Summary
No systematic review of epidemiological evidence has examined risk factors for sleep disturbances among older adults. We searched the PUBMED database combining search terms targeting the following domains (1) prospective, (2) sleep, and (3) aging, and identified 21 relevant population-based studies with prospective sleep outcome data. Only two studies utilized objective measures of sleep disturbance, while six used the Pittsburgh Sleep Quality Index (PSQI) and thirteen used insomnia symptoms or other sleep complaints as the outcome measure. Female gender, depressed mood, and physical illness were most consistently identified as risks for future sleep disturbances. Less robust evidence implicated the following as potentially relevant predictors: lower physical activity levels, African-American race, lower economic status, previous manual occupation, widowhood, marital quality, loneliness and perceived stress, preclinical dementia, long-term benzodiazepine and sedative use, low testosterone levels, and inflammatory markers. Chronological age was not identified as a consistent, independent predictor of future sleep disturbances. In conclusion, prospective studies have identified female gender, depressed mood, and physical illness as general risk factors for future sleep disturbances in later life, although specific physiological pathways have not yet been established. Research is needed to determine the precise mechanisms through which these factors influence sleep over time.
Keywords: Aging, insomnia, prospective research, risk factors, sleep disturbance, sleep quality
Prospectively established risk factors for sleep disturbances in older adults
The percentage of the global population aged 65 years or older is expected to double by 2040 1, and 36-69% of older adults report sleep disturbances2. Sleep disturbances are not only intrinsically detrimental to quality of life, they can cause or complicate physical 3 and mental 4 illness and raise the risk of mortality 5. Identifying the specific factors which increase the risk of developing sleep disturbances can help target interventions and in turn improve the overall health of our aging population.
Sleep aging research spans multiple disciplines and approaches including both human and animal studies as well as both cross-sectional and prospective designs. The wide range of topics covered includes: aging-related changes in sleep brain electrophysiology 6, circadian processes 7, and the associations of sleep with hormonal 8, immunological 9, neurodegenerative 10, and psychosocial factors 11, 12. Prior reviews have tended to draw evidence from multiple methods and sources and offer comprehensive reports on late-life sleep disturbances and factors involved in their etiology 13-15. To our knowledge, however, no prior review has systematically evaluated prospectively established epidemiologic evidence for risk factors associated with the broad category of sleep disturbances that occur in late life.
Some research indicates effects of both chronological as well as non-chronological aging-related risk factors (such as chronic health conditions) on sleep 16. Therefore a major task of sleep aging research is to examine factors associated with the development of sleep disturbances apart from the potential impact of chronological aging itself 15, 17. However, most existing sleep aging research use cross-sectional designs which are not suited to make temporal inferences regarding risk relations. Studies have attempted to isolate the effect of age by comparing “healthy” older adults (that is, those lacking a diagnosis of a major medical condition) to younger adults. However this approach is limited by the fact that subsyndromal disease processes are prevalent and poorly captured by traditional methods in older adults 18, and therefore these designs also fail to isolate disease processes which are more prevalent among older adults from the effect of chronological aging itself. Prospective studies have the advantage of comparing participants who all experience comparable chronological aging throughout the study’s follow-up period, thereby isolating effects of non-chronological aging risk factors from the potential role of chronological aging.
A systematic assessment of prospective studies may therefore provide an accurate view of what is currently known regarding temporally anteceding risk factors for sleep disturbances. Our objective is to systematically document the determinants of sleep outcomes in older adults identified through prospective research, focusing on a broad array of sleep disturbances including sleep quality and insomnia. Our goal is to provide the reader a sense of the breadth, strengths, and limitations of the current longitudinal evidence-base in order to document what is known and where future efforts are required.
Methods
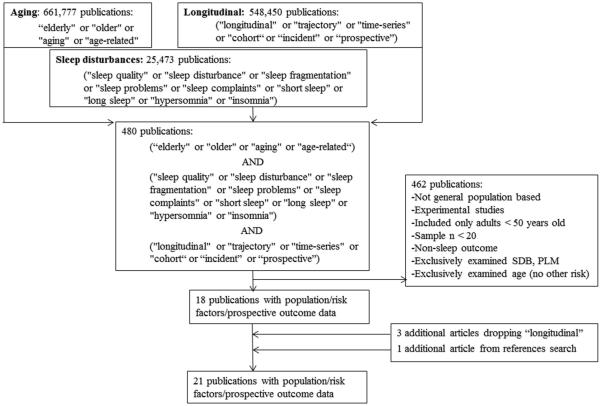
A search strategy was developed after establishing inclusion/exclusion criteria (Figure 1). We did not include studies which examined sleep disordered breathing (SDB) and periodic leg movements (PLM) as the only outcome for the following reasons: (1) the classification of SDB and PLM require objective sleep measures thus limiting the existing prospective evidence-base, and (2) the etiology of SDB and PLM involves physical health characteristics (i.e. SDB: adiposity/upper airway structure, and PLM: medication use/specific diseases), whereas the broad category of sleep disturbances examined herein may have both physical and psychological origins.
Figure 1.
