Abstract
Purpose
We hypothesized exercise vasodilation would be greater in women due to nitric oxide synthase (NOS) and cyclooxygenase (COX) signaling.
Methods
45 healthy adults (23 women, W, 22 men, M, 26 ± 1 years) completed two 10-min trials of dynamic forearm exercise at 15 % intensity. Forearm blood flow (FBF; Doppler ultrasound), arterial pressure (brachial catheter), and forearm lean mass were measured to calculate relative forearm vascular conductance (FVCrel) = F BF 100 mmHg−1 100 g−1 lean mass. Local intra-arterial infusion of L-NMMA or ketorolac acutely inhibited NOS and COX, respectively. In Trial 1, the first 5 min served as control exercise (CON), followed by 5 min of L-NMMA or ketorolac over the last 5 min of exercise. In Trial 2, the remaining drug was infused during 5–10 min, to achieve combined NOS–COX inhibition (double blockade, DB).
Results
Are mean ± SE. Women exhibited 29 % greater vasodilation in CON (AFVCrel, 19 ± 1 vs. 15 ± 1, p = 0.01). L-NMMA reduced AFVCrel (p < 0.001) (W: Δ −2.3 ± 1.3 vs. M: Δ −3.7 ± 0.8, p = 0.25); whereas, ketorolac modestly increased ΔFVCrel (p = 0.04) similarly between sexes (W: Δ 1.6 ± 1.1 vs. M: Δ 2.0 ± 1.6, p = 0.78). DB was also found to be similar between the sexes (p = 0.85).
Conclusion
These data clearly indicate women produce a greater exercise vasodilator response. Furthermore, contrary to experiments in animal models, these data are the first to demonstrate vascular control by NOS and COX is similar between sexes.
Keywords: Exercise, Sex differences, Nitric oxide synthase, Cyclooxygenase
Introduction
Investigation of sex differences in physiological mechanisms is very important and a surprisingly uncommon area of research, despite strong epidemiologic evidence of sex-specific differences in cardiovascular risk as well as responses to physiologic and mental stress (Shaw et al. 2006; Blauwet and Redberg 2007; Moro et al. 2011; Chen et al. 2012; Taylor et al. 2014). Our understanding of sex-specific cardiovascular control mechanisms is limited due to a predominance of male-only human and animal studies (Huxley 2007; Arain et al. 2009; Miller 2010, 2014), as well as, a lack of mechanistic studies directly comparing men and women. Within this context, exercise elicits a robust local vasodilation, offering an excellent model to examine dynamic vascular control mechanisms between the sexes.
The few studies comparing exercise vasodilation between the sexes have produced conflicting results. For example, healthy young women demonstrated greater vascular conductance and blood flow (relative to limb muscle mass) during graded single knee-extensor exercise than men (Parker et al. 2007), which could not be explained by differences in hemoglobin concentration or mechanical impedance during contraction. Women also have demonstrated greater vascular conductance during forearm exercise across a range of workloads (Gonzales et al. 2007; Saito et al. 2008). In contrast, FBF and FVC responses (relative to forearm volume) to rhythmic forearm exercise at the same % maximum voluntary contraction (MVC) did not differ between men and women (Limberg et al. 2010; Casey et al. 2013). Taken together, data make it difficult to interpret whether or not exercise vasodilation is greater in women. Whether exercise-induced vasodilation is similar or different, investigation of sex-specific mechanistic control of peripheral vasculature remains largely unexplored.
To our knowledge, only a single animal study has addressed sex-specific control of skeletal muscle blood flow (Rogers and Sheriff 2004). Those data indicate female rats display greater femoral vascular conductance during treadmill exercise, an effect that was abolished by combined inhibition of cyclooxygenase (COX) and nitric oxide synthase (NOS) (via indomethacin and L-NAME, respectively) (Rogers and Sheriff 2004). The translational relevance of one animal study is unclear due to use of ovarectomy followed by supraphysiologic hormone supplementation (Rogers and Sheriff 2004). Furthermore, the individual roles of NOS or COX in skeletal muscle of exercising women remains untested, leaving a large gap in knowledge regarding potential sex differences in vascular control during dynamic exercise.
Given the conflicting data on exercise vasodilator responses, and the lack of mechanistic insight comparing men and women, we designed the present study to test for sex-specific differences in skeletal muscle vascular control in healthy young men and women. Based on the work of Parker and Rogers and Sheriff, we hypothesized young healthy women would exhibit a larger skeletal muscle vasodilator response to exercise, and that inhibition of NOS and COX would eliminate the sex differences.
Materials and methods
Subjects
45 healthy younger (18–40 years) subjects participated in the study (women n = 23, men n = 22). Subjects were matched for age and physical activity (Table 1). All subjects were healthy, lean (BMI < 25), non-smokers, and were not taking any medications. Female subjects were not pregnant and were studied during the early follicular phase (days 1–5) of the menstrual cycle. Hormonal contraception was allowed and women on contraception were studied during the placebo phase (n = 9). Subjects were instructed to refrain from exercise, non-steroidal anti-inflammatory drugs (NSAIDs), acetylsalicylic acid (ASAs), alcohol, and caffeine for 24 h prior to the study day. Subjects also fasted 12 h before participating in the study. Written informed consent was obtained from all subjects. All procedures were approved by the Institutional Review Board at the University of Wisconsin, and conformed to the standards set by the Declaration of Helsinki.
Table 1.
Subject characteristics
| Females (n = 23) | Males (n = 22) | |
|---|---|---|
| Age (years) | 26 ± 1 | 27 ± 1 |
| Height (cm) | 166 ± 1 | 177 ± 2* |
| Weight (kg) | 66 ± 4 | 75 ± 4 |
| BMI (kg m−2) | 22 ± 0.4 | 23 ± 0.4 |
| Waist (cm) | 76 ± 1 | 81 ± 1* |
| Body fat (%) | 34 ± 1 | 21 ± 1* |
| Lean forearm mass (g) | 596 ± 15 | 956 ± 33* |
| MVC (kg) | 30 ± 1 | 44 ± 2* |
| 15 % MVC (kg) | 4.5 ± 0.1 | 6.5 ± 0.3* |
| Resting FBF (ml min−1) | 35 ± 2 | 62 ± 6* |
| Resting FVC (ml min−1 100 mmHg−1) |
41 ± 3 | 74 ± 7* |
| MAP (mmHg) | 83 ± 2 | 83 ± 3 |
| Glucose (mg dL−1) | 71 ± 2 | 70 ± 2 |
| Total cholesterol (mg dL−1) | 167 ± 7 | 145 ± 5* |
| Triglyceride (mg dL−1) | 74 ± 6 | 77 ± 8 |
| Physical activity (kcal/week) | 1190 ± 166 | 1286 ± 184 |
Data are mean ± SE
p < 0.05 between women and men
Measurements
Subject characteristics
Weight and height were measured and body composition was determined by waist circumference, body mass index (BMI, kg m−2), and dual-energy X-ray absorptiometry (DEXA, GE Lunar Prodigy; Milwaukee, WI). Lean forearm mass of the experimental limb was also determined from DEXA measurements. Maximal voluntary contraction (MVC, kg) of the experimental limb was determined as the average of the two highest measurements from five trials using an isometric hand dynamometer. Arterial blood was collected after a 12-h fast and levels of triglycerides, total cholesterol, and glucose were measured immediately (CardioChek; PTS Panels; Indianapolis, IN, USA). Physical activity levels were estimated using the Paffenbarger (1993) questionnaire.
