.83, 1.14) | 1.06 (0.77, 1.47) | 1.03 (0.74, 1.43) |
| February | 1.07 (0.98, 1.15) | 1.06 (0.97, 1.15) | 1.06 (0.91, 1.23) | 1.04 (0.90, 1.21) | 1.01 (0.73, 1.40) | 1.00 (0.72, 1.38) |
| March | Reference | Reference | Reference | Reference | Reference | Reference |
| April | 1.01 (0.94, 1.10) | 1.01 (0.93, 1.10) | 1.06 (0.91, 1.22) | 1.04 (0.90, 1.21) | 1.2 (0.88, 1.63) | 1.18 (0.87, 1.61) |
| May | 1.08 (0.99, 1.17) | 1.10 (1.01, 1.20) | 1.12 (0.97, 1.30) | 1.15 (0.99, 1.34) | 1.11 (0.81, 1.52) | 1.15 (0.84, 1.58) |
| June | 1.12 (1.04, 1.21) | 1.14 (1.05, 1.24) | 1.12 (0.97, 1.30) | 1.14 (0.98, 1.32) | 1.15 (0.84, 1.57) | 1.16 (0.85, 1.59) |
| July | 1.12 (1.02, 1.22) | 1.13 (1.03, 1.24) | 1.13 (0.96, 1.32) | 1.14 (0.97, 1.34) | 1.22 (0.88, 1.71) | 1.24 (0.88, 1.73) |
| August | 1.09 (1.00, 1.18) | 1.09 (1.00, 1.19) | 1.13 (0.97, 1.32) | 1.13 (0.97, 1.33) | 1.07 (0.77, 1.50) | 1.07 (0.76, 1.50) |
| September | 1.01 (0.92, 1.10) | 1.03 (0.94, 1.12) | 1.08 (0.92, 1.26) | 1.09 (0.93, 1.29) | 1.31 (0.95, 1.81) | 1.33 (0.96, 1.85) |
| October | 1.03 (0.94, 1.12) | 1.05 (0.96, 1.15) | 1.07 (0.91, 1.25) | 1.10 (0.93, 1.29) | 1.24 (0.89, 1.71) | 1.28 (0.92, 1.77) |
| November | 1.05 (0.96, 1.14) | 1.07 (0.97, 1.17) | 1.07 (0.91, 1.25) | 1.09 (0.93, 1.28) | 0.94 (0.66, 1.34) | 0.96 (0.67, 1.37) |
| December | 1.08 (0.99, 1.17) | 1.07 (0.98, 1.17) | 0.92 (0.78, 1.09) | 0.92 (0.77, 1.09) | 1.11 (0.79, 1.56) | 1.11 (0.79, 1.56) |
Adjusted for age and sex.
Number of patients meeting case group definition
Table 4.
Association between month of diagnosis and case definitions of increasing specificity for eosinophilic esophagitis
| Month | Esophageal eosinophiliaa with dysphagia (n=7197)e
|
Esophageal eosinophilia and exclusion of competing conditionsb (n=3954)e
|
Esophageal eosinophilia, exclusions of competing conditions, and eosinophilic microabscesses (n=1352)e
|
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | aORd (95% CI) | OR (95% CI) | aORd (95% CI) | OR (95% CI) | aORd (95% CI) | |
| January | 1.15 (1.03, 1.29) | 1.14 (1.01, 1.28) | 1.22 (1.06, 1.41) | 1.20 (1.03, 1.39) | 1.14 (0.90, 1.45) | 1.12 (0.88, 1.42) |
