Abstract
Neuroticism and extraversion are multifaceted affective-laden personality traits that have been associated with Major Depressive Disorder (MDD). Research and theory have argued that extraversion, and particularly its facet positive emotionality, is specific to MDD, while neuroticism is common across internalizing disorders. Converging evidence has suggested that MDD is associated with reduced engagement with emotional stimuli, but it remains unclear whether either extraversion, neuroticism, or both modulate reactivity to emotional cues. The late positive potential (LPP) is an event-related brain potential that is uniquely suited to assess engagement with emotional stimuli because it reflects sustained attention toward emotional content. The current study examined the LPP in relation to personality traits that may confer risk for depression, by examining the relationship between the LPP and both neuroticism and extraversion in never-depressed adolescent girls. Specifically, 550 girls aged 13.5-15.5 with no lifetime history of depression completed an emotional picture-viewing task and the LPP was measured in response to neutral, pleasant, and unpleasant pictures. Personality traits were gathered via self and informant report. Results indicated that high extraversion was associated with a potentiated LPP to emotional pictures—and this effect was accounted for by positive emotionality in particular. In contrast, there was no association between the LPP and neuroticism or its facets. The present study is one of the first to demonstrate that extraversion is associated with variation in neural indices of emotional picture processing, similar to what has been observed among individuals with depression and at high risk for depression.
Keywords: adolescence, personality, extraversion, neuroticism, positive emotionality, LPP
Neuroticism and extraversion, two personality traits related to affective reactivity, figure prominently in structural and dimensional models of psychopathology (Clark, 2005; Clark, Watson, & Mineka, 1994; Watson, Clark, & Harkness, 1994). Neuroticism reflects stable individual differences in the tendency to experience negative emotions; it has been conceptualized in terms of emotional instability and heightened reactivity to stress and aversive environmental stimuli (John, Naumann & Soto, 2008; Watson et al., 1994). On the other hand, extraversion is characterized by energetic engagement with the world; extraverts are social, active, and tend to experience high levels of positive emotions (John et al., 2008; Watson et al., 1994). Both general constructs consist of narrower facet traits. As of yet, hierarchical models differ on the number and nature of the facets within each trait; previous investigations have included anxiousness and melancholia as facets of neuroticism—and positive emotionality, sociability, ascendance, and venturesomeness as facets of extraversion (Simms, 2009; Naragon-Gainey, Watson, & Markon, 2009).
Aspects of greater neuroticism and lower extraversion are apparent in several forms of psychopathology. For example, major depressive disorder (MDD) is defined clinically as a dysfunction in mood that involves pronounced feelings of sadness, or loss of pleasure in activities (i.e., anhedonia), or both (American Psychiatric Association, 2013). In this way, MDD involves the combination of high neuroticism and low extraversion (especially, positive emotionality). Notably, levels of neuroticism and extraversion have demonstrated rank-order stability, even after remittance of depressive symptoms, indicating that they are trait-like risk factors that continue to be elevated and decreased, respectively, in remission (De Fruyt, Van Leeuwen, Bagby, Rolland, & Rouillon, 2006; Ormel, Oldehinkel, & Vollebergh, 2004). This personality-based view of MDD is consistent with a substantial body of converging evidence (Klein, Kotov, & Bufferd, 2011). Indeed, multiple models of depression have hypothesized that deficits in extraversion are unique to MDD, while high neuroticism is common across both depression and anxiety disorders (Shankman & Klein, 2003).
Emotional stimuli prompt a host of changes in central and peripheral nervous system activity, and these physiological measures can be used as dependent measures of emotional processing in relationship to psychopathology (Tracy, Klonsky, & Proudfit, 2014). From the perspective of personality traits, it stands to reason that depressed individuals might be both highly reactive to unpleasant stimuli (reflecting high neuroticism) and hypo-reactive to pleasant stimuli (reflecting low extraversion). However, across a large array of data and multiple physiological measures, depressed individuals show attenuated reactivity to both pleasant and unpleasant stimuli (Rottenberg & Gotlib, 2004; Rottenberg, Gross, & Gotlib, 2005). Rottenberg and colleagues proposed the Emotion Context-Insensitivity (ECI) model of depression, which suggests that emotional dysfunction in MDD may be understood in terms of a lack of engagement with emotional stimuli in the environment—a view consistent with low extraversion as a defining feature. Indeed, positive emotionality reflects not only affect but also approach motivation (Watson, Wiese, Vaidya, & Tellegen, 1999).
Electrocortical measures of emotional processing derived from the event-related brain potential (ERP) are uniquely suited to study engagement with emotional stimuli—and may be ideal for studying individual differences related to psychopathology and personality (Weinberg, Ferri, & Hajcak, 2013). Specifically, the late positive potential (LPP) is a positive deflection that is maximal at posterior midline recording sites and begins approximately 200 ms after visual stimuli are presented; this positivity is enhanced for pleasant and unpleasant compared to neutral stimuli, and this emotion-related increase in the LPP is sustained for the duration of a stimulus presentation (Hajcak & Olvet, 2008). Functionally, the LPP appears to index sustained attention toward, and engagement with, emotional content (Hajcak, MacNamara, & Olvet, 2010). Often, studies utilize emotional pictures to elicit the LPP for their relative ease of presentation and standardization (Hajcak et al., 2010). Importantly, the LPP is sensitive to changes in stimulus meaning; for instance, the LPP is larger when pictures are preceded by more emotionally arousing descriptions (Foti & Hajcak, 2008; MacNamara, Foti, & Hajcak, 2009), and when participants focus on more arousing aspects within unpleasant pictures (Dunning & Hajcak, 2009; Hajcak, Dunning, & Foti, 2009; Hajcak, MacNamara, Foti, Ferri, & Keil, 2013).