Brachial artery catheterization, and hemodynamic monitoring
A 20-gauge, 5-cm catheter was placed in the brachial artery of the non-dominant arm under aseptic conditions and after local anesthesia (2 % lidocaine). In three subjects (two male, one female) the catheter was inserted in the dominant forearm due to an inability to catheterize the non-dominant arm. The catheter was flushed at 3 ml h−1 with saline. Mean arterial pressure (MAP) was measured with indwelling pressure transducer and monitoring kit (Hospira, INC, Lake Forest, IL, USA). Beat-by-beat heart rate (ECG; Datex-Ohmeda, Helsinki, Finland) and arterial pressure measurements were collected throughout the study.
Blood flow
Forearm blood flow was calculated from blood velocity and artery diameters measured using Doppler ultrasound (Vivid 7, General Electric; Milwaukee, WI, USA). The ultrasound probe (12 MHz probe) was placed medial to the biceps brachii muscle. Measurements were made with a fixed insonation angle of ≤60°, with the sample volume adjusted to cover the width of the brachial artery (Limberg et al. 2010, 2013). The audio signal from the Vivid 7 was sampled real-time by a custom-made device which converted velocity information into a digital signal using fast Fourier transform, which was calibrated to a specific pulsed Doppler frequency (5 MHz) (Herr et al. 2010). Brachial arterial diameter was measured on B-mode images in the part of the artery running perpendicular to the ultrasound beam (Limberg et al. 2010, 2013). Vessel diameter was measured from digital video recordings of the artery and diameters were selected as the median of five measurements in late diastole during the timeframes indicated in Fig. 1. All measurements were assessed off-line. A mark was made on the skin ensured artery measurements were taken in the same anatomical position for each trial.
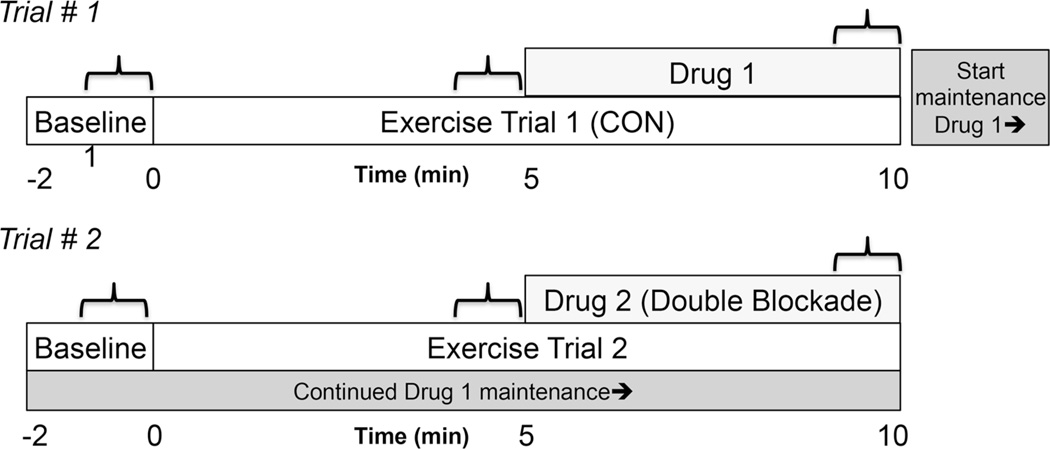
Fig. 1.
Schematic of experimental protocol. Trial #1, exercise at 15 % MVC without inhibition (CON) to a single inhibition (Drug 1) of either L-NMMA or KETO. Trial #2, exercise at 15 % MVC with single inhibition to double blockade (drug 2, DB).  indicates where measurements are taken. CON n= 23 W, 22 M, L-NMMA n= 9 W, 11 M, KETO n= 14 W, 11 M, DB n= 23 W, 22 M
indicates where measurements are taken. CON n= 23 W, 22 M, L-NMMA n= 9 W, 11 M, KETO n= 14 W, 11 M, DB n= 23 W, 22 M
FBF was calculated from mean blood velocity (MBV, cm s−1, during the last 30 s of steady-state rest and exercise) and vessel cross-sectional area (using diameter in centimeter) measurements and reported in ml min−1 [FBF = MBV π (brachial artery diameter cm 2−1)2 (60 s min−1)].
Forearm exercise
Subjects completed dynamic and rhythmic forearm exercise at a contraction intensity equal to 15 % MVC. The advantages of using this intensity minimizes increases in MAP and sympathetic nerve activity (Limberg et al. 2014) to allow for direct comparison of local vasodilation. In order to assess cardiovascular control mechanisms, which are exercise intensity-dependent, we compared sexes at the same relative exercise intensity (15 %) (Laughlin et al. 2012). It is important to emphasize this study is focused on mechanistic control of vasodilation, not oxygen delivery per se. That question is a distinct but related study, where comparing the same absolute intensity would in theory match muscle metabolism, but lead to differences in exercise intensity domains and therefore different mechanistic control.
Subjects were in a supine position with the non-dominant arm extended to the side, at heart level while raising and lowering a weight 2.5 cm over a pulley. Exercise was completed at a rate of 20 times per minute (at a duty cycle of 1 s contraction:2 s relaxation) to the rhythm of a metronome (Schrage et al. 2004; Limberg et al. 2010; Casey et al. 2013). The pulley system is instrumented with a sensor and indicator light that alerts the subject when the 2.5 cm distance had been completed to ensure standardized the work rate between subjects.
Study protocols
The study required one screening visit and one study visit. Procedures completed as part of the present study were part of a larger protocol to test resting endothelial function, where all subjects received endothelium-dependent and independent agonists (in random order), but both exercise bouts were conducted at the same study time point in all trials. The focus of this study was to test active vascular control during steady-state exercise, and to determine whether the individual and combined contributions of NOS and COX differ between men and women. Thus, the overall approach required two bouts of exercise, where the order of NOS or COX inhibition was randomized. In order to test the active control of vasculature during exercise, while avoiding possible confounding signals from the rest-to-exercise transition, the research design was limited to exercise at a single intensity. All exercise bouts were preceded by 2 min of resting data (baseline). Two study conditions were conducted: (1) 10 min of dynamic forearm exercise, with L-NMMA (n = 9 women, 11 men) or ketorolac (KETO) (n = 14 women, 11 men) administered during the last 5 min, (2) 10 min of dynamic forearm exercise during a continuous maintenance dose of L-NMMA or KETO during the first 5 min, with the addition of the remaining drug to achieve a double blockade (DB) for the final 5 min. Trials were separated by a minimum of 30 min. Time control experiments demonstrate vascular responses during 10 min of steady-state exercise at 15 % MVC (ΔFBFrel and ΔFVCrel) are not different between 5 and 10 min of exercise, p = 0.23, n = 7, 3 women, 4 men, unpublished observations.
