Abstract
Objective
To determine whether addition of an electronic sepsis evaluation and management tool to electronic sepsis alerting improves compliance with treatment guidelines and clinical outcomes in septic intensive care unit patients.
Design
A pragmatic randomized trial.
Setting
Medical and surgical intensive care units of an academic, tertiary care medical center
Patients
Four hundred and seven patients admitted during a 4-month period to the medical or surgical intensive care unit with a diagnosis of sepsis established at the time of admission or in response to an electronic sepsis alert.
Interventions
Patients were randomized to usual care or the availability of an electronic tool capable of importing, synthesizing, and displaying sepsis-related data from the medical record, using logic rules to offer individualized evaluations of sepsis severity and response to therapy, informing users about evidence-based guidelines, and facilitating rapid order entry.
Measurements and Main Results
There was no difference between the electronic tool (218 patients) and usual care (189 patients) with regard to the primary outcome of time to completion of all indicated Surviving Sepsis Campaign 6 hour Sepsis Resuscitation Bundle elements (Hazard Ratio 1.98, 95% Confidence Interval 0.75 – 5.20, p=0.159) or time to completion of each element individually. ICU mortality, ICU-free days, and ventilator-free days did not differ between intervention and control. Providers used the tool to enter orders in only 28% of available cases.
Conclusions
A comprehensive electronic sepsis evaluation and management tool is feasible and safe but did not influence guideline compliance or clinical outcomes, perhaps due to low utilization.
Keywords: Sepsis, Resuscitation, Early Goal Directed Therapy, Protocol, Electronic, Alert
INTRODUCTION
Sepsis is a common and lethal illness frequently managed in the intensive care unit (ICU) (1–3). Early resuscitation (4, 5) and prompt antibiotic administration (6–8) improve mortality. To aid clinicians in consistent implementation of these interventions, the Surviving Sepsis Campaign (SSC) outlined in 2005 a 6-hr Sepsis Resuscitation Bundle incorporating rapid sepsis recognition, early cultures and antibiotics, and goal-directed fluid administration and hemodynamic support (9, 10). Implementation of SSC 6-hr Resuscitation Bundle elements using a written protocol has been shown to improve compliance with recommendations (11–13) and mortality (14). However, in the absence of ongoing feedback to clinicians, even after intensive education in sepsis detection and management, compliance with guidelines remains low (15). The use of electronic tools to address this challenge interests physicians and hospitals. Electronic tools have been successfully employed in the ICU for ventilator weaning (16) and identification of ARDS (17). One prior study in sepsis has evaluated a computerized translation of a written protocol for early resuscitation (18). With recent advances in information technology, a single electronic tool can now couple real-time monitoring of the medical record to identify patients with potential sepsis (19) with decision-support to guide clinicians through severity evaluation, provide education about sepsis guidelines generally and identify interventions indicated in a specific patient, facilitate rapid entry of sepsis-management orders, and monitor the patient’s response to interventions throughout the ICU course. We hypothesized that, in adult ICU patients with sepsis, implementation of such an electronic evaluation and management tool would improve compliance with sepsis treatment guidelines and clinical outcomes.
MATERIALS AND METHODS
Definitions and Terms
Sepsis
The co-occurrence of suspected infection and two or more of the systemic inflammatory response syndrome (SIRS) criteria: 1. Temperature > 38 or < 36 degrees Celsius. 2. Heart rate > 90 beats/min. 3. Respiratory Rate > 20 breaths/min or PaCO2 < 32mm Hg. 4. White Blood Cell count (WBC) > 12,000 cells/mm3 or < 4,000 cells/mm3, or > 10% immature (band) forms.
Modified SIRS Criteria
Two or more SIRS criteria met within a rolling 24 hour window, with at least one being abnormal temperature or WBC count.
Listening Application
An electronic tool that monitors patient data in real-time, evaluates data against diagnostic and alerting rules to identify patients who newly meet modified SIRS criteria, and communicates with the alerting system to notify providers (19).
Alerting System
An electronic tool that receives information from the listening application on patients who have met modified SIRS criteria, notifies providers of the finding, and solicits an assessment to determine if the patient clinically meets criteria for sepsis (19).
Integrated Sepsis Assessment and Management Tool
A software program within the electronic medical record (EMR) designed to import, synthesize, and display sepsis-related data from different portions of the record, use logic rules to offer an up-to-date, individualized evaluation of sepsis severity and response to therapy, inform users about evidence-based guidelines, and facilitate rapid order entry.
Clinical Provider
ICU resident physician or nurse practitioner primarily responsible for patient management and order entry.
Screening and Enrollment
From April 1st to July 31st of 2012, we conducted a pragmatic, open-label, parallel-group randomized trial in the medical and surgical intensive care units of Vanderbilt University Hospital. This study was approved by the Institutional Review Board with waiver of informed consent.
