Abstract
Background
The field of HIV prevention research has recently experienced some mixed results in efficacy trials of pre-exposure prophylaxis (PrEP), vaginal microbicides, and HIV vaccines. While there have been positive trial results in some studies, in the near term, no single method will be sufficient to quell the epidemic. Improved HIV prevention methods, choices among methods, and coverage for all at-risk populations will be needed. The emergence of partially effective prevention methods that are not uniformly available raises complex ethical and scientific questions regarding the design of ongoing prevention trials.
Methods
We present here an ethical analysis regarding inclusion of PrEP in an ongoing phase IIb vaccine efficacy trial, HVTN 505. This is the first large vaccine efficacy trial to address the issue of PrEP, and the decisions made by the protocol team were informed by extensive stakeholder consultations. The key ethical concerns are analyzed here, and the process of stakeholder engagement and decision-making described.
Discussion
This discussion and analysis will be useful as current and future research teams grapple with ethical and scientific study design questions emerging with the rapidly expanding evidence base for HIV prevention.
Background
The field of HIV prevention research has recently experienced some mixed results. Several biomedical interventions, including pre-exposure prophylaxis (PrEP),1 treatment as prevention,2 vaccines,3 and vaginal microbicides,4 have shown some success in clinical trials, however, negative trial results have complicated the picture. In the near term, no single method will be sufficient to quell the epidemic. Improved HIV prevention methods, choices among methods, and coverage for all at-risk populations will be needed. As biomedical interventions are tested and approved by regulatory bodies, questions arise as to whether they should be incorporated into ongoing prevention trials, raising complex scientific and ethical questions. We present here an ethical analysis regarding inclusion of PrEP in an ongoing vaccine efficacy trial, HVTN 505. This is the first large vaccine efficacy trial to address the issue of PrEP, and the decisions made by the protocol team were informed by extensive stakeholder consultations. The key ethical concerns are analyzed here, and the process of stakeholder engagement and decision-making described. This discussion and analysis will be useful as current and future research teams grapple with the rapidly expanding evidence base for HIV prevention.
Prevention package in HIV clinical trials
Clinical trials of biomedical HIV prevention methods have always included a prevention package designed to help reduce HIV acquisition. This practice developed from stakeholders’ consensus during the 1990s that investigators have an ethical obligation to provide behavioral risk reduction counseling to participants in HIV prevention trials.5 The prevention package typically also includes counseling about post-exposure prophylaxis, free male and female condoms, lubricant, and diagnosis and treatment of (or referrals for) sexually transmitted diseases (STDs). The intent is to provide benefit to participants by helping them reduce HIV risk. At the same time, any reduced risk from the prevention package will also lead to increased clinical trial size, since it will reduce the overall rate of HIV incidence in the trial. The same issues arise in principle in HIV prevention trials involving people who inject drugs (PWID), for whom both sexual and parenteral HIV transmission are relevant. Different risk reduction methods for drug injection, such as provision of clean syringes, have also been advocated as part of a prevention package in trials involving PWID.6 Our discussion in this case centers around prevention of sexual transmission of HIV, as that is the main focus of HVTN 505.
Initial concerns about the need for risk reduction counseling arose in early HIV vaccine trials, as researchers became aware that many study participants falsely assumed (or hoped) that they would be protected from infection by as-yet unproven products, and may have been tempted to engage in riskier behavior.7 The concern about risk disinhibition is further fueled by the observed phenomenon that many trial participants do not fully accept that they may receive a placebo,8 even when randomization is emphasized during the informed consent process. The provision of a prevention package is part of the obligation to minimize risks in clinical trials, namely the risk of behavioral disinhibition. Failure to provide the package might be viewed as intentionally allowing or even increasing the risk of HIV negative individuals being exposed to HIV.9,10 In addition, the emergence of increased risk of HIV acquisition in a previous vaccine trial, the STEP trial (HVTN 502),11,12 was a reminder that all research involves uncertainty about product safety as well as efficacy, and this risk of unintended adverse effects provides further rationale for concern for protection of study participants.
