Summary
BRAF inhibitor treatment (BRAFi) enhances anti-tumor immunity, but is associated with increased intra-tumoral PD-L1 expression. MEK inhibitors (MEKi) may alter T-cell function, however recent studies demonstrate preserved T-cell infiltrate during treatment with BRAFi/MEKi. These data have important implications for combining BRAFi/MEKi and checkpoint blockade in the treatment of melanoma.
In this issue of Clinical Cancer Research, Kakavand and colleagues report that treatment of melanoma patients with combined BRAF inhibitors (BRAFi) and MEK inhibitors (MEKi) is associated with an increase in intra-tumoral lymphocytes (CD4+ and CD8+), which is no different than that observed when patients are treated with BRAFi monotherapy (1). The results were observed on analysis of longitudinal tumor samples from 40 patients treated with either BRAFi or combined BRAFi/MEKi, in an effort to better understand the intratumoral immune effects of these agents. In addition to their observations regarding preserved T cell infiltrate in BRAFi versus BRAFi/MEKi, the group also reported that patients with high PD-L1 expression was associated with increased TIL in pre-treatment biopsies. Furthermore, these patients had the largest increase in TIL and PD-1 on treatment compared to those with low PD-L1 expression in the pre-treatment sample. Of note, there was no significant difference in survival in these groups though the sample size was relatively small. Overall, these results are important and provide additional rationale for combining immune checkpoint blockade with BRAFi/MEKi to enhance responses to melanoma therapy, and also speak to the importance of timing and sequence of therapy.
Over the past several years, there have been significant advances in melanoma treatment through the use of targeted therapy and immune checkpoint blockade, however each of these strategies has limitations as monotherapy. Treatment with BRAF inhibitor monotherapy or combined BRAFi/MEKi results in a survival benefit in patients with melanoma, which led to the FDA approval of these regimens (2), however responses are not durable in the majority of patients. Treatment with immune checkpoint blockade (such as anti-CTLA-4 and anti-PD-1) also results in improved survival which led to their FDA approval (3, 4) though a significant proportion of patients do not benefit from therapy.
There is a strong clinical rationale for combining targeted therapy and immune checkpoint blockade in the treatment of melanoma, and a growing scientific rationale supports such combinations. The first scientific data supporting this notion was published in 2010, and demonstrated that blocking oncogenic BRAF activity through targeted MAP kinase pathway inhibition in vitro led to increased melanoma antigen expression and enhanced reactivity to antigen-specific T lymphocytes (5). Importantly, this was observed with BRAFi in melanomas harboring a BRAFV600E mutation, but was also observed in BRAF wild-type cell lines upon treatment with a MEKi. However treatment of T cells in vitro with a MEKi resulted in impaired T cell function, whereas treatment with a BRAFi had no effect on T cell function. Several groups then studied immune effects of BRAFi +/- MEKi in patients with melanoma on therapy, demonstrating enhanced T cell infiltrate (6) as well as a more favorable tumor microenvironment overall within 2 weeks of treatment initiation – with a decrease in immunosuppressive cytokines and VEGF (6, 7). However there was a concurrent increase in expression of PD-L1 early on-treatment, suggesting a possible immune mechanism of resistance (6). Interestingly, BRAFi may even stimulate T cell function through paradoxical signaling via the RAS-RAF pathway (8).
Early clinical studies combining immunotherapy with targeted therapy have largely used BRAF inhibitors as a backbone for combinations given the potential for MEKi to alter T cell function in vitro (5). However more recently, MEK inhibitors have been added to BRAF-targeted therapy in combination with immune-based strategies, and there is growing evidence that it may not “MEK” a difference (9). This has been studied in vitro, and groups have shown that treatment of BRAF wild-type cell lines with MEKi is associated with enhanced melanoma antigen expression (5, 9) and apoptosis in tumor cell lines with increased expression of HLA I and/or II (9) Importantly, investigators have reported a partial but transient inhibition of T cell proliferation and function upon MEK inhibition (9), which likely relates to T cell activation status at time of treatment. Furthermore, synergy is demonstrated synergy when combining the MEKi, trametinib, with immune checkpoint blockade (anti-PD-1, anti-PD-L1, and anti-CTLA4) in murine models. The findings in patients reported by Kakavand and colleagues are supportive of this notion, and suggest little to no deleterious effect of MEK inhibition in combination with BRAF-targeted therapy in patients with melanoma (1).
Together, these findings have important potential clinical implications in the care of patients with melanoma, and also with non-melanoma malignancies. In patients with melanoma harboring a BRAFV600E mutation, the addition of MEKi to a backbone of BRAF-targeted therapy does not appear to significantly alter T cell infiltrate (though function was not completely evaluated by Kakavand and colleagues (1)). In patients with BRAF wild-type melanoma, it may be possible to treat concurrently with a MEKi and immune checkpoint blockade, though this concept must be tested in the context of pre-clinical studies and clinical trials. Similarly, MEKi or other targeted agents may potentially be used in combination with immune checkpoint blockade in the treatment of non-melanoma malignancies (Fig. 1). This concept is not novel, as pre-clinical data suggests that treatment with a c-kit inhibitor in gastrointestinal stromal tumors (GIST) enhances T cell infiltrate in a murine model (10). In this model, treatment of mice with GIST using combined imatinib and anti-CTLA-4 demonstrated synergy with delayed tumor outgrowth and prolonged survival. This concept is now being tested in clinical trials.