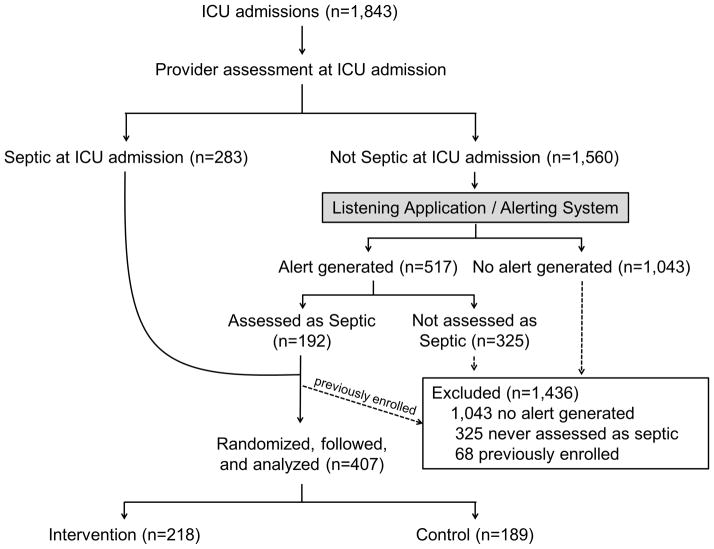
All patients admitted to a study ICU and assessed by their clinical provider as having sepsis were enrolled (Figure 1). Patients assessed as septic via an automatic prompt offered at ICU admission were enrolled immediately. For all patients without sepsis at ICU admission, electronic monitoring was employed to facilitate early detection of sepsis development (Supplemental Digital Content: Figure S1. Schematic of the Listening, Alerting, and Provider Assessment systems). A previously-established listening application screened real-time data from the nursing documentation, electronic health record, and lab system for modified SIRS criteria (19). If modified SIRS criteria developed in an ICU patient who had not previously been assessed by a clinical provider as septic, an alerting system notified the clinical provider via text page and a flag appeared next to the patient’s name on the clinical provider’s electronic patient list. Using this flag, clinical providers recorded a revised sepsis assessment. If an assessment was not recorded within one hour, a reminder was sent. No management recommendations were given by the listening application or alerting system. If patients were assessed not to be septic, further alerts were suppressed for 48 hours, and then electronic monitoring resumed. If patients were assessed as septic they were enrolled in the study and no further alerts were delivered for that hospitalization. Study enrollment occurred at the time the first prompt to which the clinical provider confirmed the presence of sepsis was offered. Patients were excluded if they were never assessed as septic by their clinical provider or had been previously enrolled during the same hospitalization. Patients with orders limiting resuscitation were not excluded.
Figure 1. Enrollment and Randomization.
Of 475 patients assessed as septic, 68 had been enrolled in the study previously in the hospitalization and were excluded. The remaining 407 were randomized, followed, and included in the analysis.
Randomization and Masking
All ICU patients assessed by a provider as septic were randomized by a computerized algorithm without use of permuted blocks or stratification to usual care (control) or the availability of an integrated, electronic sepsis assessment and management tool (intervention). Clinical providers were aware of group assignment. Separate study personnel remained blinded until the completion of data collection.
Intervention
In the intervention group, when a provider’s assessment confirmed the presence of sepsis, the integrated sepsis assessment and management tool (Supplemental Digital Content: Video 1. Overview of the electronic sepsis tool) opened automatically and remained available to all providers throughout the ICU admission. The tool resided directly within the EMR and contained a divided display. Half presented graphs of current value, trend, and goal range for temperature, heart rate, mean arterial pressure, central venous pressure, white blood cell count, hemoglobin, platelet count, prothrombin time, lactate, and Richmond Agitation and Sedation Score. The other half contained a set of tabs dedicated to evaluation of the patient condition and management. The assessment tab offered providers an evaluation of sepsis severity using an algorithm in which sepsis was defined as previous confirmation of sepsis by a clinical provider in response to a prompt, severe sepsis was sepsis plus mean arterial pressure ≤ 60 mmHg or a lactate above the laboratory upper limit of normal, and septic shock was sepsis plus vasopressor use or either mean arterial pressure ≤ 60 mmHg or a lactate ≥ 4 mmol/L despite an order for ≥ 1,000 mLs of IV crystalloid. The provider could agree with or modify the severity assessment which would then propagate into the management tabs allowing the tool to highlight interventions recommended for that specific patient. The management tabs were displayed in a workflow modeled on the SSC 6-hr Resuscitation Bundle beginning with a diagnostics panel addressing basic labs, cultures, and imaging for source control, followed by a therapeutics panel addressing antibiotics and goal-directed resuscitation including fluid boluses, CVP, vasopressors, serial lactate measurements, inotrope use, and blood transfusion, and concluded by a supportive care panel addressing lung-protective ventilation, adrenal insufficiency, glucose control, and venous thromboembolic and stress ulcer prophylaxis. Each management tab contained evidence-based information and “single-click” order entry enabling providers to place related orders while reviewing the guidelines. The tool was integrated with electronic order entry from all parts of the hospital so that providers could see via a color-coded system not only which orders were recommended but which orders had already been completed in the ICU or prior to ICU admission. The tool could be minimized at any time to review the medical record and could be closed and reopened any time throughout the admission. Technical aspects of the sepsis tool’s development, architecture, and user interface have been published previously (20–23, 19) and are summarized in the online supplement (Supplemental Digital Content: eMethods).