To maximize local muscle inhibition and minimize systemic effects of drugs, L-NG-monomethyl arginine (L-NMMA, 250 mg Clinalfa U-1090, Bachem, Germany) and Ketorolac (KETO, 30 mg, Hospira, INC, Lake Forest, IL, USA) were infused into the brachial artery of the exercising limb. Drugs were mixed specifically for each study visit. L-NMMA (5 mg ml−1) was infused at 10 mg min−1 for 5 min followed by a 1 mg min−1 maintenance infusion. This dose is greater than or equal to previous studies, where intra-arterial L-NMMA reduced vasodilation to acetylcholine infusion (Dyke et al. 1995; Shoemaker et al. 1997; Duffy et al. 1999; Casey and Joyner 2009; Casey et al. 2010) and steady-state exercise (Casey and Joyner 2009). KETO (0.5 mg ml−1) was infused at 1.2 mg min−1 for 5 min followed by a 0.1 mg min−1 maintenance infusion. This dose of KETO is also greater than previous experiments using similar experimental procedures (Schrage et al. 2004, 2010) and those that have determined the efficacy of ketorolac to inhibit COX during forearm exercise (Dinenno and Joyner 2004). A larger dose of ketorolac was used to ensure complete inhibition of COX during the entirety of the experiment, as previous experiments have only found a transient (Schrage et al. 2004) or negligible effect on exercising vasodilation. Experimental schematic can be observed in Fig. 1.
Data acquisition and analysis
All hemodynamic data were recorded at 400 Hz on a dedicated computer. Post-processing using PowerLab’s Chart7 application package yielded MBV, arterial pressures, and heart rates. Hemodynamic variables were taken as 30 s averages at baseline, steady-state exercise (4:30–5 min of exercise) and drug infusion (9:30–10 min exercise), as indicated by brackets in Fig. 1. All hemodynamic values were measured continuously throughout the 30-s sampling period including both contraction and relaxation phases of the duty cycle. Hemodynamic variables were averaged into 10-s bins to examine a possible transient effect of ketorolac (COX inhibition) (Schrage et al. 2004).
The primary analysis was to test whether vasodilation responses to exercise were different between sexes, and whether responses to NOS or COX inhibition in exercising muscle were different between sexes. To assess exercise vasodilation the main dependent variable was the change in forearm vascular conductance from baseline (ΔFVCrel) [Δ FBF (ml min−1) normalized for arterial pressure and lean forearm mass (Δ FBF (ml min−1) 100 MAP−1 100 lean forearm mass−1 = ΔFVCrel ml min−1 100 mmHg−1 100 g−1)]. Comparisons using Δ FBFrel were also conducted (Δ FBFrel = min−1 100 g−1). Baseline refers to the resting period prior to the onset of the exercise bout in any given trial. Therefore, Trial 1 CON, L-NMMA, and KETO values are calculated as ΔFVCrel = FVCrel CON, L-NMMA, or KETO - FVCrel baseline no drug infused. Trial 2, double blockade was calculated as ΔFVCrel DB = FVCrel DB - FVCrel baseline following loading and maintenance dose of L-NMMA or KETO received in Trial 1.
Comparing blood flow values at absolute or relative work rates has recently been challenged (Garten et al. 2014). We propose ΔFVCrel is the most appropriate approach, as sexes differ in resting conductance/blood flow and muscle mass (Table 1). Second subjects exercised at the same relative intensity, which should activate similar control mechanisms at any given intensity (Laughlin et al. 2012) (Table 1). Third, most of the change in FBF and FVC is directed to active muscle (Moore et al. 2010; Bagher and Segal 2011). Therefore, when comparing vascular responses to exercise, the rise in exercising limb flow (or conductance) relative to lean or muscle mass is the most appropriate.
Statistical analysis
Statistical analysis was completed using SigmaPlot Version 12.0 (Systat Software, Inc.). Non-parametric statistical tests were used as a more conservative statistical approach considering the distribution of vascular responses (i.e., ΔFVCrel) to exercise, and exercise with the addition of pharmacological agents was unknown. This approach also avoids meaningless comparisons encompassed in ANOVA comparisons (e.g., men L-NMMA exercise response compared to women KETO exercise response). Subject characteristics and hemodynamic values during the first 5 min of exercise (CON, without drug infusion) were analyzed using a Mann–Whitney rank sum test to determine if there were differences between groups (sexes). Effects of drug treatments (L-NMMA, KETO, DB) were examined by subtracting the hemodynamic value (AFVCrel) obtained during CON from the hemodynamic values obtained during treatment (e.g., AFVCrel L-NMMA - ΔFVCrel CON). These values were then tested with one-sample Wilcoxon signed-rank test to determine if the values are different than zero (zero would represent no drug effect). Differences between sexes in response to drug treatments were assessed by testing the aforementioned drug responses between groups using a Mann–Whitney rank sum test (null hypothesis, women [ΔAFVCrel “drug” - AFVCrel CON] compared to men [ΔAFVCrel “drug” - ΔFVCrel CON]). Mann–Whitney rank sum test was also applied to detect if drug order affected (L-NMMA to KETO n = 20, KETO to L-NMMA n = 24) values observed in DB. All data are presented as mean ± standard error, and significance was determined a priori at p < 0.05.
Results
Subject characteristics are summarized in Table 1. Subjects exhibited similar age, BMI, blood pressure, fasting glucose, and physical activity levels. Men displayed greater lean forearm mass and thus greater forearm strength (MVC).