Only a small group of studies have utilized the LPP to examine emotional processing in MDD, but findings have suggested that the LPP is reduced in MDD (Foti et al. 2010; Kayser, Bruder, Tenke, Stewart, & Quitkin, 2000; Weinberg, Kotov & Proudfit, under review). A blunted LPP in MDD is consistent with the ECI model, which posits that aberrant emotional processing in MDD is the result of global disengagement from the environment (Rottenberg et al., 2005)—a conceptualization that overlaps considerably with low extraversion. Thus, the reduced LPP in MDD may reflect broad personality-related differences in extraversion. Given that neuroticism is increased in MDD, it is also possible that reduced engagement with emotional stimuli reflected in the LPP relates to increased neuroticism—although few studies have examined the relationship between personality dimensions and emotional processing, and even fewer have done so using ERPs.
Neuroimaging studies that utilize functional magnetic resonance imaging (fMRI) have shown an association between high extraversion and increased activation in response to pleasant stimuli, relative to unpleasant (Canli et al., 2001; Canli, 2004). Studies by Yuan and colleagues (2009, 2012) found that extraversion was related to an increased LPP to a range of pleasant compared to neutral stimuli. An increased LPP to pleasant words, compared to both unpleasant and neutral words, has also been found in extraverts (Bartussek, Becker, Diedrich, Naumann, & Maier, 1996). On the other hand, De Pascalis and Speranza (2000) found that extraversion was associated with an increased LPP to pleasant, unpleasant, and neutral emotional words during a dot-probe task, suggesting that the increased LPP to emotional words in relation to extraversion may be valence-independent.
Neuroimaging studies have suggested that high neuroticism is associated with a bias towards negative relative to positive stimuli, and decreased responsiveness to positive stimuli more generally (Canli et al., 2001; Kehoe, Toomey, Balsters, & Bokde, 2012). On the other hand, Bartussek and colleagues (1996) found that high levels of neuroticism were associated with a “flat” LPP across all stimuli, suggesting that individuals with high neuroticism did not differentially process emotional compared to neutral stimuli. These data suggest that high neuroticism may relate to decreased engagement with emotional content – hence neuroticism could account for ECI-like effects among individuals with MDD. However, few studies have examined the LPP in relation to neuroticism—and it is unclear if neuroticism is associated with blunted responsiveness to emotional stimuli, or enhanced responsiveness to unpleasant stimuli. Moreover, no studies have simultaneously assessed the LPP in relation to extraversion and neuroticism. Given that these personality characteristics seem to play a role in the development of MDD, possibly through aberrant emotional processing, it is necessary to investigate the relationship between personality and emotional processing before MDD onset; the presence of MDD may produce changes in personality and emotional processing that make it impossible to elucidate any pre-existing individual differences that contributed to onset (Klein, et al., 2011).
Our broader goal in the current study is to examine neural correlates of emotional processing in relation to personality traits that may confer risk for depression by examining the relationship between the LPP and both neuroticism and extraversion in a large never-depressed sample of adolescent girls. By studying never-depressed girls, we are able to examine the association between the LPP and personality without the obscuring effect of MDD, which could alter personality (Klein et al., 2011). In addition, adolescence is a period characterized by increased emotionality, heightened responsiveness to emotional information, and increased risk for depression - particularly for females (Nelson, Leibenluft, McClure, & Pine, 2005; Hankin, Abramson, Moffitt, Silva, McGee, & Angell, 1998). Therefore, this developmental period may be particularly relevant for examining the relationship between emotional processing and personality traits linked to depression and risk.
In addition to examining extraversion and neuroticism broadly, the current study probed facets of both extraversion and neuroticism. Part of the difficulty in obtaining consistent findings across studies of extraversion and neuroticism may be the heterogeneity of these broad, higher-order constructs (Klein et al., 2011). Kotov and colleagues (2010) have argued for the importance of considering specific lower-level traits that comprise these broad constructs; specific facets may reveal stronger effects than more general traits. For example, Naragon-Gainey and colleagues (2009) examined four facets of extraversion: sociability, positive emotionality, ascendance and fun-seeking; they found that only low positive emotionality was significantly and strongly related to depression. Furthermore, low positive emotionality was found to be uniquely related to risk for depression in a sample of pre-school aged children (Durbin, Klein, Hayden, Buckley, & Moerk, 2005). Thus, previous research suggests that the positive emotionality factor of extraversion may be a specific personality facet to explore in relation to emotional-processing abnormalities.
The current study is part of a large longitudinal study of personality traits as predictors of subsequent first-onset depressive episodes in adolescent females. We examined the relationship between emotional information processing indexed by the LPP and individual differences in extraversion and neuroticism. Based on previous work, we hypothesized that both high neuroticism and low extraversion will be associated with an attenuated LPP to emotional stimuli. In addition, any significant associations between these broad personality traits and the LPP will be further probed on the level of facets to investigate the extent to which more specific traits relate to the LPP. We hypothesize that positive emotionality, in particular, may relate to a larger LPP to emotional stimuli.
Method
Participants
The sample included 550 adolescent females aged 13.5-15.5 (M = 14.39, SD = 0.63) and a biological parent (93.1% mothers) who participated as part of the Adolescent Development of Emotions and Personality Traits (ADEPT) project. ADEPT is a longitudinal study of adolescent development and psychological well-being, and focuses on adolescent females because they are the demographic group at highest risk for developing depression (Hankin et al., 1998). The ethnic distribution was 80.5% Caucasian, 5.1 % African-American, 8.4% Latino, 2.5% Asian, 0.4% Native American, and 3.1% ‘Other’.
The present study utilized data from the initial laboratory visit. Participants were recruited from the community using local referral sources (e.g. school districts), online classified advertisements, postings in the community, and a commercial mailing list targeting homes with a female child aged 13 to 15 years old. Families received financial compensation for their participation. Inclusion criteria were English fluency, ability to read and comprehend questionnaires, and a biological parent consenting to participate in the study. Exclusion criteria were a lifetime history of a major depressive episode (MDE) or dysthymia, or an intellectual disability. Adolescent lifetime history of depression was evaluated using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children, Present and Lifetime Version (K-SADS-PL, Kaufman et al., 1997). The K-SADS-PL was conducted by trained interviewers supervised by clinical psychologists (R.K. and D.K.), who confirmed that none of the participants had a lifetime history of a MDE or dysthymia.