Flowchart indicating the results of the systematic review
The PUBMED database was searched by targeting the following domains: (1) aging (search terms “aging,” “age-related,” “age-related changes,” “elderly,” or “older” returned 661,777 publications), (2) sleep disturbances (search terms "sleep quality," "sleep disturbance," "sleep fragmentation," "sleep problems," "sleep complaints," "short sleep," "long sleep," "hypersomnia" or "insomnia" returned 25,473 publications), and (3) prospective (search terms "longitudinal," "trajectory," "time-series," "cohort,“ or “incident” or “prospective” returned 548,450 publications). When these terms were combined with the “AND” operator, 480 articles returned. Titles and abstracts were then examined to determine if these articles met inclusion/exclusion criteria (listed in Figure 1). Studies which examined only age were excluded because they are unable to distinguish effects of age from those of factors related to age (like disease) and this is a major aim of the review.
Articles were initially excluded if they were not conducted among older adults, if their design was cross-sectional, or if sleep variables were used as predictors instead of the outcome. Abstracts were read when titles were insufficient for determining if the article met all inclusion/exclusion criteria. This process identified 18 studies. Studies were required to include older adults (>50 years of age), however three studies also included participants <50 years of age and tested associations of risk factors with sleep disturbances separately among a separate subgroup meeting the age criteria. A reliability study was performed by independent raters who reviewed a randomly selected subset (20%, n=96) of articles retrieved in the database search. Comparing these rater’s selections to the initial reviewer’s determinations indicated complete rater agreement.
For the sake of completeness, the search process was repeated without the addition of the “longitudinal” search terms, and 3,000 articles were returned. This technique identified all studies detected with the original method, as well as two additional studies which were included. Finally, one additional article was identified by examining the reference lists of included studies resulting in a total of 21 papers in this review. We report all predictors considered as well as which were significantly related to sleep outcomes. When a factor was identified as a significant predictor, we report all other studies including the factor regardless of significance level. When a factor is examined in several (>3) studies we report the portion of positive or null findings identified.
Results
The 21 articles were grouped based on their outcome measures. Self-reported sleep complaints/insomnia symptoms(n = 13) were the most common outcome measures, followed by global subjective sleep quality (n = 6). Objectively measured sleep characteristics were the least common of the identified outcome measures (n = 2). The studies utilizing the Pittsburgh sleep quality index (PSQI) assessed subjective sleep. Although this questionnaire includes items utilized in studies reviewed in the Self-reported sleep complaints/insomnia symptoms section, the PSQI is designed to capture sleep quality globally and is therefore considered separately.
Self-reported sleep complaints/insomnia symptoms (n = 13)
Identified studies of insomnia symptoms were published between 1997 and 2013 (Table 1). More than half of these studies (8/13) were conducted internationally (three from the United Kingdom and one from each of: Germany, Scotland, South Korea, Japan, and Nigeria), with 5/13 conducted in the United States. Among these thirteen studies, insomnia was heterogeneously defined, although most studies considered insomnia to be simply the presence of difficulty initiating sleep (DIS), difficulty maintaining sleep (DMS), or early morning awakening (EMA). One study defined insomnia symptoms by asking if participants had “trouble” sleeping 19, while two asked if participants had “problems” sleeping 20, 21, and another asked “how satisfied are you with your sleep?” 22. One study also examined hypersomnia (sleeping too much) in addition to DIS/DMS complaints 23. Only three studies required insomnia symptoms to be present for a certain duration (“often” in 21; at least 3 nights per week in 24; and lasting ≥2 weeks in 25), which is a key criteria required for an insomnia diagnosis.
Table 1.
Longitudinal studies (n = 13) of self-reported sleep complaints/insomnia symptoms
| First Author (year) |
Follow-up time/ sample size |
Country/ Recruitment |
Prospective Design |
Predictors / Covariates | Outcome | Independent Predictors of Prospective Sleep Outcome |
|---|---|---|---|---|---|---|
| Morgan21
(1997) |
8 years/ n=1042 |
United Kingdom/ random sample |
Excluded prevalent insomnia in incident analysis |
Demographic: Age, gender, body weight; Psychosocial: Morale, bereavement, anxiety, depression, living alone; Physical: Physical health status, physical activity |
Insomnia defined as “having problems sleeping” often or all of the time in the past week |
Depression, intermediate or low physical activity, and physical health status were associated with insomnia incidence; Female gender and age were not related to insomnia risk. |
| Foley26
(1999) |
3 years/ n=4956 |
United States/total community census or random sample |
Excluded prevalent insomnia in incident analysis |
Demographic: age, gender, study site, income, education; Psychosocial: clinically significant depressive symptoms, cognitive impairment (global cognitive function); Physical: self- reported chronic medical conditions, smoking, alcohol consumption, activity of daily living disability, respiratory symptoms, sedative use |
Any DIS, EMA symptom defined as insomnia |
Depressed mood, respiratory symptoms, worse perceived health, physical disability, sedative use, and widowhood. |
| Foley27
(1999) |
3 years/ n=2971 |
United States/ random sample |
Excluded prevalent insomnia in incident analysis |
Demographic: age, gender, race; Psychosocial: affective well-being; Physical: self- reported health, disability, respiratory symptoms, sedative use |
Any DIS, EMA symptom defined as insomnia |