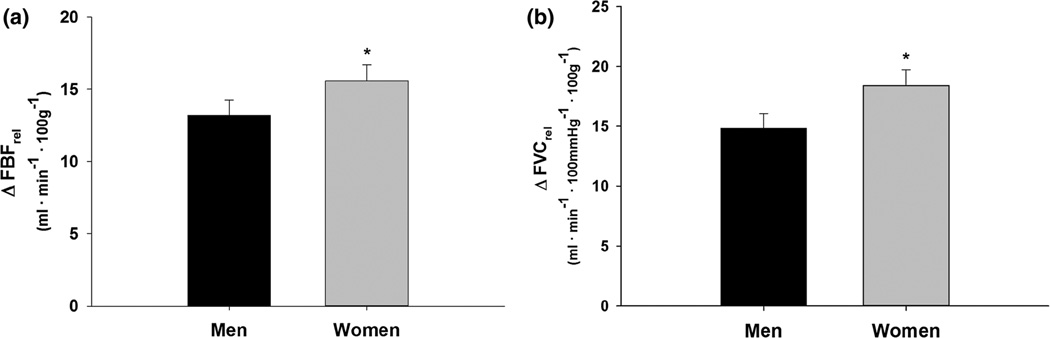
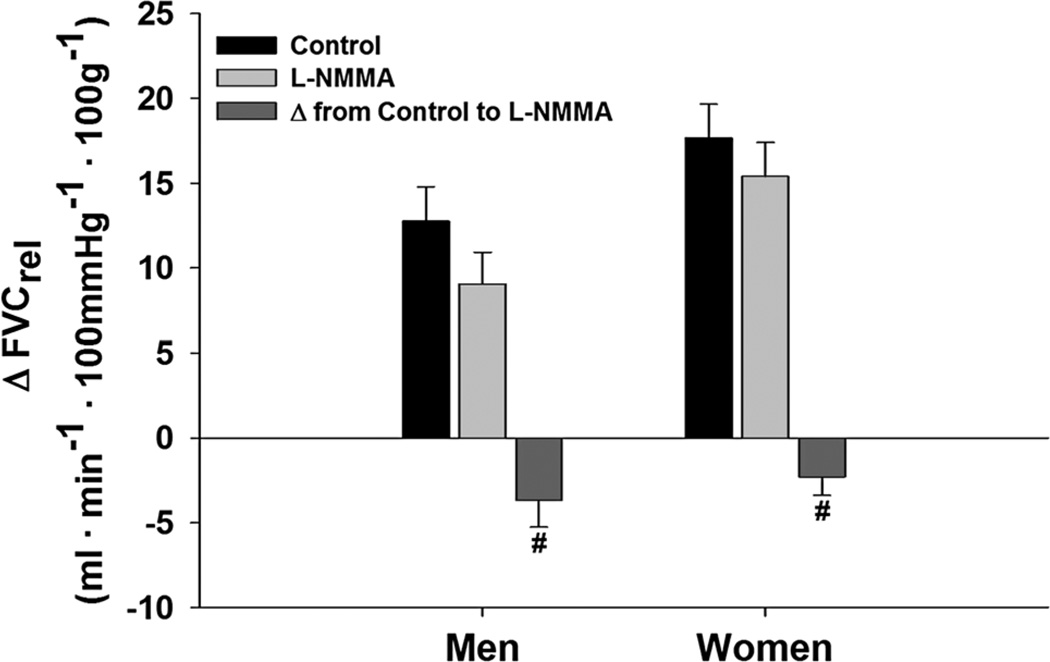
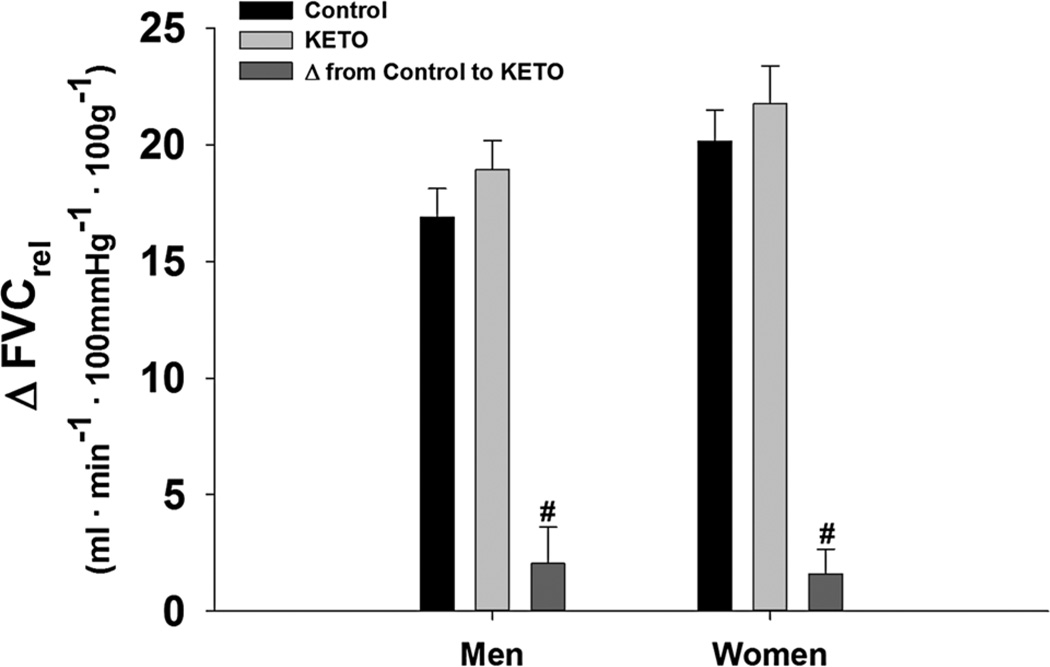
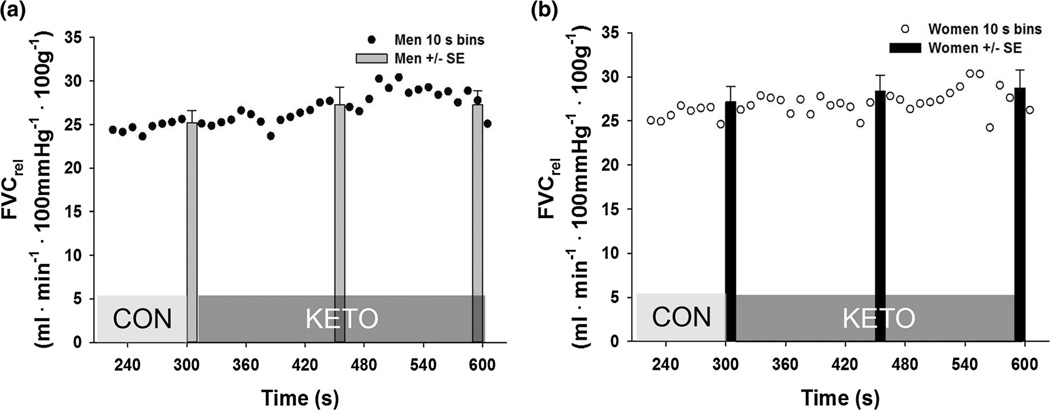
On average, women displayed a 23 % larger relative forearm blood flow response (ΔFBFrel, p = 0.03) and a 29 % larger relative forearm vascular conductance response (ΔFVCrel, p = 0.02) to 5 min of exercise at 15 % MVC when compared to men (Fig. 2). When L-NMMA was infused during steady-state exercise, there was an overall effect of L-NMMA (p < 0.001); however, ΔFVCrel decreased similarly in both sexes (W: Δ −2.3 ± 1.3 vs. M: Δ −3.7 ± 0.8, p = 0.25) (Fig. 3). Ketorolac increased ΔFVCrel (p = 0.04). The increases with KETO were not different between sexes in absolute (W: Δ 1.6 ± 1.1 vs. M: Δ 2.1 ± 1.6, p = 0.78) or relative terms (p = 0.46) (Fig. 4). Close inspection of beat-by-beat data provided no support for a transient effect of ketorolac (Fig. 5).
Fig. 2.
Change in forearm blood flow (FBF, a) and forearm vascular conductance (FVC, b) relative to lean forearm mass from baseline during exercise at 15 % MVC. Data are mean ± SE. Women = 22, men = 23, asterisk women greater than men p < 0.05
Fig. 3.
Change in relative forearm vascular conductance (FVC) from baseline during exercise at 15 % MVC with and without NOS inhibition (L-NMMA). Data are mean ± SE. Women = 9, men = 11, hash effect of L-NMMA p < 0.001, no difference between sexes p = 0.25
Fig. 4.
Change in relative forearm vascular conductance (FVC) from baseline during exercise at 15 % MVC with and without COX inhibition (KETO). Data are mean ± SE. Women = 14, men = 11, hash effect of KETO p = 0.04, no difference between sexes p = 0.78
Fig. 5.