Adolescent Personality
Big Five Inventory (BFI; John et al.,, 2008; John, Donahue & Kentle, 1991)
The BFI is a 44-item factor-analytically derived measure of extraversion, neuroticism, agreeableness, conscientiousness and openness. Each item consists of short descriptive phrases that are rated on a five-point Likert scale ranging from 1 (disagree strongly) to 5 (agree strongly). The BFI has demonstrated good internal consistency, test-retest reliability, and convergent and discriminant validity (John et al., 2008; Rammstedt & John, 2007). The present study focused on the extraversion (6-items) and neuroticism (8-items) scales. Both the participant and their biological parent completed the BFI regarding the child's personality (see Table 1).
Table 1.
Descriptive statistics and correlations between child-report and informant-report measures of extraversion and related facets
| Conditions | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Child Report | |||||||||||
| 1. | BFI Ext | ||||||||||
| 2. | FI-FFM PE | .62 | |||||||||
| 3. | FI-FFM Asc | .61 | .43 | ||||||||
| 4. | FI-FFM Soc | .58 | .54 | .39 | |||||||
| 5. | FI-FFM Ven | .55 | .55 | .49 | .48 | ||||||
| Parent Report | |||||||||||
| 6. | BFI Ext | .61 | .38 | .43 | .34 | .35 | |||||
| 7. | FI-FFM PE | .47 | .44 | .34 | .38 | .36 | .62 | ||||
| 8. | FI-FFM Asc | .55 | .34 | .55 | .29 | .34 | .66 | .61 | |||
| 9. | FI-FFM Soc | .49 | .34 | .30 | .48 | .41 | .66 | .60 | .46 | ||
| 10. | FI-FFM Ven | .42 | .28 | .28 | .31 | .44 | .54 | .60 | .54 | .63 | |
| M | 3.76 | 4.04 | 3.41 | 3.75 | 4.05 | 3.59 | 3.96 | 3.40 | 3.77 | 3.88 | |
| SD | .78 | .66 | .91 | .80 | .77 | .88 | .70 | .94 | .80 | .77 | |
| Cronbach's α | .80 | .84 | .85 | .79 | .81 | .84 | .83 | .80 | .80 | .80 | |
Note: Controlling for age; all correlations significant at 0.001 level; Bold-faced type indicates r values ≥ .50; BFI Ext: Big Five Inventory Extraversion; FI-FFM: Faceted Inventory of the Five-Factor Model; PE: positive emotionality; Asc: ascendance; Soc: sociability; Ven: venturesomeness
Faceted Inventory of the Five-Factor Model (FI-FFM; Simms, 2009)
The FI-FFM was factor-analytically derived and developed specifically to assess facets of the five-factor model. Similar to the BFI, the items consist of short descriptive phrases and are rated on a five-point Likert scale. The FI-FFM facets have demonstrated good internal consistency and discriminant and convergent validity with other measures of personality that follow the five-factor model (Simms, 2009). This study included the four facets of extraversion: positive emotionality, ascendance, sociability, and venturesomeness—as well as two facets of neuroticism: anxiousness and melancholy. The FI-FFM was completed by both the participant and their biological parent regarding the child's personality (see Tables 1 & 2).
Table 2.
Descriptive statistics and correlations between child-report and informant-report measures of neuroticism and related facets
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| Child Report | |||||||
| 1. | BFI Neu | ||||||
| 2. | FI-FFM Anx | .85 | |||||
| 3. | FI-FFM Mel | .68 | .65 | ||||
| Parent Report | |||||||
| 4. | BFI Neu | .51 | .43 | .36 | |||
| 5. | FI-FFM Anx | .48 | .46 | .27 | .84 | ||
| 6. | FI-FFM Mel | .41 | .34 | .39 | .73 | .68 | |
| M | 2.75 | 2.19 | 2.95 | 2.7 | 1.88 | 2.75 | |
| SD | .81 | .86 | .88 | .90 | .85 | .90 | |
| Cronbach's α | .83 | .87 | .86 | .84 | .85 | .82 | |
Note: Controlling for age; all correlations significant at p < 0.001 level; Bold-faced type indicates r values ≥ .50; BFI Neu: Big Five Inventory Neuroticism; FI-FFM: Faceted Inventory of the Five-Factor Model; Anx: anxiousness; Mel: melancholy
Procedure
A modified version of the emotional interrupt task was used to elicit the LPP (Mitchell, Richell, Leonard, & Blair, 2006; Weinberg & Hajcak, 2011; Kujawa, Klein, & Hajcak, 2012). Each trial began with a fixation point presented for 800 ms, followed by a neutral, pleasant or unpleasant picture for 1000 ms, followed by either a left or right arrow (i.e., the target) for 150 ms, followed by the same picture that had preceded the target for 400 ms. The intertrial interval (ITI) was a blank screen that ranged in duration from 1500-2000 ms. Pictures were taken from the International Affective Picture System (IAPS; Lang, Bradley & Cuthbert, 2008); a total of 120 trials were presented (40 neutral, 40 pleasant, and 40 unpleasant) in a random order. Pictures were selected to be age-appropriate and included 20 neutral pictures displaying objects or scenes with people, 20 pleasant pictures displaying affiliative scenes or cute animals, and 20 unpleasant pictures displaying sad or threatening scenes1. All pictures were presented twice during the task. Participants were instructed to respond as quickly as possible to the target by clicking the corresponding left or right mouse button. The emotional interrupt task was chosen over a passive picture-viewing paradigm to (1) ensure that participants were paying attention as indicated by a correct response to the target and (2) provide a behavioral measure of the influence of affective pictures on reaction time (RT). Specifically, longer RT following the presentation of pleasant and unpleasant relative to neutral pictures suggests interference of task-irrelevant emotional stimuli on behavioral performance (Mitchell et al., 2006; Weinberg & Hajcak, 2011; Kujawa et al., 2012). Similar to previous studies, trials with RT less than 150 ms or greater than 1500 ms were excluded from the analysis (Mitchell et al., 2006; Weinberg & Hajcak, 2011).