| February | 1.08 (0.97, 1.21) | 1.07 (0.95, 1.20) | 1.01 (0.87, 1.18) | 0.99 (0.85, 1.16) | 0.87 (0.68, 1.12) | 0.86 (0.66, 1.10) |
| March | Reference | Reference | Reference | Reference | Reference | Reference |
| April | 1.06 (0.95, 1.18) | 1.05 (0.94, 1.17) | 1.08 (0.93, 1.25) | 1.06 (0.91, 1.23) | 0.94 (0.73, 1.19) | 0.91 (0.72, 1.17) |
| May | 1.08 (0.97, 1.21) | 1.11 (0.99, 1.24) | 1.06 (0.91, 1.23) | 1.08 (0.93, 1.26) | 1.00 (0.78, 1.27) | 1.03 (0.81, 1.32) |
| June | 1.14 (1.02, 1.27) | 1.15 (1.03, 1.29) | 1.13 (0.98, 1.31) | 1.14 (0.99, 1.33) | 1.00 (0.79, 1.28) | 1.02 (0.80, 1.31) |
| July | 1.18 (1.05, 1.33) | 1.20 (1.06, 1.36) | 1.11 (0.94, 1.30) | 1.13 (0.96, 1.33) | 1.10 (0.85, 1.42) | 1.12 (0.86, 1.45) |
| August | 1.13 (1.00, 1.27) | 1.14 (1.01, 1.28) | 1.20 (1.03, 1.40) | 1.21 (1.04, 1.42) | 1.15 (0.90, 1.47) | 1.17 (0.91, 1.50) |
| September | 1.06 (0.94, 1.19) | 1.08 (0.95, 1.22) | 1.04 (0.89, 1.22) | 1.06 (0.90, 1.25) | 0.92 (0.70, 1.20) | 0.93 (0.71, 1.22) |
| October | 1.09 (0.97, 1.22) | 1.11 (0.98, 1.25) | 1.07 (0.92, 1.26) | 1.09 (0.93, 1.29) | 0.89 (0.68, 1.16) | 0.91 (0.70, 1.19) |
| November | 1.13 (1.01, 1.28) | 1.15 (1.02, 1.30) | 1.15 (0.98, 1.34) | 1.17 (0.99, 1.37) | 0.88 (0.67, 1.15) | 0.90 (0.69, 1.19) |
| December | 1.17 (1.04, 1.32) | 1.16 (1.03, 1.31) | 1.16 (0.99, 1.35) | 1.14 (0.97, 1.34) | 1.00 (0.77, 1.30) | 0.99 (0.76, 1.29) |
≥15 eos/hpf
Competing conditions for esophageal eosinophilia included reflux/heartburn symptoms, reflux esophagitis, Barrett’s esophagus on biopsy, inflammatory bowel disease, and eosinophilic gastroenteritis
Adjusted for age and sex.
Number of patients meeting case group definition
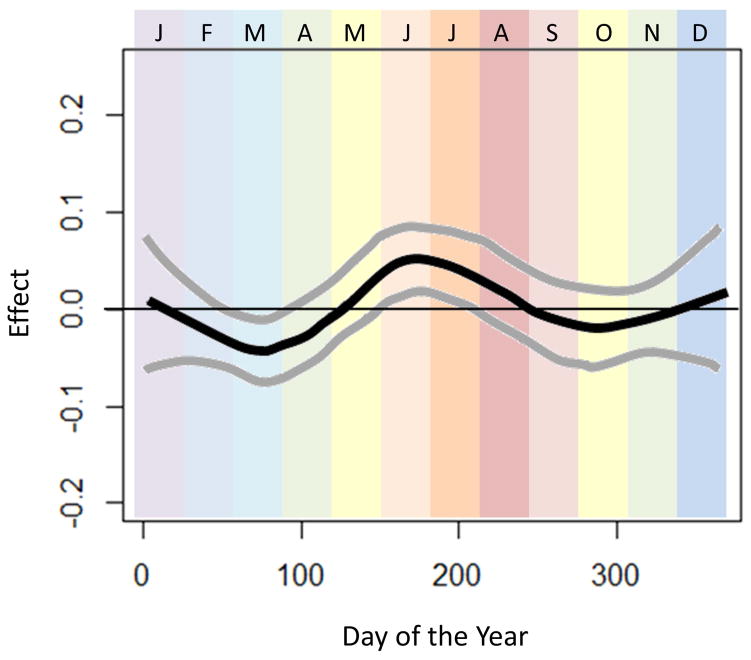
To assess the data in a more granular way, the relationship between day of the year and the odds of esophageal eosinophilia was explored with GAMs. Using a loess smoothing function, GAMs revealed that there was a significant relationship between day of the year and esophageal eosinophilia. Figure 1 displays the effect of the day of the year (log-odds ± 95% CI) on esophageal eosinophilia. There is a clear trend towards increasing esophageal eosinophil in days spanning the months of June and July, with a peak during this time frame.
Figure 1.