There are additional reasons for providing additional benefits to trial participants. Participation in research can be viewed as a contribution to society that deserves reciprocity, and a prevention package is a way of giving back to the participants.13 Others have emphasized the researcher/participant relationship as a key factor in creating ethical obligations.14 A more general reason is the ethical requirement of beneficence: a person who is able to help another person in need, without unreasonable sacrifice of her own interests, should do so. This Good Samaritan argument places a responsibility on researchers and sponsors to help research participants as long as this does not consist of unreasonable personal sacrifice.15 However general arguments for beneficence do not help determine what level of benefit is sufficient.
In spite of the many arguments in favor of providing prevention benefits, questions have been raised about providing a package of prevention services above and beyond what is locally available.5,16 Concerns expressed include high costs, potentially privileging the study population relative to the broader community, and creating undue inducement to participate in research.
While a consensus has developed over the last decade that a standard package of prevention benefits is ethically important, the interventions provided in the package have been determined pragmatically. Affordable and feasible interventions such as counseling, condoms, and STD treatments can be provided in diverse settings, do not usually interact with biomedical products or typically affect the original research questions. Counseling about post-exposure prophylaxis (PEP), used optimally as an emergency intervention following unprotected, high-risk exposure, is also incorporated into risk reduction counseling conducted in prevention studies. Uptake of this strategy has been limited in many settings, however, due to factors such as cost and challenges with medication adherence.17, 18,19,20,21,22
As new biomedical methods become validated, the prevention package question becomes more complicated. The use of new methods may affect trial design, increase clinical trial costs, or threaten scientific validity of the study, and yet failure to address use of new interventions would lead to concern about neglect of participant welfare. Inclusion of the new methods is a way of accommodating the new realities in the field, while at the same time, there is ongoing debate about when and how new methods should be used at the population level, and how governments and donors should balance their financing of different prevention and treatment options. In an uncertain environment, it may be difficult to predict whether introducing new interventions into a trial would increase or decrease relevance of the trial results for health policy decision-making.
These issues touch on two fundamental ethical commitments: ensuring the social value of clinical research, and the protection of study participants. When the prevention interventions have the potential to interfere with the scientific objectives of the trial, a tension emerges between protection of participant welfare on the one hand, and the need to reliably and efficiently answer the primary study questions on the other. In spite of the existence of international ethical guidelines23,24 and specific guidance documents on biomedical HIV prevention trials,6,25 to date no comprehensive ethical framework has been developed which can directly adjudicate this ethical tension. We describe here the decision-making about inclusion of PrEP in a major HIV vaccine trial, HVTN 505, providing an early look at the issues clinical trial stakeholders will face in future HIV prevention research.
Methods
HVTN 505
HVTN 505 is a phase 2b HIV vaccine trial evaluating two vaccine candidates26, designed to elicit both antibody and T-cell responses, consisting of three injections of DNA prime vaccine encoding proteins (clade B gag, pol, nef and env proteins from clades A, B, and C) derived from HIV followed by a single boost of a recombinant adenovirus-5 (rAd5) vector vaccine encoding five proteins (clade B gag-pol fusion protein and clades A, B, and C env) derived from HIV.a Primary study objectives include assessment of a) protection against HIV acquisition through sexual exposure, b) viral load set-point for those individuals who are infected despite receipt of the vaccine, and c) safety of the vaccine regimen. The study enrolled 2504 healthy, HIV uninfected, Ad5 neutralizing antibody (nAb) negative, circumcised US men who have sex with men (MSM) and male-to female [MTF] transgender persons who have sex with men, aged 1850 years, at high risk for HIV-1 infection through sexual exposure at 21 US study sites.