Data Collection
Patients were followed for 28 days or until hospital discharge, whichever occurred first. Demographic and baseline characteristics, vital signs and laboratory results, and clinical outcomes were collected by study personnel blinded to group assignment. The date and time at which each of the SSC 6-hr Resuscitation Bundle elements was completed underwent automated, electronic documentation in the EMR as a part of routine clinical care. These data were prospectively abstracted into a database by blinded study personnel. Listening application, alerting system, and tool usage data were collected automatically in a separate database. Retrospective review of the medical record by study physicians used all available information to adjudicate whether each patient had been septic at the time of enrollment.
The primary endpoint was time from enrollment until completion of all indicated elements of the SSC 6-hr Resuscitation Bundle – blood cultures drawn, broad spectrum antibiotics ordered, CVP measured, intravenous fluid bolus administered if indicated, vasopressors administered if indicated, and lactate measured. Lactate clearance is used in place of venous oxygen saturation in our ICUs (24). Time to completion for each element was calculated as time from enrollment until the time that each bundle element was electronically documented as completed in the EMR. Secondary endpoints included time to completion of each individual SSC 6-hr Resuscitation Bundle element, ICU mortality, days alive and free from mechanical ventilation (VFDs), days alive and out of the ICU (ICU-free days), and days alive and free from vasopressor administration (vasopressor-free days), all to study day 28.
Statistical Analysis
Based on prior data from the same setting (19), planned enrollment of 400 patients provided 80 percent statistical power to detect a one hour decrease in the primary endpoint of time to completion of all SCC 6-hr Resuscitation Bundle elements with a Type I error rate of 0.05. Demographics and baseline characteristics were summarized by median and inter-quartile range (IQR) or mean and standard deviation for continuous variables and as numbers and percentages for categorical variables across intervention and control groups. To analyze the time to completion of all SSC 6-hr Resuscitation Bundle elements and each element individually, the cumulative event probabilities were estimated and compared using Kaplan-Meier method with log rank testing and Cox proportional-hazards regression. A logistic regression model with pre-specified covariates was fit to assess what factors impacted the use of the tool to enter orders. All analyses were performed using the statistics software R version 3.0.1.
Role of the Funding Source
The National Institutes of Health, National Center for Research Resources, and National Science Foundation provided financial support for this study but were not involved in its design, data collection, analysis, interpretation, or reporting. M.W.S. had full access to all the data and had final responsibility for the decision to submit for publication.
RESULTS
Enrollment and Baseline Characteristics
Of 1,843 ICU admissions during the study period, 407 were identified by providers as having sepsis and were enrolled (Figure 1). The 218 patients randomized to the integrated sepsis assessment and management tool and the 189 patients randomized to control had similar baseline characteristics and pre-randomization management (Table 1). Patients averaged 56 years of age, were predominantly male and Caucasian, and were most frequently admitted from the emergency department and cared for in the MICU. A pulmonary source of sepsis was most common and one fifth of patients were mechanically ventilated at enrollment.
Table 1.
Baseline Characteristics
| Characteristic | Control (n=189) | Electronic Tool (n=218) |
|---|---|---|
| Age (yr) | 57±17 | 55±16 |
| Male | 55.0% (104) | 54.6% (119) |
| Race | ||
| Caucasian | 76.7% (145) | 73.9% (161) |
| African American | 15.9% (30) | 18.3% (40) |
| Asian | 1.6% (3) | 1.8% (4) |
| Unknown | 5.8% (11) | 6.0% (13) |
| Route of Admission | ||
| Emergency Department | 45.5% (86) | 46.8% (102) |
| Transfer from another hospital | 12.7% (24) | 16.5% (36) |
| Floor transfer | 25.4% (49) | 20.2% (44) |
| PACU/Recovery room | 8.5% (16) | 10.6% (23) |
| Other | 7.4% (14) | 6.0% (13) |
| MICU | 71.4% (135) | 70.6% (154) |
| Sepsis acknowledged at admission | 65.1% (123) | 54.6% (119) |
| If not at present at admission, time to development (hrs) | 1.8 [0.5–29.3] | 2.2 [0.5–49.6] |