Physiological Recording and Data Processing
Continuous EEG was recorded while participants completed the emotional interrupt task on a 21-in. computer monitor placed at eye level, at a distance of approximately 39 in. ERP activity was recorded from 34 electrodes positioned according to the 10/20 system, including FCz and Iz, using the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands). Electrodes were placed above and below the left eye to monitor vertical electrooculographic (VEOG) activity, adjacent to the outer canthi of the left and right eyes to monitor horizontal electrooculographic (HEOG) activity, and from the left and right mastoids. The EEG signal was pre-amplified at the electrode to improve signal-to-noise ratio. Data were digitized at a 24-bit resolution with a sampling rate of 512 Hz using a low pass fifth order sinc filter with a half-power cut-off of 102.4 Hz. Active electrodes were measured online with reference to a common mode sense active electrode constructing a monopolar channel. The raw EEG data were re-referenced offline to the average of the left and right mastoids and band-pass filtered from 0.1 to 30 Hz. Eye blink and ocular-movement corrections were performed using established standards described by Gratton, Coles and Donchin (1983).
A semi-automated procedure was used to identify and reject artifacts. Data for individual channels were marked for rejection if a voltage step of more than 50.0 µV between sample points was present, if a deflection of more than 300.0 µV occurred within a trial, or if a voltage difference of less than 50.0 µV was detected within 100 consecutive ms. A visual inspection of the remaining trials was then conducted to detect and reject any other artifacts, participants were included if that had at least 20 artifact-free trials in each condition.
Only ERP data associated with correct responses were included in averages to confirm that participants were paying attention to analyzed trials; the average number of non-response trials was M = .65, SD = 2.05, the average number of incorrect trials across conditions was M = 7.44, SD = 8.90, and the number of incorrect trials per condition did not differ, F(2, 1044) = .28, p = .76. The average number of neutral (M = 37.25, SD = 3.85), pleasant (M = 37.20, SD = 3.48), and unpleasant (M = 37.21, SD = 3.52) trials included in ERP averages were proportionate. The EEG was segmented for each trial beginning 200 ms before the pre-target picture and continuing for 1200ms. The LPP was scored as the average activity between 300-1000 ms after picture onset—separately for for pleasant, neutral and unpleasant trials—at both occipital (i.e., O1, Oz, O2) and parietal (i.e., P3, Pz, P4) sites. Each LPP average was baseline-corrected relative to the activity in the 200 ms before picture onset. Twenty-seven participants were excluded from analyses as the result of equipment malfunction (n = 5), excessive EEG artifacts (n = 13), >50% incorrect responses to the target stimuli (n = 2), or incomplete questionnaire data (n = 7), resulting in a final sample of 523 participants.
Data Analysis
Analyses were conducted using IBM SPSS Statistics, Version 22.0 (Armonk, NY, USA). Previous research has indicated developmental changes in the scalp distribution of the LPP. Specifically, the LPP is more distinct over occipital regions in children, becoming more apparent over centro-parietal regions in adults (Gao, Liu, Ding, & Guo, 2010; Kujawa, Klein, & Hajcak, 2012). Therefore adolescence appears to be a time when ‘frontalization’ of the LPP occurs. This change could reflect a shift in activation from basic visual regions to more fronto-parietal attentional networks; however, the precise timing and nature of this translocation is not well understood. In the present study age was significantly correlated with the occipital LPP to neutral, r(523) = -.13, p < .01, pleasant r(523) = -.22, p <.001, and unpleasant trials, r(523) = -.16, p <.001, such that older participants had a decreased LPP at occipital sites. Age was not significantly correlated with the parietal LPP (ps > .26). In order to account for the potential influence of development on the location of the LPP, age was included as covariate in all analyses.2
To examine effects of picture valence on RT and the LPP, we conducted a mixed-measure analysis of covariance (ANCOVA) with valence (neutral, pleasant, unpleasant) as the within-subjects factor and mean-centered age as a continuous covariate. For the LPP analysis, location (occipital vs. parietal) was also included as a within-subjects factor.
To examine the effects of personality on emotional processing, we calculated residual scores to isolate variance specific to emotional processing, in which the LPP and RT for neutral pictures was used to predict the LPP and RT for the affective picture averages, respectively. Emotional reactivity can be examined in multiple ways (Nelson, Shankman, Olino, & Klein, 2011). Calculating subtraction-based change scores is one method, whereby a difference score is calculated for each individual j by subtracting the raw score for the condition of interest from the comparison condition; in this case the neutral condition, Dj = X2j – X1j (Rogosa, Brandt, & Zimowski, 1982). However, the use of simple change scores has been criticized because of its dependence on the neutral condition scores; individual differences in the neutral condition may lead to misleading findings, particularly if the purpose of the change score is to eliminate the influence of the neutral variable, and only examine change as a result of the variable of interest (Cohen, Cohen, Aiken & West, 2003; Nelson et al., 2011). An alternative method of calculating change scores that are not confounded by individual differences in the neutral condition is to compute residuals, which involves regressing the variable of interest on the neutral variable and saving the residual scores from the model. In the current context, residuals would reflect the unique variance in the pleasant and unpleasant LPP that is not accounted for by the neutral LPP (McFarland & Klein, 2009; Weinberg, Venables, Proudfit, & Patrick, 2014). Arguably, for the behavioral sciences utilizing residual scores over simple change scores or raw scores provides a more reliable estimate of change (Cohen et al., 2003; Cronbach & Furby, 1970; Dubois, 1957). In addition, a recent study found that residualized change scores of the LPP to emotional pictures were more heritable and had better psychometric properties than simple subtraction-based change scores (Weinberg et al., 2014). In the current study, we conducted a valence (pleasant residual, unpleasant residual) X personality mixed-measures ANCOVA with valence as the within-subjects factor, and adolescent age and personality included as mean-centered continuous covariates; for the LPP analysis location (occipital vs. parietal) was included as within-subjects factor. For both RT and the LPP, each personality trait (i.e., extraversion/neuroticism) was analyzed separately3.