In April 2013, vaccinations in the HVTN 505 study were halted after a prescheduled Data and Safety Monitoring Board review indicated that vaccination failed either to decrease acquisition of infection or lower HIV viral load for those who became infected after vaccination. There were 71 incident HIV-1 infections (41 in vaccine and 30 in placebo recipients) 48 of which occurred at least 4 weeks after completing the vaccination regimen. There were 27 in the vaccine group and 21 in the placebo group, a difference that was not statistically significant. The conditional probability of observing a different outcome with additional vaccinations was too low to support continuing vaccinations. HVTN 505 has been modified to become an observational study with close attention to lost-to-follow-up rates and monitoring of HIV infections in both study arms.27
Background on Truvada for PrEP
In 2010 and 2011, results from three major international trials showed that daily oral Truvada® is efficacious in reducing risk of acquisition of HIV in MSM and transgender women,1 heterosexual discordant couples,28 and in heterosexual men and women.29 In August 2012 FDA approved Gilead’s submission for the use of Truvada® for HIV prevention in certain populations. These events have stimulated vigorous debate about the appropriate role of PrEP in HIV prevention programs. Many believe that more information is needed about clinical implementation of PrEP, costs, and prioritization for at-risk groups. These developments have also raised the question of whether, when, or how PrEP should be offered to trial participants in HIV prevention clinical trials. There is currently no standard of practice in the field on use of PrEP in programs or research. Decision-making is complicated by the continuing need for adequate ARV treatment for those who are already infected.
At the moment, PrEP with Truvada® is not widely used in any country, and at the time of the HVTN 505 discussions, the inclusion of PrEP in a clinical trial would go beyond available clinical care in most settings. Also at that time, CDC had released preliminary guidance on PrEP, although more recent guidance has come out from CDC and WHO.30,31
Communication, HVTN 505 participant surveys and community consultation
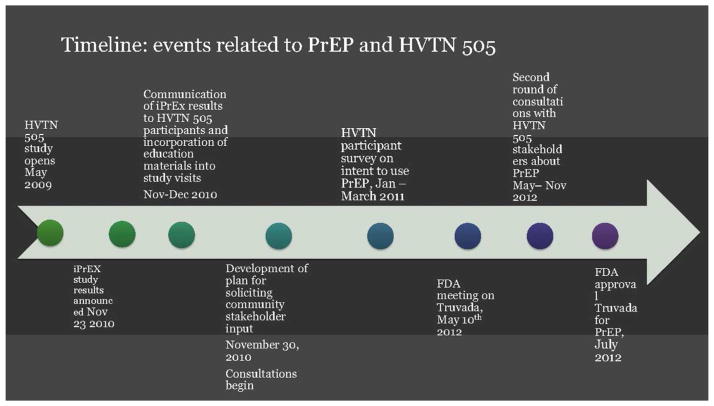
The first communication step was to discuss the results of the iPrEx study with study participants and communities, which was done via a letter to participants shortly after the release of the results to inform participants about the study, in addition to written educational materials about PrEP, individual education sessions with participants at study visits, meetings with Community Advisory Boards at every site, as well as community forums. The timeline for these activities relative to events surrounding the use of Truvada® for PrEP is shown in Figure 1. The dialogue was framed around informing people about PrEP and the results of the iPrEx study, and informing them that the HVTN 505 team is considering what the results mean to 505 and obtaining their feedback about what the results of iPrEx mean to the relevant communities and to prevention research. This process took place from November 2010, after the release of iPrEx results, through the summer of 2011.32 The HVTN 505 team also implemented an online survey33 from January to March 2011 to educate enrolled study participants about PrEP and collect data on their views of PrEP acceptability, affordability, plans to seek access to PrEP, and any concerns regarding use of PrEP by the respondents.
Figure 1.
Decision on PrEP for HVTN 505 participants
Subsequently, the team took up the question of PrEP use in the context of study participation. The team determined that it would not be feasible to redesign the study to reliably measure the combined effectiveness of PrEP plus vaccine, so the main question was whether or how PrEP might be provided as option for study participants. While the efficacy of Truvada® in MSM and transgender women populations in clinical trials is solidly established, and the risks of side effects are reasonably low, PrEP has not yet been widely implemented in any country since its recent approval by the FDA. The question arose as to whether participants in HVTN 505 should be offered PrEP, and if so, whether it should be paid for by the study or some other mechanism.
Three options were considered: a) provision of information about PrEP to study participants but no further action; b) provision of information about PrEP and referral of participants to sources of PrEP outside the study; and c) provision of both information as well as PrEP to those participants who were interested, as part of the study itself. The HVTN 505 protocol team leadership undertook extensive community consultations through HVTN-wide meetings, teleconferences, and meetings with advocacy groups when considering how to handle the PrEP decision. Ultimately, the general consensus was to provide information and educational materials about PrEP, developed specifically for vaccine trial participants, as well as referrals to providers willing to prescribe PrEP for trial participants wanting access. The protocol team approached Gilead and secured a donation of the product for HVTN 505 study participants. The coordinating center for the HVTN also established a contractual relationship with an independent mail-order pharmacy that could distribute the drug to individual participants.