Relative forearm vascular conductance (FVC) during exercise at 15 % MVC depicting the transition from steady state without COX inhibition to COX inhibition (KETO) in men (a) and women (b). Data presented in 10 s bins (dots) and 30 s averages (bars with error)
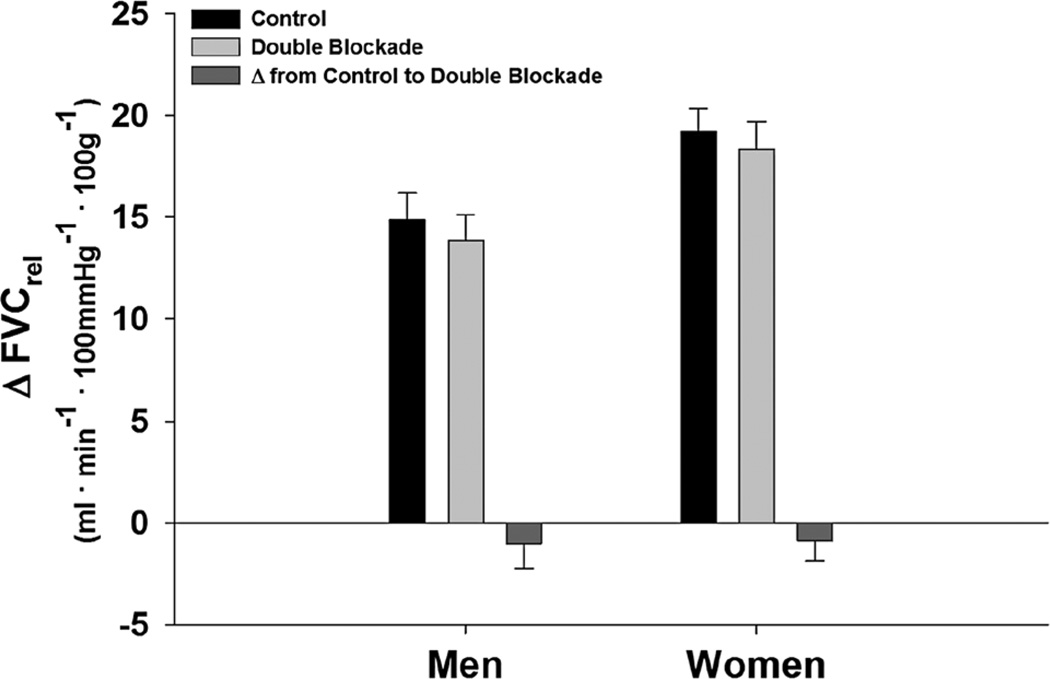
Interestingly, the effects of combined NOS–COX inhibition (DB) returned vascular responses to those observed during control exercise (without drug treatment, p = 0.28). These DB responses were similar between sexes (p = 0.85) (Fig. 6). There was also no effect of drug order in DB (p = 0.68); therefore the groups (L-NMMA in Trial 1, or KETO in Trial 1) were combined for analysis in DB. Additionally, women taking oral contraception did not differ from women without contraception in response to CON (p = 0.5), L-NMMA (p = 0.6), KETO (p = 0.2), and DB (p = 0.5). We have also considered that men and women working at different absolute workloads may affect the interpretation of our results. In subjects that worked at the exact same absolute workload (three men, three women), women still exhibited greater vasodilation response (W: 16.1 ± 1.1 vs. M: 9.7 ± 0.7, p = 0.008). Absolute measures of FBFrel, FVCrel, and MAP are summarized in Table 2. It is important to note that analysis of FBFrel, ΔFBFrel, and FVCrel provide similar conclusions of greater exercise vasodilation in women, but similar contributions of NOS and COX.
Fig. 6.
Change in relative forearm vascular conductance (FVC) from baseline during exercise at 15 % MVC with and without double blockade (DB, L-NMMA + KETO). Data are mean ± SE. Women = 23, men = 22, no effect of double blockade p= 0.28, no difference between sexes p= 0.85, no effect of drug order p= 0.68
Table 2.
Hemodynamic variables during exercise at 15 % MVC with and without NOS, (L-NMMA), COX (KETO), or combined inhibition (double blockade, DB)
| Group | Baseline | Steady-state exercise | Exercise + drug |
|---|---|---|---|
| FBFrel (ml min−1 100 g−1) | |||
| L-NMMA | |||
| W | 6 ± 1 | 22 ± 2 | 21 ± 2 |
| M | 6 ± 1 | 18 ± 2 | 16 ± 2 |
| Keto | |||
| W | 6 ± 1 | 23 ± 1 | 25 ± 2 |
| M | 7 ± 1 | 21 ± 1 | 24 ± 2 |
| DB | |||
| W | 6 ± 0 | 22 ± 1 | 23 ± 1 |
| M | 6 ± 1 | 20 ± 1 | 18 ± 1 |
| MAP (mmHg) | |||
| L-NMMA | |||
| W | 87 ± 3 | 88 ± 3 | 95 ± 4 |
| M | 89 ± 1 | 92 ± 2 | 97 ± 3 |
| Keto | |||
| W | 84 ± 2 | 84 ± 2 | 86 ± 2 |
| M | 79 ± 2 | 84 ± 2 | 88 ± 2 |
| DB | |||
| W | 85 ± 2 | 86 ± 2 | 92 ± 2 |
| M | 84 ± 2 | 88 ± 2 | 92 ± 2 |
| FVCrel (ml min−1 100 mmHg−1 100 g−1) | |||
| L-NMMA | |||
| W | 7 ± 1 | 25 ± 2 | 22 ± 2 |
| M | 7 ± 1 | 20 ± 2 | 16 ± 2 |
| Keto | |||
| W | 7 ± 1 | 27 ± 2 | 29 ± 2 |
| M | 8 ± 1 | 25 ± 1 | 27 ± 2 |
| DB | |||
| W | 7 ± 1 | 26 ± 1 | 26 ± 2 |
| M | 8 ± 1 | 23 ± 1 | 20 ± 1 |
Data are mean ± SE
L-NMMA n= 9 W, 11 M, KETO n= 14 W, 11 M, DB n= 23 W, 22 M
W women, M men
Discussion
We studied healthy young men and women to investigate whether the sexes differed in terms of exercise-mediated vasodilation, as well as whether NOS or COX regulate dynamic exercise vasodilation in a sex-specific manner. This is the first study in humans to test the concept of sex-specific vascular control mechanisms during exercise. The key findings from this study were: (1) women demonstrate robustly greater vasodilator responses to exercise; (2) NOS inhibition decreased FVC similarly between sexes; (3) COX inhibition increased FVC similarly between sexes; and (4) sex differences in FVC were still evident after NOS, COX or combined inhibition of both NOS and COX. These studies provide the mechanistic and technical rationale for follow-up studies designed to identify NOS– COX independent mechanisms. Collectively, these data provide the first mechanistic evidence that contributions of NOS and COX to vasodilation are similar between healthy young men and women during exercise, and that other novel mechanisms account for greater exercise vasodilation in women.