Depressed mood predicted insomnia incidence; African-American women had higher incidence of insomnia than African-American men or white men and women; consistently poor health and being age 74-84 (among whites only) predicted insomnia incidence |
| Roberts23
(1999) |
1 year/ n=2380 |
United States/ stratified random sample |
Excluded prevalent insomnia in incident analysis |
Demographic: age, gender, education, marital status; Psychosocial: financial strain, life events, mood disturbance; Physical: chronic health, ADL problems |
Single item for DIS/DMS defined insomnia, and single item defined hypersomnia |
Female gender, mood disturbance, and chronic medical conditions predicted incident insomnia; recent life events, mood disturbance, and chronic medical conditions predicted incident hypersomnia |
| Morgan20
(2003) |
4 or 8 years/ n=1042 |
United Kingdom/ random sample |
Excluded prevalent insomnia in incident analysis |
Demographic: age, gender; Psychosocial: social engagement, clinically significant depressive symptoms; Physical: physical health status, daytime physical activity, walking, BMI |
"Do you ever have problems sleeping?" |
Physical activity and being 75+ years old predicted incident sleep problems; clinically significant depressive symptoms was a marginally significant predictor; |
| Quan29
(2006) |
3.6 years/ n=4467 |
United States/ random sample |
Excluded prevalent insomnia in incident analysis |
Demographic: Age, gender, race, follow-up time; Psychosocial: Clinically significant depressive symptoms; Physical: Psychoactive or hypertensive medication use, chronic illnesses, self-rated health, BMI, activity of daily living impairment (ADL), instrumental activity of daily living impairment (IADL) |
DIS; DMS | Among men incident DIS was related to: current clinically significant depressive symptoms, baseline ADL impairment, current psychoactive medication use, hypertensive medication use, and incident coronary heart disease (CHD); Among women incident DIS was related to: clinically significant depressive symptoms, respiratory symptoms, baseline arthritis, and current psychoactive medication use; Among men incident DMS was associated with: clinically significant depression, BMI, and consistently poor self-reported health; Among women incident DMS was associated with: clinically significant depression, respiratory symptoms, IADL impairment, and hypertensive medication use |
| Kim24
(2009) |
2 years/ n=909 |
South Korea/ invited all residents from 2 defined areas |
Excluded prevalent insomnia in incident analysis |
Demographic: Age, gender, education, past occupation, current employment, housing (owned or rented), living area (urban or rural); Psychosocial: Life events, social support, cognitive impairment (probable dementia diagnosis), clinically significant depressive symptoms, anxiety symptoms; Physical: Chronic health conditions, physical activity, anxiety, alcohol consumption |
DIS/DMS 3 nights/week over the last month defined insomnia |
Clinically significant depressive symptoms, physical disorders, and previous manual occupation |
| Fok19
(2010) |
1 year/ n=656 |
United Kingdom/ invited all residents from one defined area |
Excluded prevalent insomnia in incident analysis |
Demographic: Age, sex, marital status, social class; Psychosocial: Depressive symptoms, social support, depression; Physical: ADL impairment, illness |
"Have you had trouble sleeping over the past month?" (sleep complaint) |
Female sex, being a widow, and depressive symptoms |
| Gureje25
(2011) |
1 year/ n=1,307 |
Nigeria/ random sample |
Excluded prevalent insomnia in incident analysis |
Demographic: Age, gender, economic status; Psychosocial: lifetime major depressive disorder (MDD), dementia, stressful life events; Physical: Functional capacity, chronic physical/pain conditions, functional limitations, BMI |
Questions regarding DIS, DMS, EMA, non-restorative sleep, daytime sleepiness, dissatisfaction with sleep; complaints lasting for ≥ 4 weeks were considered insomnia syndrome |
Females were at elevated risk for insomnia symptoms and syndrome; lower economic status and chronic medical conditions increased insomnia, lifetime MDD was marginally associated with insomnia |
| Inoue30
(2012) |
3 years/ n=3,697 |
Japan/ stratified random sample |
Excluded prevalent insomnia in incident analysis |
Demographic: Age, gender, SES; Psychosocial: Single mood question; Physical: Physical activity, pre-existing disease |
Questions on DIS, DMS, EMA, or requiring hypnotics to fall asleep; insomnia was defined as one or more symptom |
Frequent physical activity reduced the risk of DMS only, but only among individuals without pre-existing disease (other associations not reported) |
| Pedraza28
(2012) |
3 years/ n=1,085 |
United States/ probability sample |
Did not assess/exclude prevalent cases at baseline: only predicted prevalence at follow-up |
Demographic: Gender, marital status, education; Psychosocial: Cognitive impairment (global cognitive function), depressive symptoms; Physical: chronic diseases, ADL, BMI |
Sleep complaints (DIS, DMS, difficulty staying sleep, non- restorative sleep) more than 15 days in the past month, and quality of sleep measured with a single item |
DIS: depressive symptoms; DMS: diabetes, cancer, and obesity; Trouble staying sleep: diabetes, hypertension, cancer, and depressive symptoms; Non-restorative sleep: diabetes, hypertension, cancer, and depressive symptoms; poor sleep quality: female gender, being married, and depressive symptoms |
| Green31
(2012) |
20 years/ n=1,444 (~36 years old at baseline) plus n=1,551 (~56 years old at baseline) |
Scotland/ stratified random sample |
Data-driven method to examine distinct patterns of change over time (serial sleep measures used) |
Demographic: Gender, cohort, occupational class; |
Weekly occurring DIS or DMS |
Latent class growth analysis revealed four groups: (1) healthy with minimal probabilities of DIS or DMS; (2) episodic complaints, (3) developing, and (4) chronic); being a female predicted worse trajectories, and manual occupation predicted developing or chronic trajectories. |
| Lemola22
(2013) |
14,179 including ages 18-85 reported separately as < or ≥ age 60 |