Figure 1.
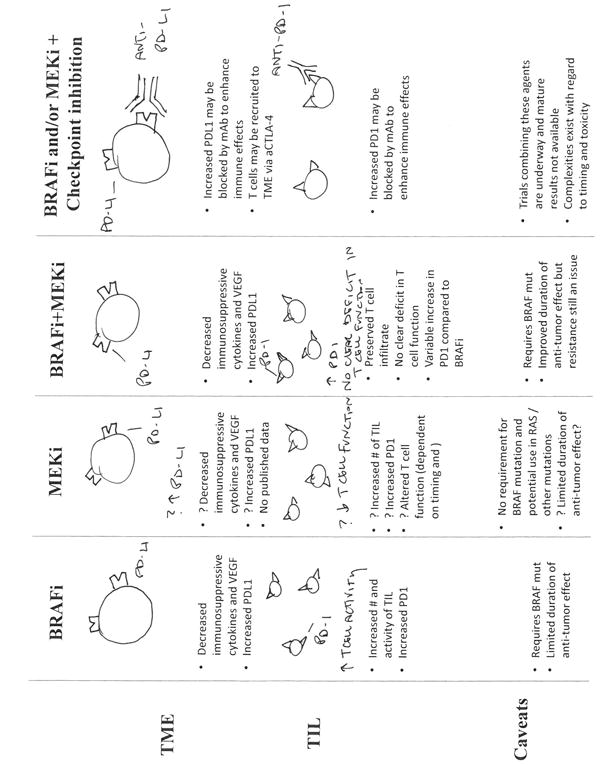
Immune effects of targeted therapy and the potential of adding immune checkpoint blockade. Treatment with a BRAF inhibitor results in favorable effects such as an increase in antigen expression and CD8+ T cell infiltrate and a decrease in immunosuppressive cytokines and VEGF. However concurrently, there is an increase in expression of immunomodulatory molecules (PD-1 and PD-L1). Importantly, this therapy requires a BRAFV600E mutation and the anti-tumor effect is limited. Treatment with MEKi monotherapy is not as well studied as there is no published data on immune effects of MEKi on the tumor microenvironment in melanoma patients, however, in vitro studies suggest a transient altered phenotype in T-cells after MEKi monotherapy. In MEKi monotherapy, there is no requirement for a BRAF mutation and MEKi can be used in non-BRAF (e.g., RAS) mutant tumors. Treatment with combined BRAFi + MEKi has the same favorable effects of BRAFi, with similar changes in PD-1 and PD-L1 expression. The addition of immune checkpoint blockade to a backbone of BRAFi and/or MEKi is hypothesized to enhance immune response and overall response to therapy via recruitment and activation of TIL (anti-CTLA-4) and through blocking of PD-1 or its ligand.
Despite the enthusiasm for combining these approaches, several caveats exist. First, appropriate timing and sequence is unknown, though recent studies would suggest that the immune response to targeted therapy is early and transient (11) and that these therapies should be given concurrently. This is supported by data in the article by Kakavand and colleagues, which suggests a “window of opportunity” for the addition of immune checkpoint blockade onto a backbone of combined BRAF/MEK inhibition (1). Second, unexpected toxicities have been observed in some of the clinical trials combining targeted therapy and immune checkpoint blockade (12), thus potential toxicity of these combinations must be taken into consideration and ideally patients should be treated with these strategies in the context of close monitoring on a clinical trial. Third, potential synergy (and related toxicity) of targeted therapy and immune checkpoint blockade in BRAF wild-type melanoma and non-melanoma malignancies is also unclear, thus such treatment strategies should also be used in the context of close monitoring on clinical trials. Finally, it is critical for us as clinicians and scientists to optimally understand responses to therapy through longitudinal blood and tumor sampling from patients on therapy (as suggested by Kakavand and colleagues), as well as through the use of translational studies in murine models. Ultimately, ideal combination strategies will be built on a deep understanding of the molecular and immune effects of each of these strategies in isolation, as well as in combination.
Acknowledgments
Grant Support: J.A. Wargo was supported by the NIH under award numbers K08CA160692 and U54CA163125, the Melanoma Research Alliance Team Science Award, and the generous philanthropic support of several families whose lives have been affected by melanoma.
Footnotes
Disclosure of Potential Conflicts of Interest: J.A. Wargo reports receiving speakers bureau honoraria from DAVA Oncology, and is a consultant/advisory board member for GlaxoSmithKline and Roche/Genentech. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Kakavand H, Wilmott JS, Menzies AM, Vilain R, Haydu LE, Yearley JH, et al. PD-L1 expression and tumor-infiltrating lymphocytes define different subsets of MAPK inhibitor treated melanoma patients. Clin Cancer Res. 2015 Jan 21; doi: 10.1158/1078-0432.CCR-14-2023. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 6.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–31. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan MK, Masters G, Pratilas CA, Ariyan C, Katz J, Kitano S, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol Res. 2014;2:70–9. doi: 10.1158/2326-6066.CIR-13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD1, PD-L1 and CTLA-4. Clin Cancer Res. 2015 Jan 14; doi: 10.1158/1078-0432.CCR-14-2339. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper ZA, Juneja VR, Sage PT, Frederick DT, Piris A, Mitra D, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res. 2014;2:643–54. doi: 10.1158/2326-6066.CIR-13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]