Vascular control differences between sexes during exercise
Our data agree with previous findings from Parker et al. (2007) and Saito et al. (2008), where healthy young women demonstrated greater exercise vascular conductance in leg and forearm muscles, respectively (Parker et al. 2007; Saito et al. 2008). In further support of our results, when subjects that are matched for absolute workload were compared, women were still observed to have a substantially greater vascular response to exercise. These particular observations are in congruent with Gonzales et al. (2007), who observed greater FVC in women at submaximal intensities. The current findings are in contrast to similar vascular responses during forearm exercise between the sexes ranging from 10 to 30 % MVC (Limberg et al. 2010; Casey et al. 2013). This discrepancy might be due to blood flow variables made relative to forearm volume (Limberg et al. 2010; Casey et al. 2013) rather than lean mass. Together, our data along with a handful of recent work (Parker et al. 2007, 2008), strongly suggests exercise vasodilation is greater in young healthy women. Additionally, our design allowed for the evaluation of NOS and COX pathways mediating this sex difference.
Is NOS signaling in steady-state exercising muscle greater in women than men?
Contrary to our hypothesis, NOS inhibition reduced FVCrel equally in both sexes (Fig. 3). This study is the first to directly address this question, and results indicate NOS-mediated vasodilation was similar between men and women, such that, differences in exercise vasodilation between sexes are not NOS mediated. This was surprising, given the common link between female sex hormones and greater NOS expression in both skeletal muscle and endothelium (Fadel et al. 2003; Gavin et al. 2009). Estradiol levels increase through the luteal phase and increasing levels of estrogen may create a NOS-mediated rise in exercise vasodilation, although progesterone may offset estrogen-mediated enhancements (Miner et al. 2011). Similarly to Parker et al. (2007) we studied women in days 1–5 of the menstrual cycle, to minimize sex differences in hormones. Thus, the sex differences observed in Fig. 2 are not due to short-term estrogen effects. These data convincingly demonstrate NOS signaling during exercise is similar between healthy young men and women when not influenced by hormone fluctuations, yet women still demonstrate a greater vascular response to exercise.
Nitric oxide has been found to significantly contribute to steady-state exercise vasodilation but is not obligatory (Schrage et al. 2004). Using a lower exercise intensity (10 % MVC) and identical exercise device and contraction cycle, we previously observed ~20 % decrease in exercise vasodilation with NOS inhibition (Schrage et al. 2004). Using the current protocol we observed ~25 % decrease at 15 % MVC (Fig. 3). Together the data support the concept that NOS contribution to steady-state vasodilation may increase with exercise intensity (Wray et al. 2011). Nevertheless, at the exercise intensity examined, the sex-specific role of NOS appears similar.
Is COX signaling in steady-state exercising muscle different in women and men?
These new data indicate the COX enzyme regulates exercise vasodilation similarly in men and women (Figs. 4, 5). Prostanoid contributions to exercise hyperemia appears to be negligible or relatively modest (Shoemaker et al. 1996; Schrage et al. 2004, 2010; Mortensen et al. 2007). Inhibition of COX prior to exercise onset does not affect vascular response to exercise (Shoemaker et al. 1996; Mortensen et al. 2007). However, when COX is inhibited during steady-state exercise there is a small, transient decrease in vascular conductance (Schrage et al. 2004). The transient decrease is at odds with current data where KETO increased FVCrel similarly in both sexes (Figs. 4, 5). The present study infused ~2× larger dose of KETO which may have more effectively inhibited thromboxane production in the active muscle bed, effectively removing a vasoconstrictor stimulus to generate the greater FVC observed. Considering prostacyclin and thromboxane have been observed in the interstitium of contracting skeletal muscle, a shift in the balance between vasoconstrictor and vasodilator signals appears to be the most plausible explanation (Karamouzis et al. 2001a, b). While the impact of COX inhibition may depend on dosing and delivery route, present data indicate the functional roles of COX products are similar between the sexes during exercise.
Does combined inhibition of NOS and COX in steady-state exercising muscle abolish sex differences?
Concurrent inhibition of NOS and COX have been observed to attenuate blood flow and vascular conductance during some (Boushel et al. 2002; Schrage et al. 2004; Mortensen et al. 2007, 2009; Heinonen et al. 2011), but not all (Schrage et al. 2004, 2010; Crecelius et al. 2011) small muscle mass exercise. This discrepancy may be an artifact of differences in presentation of data. For example, Heinonen et al. (2011) reported significant differences in vascular conductance relative to muscle mass with NOS and COX inhibition under resting conditions, but not during exercise (Heinonen et al. 2011). Therefore, the changes from baseline may also be similar which could be interpreted as combined NOS–COX inhibition only affects resting values. During forearm exercise, combined NOS–COX inhibition only appears to reduce hyperemia if drugs are added during steady-state exercise, but not if NOS or COX is inhibited from exercise onset (Shoemaker et al. 1996; Schrage et al. 2004). The latter finding is consistent with the current experiment, where DB did not elicit a different vascular response to control exercise (Fig. 6). When starting from a resting state these data suggests mechanistic insight to exercise vasodilation is distinctly dependent on the timing and method of pharmacologic intervention.
Previous data suggest that there is a synergistic NOS– COX interaction during leg exercise (Boushel et al. 2002; Mortensen et al. 2009; Hellsten et al. 2012). If compensatory mechanisms do not explain the lack of DB effect presently (Fig. 6), then the directionally opposite effects of L-NMMA (Fig. 3) and KETO (Fig. 4) seen in the present study, may indicate NOS and COX work in opposition during dynamic forearm exercise. While our single inhibition data (Figs. 3, 4) clearly support this interpretation for DB (Fig. 6), our data are at odds with Boushel et al. (2002), who reported an exercise intensity-dependent reduction in leg blood flow during combined NOS–COX inhibition. Beyond the potential for limb differences, inhibition protocols may explain the differing conclusions. Boushel et al. (2002) used oral Indomethacin 16 h prior to exercise, rather than acute arterial infusion of a COX inhibitor (KETO) used in the current study. Second, the present KETO dose was much higher, per kilogram lean mass, and may account for some of the differences. Without direct comparison of arm and leg in the same subjects, exercise intensity, and drugs, the role of COX or COX–NOS interactions in regulating exercise dilation remains elusive. However, these data provide the first mechanistic evidence that while NOS and COX actively regulate exercise vasodilation (Figs. 3, 4, 5), they also indicate both men and women can achieve steady-state exercise responses in the absence of NOS and COX signaling. An interesting future study might test whether the mechanism(s) which allow for compensatory exercise vasodilation (from resting DB condition) are sex-specific.
An unexpected finding from the current study is that a substantial sex difference in ΔFVCrel remained after NOS, COX, or combined NOS–COX inhibition (Fig. 6). These results contrast starkly from exercising rats, where sex differences in exercise vasodilation were abolished with combined NOS–COX inhibition (Rogers and Sheriff 2004). A key difference was the use of estrogen supplementation (after ovarectomy), such that, female rats displayed supraphysiologic plasma estrogen concentrations ~fivefold greater than control animals (Rogers and Sheriff 2004). In contrast, we studied women during menstruation, minimizing acute effects of estrogen, such that chronic, cyclic exposure to physiologic levels of sex hormones likely account for the current sex differences in vascular conductance observed in control exercise conditions. While we did not manipulate sex hormones in the present study, our data provides the first direct evidence in humans that greater exercise vasodilator responses in women are: (1) apparent when female sex steroids are least different than men, and (2) not explained by NOS and COX signaling. Identifying the mechanisms driving this sex difference represents an attractive future area of research, which may provide important explanations for altered vasodilation responses in disease conditions, which exhibit a sex-specific pathophysiology.