Germany/ nationally representati ve sample |
Repeated measures from four time points used in cross- lagged panel model |
Demographic: Gender, age; Psychosocial: Retirement; Physical: Self-reported health |
“How satisfied are you with your sleep?” |
Self-reported health was independently related to future sleep quality among older and young adults; retirement was associated with an increase in subjective sleep quality; |
Female gender 19, 23, 25-29, depressed mood 19, 20, 24, 26-29 and poor physical health 21-24, 26-29were most consistently reported as independent predictors of these sleep outcomes. Four studies examined physical activity with mixed findings (see discussion)20, 21, 24, 30. These studies also examined demographic and other psychosocial factors: African-American race (compared to white race)27, previous manual occupation 24, 31, widowhood 19, 26, and lower economic status 25 predicted incident insomnia symptoms; recent life events predicted hypersomnia but not insomnia in one study 23.
Independent estimates of the effects of age were reported in the minority of these studies (5/13) 20, 21, 23, 27, 28, in two cases presumably due to the fact that age was not associated with future sleep disturbance in univariate analyses 19, 25. Of the studies examining the adjusted effect of age, one identified increased risk for older (75+ years) adults18, while another study found significantly increased risk for incident insomnia symptoms for white, but not black adults aged 75-84 27. The other three studies reporting found no age effect 21, 23, 28.
PSQI assessed subjective sleep (n = 6)
Reports examining subjective sleep quality with the PSQI were published between 2010 – 2014 (n = 6; Table 2). These studies were conducted in South Korea, Taiwan, the United States, Canada, France, and Ireland. Benzodiazepine use duration was associated with a faster rate of sleep quality decline 32; hypnotic use predicted lower odds of sleep measures improving over time 33.
Table 2.
Longitudinal studies using the Pittsburgh Sleep Quality Index (PSQI) (n = 6)
| First Author |
Follow-up time/sample size |
Country/ Recruitment |
Prospective Design |
Predictors / Covariates | Outcome | Independent Predictors of Prospective Sleep Outcome |
|---|---|---|---|---|---|---|
| Dowd37
(2010) |
6 years/ n=1,020 |
Taiwan/ random sample |
Did not assess/exclude prevalent cases at baseline: only predicted prevalence at follow-up |
Demographic: age, sex; Physical:
IL-6, albumin, WBC, CRP, e- selectin, sICAM-1 |
PSQI derived sleep duration |
Increases in IL-6 and s- ICAM-1 and decreases in albumin were associated with risk for future long sleep only; PSQI sleep quality not examined prospectively |
| Phelan35
(2010) |
8 or 10 years/ n=115 |
United States/ convenience sample |
Growth curve and growth mixture models were constructed using three serial sleep measures |
Demographic: age (study only included women); Psychosocial: psychological well-being, clinically significant depressive symptoms, anxiety symptoms; Physical: self-rated health, number of illness |
PSQI continuous scores |
Psychological well- being, fewer illnesses, and absence of clinically significant depressive symptoms predicted lower risk for disturbed sleep over time |
| Béland 32
(2011) |
1 year/ n=892 |
Canada/ random sample |
Rate of change in sleep assessed over two time points in a structural equation model |
Demographic: gender; Physical:
Benzodiazepine (BZ) use and duration |
PSQI (French version) defined poor sleep |
BZ use was associated with slower increases in sleep quality; female gender was associated with more sleep complaints |
| McHugh 36
(2012) |
2 years/ n=447 |
Ireland/ convenience sample |
Controlling for baseline sleep measure, regression modeling predicted sleep at follow-up |
Demographic: age, gender; Psychosocial: Perceived stress and loneliness; Physical: co-morbidity index |
PSQI defined poor sleep |
Emotional loneliness predicted sleep quality, and this relationship was partially mediated by emotional stress; the co-morbidity index was not associated with sleep over at follow-up |
| Yang 34
(2013) |
4 years/ n=680 aged 45-54, plus n=401 aged 55-74 |
South Korea/all residents in one area invited |
Controlling for baseline sleep measure, regression modeling predicted sleep at follow-up |
Demographic: age, gender, education, employment status, presence of children in household, smoking status, alcohol use; Psychosocial: Marital status, depressive symptoms; Physical: Menopausal status, regular exercise, psychotropic or sleep medication use, self-reported health |
PSQI continuous scores |
Among older (55-75 years old) but not younger (45-54 years old) participants, marital status predicted sleep disturbances at follow-up |
| Martin 33
(2014) |
3 years/ n=314 |
France/all residents in one area invited |
Regression models predicted were characterized as stable, increasing, or decreasing sleep disturbances |
Demographic: Gender; Psychosocial: Anxiety, depression, Daytime sleepiness; Physical: BMI, change in BMI Oxygen desaturation index, apnea plus hypopnea index, hypnotic intake |
Stable sleep vs. either worsening (PSQI or hypnotic intake increased or sleep duration became shorter) or improving (PSQI or hypnotic intake decreased or sleep duration became longer) sleep |
Hypnotic use predicted lower odds of improving sleep; marginal association between anxiety and depression with worsening; Female gender was not associated with worsening (but overall levels by gender not reported) |
Depressed mood was associated with disrupted sleep 34, 35. One study also showed that greater psychological well-being and fewer illnesses were associated with reduced risk for disturbed sleep35. McHugh 36 found an association between emotional loneliness and future sleep quality which was partially mediated by emotional stress. Marital quality was related to sleep at follow-up among older (55-75) but not younger (45-54) participants 34. In one of the few studies identified which examined a biomarker, Dowd 37 found that higher levels of the inflammatory markers (interleukin-6 and soluble intercellular adhesion molecule-1) predicted future long (> 8 hours) but not short (<6 hours) sleep duration.