| Time from sepsis prompt to positive assessment (min) | 48.5 [16.5–152.0] | 47.5 [18.0–154.0] |
| Sepsis confirmed on review | 88.4% (167) | 84.4% (184) |
| Source of Sepsis | ||
| Pulmonary | 30.2% (57) | 22.5% (49) |
| Urinary | 14.3% (27) | 14.7% (32) |
| Abdominal | 22.2% (42) | 21.1% (46) |
| Skin and soft tissue | 7.4% (14) | 7.3% (16) |
| Bacteremia | 16.4% (31) | 16.1% (35) |
| Other | 9.5% (18) | 18.3% (40) |
| Fluid in 6 hours pre-enrollment (mL) | 858±983 | 777±800 |
| Vasopressors in 6 hours pre-enrollment | 6.3% (12) | 10.1% (22) |
| Mechanically Ventilated | 20.7% (39) | 21.6% (47) |
| APACHE II score | 20.6±8.5 | 20.2±8.6 |
Data given as mean ± SD, median [25th – 75th percentile], or percentage (number)
Main Outcomes
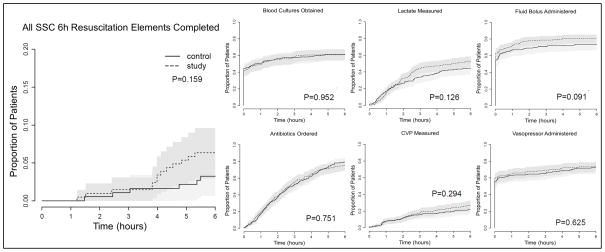
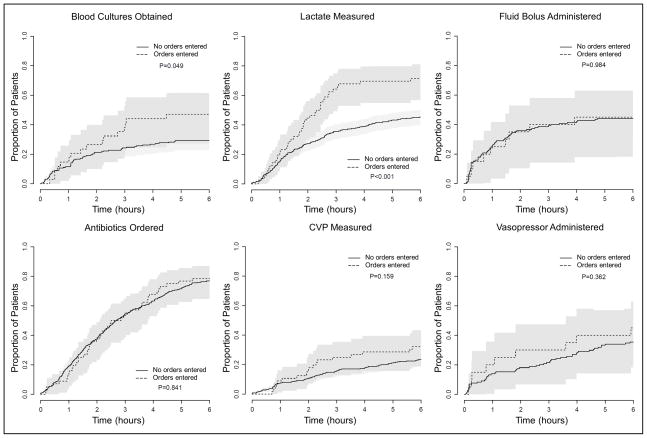
There was no significant difference between the integrated sepsis assessment and management tool and control with regard to the primary outcome of time to completion of all indicated SSC 6-hr Resuscitation Bundle elements or the secondary outcome of time to completion of each element individually (Figure 2). There was no difference between the groups in ICU mortality, ICU-free days, ventilator-free days, or Vasopressor-free days (Table 2). Sensitivity analyses showed no difference in the primary outcome between intervention and control in the subgroups with sepsis at ICU admission (Hazard Ratio 1.60, 95% Confidence Interval 0.45 – 5.67, p=0.462) or sepsis recognized after ICU admission in response to an alert (Hazard Ratio 2.53, 95% Confidence Interval 0.52 – 12.16, p=0.231). In a prospectively-planned per-protocol analysis, patients for whom one or more orders were placed using the tool were more likely to have blood cultures and lactate drawn in the first 6 hours than patients for whom the tool was not used to place orders (Figure 3) but there was no difference in ICU mortality (14.3% versus 14.9%, p=0.905), ICU-free days (17.9±1.4 versus 19.0±0.5, p=0.473), VFDs (22.3±1.3 versus 23.0±0.5, p=0.576), or vasopressor-free days (22.2±1.3 versus 22.6±0.5, p=0.789).
Figure 2. Completion of Surviving Sepsis Campaign 6 hour Sepsis Resuscitation Bundle Elements for Patients Randomized to the Electronic Tool (study intervention) versus Usual Care (control).
Proportion of patients for whom indicated bundle elements were completed over the 6 hours after enrollment. Lactate measurement, CVP measurement, and antibiotic administration were considered indicated in all patients during their first 6 hours in the ICU. Blood cultures were considered indicated in all patients but deemed completed at enrollment if obtained in the 6 hours prior to enrollment. Fluid bolus administration and vasopressor administration were considered indicated only in patients who met the hemodynamic criteria for each intervention as specified in the SSC 6-hr Resuscitation Bundle. There was no difference between the electronic tool and usual care with regard to time to completion of the SSC 6-hr Resuscitation Bundle elements.
Table 2.
Clinical Outcomes
| Control (n=189) | Electronic Tool (n=218) | p | |
|---|---|---|---|
| ICU mortality | 15.9% (30) | 13.8% (30) | p=0.549 |
| ICU-free days to day 28 | 18.7±0.7 | 18.9±0.7 | p=0.901 |
| Ventilator-free days to day 28 | 23.0±0.7 | 23.0±0.7 | p=0.940 |
| Vasopressor-free days to day 28 | 22.4±0.8 | 22.5±0.7 | p=0.909 |
Figure 3. Completion of Surviving Sepsis Campaign 6 hour Sepsis Resuscitation Bundle Elements by Use of Electronic Tool for Order Entry.
Proportion of patients for whom indicated bundle elements were completed over the 6 hours after enrollment for the subgroups of patients for whom the tool was used to enter orders versus patients for whom it was not used to enter orders. Lactate measurement, CVP measurement, and antibiotic administration were considered indicated in all patients during their first 6 hours in the ICU. Blood cultures were considered indicated in all patients but deemed completed at enrollment if obtained in the 6 hours prior to enrollment. Fluid bolus administration and vasopressor administration were considered indicated only in patients who met the hemodynamic criteria for each intervention as specified in the SSC 6-hr Resuscitation Bundle. Graphs in this figure display patients for whom the resuscitation bundle element was indicated during the 6 hours after enrollment and exclude patients for whom the element was completed before enrollment or not indicated. Patients for whom the tool was used to enter orders were more likely to have blood cultures obtained and lactate measured.