Seasonal variation of esophageal eosinophilia. Adjusted associations between biopsy date and esophageal eosinophilia (adjusted for categorical age and sex) are presented. Day of the year is displayed across the horizontal-axis, with colored bands representing the month of the year. The vertical-axis displays the effect (log-odds) of day of the year on diagnosis of esophageal eosinophilia. Smoothed data were generated using the optimal degree of smoothing of the analysis including patients from all climate zones (n=104983; optimal span size=0.40). 95% confidence bands are displayed around each association.
Secondary analyses accounting for climate zone
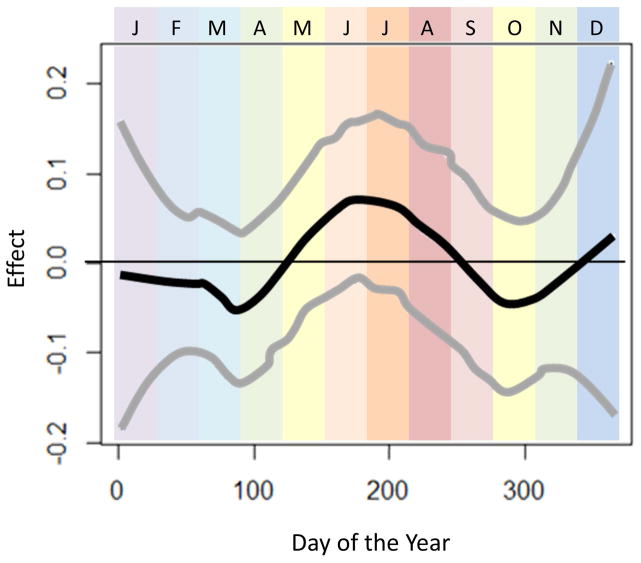
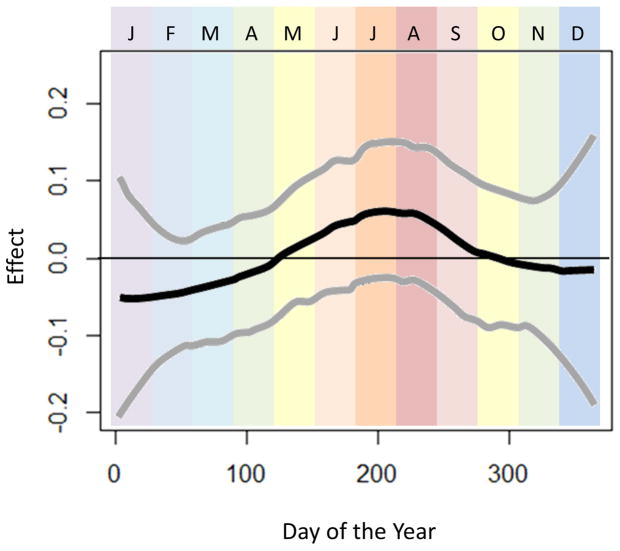
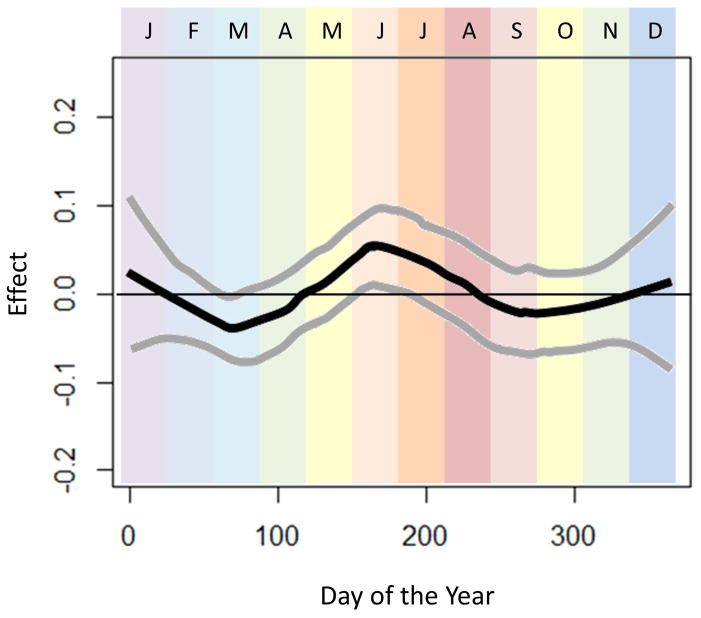
In sensitivity analyses assessing for modification of effect by climate zone (Figures 2–4), we observed that seasonal variation in esophageal eosinophilia was strongest in cold climates (Figure 4). Furthermore, while the peak odds in the temperate and arid climates was observed in June, in cold climates, the peak odds of diagnosis was shifted slightly later, to July and August.