In both studies reporting adjusted associations, age was not a significant independent predictor of future sleep quality34, 36.
Objectively measured sleep characteristics (n = 2)
Two studies utilized actigraphy to objectively measure sleep characteristics, and both were conducted in the United States (Table 3). In the Study of Osteoporotic Fractures (SOF) of community dwelling older women, cognitive decline (global function and performance on the Trail Making Test) was associated with prolonged sleep latencies and greater sleep fragmentation at follow-up; cognitive decline did not predict sleep duration 38. In the Osteoporotic Fractures in Men (MrOS) Sleep Study, which examined older men with both actigraphy and polysomnography, participants with lower testosterone levels at baseline had greater sleep fragmentation and less slow wave sleep at follow-up; however, these findings were attributable to levels of adiposity. Low levels of testosterone did not predict future sleep duration 39. It should be noted that these studies did not objectively assess sleep at baseline, and thus could not make conclusions regarding changes to sleep disturbances from study baseline.
Table 3.
Longitudinal studies of objective sleep characteristics (n = 3)
| First Author |
Follow-up time / sample size |
Country/ Recruitment |
Prospective Design |
Predictors / Covariates |
Outcome | Independent Predictors of Prospective Sleep Outcome |
|---|---|---|---|---|---|---|
| Yaffe38
(2007) |
13/15 years, n=2474 |
United States/ population- based listing |
Did not assess/exclude prevalent cases at baseline: only predicted prevalence at follow-up |
Demographic: age, (study only included women), education; Psychosocial: cognitive performance (global cognitive function and Trail Making Test), clinically significant depressive symptoms; Physical: walking, self-reported health, smoking, stroke, sleep medication use |
Actigraph sleep fragmentation and total sleep duration |
Cognitive decliners (both global cognition and on the Trial Making Test) were more likely than non- decliners to experience worse sleep fragmentation but not duration; other associations not reported |
| Barrett- Connor 39 (2008) |
3.4 years, n=1312 |
United States/ population- based listing |
Did not assess/exclude prevalent cases at baseline: only predicted prevalence at follow-up |
Demographic: age, race, education, marital status; Physical: testosterone (T), chronic diseases, self-reported health, physical activity, smoking status, physical activity, BMI, medication use |
Actigraph sleep fragmentation and total sleep duration; sleep architecture |
men with lower T had lower sleep efficiency, increased nocturnal awakenings and less slow-wave sleep; lower T was not related to sleep duration |
Neither of these studies reported adjusted estimates of the effects of age.
Findings summarized across outcomes
Age was consistently entered as a covariate in the articles reviewed, however the multivariable adjusted effect of age was reported only in 10/21 of the studies. Of these studies reporting on the potential independent effect of chronological age, few (2/10) found that age itself was a significant predictor of future sleep disturbance. One of these studies reported that, only among whites, being 75-84 years old (but not 85+) was associated with 1.73 times the odds of incident symptoms27; the other positive finding was that adults 75+ had 1.8 times the odds of incident symptoms20.
The factors most consistently reported on across outcomes were: female gender 19, 21, 23-25, 27-29, 31-34, 36, depressed mood 19-21, 23-29, 33-35, and poor physical health 23-26, 28 (Table 4).
Table 4.