Results
Personality Measures
As shown in Tables 1 and 2, self and informant-reports of extraversion and neuroticism and their related facets showed substantial consistency (r = .39 to .61). Therefore, to reduce source variance and simplify analyses (Kandler, Riemann, Spinath, & Angleitner, 2010), we standardized and then averaged together self and informant-report versions of the BFI and FIFFM scales. Subsequent analyses used the composite scores4.
Behavioral Data
We found that RT varied by valence, F(2, 1042) = 10.16, p < .001, ηp2 = .02 such that RT was slower following unpleasant (M = 503.78 ms, SD = 147.03) relative to neutral pictures (M = 495.87 ms, SD = 145.38), t(522) = 4.43, p < .001, but was not different from pleasant pictures (M = 500.52 ms, SD = 143.53), t(522) = 1.83, p > .05, and RT was slower following pleasant compared to neutral pictures, t(522) = 2.70, p < .01. Furthermore, RT was not associated with extraversion or neuroticism (ps > .09 in each analysis).
ERP Data
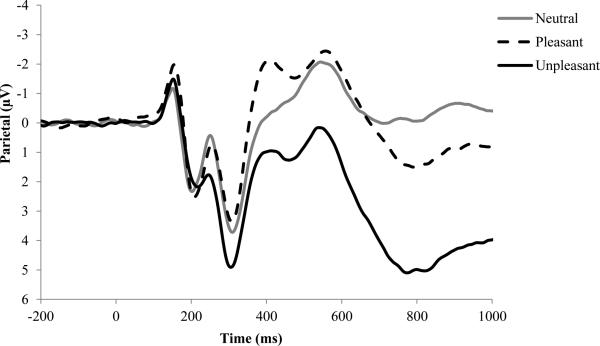
Figure 1 presents the LPP waveforms for neutral, pleasant, and unpleasant pictures at parietal sites. As expected, the LPP was modulated by picture valence, F(2, 1042) = 110.81, p < .001, ηp2 = .18, such that the LPP was larger for unpleasant (M = 7.65 µV, SD = 6.41) compared to neutral (M = 4.98 µV, SD = 5.52), t(522) = 13.08, p < .001, and pleasant pictures (M = 5.42 µV, SD = 6.21), t(522) = 12.27, p < .001, and larger for pleasant compared to neutral pictures, t(522) = 2.31, p < .05. Pleasant and unpleasant pictures relative to neutral pictures evoked a broadly distributed positivity that appeared more evident in centro-parietal regions for unpleasant pictures compared to pleasant pictures.
Figure 1.
LPP waveforms for neutral, pleasant, and unpleasant stimuli. The LPP waveforms were pooled across parietal (Pz, P3, and P4) electrodes.
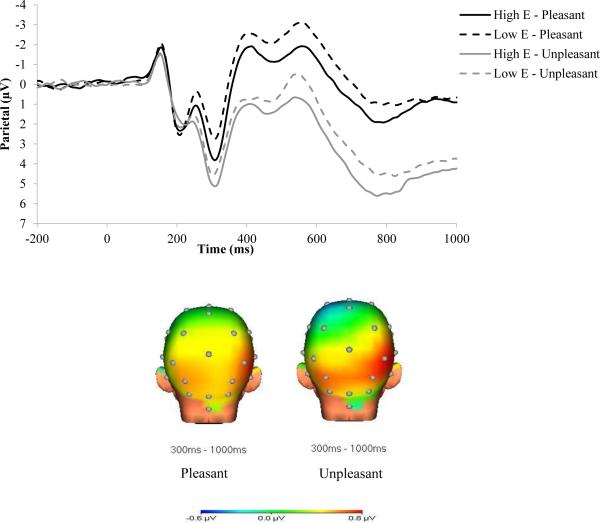
As depicted in Figure 2, extraversion was positively associated with LPP to emotional stimuli, F(1, 520) = 6.03, p < .05, ηp2 = .01. There was also an interaction with location, F(1, 520) = 3.84, p = .05, ηp2 = .01, due to an association between emotional modulation of the LPP and extraversion over parietal sites, F(1, 521) = 5.78, p < .05, that was not present over occipital sites, F(1,521) = 1.73, ns5. In contrast, there were no main effects or interactions for neuroticism, (ps > .20).
Figure 2.
LPP waveforms and head maps for pleasant and unpleasant stimuli at high and low levels of extraversion (E). The LPP waveforms were pooled across parietal (Pz, P3, and P4) electrodes. The left head map displays the difference between high and low extraversion for pleasant stimuli, and the right head map displays the difference between high and low extraversion for unpleasant stimuli.
To better characterize the association between extraversion and the LPP to emotional pictures, we examined facets of extraversion in relation to the LPP. We examined the association, controlling for age, between each FI-FFM extraversion facet and the LPP residual scores at parietal electrodes to pleasant and unpleasant pictures; Benjamini and Hochberg (1995) correction for multiple comparisons was used to adjust p-values. As shown in Table 3, greater positive emotionality was associated with a larger LPP to pleasant and unpleasant pictures, and was the only facet significantly related to the LPP. The three other facets of extraversion, sociability, venturesomeness and ascendance (social dominance), were not associated with the LPP.
Table 3.