Tool Utilization
Of the patients enrolled, 60% were identified as septic at ICU admission. In these, the median time from electronic prompt to provider’s completed assessment was 46 [interquartile (IQR) 20–125] minutes compared to 59 [interquartile (IQR) 16–200] minutes for those assessed as septic later in response to an alert from the listening application (p=0.982). Of 218 patients randomized to the intervention arm, the tool was opened by providers in 126 cases (57.8%). The tool was re-opened after closure 51 times in the care of 17 patients (7.8%). The tool was used to enter orders in 62 cases (28.4%) yielding 473 individual orders for 37 unique actions including 104 (38.1%) for hematologic or metabolic laboratory studies, 62 (22.7%) for cultures, 31 (11.4%) for lactate measurement, 29 (10.6%) for antibiotic administration, 16 (5.9%) for imaging studies, and the remainder for fluid administration, vasopressors, steroids, glucose control, sedation, prophylaxis, and intravenous access. Orders were entered via the tool for 67.3% of SICU patients versus 36.5% of MICU patients (p=0.001) and a multivariable analysis confirmed that surgical ICU was the only baseline factor associated with utilization of the tool to enter orders (Odds Ratio 4.65, 95% CI 2.06–11.0, p<0.001) (Supplemental Digital Content: Table S1. Multivariable regression for use of tool to enter orders). The tool was employed by 87 unique providers, primarily resident physicians (63.5% of MICU utilizations versus 7.7% of SICU utilizations, p<0.001) and nurse practitioners (35.1% of MICU utilization versus 90.4% of SICU utilization, p<0.001). Over the course of the study, each provider accessed the tool on a median of 2 occasions (range 1 to 21).
DISCUSSION
This pragmatic randomized trial of an integrated, electronic sepsis evaluation and management tool in two ICUs at a single center did not demonstrate a significant difference in the primary outcome of time until completion of all indicated SSC 6-hr Resuscitation Bundle elements. There are several potential explanations for this lack of effect.
Utilization of the tool was low. Providers opened the tool in less than 60% of available cases and placed orders through the tool in less than 30%. Prior studies of computerized clinical decision support systems (CDSS) have identified characteristics predictive of CDSS success which may be relevant to understanding our tool’s unexpectedly low utilization (25–27). Two systemic reviews (25, 26) suggest that successful CDSSs provide decision support automatically rather than relying on clinician initiative, deliver decision support at the time and location of decision making, and provide actionable recommendations rather than simply assessment. Our sepsis tool opened automatically at enrollment but, once closed, relied on providers to re-access rather than prompting with changes in patient status. The tool resided in the EMR and became available immediately after identification of sepsis by the ICU team. However, the capacity of the tool to generate orders directly from the EMR interface was novel and providers may have been more comfortable entering orders through the established computerized physician order entry system. Though our tool went beyond evaluation to provide patient-specific recommendations, impact may have been limited by the manner in which recommendations were delivered. Color-coding the recommended elements of the SSC 6-hr Resuscitation Bundle relied on the provider to actively select highlighted orders. Collating recommended orders into a discrete bundle and perhaps even employing an opt-out approach where recommended orders occurred unless providers actively disagreed may have been more effective. Other potential strengths of our tool’s design (integration with EMR and order entry, minimal clinician data entry, justification of recommendations with evidence, and on-site development in a CDSS-friendly environment via iterative refinement with support of ICU leaders) (25–27) may have been outweighed from the user’s perspective by shortcomings in either usability or content. Although system speed, clinician time savings, and clarity of user interface (25) were not barriers in pilot testing, rotations in ICU resident staffing meant each provider interacted with the tool an average of only two times during the four month study. The suggestion that the time and energy costs of learning new software may have disincentivized use is supported by the observation that the nurse practitioners, who worked continuously in the ICUs throughout the study period, were the highest utilizers of the tool. “Information overload” is particularly challenging to CDSSs targeting disease management and the amount of content presented in our tool may have overwhelmed users. Similarly, the abundance of electronic reminders in routine use in our ICUs (drug interaction checks, sedation goals, anticoagulation monitoring, insulin advisors, imaging advisors, etc.) coupled with the sepsis alerts may have precipitated “alert fatigue” encouraging providers to navigate around the electronic sepsis tool reflexively. Bypass of the tool by providers might have been minimized by providing periodic performance feedback, requiring documentation of reasoning when tool was not used or recommendations were not followed, or even electronically forcing use in the intervention arm (28). Provider acceptance of protocolized care may have influenced tool utilization. Use was higher in the surgical ICU and among nurse practitioners, provider groups that in our institution have greater preexisting familiarity with protocolized care. Finally, although the tool was available to all providers during the patient’s ICU stay, only residents and nurse practitioners received the sepsis alert page. Given that attending physicians at our institution play a supervisory role in which they are encouraged to defer order entry, initial evaluation, and initial management to trainees whenever appropriate, we feel that including attending physicians on the sepsis alerts would not have changed our findings.