Summary of significant independent factors that have been consistently associated with sleep outcomes in the literature
| Association with: | ||
|---|---|---|
|
| ||
| Risk Factor | Self-reported sleep complaints/insomnia symptoms |
Pittsburgh Sleep Quality Index (PSQI) |
| Gender | Elevated odds for the sleep outcome were found for females in the majority of studies reporting: OR = 1.4425, 1.5827 (among African– Americans), 1.5823, 1.5931, 1.6728, 2.4419; for men OR=0.52 29; gender did not predict future complaints in 20, 21, 24. |
Béland et al. 32 found sleep problems more commonly in women than men positive association (73.4% vs. 26.6%); gender did not predict future PSQI scores in 33, 34, 36. |
| Depression | Elevated odds for the sleep outcome were found in 8/10 studies reporting (OR = 1.5426 - 9.1819 for current depression 19, 21, 23, 24, 26, 27, 29, 35, OR = 1.07 per symptom increases28); negative findings were marginal: OR=2.30, 95% CI: 1.0- 5.20 20; OR = 1.50, 95% CI: 0.9-2.525. |
Elevated odds for the sleep outcome in 2/3 studies reporting 34, 35, with the other reporting a marginal association (OR=3.17, 95% CI: 0.95-10.59) 33. |
| Physical health | Elevated odds for the sleep outcome were found in 9/11 studies reporting: heart disease OR = 1.6826, 1.67 28, 2.58 (men only)29; stroke OR = 1.54 26 but not significant in 28; hip fracture was not related to sleep outcomes in 26, 28; 2+ physical disorders OR = 1.7 24 and 2.7723; chronic medical condition OR =2.60 25; below median on a physical health scale OR=4.3 21; perceived health was reciprocally (bi- directionally) related to sleep in 22; Not related to future sleep disturbances in 19 and 20. |
Self-reported health was related to future PSQI scores in 34, 35 but a comorbidity index was not in 36 |
Note: No studies for these risk factors were conducted using objective sleep measures
Female Gender
In 8/14 of the studies reporting gender associations, female gender was identified as an independent risk factor for future sleep disturbances 19, 23, 25, 27-29, 31, 32. The two studies conducted in South Korea found no association between gender and future sleep disturbances 24, 34, and the other studies conducted in Asia did not report gender associations 37, 40
Depressed mood
Depressed mood was associated with worse future sleep quality in 10/13 studies reporting19, 21, 23-29, 35. In one study, risk for future sleep disturbances was increased by 7% per additional symptom 28; in studies using categorical measures of clinically significant depressive symptoms, the odds of worse sleep outcomes increased from 54% 26 to 9 times the odds19. Of the three negative studies reporting on depression, two had a marginal (p=0.06) associations with worsening sleep 20, 33, and the other was the only to examine a lifetime (as opposed to current) episode of depression as a predictor25.
Physical health status
Worse physical health was independently associated with future sleep disturbances in 11/14 studies21-29, 34, 35 reporting on this factor. Measures of physical health status varied considerably, and included self-reported health status 22, 27, 34, 35, number of medical conditions/composite variables 19, 21, 23, 24 and/or specific chronic diseases 26, 28, 29. Studies examining number of medical conditions showed a 70-277% increase in risk for incident insomnia symptoms in participants with 2+ physical disorders 23, 24, or 4.3 times the odds for those below the median in a physical health status scale 21. When examining specific chronic diseases, heart disease emerged as an independent predictor, associated with a 67-68% increased risk of incident insomnia symptoms 26, 28, and one study found a strong (OR=2.58) association of CHD and future sleep disturbance only among men 29. Respiratory disturbance was identified as a risk factor among African-Americans27, and among women29 who, in the same study, were also at increased risk in the presence of arthritis.
Other relevant factors
Generally, these risk factors were examined in few studies limiting our assessment of the evidence’s consistency. African-American race 27, lower economic status 25, previous manual occupation 24, 31, widowhood 19, 26, marital quality 34, loneliness and perceived stress 36, recent life events 23, preclinical dementia 38, long-term benzodiazepine 32 and sedative use 26, 33, low testosterone levels 38, and high levels of inflammatory markers 37 were also identified as potential risk factors. It is important to note that although Dowd et al. 37 found that higher levels of inflammatory markers predicted future long (> 8 hours) sleep duration, their analysis did not consider the roles of chronic diseases, mood, or physical activity and inflammation may therefore not represent an independent risk factor. Physical activity was an independent predictor of future sleep in some 20, 21, 30 but not other studies 24, 34
Activity of daily living (ADL) impairment was identified as a risk factor for DIS among men in one study, whereas in the same study instrumental ADL impairment predicted DMS among women29. In another study, ADL impairment was related to future insomnia symptoms regardless of gender 26, although this association was only apparent among whites 27. However, in the other four studies 19, 23, 25, 28, disability was not associated with future sleep.
Discussion
This review set out to identify and assess the prospective evidence for risk factors involved in the development of sleep disturbances among older adults. The identified literature primarily (13/21 studies) relied on a few self-reported questions on sleep disturbances, with very few studies (2/21) utilizing objective measures of sleep. With increasing ease of use and decreasing costs, more studies may complement self-report measures with objective measures of sleep (like actigraphy) in the future.
Age was independently associated with future sleep disturbances in only 2/10 studies. Increases in the prevalence of sleep disturbances with age appeared to be related to an increase in non-chronological aging-related risk factors for sleep disturbances. Aging-related changes but not chronology per say may account for sleep disturbance risk among older adults; changes to sleep across the lifespan may coincide with pathological processes that generally correlate with but are independent of chronological age. Thus, disturbed sleep should not necessarily be expected as a part of normative or healthy aging. This conclusion agrees with previous reviews (i.e. 13) and cross-sectional reports 2, 41, but adds substantially by demonstrating mostly consistently null age findings in a systematic evaluation of longitudinal evidence.