Ethical considerations regarding inclusion of PrEP
In reviewing the ethical considerations associated with this decision, we considered four key areas: researchers’ obligations to study participants and communities; effects on study design; health policy considerations; and stakeholders’ opinions.
Researchers’ obligations to study participants and communities
Generally, obligations of beneficence require that researchers provide research-related health care commensurate with the relevant standard of care. In this case, PrEP had not become “standard” in the sense of widespread use and general availability. Pivotal clinical trials had been completed, but additional studies of implementation, adherence, and longer-term safety had not yet been done. Also, policies regarding insurance coverage and clinical practice were still in flux, suggesting that PrEP was not an established standard of care. Given this picture it is unclear to what obligations researchers would have regarding Truvada provision.
Also, some of the HVTN 505 trial sites are not direct care providers, making direct provision of Truvada through those sites more complicated, since use of Truvada requires medical monitoring. Some argued that sites should not provide an intervention they are not well equipped to deliver and monitor.
Since PrEP was not widely available, providing it to HVTN 505 trial participants could be seen as unfairly privileging these participants over others in the community. However, it also could be argued that study participants could be given more benefits due to their contribution to medical research and assumption of risks and burdens of study participation. In fact, UNAIDS’ Ethical Considerations in Biomedical HIV Prevention Trials states that investigators should provide all “state of the art” risk reduction methods to participants in clinical trials when they are scientifically validated or are approved by regulatory authorities.6 The document also states that plans for inclusion of new methods should be outlined in the protocol: “Mechanisms for negotiation among all research stakeholders, including the community, about the standards for enhancement of the risk reduction package during the trial as new biomedical HIV prevention modalities are scientifically validated or are approved by national authorities need to be set in the study protocol. Negotiations should take into consideration feasibility, expected impact, and the ability to isolate the efficacy of the biomedical HIV modality being tested, as other prevention activities improve.” While the guidance is helpful in outlining key issues for consideration, interpretation of the requirement for “state of the art” risk reduction methods may be challenging when some modalities have been scientifically validated but not fully implemented in host countries.
Another speculation was that use of Truvada® would encourage riskier behavior. There was no evidence of this in previous clinical trials (nor is this concern unique to Truvada). However a critical difference in the post-approval period for Truvada was that the product was then known to work and had FDA approval for use in HIV prevention. The concern about risk disinhibition with PrEP is speculative, as no systematic data exist on this point.
Another further theoretical possibility was diversion of Truvada during the trial. HVTN 505 participants could access Truvada® through the study and sell it to others via a “black market,” resulting in unmonitored use of the product. This could create negative consequences for individuals using the product, and greater potential for generating drug-resistant virus. With adequate measures, however, the risk of black market activity could be mitigated. To address this challenge, the study team developed plans to intensify counseling on use of Truvada® to emphasize that PrEP requires monitoring by qualified clinicians.
There may be added burden to research participants using Truvada who subsequently drop out of the trial: difficulty accessing the frequent HIV testing required for monitoring. HIV vaccine trial participants who received a study vaccine may require nucleic acid testing (NAT) for HIV to distinguish between true HIV infection and vaccine-induced sero-positivity (VISP). While former HVTN trial participants who exhibit VISP are offered ongoing NAT testing through the HVTN, this may be less convenient than standard testing. Therefore, counseling on longer term implications of VISP and use of PrEP was provided to HVTN 505 participants seeking PrEP.
If PrEP is provided at no cost in the research trial, does this put unreasonable pressure on a trial participant to continue in the study simply to maintain access? Participation in the trial during the follow up period involved only blood draws and HIV testing and did not pose a high risk for participants—meaning that continuation in the study did not involve unreasonable risk or burden. If the study were unreasonably risky, this calculation might be different. But as some commentators have noted,34 unreasonably risky studies should be stopped by IRBs in any case, making most arguments about undue inducement untenable. And as noted above, one of the arguments in favor of offering additional benefits to study participants is precisely because of their contribution to science through study participation—in other words, promoting reciprocity in trial benefits.