Restriction of the tool to ICUs in an academic center may have further diminished its utility. In contrast to studies in which sepsis management interventions were initiated in the emergency department (11–13, 29) or hospital floor (30), the majority of patients in our study received elements of the SSC 6-hr Resuscitation Bundle prior to enrollment. The performance of blood cultures, fluid resuscitation, and vasopressor administration before ICU admission in more than half of cases in which they were indicated may have limited the additive value of the tool. Additionally, our ICUs are staffed 24 hours a day by physicians and nurses who, because of the high incidence of sepsis in this environment, are experienced in the early recognition and guidelines-based management of septic patients. The implementation of an electronic sepsis tool in an emergency department, hospital ward, or less resource-intensive ICU may demonstrate substantially different results. Moreover, bundle compliance in the control arm may have been augmented by provider awareness of the study (Hawthorne effect) and contamination of “usual care” by exposure to the electronic tool when providers were caring for patients in the intervention and control arms concurrently (patient-level rather than cluster randomization).
Low severity of illness may have influenced the outcome of the trial. While the study was limited to patients requiring ICU admission, we included septic patients without organ dysfunction. The mean APACHE II score of 20 and overall ICU mortality of 15% are lower than prior studies of sepsis bundle implementation (4, 12–14, 31) and some of the SSC 6-hr Resuscitation Bundle elements may not have been indicated in the subgroup of less severely ill patients.
In addition to the above weaknesses, our study has several major strengths. Prior investigations have examined sepsis detection via electronic listening applications (19, 30) and implementation of sepsis management bundles via written protocols (11–13, 29, 31) or computerized translation of a written protocol (18). However, this is the first trial of a comprehensive electronic tool with sepsis detection, alerting, severity assessment, and management functions. Our study confirms that development and implementation of such a comprehensive tool for a complex disease like sepsis is feasible and safe. The finding that patients for whom the tool was used to enter orders experienced higher compliance with early sepsis treatment metrics suggests that, if utilized more consistently, the tool itself may have been capable of improving guideline adherence. Future studies of comprehensive electronic sepsis tools should target more severely ill patients in lower provider-intensity settings, immediately after sepsis presentation. Integration into the EMR should occur where providers enter orders, not where they review data. The appeal of a tool that is comprehensive and educational must be carefully weighed against the advantages of simplicity, speed, and ease of use. Finally, attention and resources may need to be shifted from the underlying algorithms to the user interface, and from development to implementation.
Since the completion of this study, the SSC has released new sepsis resuscitation guidelines and restructured sepsis bundles emphasizing performance of lactate, blood culture, antibiotics, and fluid bolus elements in the first three hours and vasopressors, central venous pressure measurement, and lactate re-measurement in the first six hours (32). Even more recently, two large multicenter trials comparing early goal-directed resuscitation with usual care failed to confirm the difference in outcome on which the early sepsis resuscitation guidelines themselves are based (33, 34). These potentially major changes in understanding early sepsis resuscitation do not undermine the potential utility of an electronic sepsis evaluation and management tool. As evidence for best practices in critical care medicine evolves, translation into routine clinical practice frequently lags on the scale of years to decades (35). In contrast, the clinical logic algorithms and evidence-based education structured into an electronic tool can be updated rapidly with minimal effort by a small number of individuals. With the proliferation of electronic medical records and the recent passage of the Health Information Technology for Economic and Clinical Health Act linking incentive payment to implementation of clinical decision support tools (36), interest in developing sophisticated electronic disease management tools for use in the ICU environment can be expected to increase. Our study emphasizes that the success of future electronic tools depends not only on addressing the technical challenges of managing increasingly large data inputs, integrating multiple complex software platforms, and providing up-to-date, evidence-driven recommendations for an individual patient at any specific moment in the clinical course, but on ensuring that providers use the tool once its built.
CONCLUSIONS
Developing and instituting a comprehensive electronic sepsis evaluation and management tool is feasible and safe. Addition of an electronic sepsis evaluation and management tool to electronic sepsis alerting in the ICU did not change guideline compliance or clinical outcomes, possibly due to low utilization.
Supplementary Material
eMethods: Overview of the Sepsis Tool’s Development, Architecture, and Interface
Figure S1. Schematic of the Listening, Alerting, and Provider Assessment systems.
Table S1. Multivariable regression for use of tool to enter orders.
Overview of the electronic sepsis tool. A video tour of the interface and capabilities of the electronic sepsis assessment and management tool narrated by Liza Weavind, MBBCh MMHC on April 7th, 2014. Author: Liza Weavind, Videographer: Andras Nadas, Participants: Liza Weavind, Length: 3min 12 sec, Size: 26.2MBs
Acknowledgments
Study supported by grants 1RC1LM010310-01 from NIH, 1 UL1 RR024975 from NCRR/NIH, and CCF- 0424422 from NSF. The authors would like to thank Rachel M. Hayes, PhD; Daniel W. Albert, MS; Stephen Clark; Norment B. Deane MS; Janos L. Mathe, MSc; Janos Sztipanovits, PhD; and Anne Miller, PhD for their contributions to making this study possible.