It should be noted that all except 4 of the studies 28, 37-39 reviewed either controlled for baseline sleep measures or excluded prevalent cases of sleep disturbance, thus providing some assurance that the effects observed were in fact related to the risk factors rather than pre-existing sleep problems. It is important to note that, in most studies examined, the follow-up durations were relatively short. Future research with a longer duration of follow-up is required to understand more fully how sleep changes across the lifespan.
Female gender was somewhat consistently associated with future sleep disturbances (in 8/14 studies). Studies conducted in Asia either did not report on 30, 37 or found no gender associations 24, 34, thus leaving open the question of whether female gender is associated with future sleep disturbances in non-western populations. Including only westernized populations, 8/12 studies found gender associations. One study with a null gender finding only reported on change relative to baseline level, and this study cannot rule out the possibility that women generally had higher levels of disturbances, despite no difference in the odds of declining 33.
Two of the western-based null gender findings included emotional loneliness 36 or marital quality in their models 34. Although not tested in any paper reviewed, it is possible that the relationship between female gender and increased risk for sleep disturbances is driven by differences in these socio-emotional characteristics. Since no other studies included adjustments for these socio-emotional factors, this suggestion is tentative and requires a future empirical evaluation. Nevertheless, positive findings from multiple studies suggest female gender is associated with both insomnia symptoms 19, 25-29, 31 and PSQI reported sleep disturbances 32, above and beyond the other risk factors examined. The underlying mechanism(s) through which gender increases future sleep disturbance risk remain unclear.
Though measures of depression varied across studies, very consistent (10/13) findings indicated a strong (see Table 4) independent association between depressed mood and future sleep disturbances. Given the strength and consistency of these associations across diverse samples, depressed mood appears to be a robust predictor of future sleep disturbances. The only study examining lifetime depression found no independent association of mood and future sleep 25, indicating that the effect of depressed mood may dissipate over time, such that earlier depressive episodes are not necessarily a risk factor for late life sleep disturbances. Future research is needed to examine how mood influences risk for declining sleep quality through putative behavioral and physiological pathways. In the meantime, because sleep disturbances have long been recognized to increase the risk of depression 42, treatment strategies should account for these reciprocal relations by assessing and treating both to prevent the persistence of either.
Similarly consistent (9/11 studies) and strong (see Table 4) were associations of physical disease with future sleep. On the other hand, evidence for an impact of physical activity on future sleep disturbances is considerably less clear. Further, putative inter-relations with effects of mood and physical illness require future elucidation. Five studies 20, 21, 24, 30, 34 considered all three of these factors (physical activity, mood, and physical health) with 3/5 18, 25, 32 finding that physical activity had an independent association with future sleep20, 21, 30. One of these showed that the protective effect of physical activity on sleep was present only in older adults without pre-existing disease 30. Conceptually, the more consistent associations of mood and physical health with sleep may be mediated or moderated by levels of physical activity. Few prospective studies of sleep have examined the role of physical activity, and it is important to note that existing studies used different methods to measure physical activity and sleep, and also considered different covariates (i.e. how medical burden was assessed). Further research is needed to elucidate relations between mood, physical health, physical activity, and sleep. Studies with objective measures of these factors, for example using actigraphy, may be particularly clarifying.
An important limitation of our review is the exclusion of studies which focused exclusively on SDB. The studies we included did not exclude participants with prevalent SDB. Indeed, objective assessments of SDB were seldom included; accordingly, SDB was almost never considered as a covariate/predictor of future sleep disturbances. Although unmeasured in these studies, it is likely the many individuals with SDB were included. We are unable to determine the role of SDB in the development of these sleep disturbances. However, the intermittent hypoxic events/arousals which characterize SDB may have a causal role in the development the sleep disturbances examined (for example, including sleep fragmentation or poor perceived sleep quality). Future research with PSG is required to address the role of SDB in relation to other aspects of sleep over time.
Conclusions and future directions
Given the population-based, prospective nature of the studies discussed here, along with the strength and consistency of these associations across various samples, the current literature strongly suggests that female gender, depressed mood, and physical illness predict future sleep disturbances among older adults. However, the mechanisms linking these factors to the development of sleep disturbances in older adults are currently unknown. This is especially the case when compared to the number of candidate mechanisms implied in cross-sectional and case-controlled research; the breadth of cross-sectional research findings dramatically overshadows what is currently known from longitudinal research.
The existing prospective studies constitute a valuable knowledge base from which to broadly understand risk for developing sleep disturbances. For every question answered, however, many more regarding how sleep changes in late-life can be raised. For example, why does female gender appear to be a risk factor? Is it related to sex steroid hormones? What aspects of physical illness are most relevant, when, and for whom? How do physical illness, mood, and physical activity interact in relation to sleep in older adults? What about other lifestyle factors such as alcohol consumption? The prospective evidence reviewed here mainly supports general psychosocial and physical health risk factors. The factors identified could themselves be considered conglomerates or sets of physiological states. Cross-sectional and animal research suggests biologically plausible answers to many of these questions. Informed by these other methodologies, prospective research including markers of putative biopsychosocial pathways is now needed to examine the relative and temporal roles of the known correlates of sleep disturbances. Such research may improve our mechanistic understanding of the development of sleep disturbances on physiological, psychological, and behavioral levels.