After analyzing direct effects of provision of PrEP with Truvada to study participants along with potential effects on communities, it seems reasonable, but not ethically required, to offer free access to participants, even when this access is not available to the broader community. This approach is consistent with the spirit of the UNAIDS guidelines in that the process of consultation identified potential benefits to participants and views in favor of PrEP provision, in spite of the fact that PrEP has not been widely implemented in the US or in any country at present
Effects on study design and health policy considerations
The ethical conduct of clinical research depends on carrying out scientifically sound, useful studies. Introducing new prevention methods into a clinical trial may affect study efficiency, potential for answering the primary research question(s), or usefulness for further decision making in regulatory, policy, or research domains. Therefore, ethical assessment must include evaluation of the impact of including PrEP in HVTN 505 on the study’s scientific viability and usefulness.
If a large number of HVTN 505 participants used PrEP, this would likely reduce HIV incidence in the trial, leading to the need for a larger sample size. Prior to FDA approval of PrEP but after publication of the iPrEx trial results, a previous HVTN 505 study protocol amendment had already included an allowance for larger study size to accommodate an estimate that 20% of the HVTN 505 participants might be using PrEP, or might use post-exposure prophylaxis repeatedly. Therefore, the study team was not concerned that PrEP use would lead to an underpowered trial in this case.
It is important to consider the overall usefulness of the trial’s primary results for future decision making in research and policy decisions. In this case, allowing use of PrEP in the trial neither significantly enhances nor detracts from the usefulness of the primary results, since the study was not powered to measure the combined effect of the vaccine regimen and PrEP, and there would be no combination strategy developed on the basis of this trial. The usefulness of the primary objectives, vaccine efficacy and lowering viral load set-point, will not be significantly altered. If the study’s scientific integrity had been significantly reduced by provision of PrEP, scientific goals could conflict with the goal of providing more benefits to trial participants, creating a stark dilemma. Fortunately, these circumstances did not lead to such an ethical impasse.
One theoretical possibility is biological interaction between PrEP and vaccine products, which could affect either safety or efficacy in the study. Due to the separate and unrelated mechanisms of action of the two products, it is unlikely that significant interactions would occur. A more plausible possibility would be synergistic action in reducing HIV acquisition or viral load set-point, making it difficult to identify independent vaccine-specific effects. The HVTN 505 protocol is collecting self-report of Truvada use and is monitoring blood levels of Truvada in a sample of participants to approximate the true uptake of PrEP during the trial, which may be helpful for future analysis of the interaction of the two products, although this protocol is not powered to measure interaction statistically. Also, the additional data to be collected about safety and reported adherence to PrEP are informative for the prevention field, the pharmaceutical sponsor, and future implementation decisions, as well as informing future study design decisions.
In sum, the study integrity or ability to detect an effect of the HIV vaccine regimen would not be significantly diminished by the addition of PrEP. While there are cases in which additional prevention modalities have significant impacts on a study’s ability to generate endpoints or draw conclusions about the intervention of interest, in this case the addition of PrEP was not deemed a threat to the study’s design. It is important to note that in general, the integrity of the study and the ability to answer the research questions must always be considered when adding or changing prevention modalities in any HIV prevention trial.
Opinions/views of key stakeholders
The views of stakeholders are pragmatically and ethically important. First, it may be simply impossible to conduct the study if key stakeholders do not agree on basic arrangements. Second, even if it is possible to carry out the study over the objections of some parties, these kinds of actions can create lasting damage to important relationships and foster distrust among key stakeholders. Third, there is inherent value in the respectful treatment of stakeholders and participants in research enterprise, regardless of specific outcomes.
In anticipation of the FDA decision regarding use of Truvada as PrEP, the HVTN 505 protocol team held a series of consultations with site investigators, leadership at the National Institute of Allergy and Infectious Diseases (NIAID), Institutional Review Boards, advocacy group members, including the NIAID Be The Generation Bridge Partners, and community stakeholders. During the consultations it was evident that majority favored PrEP provision and education about PrEP in the trial, and only minority opposed provision of PrEP. Key considerations in favor of providing PrEP were (1) some considered it unethical not to provide an option that was proven effective; and (2) providing PrEP broadens the choice of HIV prevention options for participants.