Correlation coefficients for the association between personality traits and the late positive potential residual score for emotional pictures
| Picture Valence | ||
|---|---|---|
| Pleasant | Unpleasant | |
| BFI-Neu | −.09 | −.02 |
| FI-FFM Anx | −.05 | −.00 |
| FI-FFM Mel | −.09 | −.05 |
| BFI-Ext | .10* | .12* |
| FI-FFM Ast | .06 | .06 |
| FI-FFM PE | .14* | .11* |
| FI-FFM Soc | .09 | .07 |
| FI-FFM Ven | .07 | −.02 |
Note. Controlling for age. Correlations were corrected for each personality domain using the Benjamini & Hochberg (1995) method for multiple comparisons. The LPP at pooled parietal electrode sites (Pz, P3, P4) was used for these analyses. BFI: Big Five Inventory; Neu: neuroticism; Ext: extraversion; FI-FFM: Faceted Inventory of the Five-Factor Model; PE: positive emotionality; Asc: ascendance; Soc: sociability; Ven: venturesomeness.
Correlation significant at the .05 level (2-tailed).
Discussion
The current study examined the relationship between the broad personality traits of extraversion and neuroticism and the LPP, a neural correlate of emotional information processing and putative index of engagement with emotional stimuli, in a large sample of never-depressed adolescent girls. RT analysis revealed the expected pattern of results in which emotional compared to neutral pictures prolonged reaction times to a subsequently presented target; there were no associations between RT measures and personality. Our hypothesis that extraversion would modulate the LPP was supported: higher extraversion was associated with an increased parietal LPP to both pleasant and unpleasant emotionally evocative pictures. In addition, the positive emotionality facet of extraversion appeared to uniquely relate to the observed potentiation of the LPP to emotional pictures. On the other hand, there were no significant associations between the LPP and neuroticism. This study was the first to demonstrate a specific association between individual differences in extraversion, especially the facet of positive emotionality, and variation in emotional information processing as assessed by the LPP.
The association between extraversion and the LPP was valence-independent, indicating that extraversion—and positive emotionality more specifically—relates to increased neural response to both unpleasant and pleasant stimuli. The specificity of these findings to emotional modulation of the LPP is highlighted by the fact that we utilized residualized change scores, which controls for differences in response to neutral pictures. Conceptually, these findings are consistent with descriptions of extraversion that focus on increased engagement with one's environment (Watson et al., 1999). Insofar as low levels of extraversion characterize depression (Kotov et al., 2010), the current findings suggest that low extraversion specifically may explain the reduced LPP observed in depressed individuals (Foti et al., 2010; Kayser et al., 2000; Weinberg et al., under review).
The current study found that positive emotionality was the only facet of extraversion that significantly related to the LPP. Interestingly, the low positive emotionality facet of extraversion has been shown to demonstrate the strongest relationship to depression (Naragon-Gainey et al., 2009), and MDD may be associated with a blunted LPP due to low positive emotionality specifically. The current study also complements and extends the ECI model of depression by suggesting that low extraversion, and positive emotionality specifically, may contribute to the lack of approach motivation and global disengagement with one's environment in depression (Rottenberg et al., 2005).
Our results also have important implications for understanding the development of depression because low extraversion has been linked to depression onset (Kendler et al., 2006). It may be that low extraversion and a blunted LPP are preexisting risk factors for depression, and could provide important insights into the pathophysiology of depression or identify at-risk youth for intervention prior to the onset of MDD. This notion is also consistent with a recent study which reported a reduced LPP among children of depressed mothers as early as age 6 (i.e., 6 year-olds at risk for depression; Kujawa, Hajcak, Torpey, Kim, & Klein, 2012). Of course, both Kujawa et al. (2012) and the current study used a cross-sectional design—and longitudinal designs are needed to clarify the relationship between the LPP, extraversion, and the subsequent development of depression across adolescence.
Conversely, the current study does not support a relationship between neuroticism and emotional processing as reflected in the LPP. Previous neuroscience research on neuroticism has been mixed. Neuroimaging studies have suggested that neuroticism is related to increased processing of unpleasant stimuli and decreased processing of pleasant stimuli (Canli et al., 2001; Kehoe et al., 2012). On the other hand, previous ERP research has found a negative association between LPP magnitude to emotional information and neuroticism (Bartussek et al., 1996). The current study suggests that neuroticism and emotional modulation of the LPP are not associated. However, the relationship between neuroticism and emotional processing may be modulated by context, which could account for the discrepancies in previous studies. For instance, stimuli that are self-relevant may have a differential impact on emotional processing compared to normative stimuli.
There are several limitations to the current study that should be noted. First, the sample was limited to 13.5-15.5 year-old females, and findings may not generalize to boys or to a different age group. Second, this study utilized pictures of emotionally evocative scenes to elicit emotional responses; it is unclear if the same pattern of results would be found if more idiographic, self-relevant stimuli had been used. It should be noted that previous investigations of the LPP in relation to depression and depression risk used pictures of emotional faces, which have been shown to be less arousing than emotional scenes (Kujawa, Klein, & Hajcak, 2012; Britton, Taylor, Sudheimer, & Liberson, 2005). The current study demonstrated that individual differences in the LPP can be observed with more arousing and more complex stimuli. It should be noted that the current study did not examine differences between picture types within each valence category (e.g. social vs. animal pictures), and it is possible that personality differentially modulates the LPP to specific picture types. The current study included two facets of neuroticism, anxiousness and melancholia, however, anger/hostility is a third facet that has been included by previous research and may have a relationship to depression risk (Bagby, Kennedy, Dickens, Minifie, & Schuller, 1997). Lastly, the significant effects reported in this study were relatively modest and may not be observable in studies with smaller sample sizes. However, the observed correlations do not share method variance, and can be expected to be smaller and more modest compared to correlations across measures from the same domain (Patrick, Venables, Yancey, Hicks, Nelson, & Kramer, 2013).
In conclusion, the present study found that extraversion was associated with increased neural responses to emotional stimuli in adolescents. The LPP to both pleasant and unpleasant pictures was positively correlated with extraversion, and these relationships were most likely driven by the more specific facet of positive emotionality. Interestingly, there was no relationship between the LPP and neuroticism. Future research should assess whether picture content impacts the relationship between the LPP and personality. In addition, future research should examine if the association between extraversion and the LPP extends to other populations (e.g. boys), and whether individual differences in the LPP predict increases in depressive symptoms and the onset of depression prospectively.