Footnotes
Institution where study was performed: Vanderbilt University Medical Center, Nashville, TN
Author Contributions:
M.W.S., L.W., M.H.H., T.W.R, J.B.M., G.R.B., and A.P.W., study concept and design. M.W.S., L.W., M.H.H., S.S., A.N., and A.P.W., acquisition of the data. M.W.S., L.W., T.W.R, S.S., A.N., Y.S., G.R.B., and A.P.W. data analysis and interpretation. M.W.S., L.W., T.W.R, and A.P.W. manuscript preparation and drafting. M.W.S, L.W., S.S., Y.S., and T.W.R, statistical methods, statistical data analysis. M.W.S., L.W., M.H.H., T.W.R, S.S., A.N., Y.S., J.B.M., G.R.B., and A.P.W, manuscript critique and review. All authors approved the manuscript submitted.
Conflicts of Interest and Source of Funding: Study supported by grants 1RC1LM010310-01 from NIH, 1 UL1 RR024975 from NCRR/NIH, and CCF- 0424422 from NSF. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. M.H.H. reported prior consulting for Biomereux and T.W.R. reported serving on an advisory board for Avisa Pharma, LLC and as a DSMB member for GlaxoSmithKline. Otherwise, the authors declare no potential conflicts of interest.
Copyright form disclosures: Drs. Semler, Weavind, Gowda, Nadas, Song, Martin, and Bernard received support for article research from the National Institutes of Health (NIH) (1RC1LM010310-01), NCRR/NIH (1 UL1 RR024975), and NS (CCF- 0424422). Their institutions received grant support from the NIH (1RC1LM010310-01), NCRR/NIH(1 UL1 RR024975), and NSF (CCF- 0424422). Dr. Hooper consulted for Biomereux and received support for article research from the National Institutes of Health (NIH) (1RC1LM010310-01), NCRR/NIH (1 UL1 RR024975), and NS (CCF- 0424422). His institution received grant support from the NIH (1RC1LM010310-01), NCRR/NIH(1 UL1 RR024975), and NSF (CCF- 0424422). Dr. Rice consulted for Avisa Pharma, LLC (Advisory Board) and GlaxoSmithKline, LLC (DSMB Member). He received support for article research from the National Institutes of Health (NIH) (1RC1LM010310-01), NCRR/NIH (1 UL1 RR024975), and NS (CCF- 0424422). His institution received grant support from the NIH (1RC1LM010310-01), NCRR/NIH(1 UL1 RR024975), and NSF (CCF- 0424422). Dr. Wheeler consulted for Cumberland Pharmaceuticals; lectured for numerous grand rounds presentations at academic institutions; received royalties for a textbook; has stock in Cumberland Pharmaceuticals; and received support for article research from the National Institutes of Health (NIH) (1RC1LM010310-01), NCRR/NIH (1 UL1 RR024975), and NS (CCF- 0424422). His institution received grant support from the NIH (1RC1LM010310-01), NCRR/NIH (1 UL1 RR024975), and NSF (CCF- 0424422) and received grant support (Investigator on numerous NIH grants not related to this project).
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA J Am Med Assoc. 1995;273(2):117–123. [PubMed] [Google Scholar]
- 3.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulmé R, Lepage E, Le Gall R. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28(2):108–121. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Jones AE, Brown MD, Trzeciak S, Shapiro NI, Garrett JS, Heffner AC, Kline JA Emergency Medicine Shock Research Network investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med. 2008;36(10):2734–2739. doi: 10.1097/CCM.0b013e318186f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 7.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31(12):2742–2751. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]
- 8.MacArthur RD, Miller M, Albertson T, Panacek E, Johnson D, Teoh L, Barchuk W. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;38(2):284–288. doi: 10.1086/379825. [DOI] [PubMed] [Google Scholar]
- 9.Severe sepsis bundles. [Accessed December 15, 2009];Resuscitation bundle. Available at: http://www.survivingsepsis.org/Bundles/Pages/default.aspx.