Key considerations mentioned against provision of PrEP were (1) concerns about side effects, (2) challenges with adherence, (3) the risk of generating HIV-1 resistance to Truvada, and (4) the potential for behavioral disinhibition. The HVTN 505 protocol team also conducted a survey of 400 study participants asking about their views on PrEP. Results showed that about 30% of participants were moderately or very interested in using PrEP; although a majority (68%) expressed that they were unlikely to take it at their own expense.33
At the end of the consultations, there was agreement that the preferred option was to reintensify education and counseling about pre-exposure prophylaxis and develop a referral system rather than to provide the drug directly at trial sites as part of the study. This option was viewed by all stakeholders as empowering the participants to make informed decisions about PrEP independent of their study participation, creating a flexible system that would be compatible with variety of infrastructures at trial sites, and feasible given diverse levels of expertise and experience with PrEP at the sites. Most importantly, the referral plan ensures that participants have a relationship with a primary care provider who can prescribe PrEP and monitor its use after the conclusion of HVTN 505.
If Truvada had been purchased through HVTN 505 study funds, this would have increased study costs significantly. The possibility of free access to PrEP through a HVTN-supported mail-order pharmacy mechanism and donation from Gilead alleviates this burden on the sponsor and the team. However, the most important consideration for the study participants is the ability to make a choice about whether or not to use PrEP.
The decision by the research team to include PrEP in HVTN 505 can affect longer term relationships amongst stakeholders such as community members and researchers. On the one hand, provision of PrEP may positively affect community members’ views of the research. On the other hand, offering the options of accessing PrEP may set a precedent for future vaccine trials. Provision of PrEP in HVTN 505 depended on the manufacturer’s willingness to provide the product, which may not always be available in future trials. Therefore, issues regarding costs and procurement may become more complex in future cases.
Discussion
The discussions about PrEP in HVTN 505 offer useful lessons. First, regarding the process, the team’s extensive consultations with community representatives and other parties facilitated full consideration of stakeholder views and transparent communication. These steps will be essential in all HIV vaccine and prevention efficacy trials. The tradition of robust community engagement in HIV vaccine research has provided a good foundation for discussions of complex new issues involving emerging prevention methods, and the design and conduct of clinical trials. That said, diverse opinions may be expressed, and there will not always be consensus on the best way to move forward in a trial as stakeholders grapple with new evidence and policy changes in the field. In this case, consensus was reached on how to move forward.
Second, the most contentious ethical dilemmas did not arise in full force in this case, since a) PrEP could be offered in the trial without unduly disrupting the scientific integrity of the study; b) a supply of PrEP was made freely available by Gilead, obviating any concerns about barriers to access; and c) the stakeholders consulted approved of the plan. Also, this trial was conducted exclusively in the US, thus not raising further complexities about different standards of care in limited-resource settings and countries and different regulatory standards and approvals. Future trials will likely encounter these knotty ethical challenges. In the near future, more difficult trial design decisions will emerge as clinical trial teams face an array of partially effective biomedical prevention methods. The feasibility and desirability of incorporating different prevention interventions into clinical trials will have to be explored with attention to stakeholder views and interests, scientific soundness of the trial, utility of the trial for decision-making, local standards of care, and ethical acceptability. In the most difficult cases, participant benefit conflicts directly with scientific goals of the study.
Our analysis should be helpful for these future scenarios in that we have identified key domains for discussion and debate. At a minimum, scientific integrity must be protected for a trial to go forward, and stakeholder consultation should identify key areas of consensus or disagreement in considering different elements of prevention packages. The relationship of the prevention package to the local or national standard of care will also be a subject of discussion and will impact the overall utility of each prevention trial in informing policy decisions. Frequently, local and national standards of care differ among different countries that may be involved in a multisite trial, complicating this analysis. There is frequently no easy formula for balancing competing ethical priorities in providing participant benefits and advancing scientific inquiry. Further conceptual analysis will be required to fully explicate ethical responsibilities of researchers and funders in these scenarios.