Figure 2.
Seasonal variation of esophageal eosinophilia in the arid climate zone (n=13280). Adjusted associations between biopsy date and esophageal eosinophilia (adjusted for categorical age and sex) are presented. Day of the year is displayed across the horizontal-axis, with colored bands representing the month of the year. The vertical-axis displays the effect (log-odds) of day of the year on diagnosis of esophageal eosinophilia.
Figure 4.
Seasonal variation of esophageal eosinophilia in the cold climate zone (n=16826). Adjusted associations between biopsy date and esophageal eosinophilia (adjusted for categorical age and sex) are presented. Day of the year is displayed across the horizontal-axis, with colored bands representing the month of the year. The vertical-axis displays the effect (log-odds) of day of the year on diagnosis of esophageal eosinophilia.
Discussion
The rapidly changing incidence and prevalence of EoE demonstrates the potential importance of environmental factors.9, 10 While food triggers have clearly been shown to be contributors in disease pathogenesis,11, 14, 20 there are strong associations between EoE and other atopic diseases.14, 15, 19 There is also an increasing awareness of the possibility of aeroallergens playing a role.17 Evaluating seasonal variation in diagnosis of EoE is one way that environmental influences in disease pathogenesis can be assessed. To date, however, reports of seasonal variation are limited to single centers and some reports have been conflicting.5, 6, 17, 25–31 In the present study, we aimed to evaluate seasonal variation in the diagnosis of esophageal eosinophilia by analyzing a large, national pathology database. Our findings revealed a weak, but persistent association between season and month of endoscopy, specifically that esophageal eosinophilia is increased in summer months such as June and July. The magnitude of the association was generally consistent across levels of eosinophilia as well as with more restrictive case definitions of EoE. Moreover, in examining associations by climate zone, we found greater seasonal variation in temperate and cold climates, as might be expected given the more prominent seasonality experienced in these areas compared to arid or tropical climates.
The seasonal variation of EoE has been reported in prior studies. In one of the first reports, Wang et al performed a retrospective chart review of 234 children with EoE over a 6 year period and showed increased diagnosis in the spring, summer, and autumn.25 Subsequent reports showed similar findings with increases in diagnosis of EoE during the spring,17 spring and summer,26, 28 summer,5 summer and autumn,27 and autumn.29 There has also been a report of increased food impaction in summer and autumn months.39 These trends could be explained by increased environmental allergies or aeroallergens during these time frames, and there are data to support correlation between allergen or pollen exposure and increased EoE diagnosis or activity.16, 17, 23 However, the results are not consistent in the literature as there are several studies showing no seasonal variation in EoE.6, 30, 31 This discrepancy might be explained by the lack of comparator group in many of these studies, and the variation in the geographic and climate characteristics between the various studies. Our study, which was conducted using data on a large number of patients from throughout the United States, accounted for climate and geographic variability, and shows a consistent, though weak association between esophageal eosinophilia and seasonality. While our data cannot directly be used to draw etiologic conclusions, an aeroallergen hypothesis related to EoE pathogenesis in some patients is intriguing. Future studies assessing data sources with more granular patient and exposure information will be needed to further investigate this hypothesis.
There are some limitations to this study. Because we used a pathology database, not all clinical data were available. Because of this, we were unable to confirm whether patients were diagnosed with EoE per the consensus guidelines. However, we anticipated this potential limitation during our study design, and therefore focused on those with esophageal eosinophilia as our primary cases of interest. For additional analyses, we created increasingly specific EoE case definitions with the data that were available, an approach we have successfully used previously.32–35 We note that the precision of our estimates did diminish with the smaller number of cases identified with the more stringent case definitions. Limited clinical data also precluded assessment of symptom onset. This is important because there is a conceptual difference in when symptoms started and when a patient could have a health system encounter that would allow EoE to be diagnosed. The general finding of seasonal variation in diagnosis is likely robust to diagnostic delay, although there is a potential that the peak in clinical manifestations of the disease may be shifted a month or two earlier. Additionally, the study design cannot control for a number of potential unmeasured factors (e.g. differences in health seeking behavior; vacation days; food intake; travel; time spent outdoors; etc) that may have impacted the results.