- 10.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro NI, Howell MD, Talmor D, Lahey D, Ngo L, Buras J, Wolfe RE, Weiss JW, Lisbon A. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34(4):1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 12.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132(2):425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, Edwards J, Cho TW, Wittlake WA. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35(4):1105–1112. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 14.Lin S-M, Huang C-D, Lin H-C, Liu C-Y, Wang C-H, Kuo H-P. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock Augusta Ga. 2006;26(6):551–557. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer R, Artigas A, Levy MM, Blanco J, González-Díaz G, Garnacho-Montero J, Ibáñez J, Palencia E, Quintana M, de la Torre-Prados MV Edusepsis Study Group. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA J Am Med Assoc. 2008;299(19):2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 16.Lellouche F, Mancebo J, Jolliet P, Roeseler J, Schortgen F, Dojat M, Cabello B, Bouadma L, Rodriguez P, Maggiore S, Reynaert M, Mersmann S, Brochard L. A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med. 2006;174(8):894–900. doi: 10.1164/rccm.200511-1780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herasevich V, Yilmaz M, Khan H, Hubmayr RD, Gajic O. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35(6):1018–1023. doi: 10.1007/s00134-009-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinley BA, Moore LJ, Sucher JF, Todd SR, Turner KL, Valdivia A, Sailors RM, Moore FA. Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. J Trauma. 2011;70(5):1153–1166. doi: 10.1097/TA.0b013e31821598e9. discussion 1166–1167. [DOI] [PubMed] [Google Scholar]
- 19.Hooper MH, Weavind L, Wheeler AP, Martin JB, Gowda SS, Semler MW, Hayes RM, Albert DW, Deane NB, Nian H, Mathe JL, Nadas A, Sztipanovits J, Miller A, Bernard GR, Rice TW. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit*. Crit Care Med. 2012;40(7):2096–2101. doi: 10.1097/CCM.0b013e318250a887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin JB, Mathe JL, Miller P, Ledeczi A, Weavind L, Miller A, Maron D, Nadas A, Sztipanovits J. A Model-Integrated Approach to Implementing Individualized Patient Care Plans Based on Guideline-Driven Clinical Decision Support and Process Management - A Progress Report. 2008. [Google Scholar]
- 21.Mathe JL, Ledeczi A, Nadas A, Sztipanovits J, Martin JB, Weavind LM, Miller A, Miller P, Maron DJ. A Model-Integrated, Guideline-Driven, Clinical Decision-Support System. IEEE Softw. 2009;26(4):54–61. doi: 10.1109/MS.2009.84. [DOI] [Google Scholar]
- 22.Nadas A, Mathe J, Weavind L, Semler M, Sztipanovits J. Lessons Learned from the Development of aModel-integrated Decision-support Application for Sepsis. Washington (DC): 2014. [Google Scholar]
- 23.Miller A. Alerts and reminders: Is this all there is to clinical decision support? 2011 Available at: www.cs.umd.edu/hcil/sharp/workshop2011/files/Miller,Anne-EHR-HCILseminar.pdf#sthash.fY1CVR4W.dpuf.
- 24.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA Emergency Medicine Shock Research Network (EMShockNet) Investigators. . Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA J Am Med Assoc. 2010;303(8):739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg AX, Adhikari NKJ, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 27.Niès J, Colombet I, Degoulet P, Durieux P. Determinants of success for computerized clinical decision support systems integrated in CPOE systems: a systematic review. AMIA Annu Symp Proc AMIA Symp AMIA Symp. 2006:594–598. [PMC free article] [PubMed] [Google Scholar]
- 28.Durieux P. Electronic medical alerts--so simple, so complex. N Engl J Med. 2005;352(10):1034–1036. doi: 10.1056/NEJMe058016. [DOI] [PubMed] [Google Scholar]
- 29.MacRedmond R, Hollohan K, Stenstrom R, Nebre R, Jaswal D, Dodek P. Introduction of a comprehensive management protocol for severe sepsis is associated with sustained improvements in timeliness of care and survival. Qual Saf Health Care. 2010;19(5):e46. doi: 10.1136/qshc.2009.033407. [DOI] [PubMed] [Google Scholar]
- 30.Sawyer AM, Deal EN, Labelle AJ, Witt C, Thiel SW, Heard K, Reichley RM, Micek ST, Kollef MH. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473. doi: 10.1097/CCM.0b013e318205df85. [DOI] [PubMed] [Google Scholar]
- 31.Gurnani PK, Patel GP, Crank CW, Vais D, Lateef O, Akimov S, Balk R, Simon D. Impact of the implementation of a sepsis protocol for the management of fluid-refractory septic shock: A single-center, before-and-after study. Clin Ther. 2010;32(7):1285–1293. doi: 10.1016/j.clinthera.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent J-L, Moreno R Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. . Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 33.ProCESS Investigators. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The ARISE Investigators and the ANZICS Clinical Trials Group. Goal-Directed Resuscitation for Patients with Early Septic Shock. N Engl J Med. 2014 doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 35.Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, Raymondos K, Rios F, Nin N, Apezteguía C, Violi DA, Thille AW, Brochard L, González M, Villagomez AJ, Hurtado J, Davies AR, Du B, Maggiore SM, Pelosi P, Soto L, Tomicic V, D’Empaire G, Matamis D, Abroug F, Moreno RP, Soares MA, Arabi Y, Sandi F, Jibaja M, Amin P, Koh Y, Kuiper MA, Bülow H-H, Zeggwagh AA, Anzueto A. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188(2):220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 36.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501–504. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods: Overview of the Sepsis Tool’s Development, Architecture, and Interface
Figure S1. Schematic of the Listening, Alerting, and Provider Assessment systems.
Table S1. Multivariable regression for use of tool to enter orders.
Overview of the electronic sepsis tool. A video tour of the interface and capabilities of the electronic sepsis assessment and management tool narrated by Liza Weavind, MBBCh MMHC on April 7th, 2014. Author: Liza Weavind, Videographer: Andras Nadas, Participants: Liza Weavind, Length: 3min 12 sec, Size: 26.2MBs