Conclusion
The question of how to design and conduct future HIV prevention trials will become increasingly complex as more partially effective prevention methods are developed.35 Ethical and scientific challenges may intensify as new interventions become widely distributed. The key domains considered in the case of PrEP introduction during the HVTN 505 efficacy trial will be essential aspects to consider in future cases. The approach taken by the HVTN 505 leadership team highlights the importance of thorough consultations with all stakeholders. As the HIV prevention standard of care evolves and clinical trial design becomes increasingly challenging, both ethically and scientifically, communication and transparency with all stakeholders will continue to be essential.
Acknowledgments
The authors and the HVTN 505 team gratefully acknowledge the input of study participants, site staff, investigators, Community Advisory Board Members, community stakeholders and advocates who participated in the consultations. The HVTN 505 team also gratefully acknowledges support from Gilead Sciences for the Truvada® used in the Referral Program and for the commitment to the development and implementation of the Referral Program.
No specific funding was provided for the preparation of this article. The HVTN 505 clinical trial was supported by grants from the National Institute of Allergy and Infectious Diseases of the NIH (UM1AI68614, UM1AI068618, UM1AI68635, UM1AI069496, UM1AI69452, UM1AI069412, UM1AI069470, UM1AI069481, UM1AI069439, UM1AI069534, UM1AI069511, UM1AI069554, UM1AI069418, UM1Al069532, UM1AI069424, UM1AI069501, and UM1AI036219 and contract HHSN272200800014C), Columbia University’s Clinical and Translational Science Award from the National Center for Advancing Translational Sciences, NIH (UL1TR000040), the NIH Intramural Research Program, Emory Center for AIDS Research (P30AI50409), the Harvard Center for AIDS Research (P30AI06354), the Baylor–University of Texas Houston Center for AIDS Research (UM1AI036211), the Colorado Clinical Translational Science Institute (TR000154), and the National Center for Advancing Translational Science, NIH (UL1TR000451).
The views expressed in this article are those of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Footnotes
Full title of HVTN 505: Phase 2b, randomized, placebo-controlled test-of concept trial to evaluate the safety and efficacy of a multiclade HIV-1 DNA plasmid vaccine followed by a multiclade HIV-1 recombinant adenoviral vector vaccine in HIV-uninfected, adenovirus type 5 neutralizing antibody negative, circumcised men and male-to-female (MTF) transgender persons, who have sex with men. The study was reviewed by appropriate IRBs at participating institutions and informed consent was obtained from all trial participants.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with Alvac and Aidsvax to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 4.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Campaign for Microbicides. Rethinking the Ethical Roadmap for Clinical Testing of Microbicides: Report on an International Consultation. Washington, DC: 2005. [Google Scholar]
- 6.UNAIDS and WHO. Ethical considerations in biomedical HIV prevention trials. 2012. [Google Scholar]
- 7.Chesney MA, Chambers DB, Kahn JO. Risk behavior for HIV infection in participants in preventive HIV vaccine trials: a cautionary note. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:266–271. doi: 10.1097/00042560-199712010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Ndebele PM, Wassenaar D, Munalula E, et al. Improving understanding of clinical trial procedures among low literacy populations: an intervention within a microbicide trial in Malawi. BMC Med Ethics. 2012;13:29. doi: 10.1186/1472-6939-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thapinta D, Jenkins RA, Celentano DD, et al. Evaluation of behavioral and social issues among Thai HIV vaccine trial volunteers. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:308–314. doi: 10.1097/00042560-199903010-00015. [DOI] [PubMed] [Google Scholar]