If not NOS or COX, what signals explain the greater exercise-mediated vasodilation in women?
Endothelium-derived hyperpolarizing factor (EDHF), potassium channels, and functional sympatholysis are all mechanisms that have been found to contribute to exercise hyperemia, and could potentially be different between sexes. Sex differences in resting α and β-adrenergic responses must be considered as possible explanations for the greater vasodilation in women (Kneale et al. 2000; Hart et al. 2011). This seems an unlikely explanation for observations in the current study as the exercise intensity selected does not increase muscle sympathetic nervous activity (Limberg et al. 2014), and similar vascular responses to arterial infusion of α-adrenergic agonists using an identical forearm exercise model have been observed (Limberg et al. 2010). Additionally, arterial epinephrine does not appear to change with exercise and with propranolol (Wilkins et al. 2008; Casey et al. 2011). Inhibition of EDHF or cytochrome P450 2C9 has been found to not affect blood flow during exercise; however, these mechanisms have only been tested in small studies in young men (Hillig et al. 2002; Mortensen et al. 2007), and only in knee-extensor exercise models. Animal models indicate endothelium-dependent dilation is heavily reliant on EDHF in females (Huang et al. 2001; Pak et al. 2002; Scotland et al. 2005; Villar et al. 2008), providing rationale to pursue EDHF contributions as potential mechanisms for the sex differences observed. In part via EDHF, potassium (K+) channels hold great influence over vascular tone (Jackson 2005) but studies investigating responses to contraction do not directly compare men to women (Crecelius et al. 2013, 2014). Thus, K+ channel involvement in regulating steady-state exercise vasodilation between the sexes is unclear in humans. Interestingly, recent studies indicate female animals have an increased expression of large conductance K+ channels (BKCa) (Khan et al. 2010). As our data clearly identified dynamic vascular control via NOS and COX mechanisms are similar between the sexes, these findings set the stage for future studies directly testing sex differences in other pathways like EDHF and/or K+ channels.
Experimental considerations
There are several design strengths in the present study which offer novel insight into the research question, including (1) relatively large sample size for invasive human physiology experiments; (2) subjects were well matched for age and physical activity; (3) volumetric blood flow measures which can be made relative to a direct measure of lean mass; and (4) direct local inhibition of NOS and COX during active exercise to test active exercise mechanisms.
However, it is important to consider some potential limitations. First, arm and leg vascular control may be different, such that these novel mechanistic findings may not apply to leg vascular control. Second, it is possible that the doses of L-NMMA and KETO selected to inhibit NOS and COX were not adequate to achieve complete inhibition of these mechanisms. However, our dose of L-NMMA is common in the literature (Casey et al. 2010, 2011) and our dose of KETO was greater than those who have used similar experimental design (Schrage et al. 2004; Crecelius et al. 2011) or similar to previous studies using forearm exercise model (Markwald et al. 2011; Crecelius et al. 2011). Furthermore, in order to test active vascular control mechanisms during steady-state exercise, we only evaluated NOS and COX at one exercise intensity. This is an important design of study, since starting exercise from an “inhibited state” will mask the physiologic contribution of NOS, COX, or both (Figs. 3, 4) in dynamic vascular control during exercise. Thus future work requires investigation of higher exercise intensities, which may yield other striking sex differences or similarities. Finally, future studies should study women during the luteal phase of menstrual cycle in addition to the follicular phase. It is possible that larger sex differences in exercise vascular conductance could be observed during the luteal phase that are NOS or COX mediated. However, progesterone can counter many vascular effects of estrogen in humans (Miner et al. 2011).
Summary and conclusions
The present study strengthens experimental evidence for greater exercise vasodilation in skeletal muscle of healthy young women relative to men. In contrast to animal studies, NOS and COX signaling does not explain sexes differences in humans justifying the need for future studies in humans aimed at uncovering novel mechanisms of sex-mediated vascular control. In conclusion, our results show for the first time in humans, that vascular control via NOS and COX are remarkably similar between the sexes.
Acknowledgments
We would like to thank the subjects for their time and effort. We would also like to acknowledge Meghan Crain, Garrett Peltonen, Joshua Trierweiler, and Cameron Rousseau, for their help in data collection. The Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), supported the described project grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Funding: NIH HL-105820.
Abbreviations
- ASAs
Acetylsalicylic acid
- BMI
Body mass index
- CON
Control exercise
- COX
Cyclooxygenase
- DEXA
Dual-energy X-ray absorptiometry
- DB
Double blockade, NOS–COX inhibition condition
- ECG
Electrocardiogram
- EDHF
Endothelium-derived hyperpolarizing factor
- FBF
Forearm blood flow
- FVC
Forearm vascular conductance
- KETO
Ketorolac, COX inhibition condition
- L-NMMA
L-NG-monomethyl arginine, NOS inhibition condition
- MAP
Mean arterial pressure
- MBV
Mean blood velocity
- MVC
Maximal voluntary contraction
- NOS
Nitric oxide synthase
- NSAIDs
Non-steroidal anti-infammatory drugs acetyl-salicylic acid
Footnotes
Conflict of interest The authors have no conflicts of interests and nothing to disclose.