This study also has several strengths. It examines the relationship between EoE diagnosis and season in a large, national database representative of the various geographical and climatic differences across the United States. The modeling approach employed offered an improved means of assessing seasonal variation, incorporating data on timing of endoscopy into daily increments, which allowed a more flexible assessment of variation over time, rather than relying on artificial season constructs. In accounting for climate zone, we were able to observe that climate zones with greater variation in seasons demonstrated stronger seasonal variation in diagnoses. This finding supports the assertion that aeroallergens may contribute to EoE, and also may explain some of the discordant results previously reported.
In conclusion, we report a weak but consistent seasonal variation in the diagnosis of esophageal eosinophilia or EoE, with more cases more frequently diagnosed during summer months, and June and July specifically. These findings take into account climate and geographic differences, and indirectly suggest that aeroallergens may contribute to disease development or flare. This may also have implications for therapeutic approaches in disease treatment and monitoring. Patients refractory to dietary or pharmacologic approaches to EoE may benefit from having environmental allergens assessed, and practitioners could consider monitoring for worsening of disease activity during spring and summer months.
Figure 3.
Seasonal variation of esophageal eosinophilia in the temperate climate zone (n=73847). Adjusted associations between biopsy date and esophageal eosinophilia (adjusted for categorical age and sex) are presented. Day of the year is displayed across the horizontal-axis, with colored bands representing the month of the year. The vertical-axis displays the effect (log-odds) of day of the year on diagnosis of esophageal eosinophilia.
Acknowledgments
Financial support: This research was conducted with support, in part, by NIH award K23DK090073 (ESD).
Footnotes
Competing interests: Dr. Genta is an employee of Miraca Life Sciences. None of the other authors have conflicts of interest related to this article.
Authorship statement
Guarantor of the article: Evan Dellon
Specific author contributions (note that all authors approved the final draft and the authorship list):
Jensen: Data analysis/interpretation; manuscript drafting; critical revision; approved final draft
Shah: Manuscript drafting; critical revision; approved final draft
Hoffman: Data analysis/interpretation; critical revision; approved final draft
Sonnenberg: Project conception; data interpretation; critical revision; approved final draft
Genta: Project conception/design; database creation; data interpretation; critical revision; approved final draft
Dellon: Project conception/design; data analysis/interpretation; manuscript drafting; critical revision; approved final draft
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras C, Katzka DA. ACG Clinical Guideline: Evidence based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol. 2013;108:679–92. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 3.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115(2):418–9. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Hruz P, Straumann A, Bussmann C, et al. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128(6):1349–1350. e5. doi: 10.1016/j.jaci.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7(10):1055–61. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. 2013;25(1):47–52. e5. doi: 10.1111/nmo.12009. [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Erichsen R, Baron JA, et al. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population-based estimates from Denmark. Aliment Pharmacol Ther. 2015 doi: 10.1111/apt.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12:589–596. e1. doi: 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:201–218. doi: 10.1016/j.gtc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonis PA. Putting the puzzle together: epidemiological and clinical clues in the etiology of eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29(1):41–52. doi: 10.1016/j.iac.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109(5):1503–12. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 12.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–1102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination Diet Effectively Treats Eosinophilic Esophagitis in Adults; Food Reintroduction Identifies Causative Factors. Gastroenterology. 2012;142:1451–9. e1. doi: 10.1053/j.gastro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48(1):30–6. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 15.Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The Role of Allergy Evaluation in Adults With Eosinophilic Esophagitis. J Clin Gastroenterol. 2010;44:22–7. doi: 10.1097/MCG.0b013e3181a1bee5. [DOI] [PubMed] [Google Scholar]