Acknowledgments
This study was supported by National Institute of Mental Health grant R01 MH093479 awarded to Roman Kotov.
Footnotes
IAPS pictures included neutral (2514, 2580, 5390, 5395, 5500, 5731, 5740, 5900, 7000, 7002, 7009, 7010, 7026, 7038, 7039, 7090, 7100, 7130, 7190, and 7175), pleasant (1463, 1710, 1750, 1811, 2070, 2091, 2092, 2224, 2340, 2345, 2347, 7325, 7330, 7400, 8031, 8200, 8370, 8461, 8496, and 8497), and unpleasant images (1050, 1052, 6571, 1205, 1200, 1300, 1304, 1930, 2458, 2691, 2703, 2800, 2811, 2900, 3022, 6190, 6213, 6231, 6510, and 9600). Normative ratings indicated that unpleasant pictures (valence: M = 2.67, SD = 0.81) were less pleasant than the neutral pictures (valence: M = 5.33, SD = 0.43), which were less pleasant than pleasant pictures (valence: M = 7.84, SD = 0.53). Unpleasant (arousal: M = 6.36, SD = 0.55) and pleasant (arousal: M = 5.22, SD = 0.82) pictures were more emotionally arousing compared to neutral pictures (arousal: M = 3.03, SD = 0.63).
In addition to age, pubertal stage was assessed in a subsample of participants with two self-report measures, the Pubertal Development Scale and a picture rating scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988). When a composite score of pubertal development was included as a covariate in all analyses the pattern of results did not change; all reported findings remained significant. In addition, there were no significant interactions found between puberty and personality. Therefore, in order to retain the larger sample size, we reported results using age as a covariate.
We also conducted exploratory analyses testing whether the interaction between extraversion and neuroticism was associated with the LPP to emotional pictures. To this end, two simultaneous regression analyses were conducted with the residual pleasant and unpleasant LPP values included as the dependent variable, and mean-centered age, extraversion, neuroticism, and the extraversion X neuroticism interaction term included as independent variables. Results indicated the interaction between extraversion and neuroticism was not associated with the LPP to emotional pictures (p > .20).
To examine the individual contributions of self and informant reported extraversion on the LPP, we conducted separate valence (pleasant residual vs. unpleasant residual) X location (occipital vs. parietal) X personality mixed-measures ANCOVA with valence and location as the within-subjects factors, and adolescent age and personality included as mean-centered continuous covariates. Consistent with the composite scores, there was a significant main effect of self-reported extraversion, F(1, 520) = 7.04, p < .01, ηp2 = .01, which was qualified by an interaction with location, F(1, 520) = 4.08, p < .05, ηp2 = .01, due to an association between emotional modulation of the LPP and self-reported extraversion over parietal sites, F(1, 520) = 9.46, p < .01, that was less pronounced over occipital sites, F(1, 520) = 3.83, p = .05. Alternatively, there was a trending main effect of informant reported extraversion, F(1, 520) = 3.05, p = .08, ηp2 = .01. Thus, both sources show a similar association, but self-reported extraversion appears to be the stronger contributor to variation in the emotion-modulated LPP.
To better characterize the relationship between the parietal LPP to emotional pictures and extraversion, a follow-up analysis was conducted looking at early and late components of the emotion-modulated LPP, as a previous study by Kujawa and colleagues (2012) found differential associations between early and late components of the LPP to depression risk in young children. A mixed-measures ANCOVA was conducted with time (300-600 ms vs. 600-1000 ms) and valence (pleasant residual vs. unpleasant residual) included as within-subjects factors, and mean centered age and extraversion entered as continuous covariates. There was a significant time x valence x extraversion interaction, F(1, 520) = 4.31, p < .05, ηp2 = .01. However, follow-up analyses did not indicate a significant relationship between extraversion and the LPP for the early vs. late components.
References
- American Psychiatric Association . DSM 5. American Psychiatric Association; 2013. [Google Scholar]
- Bagby RM, Kennedy SH, Dickens SE, Minifie CE, Schuller DR. Personality and symptom profiles of the angry hostile depressed patient. Journal of Affective Disorders. 1997;45(3):155–160. doi: 10.1016/s0165-0327(97)00065-7. doi:10.1016/S0165-0327(97)00065-7. [DOI] [PubMed] [Google Scholar]
- Bartussek D, Becker G, Diedrich O, Naumann E, Maier S. Extraversion, neuroticism, and event-related brain potentials in response to emotional stimuli. Personality and individual Differences. 1996;20(3):301–312. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31(2):906–919. doi: 10.1016/j.neuroimage.2005.12.050. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. Journal of Personality. 2004;72(6):1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115(1):33. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Clark LA. Temperament as a unifying basis for personality and psychopathology. Journal of Abnormal Psychology. 2005;114(4):505. doi: 10.1037/0021-843X.114.4.505. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103(1):103. [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2003. [Google Scholar]
- Cronbach LJ, Furby L. How we should measure“ change”: Or should we? Psychological Bulletin. 1970;74(1):68. [Google Scholar]
- De Fruyt F, Van Leeuwen K, Bagby RM, Rolland JP, Rouillon F. Assessing and interpreting personality change and continuity in patients treated for major depression. Psychological Assessment. 2006;18(1):71. doi: 10.1037/1040-3590.18.1.71. doi:10.1037/1040-3590.18.1.71. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry. 2009;50(11):1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascalis V, Speranza O. Personality effects on attentional shifts to emotional charged cues: ERP, behavioural and HR data. Personality and Individual differences. 2000;29(2):217–238. [Google Scholar]
- DuBois P. Multivariate Correlational Analysis. Harper; New York: 1957. [Google Scholar]