- 10.Ramjee G, Coumi N, Dladla-Qwabe N, et al. Experiences in conducting multiple community-based HIV prevention trials among women in Kwazulu-Natal, South Africa. AIDS Res Ther. 2010;7:10. doi: 10.1186/1742-6405-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchbinder SP, DMehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated Immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray GE, Allen M, Moodie Z, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11:507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Bioethics Advisory Commission. Ethical and policy issues in international research: clinical trials in developing countries. 2001. [PubMed] [Google Scholar]
- 14.Stobie M, Slack C. Treatment needs in HIV prevention trials: using beneficence to clarify sponsor-investigator responsibilities. Dev World Bioeth. 2010;10:150–157. doi: 10.1111/j.1471-8847.2009.00272.x. [DOI] [PubMed] [Google Scholar]
- 15.Lie RK, Emanuel EJ, Grady C. Circumcision and HIV prevention research: an ethical analysis. Lancet. 2006;368:522–525. doi: 10.1016/S0140-6736(06)69160-5. [DOI] [PubMed] [Google Scholar]
- 16.Philpott S, Heise L, McGrory E, et al. The challenge of defining standards of prevention in HIV prevention trials. J Med Ethics. 2011;37:244–248. doi: 10.1136/jme.2010.037176. [DOI] [PubMed] [Google Scholar]
- 17.Mayer KH, Mimiaga MJ, Cohen D, et al. Tenofovir DF plus lamivudine or emtricitabine for nonoccupational postexposure prophylaxis (NPEP) in a Boston community health center. J Acquir Immune Defic Syndr. 2008;47:494–499. doi: 10.1097/QAI.0b013e318162afcb. [DOI] [PubMed] [Google Scholar]
- 18.Pinkerton SD, Martin JN, Roland ME, et al. Cost-effectiveness of postexposure prophylaxis after sexual or injection-drug exposure to human immunodeficiency virus. Arch Intern Med. 2004;164:46–54. doi: 10.1001/archinte.164.1.46. [DOI] [PubMed] [Google Scholar]
- 19.Laporte A, Jourdan N, Bouvet E, et al. Post-exposure prophylaxis after non-occupational HIV exposure: impact of recommendations on physicians’ experiences and attitudes. AIDS. 2002;16:397–405. doi: 10.1097/00002030-200202150-00011. [DOI] [PubMed] [Google Scholar]
- 20.Scheid DC, Hamm RM, Stevens KW. Cost effectiveness of human immunodeficiency virus postexposure prophylaxis for healthcare workers. Pharmacoeconomics. 2000;18:355–368. doi: 10.2165/00019053-200018040-00004. [DOI] [PubMed] [Google Scholar]
- 21.Beymer MR, Bolan RK, Flynn RP, et al. Uptake and repeat use of postexposure prophylaxis in a community-based clinic in Los Angeles, California. AIDS Res Hum Retroviruses. 2014;30:848–855. doi: 10.1089/aid.2014.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landovitz RJ, Combs KB, Currier JS. Availability of HIV postexposure prophylaxis services in Los Angeles County. Clin Infect Dis. 2009;48:1624–1627. doi: 10.1086/598976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Medical Association. Declaration of Helsinki: ethical principles for medical research Involving human subjects. 2013. [DOI] [PubMed] [Google Scholar]
- 24.Council for International Organizations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. 2002. [PubMed] [Google Scholar]
- 25.UNAIDS and AVAC. Good participatory practice: guidelines for biomedical HIV prevention trials. 2011. [Google Scholar]
- 26.www.clinicaltrials.gov NCT00865566
- 27.Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States -2014: a clinical practice guideline. 2014. [Google Scholar]
- 31.World Health Organization. Guidance on oral pre-exposure prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. Geneva: WHO Press; 2012. [PubMed] [Google Scholar]
- 32.Broder GB, Maynard JP, Karuna ST, et al. Implementing good participatory practice (GPP) in HVTN 505 - the HIV Vaccine Trials Network (HVTN) experience. AIDS Res Hum Retroviruses. 2014;30:A105–106. [Google Scholar]
- 33.Fuchs JD, Sobieszczyk ME, Madenwald T, et al. Intentions to use preexposure prophylaxis among current phase 2B preventive HIV-1 vaccine efficacy trial participants. J Acquir Immune Defic Syndr. 2013;63:259–262. doi: 10.1097/QAI.0b013e318296df94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emanuel EJ. Undue inducement: nonsense on stilts? Am J Bioeth. 2005;5:9–13. doi: 10.1080/15265160500244959. discussion W8–11, W17. [DOI] [PubMed] [Google Scholar]
- 35.Janes H, Gilbert P, Buchbinder S, et al. In pursuit of an HIV vaccine: designing efficacy trials in the context of partially effective nonvaccine prevention modalities. AIDS Res Hum Retroviruses. 2013;29:1513–1523. doi: 10.1089/aid.2012.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]