- 16.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112(4):796–7. doi: 10.1016/s0091-6749(03)01715-9. [DOI] [PubMed] [Google Scholar]
- 17.Moawad FJ, Veerappan GR, Lake JM, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. 2010;31(4):509–15. doi: 10.1111/j.1365-2036.2009.04199.x. [DOI] [PubMed] [Google Scholar]
- 18.Wolf WA, Jerath MR, Dellon ES. De-novo onset of eosinophilic esophagitis after large volume allergen exposures. J Gastrointestin Liver Dis. 2013;22(2):205–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Dellon ES, Liacouras CA. Advances in Clinical Management of Eosinophilic Esophagitis. Gastroenterology. 2014;147(6):1238–1254. doi: 10.1053/j.gastro.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothenberg ME. Molecular, Genetic, and Cellular Bases for Treating Eosinophilic Esophagitis. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107(1):83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra A, Wang M, Pemmaraju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134(1):204–14. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rhijn BD, van Ree R, Versteeg SA, et al. Birch pollen sensitization with cross-reactivity to food allergens predominates in adults with eosinophilic esophagitis. Allergy. 2013;68(11):1475–81. doi: 10.1111/all.12257. [DOI] [PubMed] [Google Scholar]
- 24.Miehlke S, Alpan O, Schroder S, Straumann A. Induction of eosinophilic esophagitis by sublingual pollen immunotherapy. Case Rep Gastroenterol. 2013;7(3):363–8. doi: 10.1159/000355161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41(5):451–3. doi: 10.1097/01.mcg.0000248019.16139.67. [DOI] [PubMed] [Google Scholar]
- 26.Almansa C, Devault KR, Achem SR. A comprehensive review of eosinophilic esophagitis in adults. J Clin Gastroenterol. 2011;45(8):658–64. doi: 10.1097/MCG.0b013e318211f95b. [DOI] [PubMed] [Google Scholar]
- 27.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305–1313. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwanczak B, Janczyk W, Ryzko J, et al. Eosinophilic esophagitis in children: frequency, clinical manifestations, endoscopic findings, and seasonal distribution. Adv Med Sci. 2011;56(2):151–7. doi: 10.2478/v10039-011-0038-7. [DOI] [PubMed] [Google Scholar]
- 29.Sorser SA, Barawi M, Hagglund K, Almojaned M, Lyons H. Eosinophilic esophagitis in children and adolescents: epidemiology, clinical presentation and seasonal variation. J Gastroenterol. 2013;48(1):81–5. doi: 10.1007/s00535-012-0608-x. [DOI] [PubMed] [Google Scholar]
- 30.Elitsur Y, Aswani R, Lund V, Dementieva Y. Seasonal distribution and eosinophilic esophagitis: the experience in children living in rural communities. J Clin Gastroenterol. 2013;47(3):287–8. doi: 10.1097/MCG.0b013e31826df861. [DOI] [PubMed] [Google Scholar]
- 31.Elias MK, Kopacova J, Arora AS, et al. The Diagnosis of Esophageal Eosinophilia is Not Increased in the Summer Months. Dysphagia. 2014 doi: 10.1007/s00455-014-9574-1. [DOI] [PubMed] [Google Scholar]
- 32.Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. 2012;107(5):698–706. doi: 10.1038/ajg.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen ET, Hoffman K, Shaheen NJ, Genta RM, Dellon ES. Esophageal eosinophilia is increased in rural areas with low population density: results from a national pathology database. Am J Gastroenterol. 2014;109(5):668–75. doi: 10.1038/ajg.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology. 2011;141(5):1586–92. doi: 10.1053/j.gastro.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen ET, Eluri S, Lebwohl B, Genta RM, Dellon ES. Increased risk of esophageal eosinophilia and eosinophilic esophagitis in patients with active celiac disease on biopsy. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.02.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hastie T, Tibshirani R. Generalized Additive Models. New York: Chapman and Hall; 1990. [Google Scholar]
- 37.Hastie T. Gam: Generalized Additive Models, R Package, Version 0.98. 2006. 2006 [Google Scholar]
- 38.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World Map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrif. 2006;15(3):4. [Google Scholar]
- 39.Larsson H, Bergquist H, Bove M. The incidence of esophageal bolus impaction: is there a seasonal variation? Otolaryngol Head Neck Surg. 2011;144(2):186–90. doi: 10.1177/0194599810392655. [DOI] [PubMed] [Google Scholar]