References
- Arain FA, Kuniyoshi FH, Abdalrhim AD, Miller VM. Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine. Circ J. 2009;73:1774–1782. doi: 10.1253/circj.cj-09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol. 2011;202:271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwet LA, Redberg RF. The role of sex-specific results reporting in cardiovascular disease. Cardiol Rev. 2007;15:275–278. doi: 10.1097/CRD.0b013e318158b45b. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, et al. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Joyner MJ. NOS inhibition blunts and delays the compensatory dilation in hypoperfused contracting human muscles. J Appl Physiol. 2009;107:1685–1692. doi: 10.1152/japplphysiol.00680.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Madery BD, Curry TB, et al. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol. 2010;588:373–385. doi: 10.1113/jphysiol.2009.180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide-mediated vasodilation becomes independent of -adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol. 2011;110:687–694. doi: 10.1152/japplphysiol.00787.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Shepherd JRA, Joyner MJ. Sex and vasodilator responses to hypoxia at rest and during exercise. J Appl Physiol. 2013;116:927–936. doi: 10.1152/japplphysiol.00409.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dangardt F, Osika W, et al. Atherosclerosis. Atherosclerosis. 2012;220:269–274. doi: 10.1016/j.atherosclerosis.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol. 2011;589:3671–3683. doi: 10.1113/jphysiol.2011.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Luckasen GJ, et al. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. AJP: Heart Circ Physiol. 2013;305:H29–H40. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. KIR channel activation contributes to onset and steadystate exercise hyperemia in humans. AJP: Heart Circ Physiol. 2014;307:H782–H791. doi: 10.1152/ajpheart.00212.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. AJP: Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, New G, Tran BT, et al. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am J Physiol. 1999;276:H663–H670. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol. 1995;488(Pt 1):259–265. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Zhao W, Thomas GD. Impaired vasomodulation is associated with reduced neuronal nitric oxide synthase in skeletal muscle of ovariectomized rats. J Physiol. 2003;549:243–253. doi: 10.1113/jphysiol.2003.038828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten RS, Groot HJ, Rossman MJ, et al. The role of muscle mass in exercise-induced hyperemia. J Appl Physiol. 2014;116:1204–1209. doi: 10.1152/japplphysiol.00103.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94:3513–3520. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales JU, Thompson BC, Thistlethwaite JR, et al. Forearm blood flow follows work rate during submaximal dynamic forearm exercise independent of sex. J Appl Physiol. 2007;103:1950–1957. doi: 10.1152/japplphysiol.00452.2007. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, et al. Sex and ageing differences in resting arterial pressure regulation: the role of the -adrenergic receptors. J Physiol. 2011;589:5285–5297. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen I, Ilkka H, Saltin B, et al. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. AJP: Heart Circ Physiol. 2011;300:H1510–H1517. doi: 10.1152/ajpheart.00996.2010. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol. 2012;590:6297–6305. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr MD, Hogeman CS, Koch DW, et al. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. AJP: Heart Circ Physiol. 2010;298:H1626–H1632. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, et al. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2002;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Sun D, Carroll MA, et al. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. AJP: Heart Circ Physiol. 2001;280:H2462–H2469. doi: 10.1152/ajpheart.2001.280.6.H2462. [DOI] [PubMed] [Google Scholar]
- Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. AJP: Adv Physiol Edu. 2007;31:17–22. doi: 10.1152/advan.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamouzis M, Karamouzis I, Vamvakoudis E, et al. The response of muscle interstitial prostaglandin E2(PGE2), prostacyclin I2(PGI2) and thromboxane A2(TXA2) levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandins Leukot Essent Fatty Acids. 2001a;64:259–263. doi: 10.1054/plef.2001.0269. [DOI] [PubMed] [Google Scholar]
- Karamouzis M, Langberg H, Skovgaard D, et al. In situ microdialysis of intramuscular prostaglandin and thromboxane in contracting skeletal muscle in humans. Acta Physiol Scand. 2001b;171:71–76. doi: 10.1046/j.1365-201X.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- Khan LH, Rosenfeld CR, Liu XT, Magness RR. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. AJP: Endocrinol Metab. 2010;298:E222–E228. doi: 10.1152/ajpendo.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, et al. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Davis MJ, Secher NH, et al. Peripheral circulation. Compr Physiol. 2012;2:321–447. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- Limberg JK, Eldridge MW, Proctor LT, et al. Adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol. 2010;109:1360–1368. doi: 10.1152/japplphysiol.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg JK, Harrell JW, Johansson RE, et al. Microvascular function in younger adults with obesity and metabolic syndrome: role of oxidative stress. AJP: Heart Circ Physiol. 2013;305:H1230–H1237. doi: 10.1152/ajpheart.00291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg JK, Morgan BJ, Sebranek JJ, et al. Neural control of blood flow during exercise in human metabolic syndrome. Exp Physiol. 2014;99:1191–1202. doi: 10.1113/expphysiol.2014.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwald RR, Kirby BS, Crecelius AR, et al. Combined inhibition of nitric oxide and vasodilating prostaglandins abolishes forearm vasodilatation to systemic hypoxia in healthy humans. J Physiol. 2011;589:1979–1990. doi: 10.1113/jphysiol.2011.205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM. Sex-based differences in vascular function. Women’s Health. 2010;6:737–752. doi: 10.2217/whe.10.53. [DOI] [PubMed] [Google Scholar]
- Miller VM. Why are sex and gender important to basic physiology and translational and individualized medicine? AJP: Heart Circ Physiol. 2014;306:H781–H788. doi: 10.1152/ajpheart.00994.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JA, Martini ER, Smith MM, et al. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. AJP: Heart Circ Physiol. 2011;301:H1716–H1722. doi: 10.1152/ajpheart.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AW, Bearden SE, Segal SS. Regional activation of rapid onset vasodilatation in mouse skeletal muscle: regulation through -adrenoreceptors. J Physiol. 2010;588:3321–3331. doi: 10.1113/jphysiol.2010.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro P-J, Flavian A, Jacquier A, et al. Gender differences in response to cold pressor test assessed with velocity-encoded cardiovascular magnetic resonance of the coronary sinus. J Cardiovasc Magn Reson. 2011;13:54. doi: 10.1186/1532-429X-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Damsgaard R, et al. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Thaning P, et al. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension. 2009;53:993–999. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Blair SN, Lee I-M, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Pak KJ, Geary GG, Duckles SP, Krause DN. Male-female differences in the relative contribution of endothelial vasodilators released by rat tail artery. Life Sci. 2002;71:1633–1642. doi: 10.1016/s0024-3205(02)01851-9. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, et al. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol. 2007;103:1583–1591. doi: 10.1152/japplphysiol.00662.2007. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, et al. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol. 2008;104:655–664. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- Rogers J, Sheriff DD. Role of estrogen in nitric oxide- and prostaglandin-dependent modulation of vascular conductance during treadmill locomotion in rats. J Appl Physiol. 2004;97:756–763. doi: 10.1152/japplphysiol.00115.2004. [DOI] [PubMed] [Google Scholar]
- Saito Y, Iemitsu M, Otsuki T, et al. Gender differences in brachial blood flow during fatiguing intermittent handgrip. Med Sci Sports Exerc. 2008;40:684–690. doi: 10.1249/MSS.0b013e3181614327. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Wilkins BW, Johnson CP, et al. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol. 2010;109:768–777. doi: 10.1152/japplphysiol.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Madhani M, Chauhan S, et al. Investigation of vascular responses in endothelial nitric oxide synthase/ cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111:796–803. doi: 10.1161/01.CIR.0000155238.70797.4E. [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Naylor HL, Pozeg ZI, Hughson RL. Failure of prostaglandins to modulate the time course of blood flow during dynamic forearm exercise in humans. J Appl Physiol. 1996;81:1516–1521. doi: 10.1152/jappl.1996.81.4.1516. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. AJP: Heart Circ Physiol. 1997;273:H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- Taylor MK, Larson GE, Hiller Lauby MD, et al. Sex differences in cardiovascular and subjective stress reactions: prospective evidence in a realistic military setting. Stress. 2014;17:70–78. doi: 10.3109/10253890.2013.869208. [DOI] [PubMed] [Google Scholar]
- Villar IC, Hobbs AJ, Ahluwalia A. Sex differences in vascular function: implication of endothelium-derived hyperpolarizing factor. J Endocrinol. 2008;197:447–462. doi: 10.1677/JOE-08-0070. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Pike TL, Martin EA, et al. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol. 2008;586:1195–1205. doi: 10.1113/jphysiol.2007.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Witman MAH, Ives SJ, et al. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. AJP: Heart Circ Physiol. 2011;300:H1101–H1107. doi: 10.1152/ajpheart.01115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]