- Dunning JP, Hajcak G. See no evil: Directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. 2009;46(1):28–33. doi: 10.1111/j.1469-8986.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- Durbin C, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental Emotionality in Preschoolers and Parental Mood Disorders. Journal Of Abnormal Psychology. 2005;114(1):28–37. doi: 10.1037/0021-843X.114.1.28. doi:10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20(6):977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety. 2010;27:813–820. doi: 10.1002/da.20712. doi:10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Gao PX, Liu HJ, Ding N, Guo DJ. An event-related-potential study of emotional processing in adolescence. Acta Psychologica Sinica. 2010;42:342. [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80(3):333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology. 2009;120(3):505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Foti D, Ferri J, Keil A. The dynamic allocation of attention to emotion: Simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biological Psychology. 2013;92(3):447–455. doi: 10.1016/j.biopsycho.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet D. Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology. 2010;35(2):129–155. doi: 10.1080/87565640903526504. doi:10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8(2):250. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. doi:10.1037/0021-843X.107.1.128. [DOI] [PubMed] [Google Scholar]
- John OP, Donahue EM, Kentle RL. The Big Five Inventory--Versions 4a and 54. University of California, Berkeley, Institute of Personality and Social Research; Berkeley, CA: 1991. [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm Shift to the Integrative Big-Five Trait Taxonomy: History, Measurement, and Conceptual Issues. In: John OP, Robins RW, Pervin LA, editors. Handbook of Personality: Theory and Research. Guilford Press; New York, NY: 2008. pp. 114–158. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for affective disorders and schizophrenia for school-age children present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. doi:10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder G, Tenke C, Stewart J, Quitkin F. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. doi:10.1016/S0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Kehoe EG, Toomey JM, Balsters JH, Bokde AL. Personality modulates the effects of emotional arousal and valence on brain activation. Social Cognitive and Affective Neuroscience. 2012;7(7):858–870. doi: 10.1093/scan/nsr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annual Review of Clinical Psychology. 2011;7:269. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bulletin. 2010;136(5):768. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry. 2012;53:207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Hajcak G. Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience. 2012;2:458–467. doi: 10.1016/j.dcn.2012.03.005. doi:10.1016/j.dcn.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instructional manual. Unpublished manuscript. [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9(4):531. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- McFarland BR, Klein DN. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depression and Anxiety. 2009;26(2):117–122. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Richell RA, Leonard A, Blair RJR. Emotion at the expense of cognition: Psychopathic individuals outperform controls on an operant response task. Journal of Abnormal Psychology. 2006;115:559–566. doi: 10.1037/0021-843X.115.3.559. doi:10.1037/0021-0021-843X.115.3.559. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K, Watson D, Markon KE. Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. Journal of Abnormal Psychology. 2009;118(2):299. doi: 10.1037/a0015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA, Olino TM, Klein DN. Defining reactivity: How several methodological decisions can affect conclusions about emotional reactivity in psychopathology. Cognition & Emotion. 2011;25(8):1439–1459. doi: 10.1080/02699931.2010.551185. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(02):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Ormel J, Oldehinkel AJ, Vollebergh W. Vulnerability before, during, and after a Major Depressive Episode: A 3-wave population-based study. Arch Gen Psychiatry. 2004;61(10):990–996. doi: 10.1001/archpsyc.61.10.990. doi:10.1001/archpsyc.61.10.990. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal Of Abnormal Psychology. 2013;122(3):902–916. doi: 10.1037/a0032807. doi:10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Rammstedt B, John OP. Measuring personality in one minute or less: A 10-item short version of the Big Five Inventory in English and German. Journal of Research in Personality. 2007;41(1):203–212. [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: a quantitative review of longitudinal studies. Psychological Bulletin. 2000;126(1):3. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Rogosa D, Brandt D, Zimowski M. A growth curve approach to the measurement of change. Psychological Bulletin. 1982;92(3):726–748. doi:10.1037/0033-2909.92.3.726. [Google Scholar]
- Rottenberg J, Gotlib IH. Socioemotional functioning in depression. Mood Disorders: A Handbook of Science and Practice. 2004:61–77. [Google Scholar]
- Rottenberg J, Gross J, Gotlib I. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. doi:10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: an evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23(4):605–637. doi: 10.1016/s0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Simms EE. Assessment of the facets of the five factor model: Further development and validation of a new personality measure. 2009 Retrieved from THE UNIVERSITY OF IOWA. [Google Scholar]
- Tracy JL, Proudfit GH, Klonsky ED. How affective science can inform clinical science: An introduction to the special issue. Clinical Psychological Science. 2014;2(4):371–386. [Google Scholar]
- Watson D, Clark LA, Harkness AR. Structures of personality and their relevance to psychopathology. Journal of Abnormal Psychology. 1994;103(1):18. [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76(5):820. [Google Scholar]
- Weinberg A, Ferri J, Hajcak G. Interactions between attention and emotion. In: Robinson MD, Watkins ER, Harmon-Jones E, editors. Handbook of Cognition and Emotion. Guilford; New York: 2013. pp. 35–54. [Google Scholar]
- Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience. 2011;23:2994–3007. doi: 10.1162/jocn.2011.21630. doi:10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Venables NC, Proudfit GH, Patrick CJ. Heritability of the neural response to emotional pictures: Evidence from ERPs in an adult twin sample. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu059. doi: 10.1093/scan/nsu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, He Y, Lei Y, Yang J, Li H. Event-related potential correlates of the extraverts' sensitivity to valence changes in positive stimuli. NeuroReport. 2009;20(12):1071–1076. doi: 10.1097/WNR.0b013e32832e7d55. [DOI] [PubMed] [Google Scholar]
- Yuan J, Zhang J, Zhou X, Yang J, Meng X, Zhang Q, Li H. Neural mechanisms underlying the higher levels of subjective well-being in extraverts: Pleasant bias and unpleasant resistance. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(1):175–192. doi: 10.3758/s13415-011-0064-8. [DOI] [PubMed] [Google Scholar]