It is crucial for researchers and clinicians to understand and be able to distinguish between what is known from the evidence, what is implied, and what questions remain. While more has been established regarding the physiological correlates of mood, physical illness, and sleep, currently next to no evidence exists relating these dynamic processes, over time, to sleep outcomes in older adults. Knowledge of the interrelating physiological antecedents driving changes in sleep has the potential to increase our understanding of the functional basis and role of sleep. Importantly, such knowledge may form the basis for early detection of at risk individuals and the prevention of sleep disturbances in late life. With sleep conceived as central to quality of life as well as the maintenance of health, establishing a mechanistic understanding of the causes of sleep disturbances will have far reaching public health implications.
Practice Points.
Sleep disturbances are not a normal part of aging:
According to the available prospective evidence, chronological aging itself may not independently increase the risk of sleep disturbances;
Female gender, depressed mood, and physical illnesses are the most consistently identified risk factors and may be used to identify those at risk for sleep disturbances;
Because sleep disturbances may also worsen depression and some physical illnesses, it is especially important to treat sleep problems in order to prevent a cycle of risk relations detrimental to overall health.
Research Agenda.
The current evidence can be used to identify older adults who may be at increased risk, but future research is needed to:
Clarify the role of physical activity in relation to known risk factors (depression, physical illness) and sleep disturbances;
Understand the behavioral and biological mechanisms underlying the development of sleep disturbances;
Target novel pathways for the prevention and treatment of sleep disturbances.
Acknowledgements
SFS is supported by T32 AG000181.
Abbreviations
- DIS
Difficulty initiating sleep
- DMS
Difficulty maintaining sleep
- EMA
Early morning awakening
- MrOS
Osteoporotic Fractures in Men Study
- PLM
Periodic leg movements
- PSQI
Pittsburgh sleep quality index
- SDB
Sleep disordered breathing
- SOF
Study of Osteoporotic Fractures
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- 1.Cauley J. The Demography of Aging. In: Newman AC, editor. The Epidemiology of Aging. Springer; 2013. pp. 3–14. J. [Google Scholar]
- *2.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. Journal of psychosomatic research. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Luyster FS, Strollo PJ, Jr., Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–34. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaussent I, Bouyer J, Ancelin ML, Akbaraly T, Peres K, Ritchie K, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34:1103–10. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raju DV, Radtke RA. Sleep/Wake Electroencephalography Across the Lifespan. Sleep Medicine Clinics. 2012;7:13–22. [Google Scholar]
- 7.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiology international. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 8.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA : the journal of the American Medical Association. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 9.Zielinski MR, Krueger JM. Sleep and innate immunity. Frontiers in bioscience (Scholar edition) 2011;3:632–42. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singletary KG, Naidoo N. Disease and Degeneration of Aging Neural Systems that Integrate Sleep Drive and Circadian Oscillations. Frontiers in neurology. 2011;2:66. doi: 10.3389/fneur.2011.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paudel ML, Taylor BC, Diem SJ, Stone KL, Ancoli-Israel S, Redline S, et al. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56:1228–35. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poon CY, Knight BG. Impact of childhood parental abuse and neglect on sleep problems in old age. The journals of gerontology Series B, Psychological sciences and social sciences. 2011;66:307–10. doi: 10.1093/geronb/gbr003. [DOI] [PubMed] [Google Scholar]
- *13.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep medicine. 2009;10(Suppl 1):S7–11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- *14.Vitiello MV, Prinz PN, Avery DH, Williams DE, Ries RK, Bokan JA, et al. Sleep is undisturbed in elderly, depressed individuals who have not sought health care. Biological psychiatry. 1990;27:431–40. doi: 10.1016/0006-3223(90)90553-e. [DOI] [PubMed] [Google Scholar]
- *15.Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. Handbook of clinical neurology. 2011;98:653–65. doi: 10.1016/B978-0-444-52006-7.00041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unruh ML, Redline S, An MW, Buysse DJ, Nieto FJ, Yeh JL, et al. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56:1218–27. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 17.Bilwise DL. Normal Aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier Saunders; Saint Louis, Missouri: 2011. pp. 16–26. [Google Scholar]
- 18.Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. A physiologic index of comorbidity: relationship to mortality and disability. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63:603–9. doi: 10.1093/gerona/63.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fok M, Stewart R, Besset A, Ritchie K, Prince M. Incidence and persistence of sleep complaints in a community older population. International journal of geriatric psychiatry. 2010;25:37–45. doi: 10.1002/gps.2295. [DOI] [PubMed] [Google Scholar]
- *20.Morgan K. Daytime activity and risk factors for late-life insomnia. Journal of sleep research. 2003;12:231–8. doi: 10.1046/j.1365-2869.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- 21.Morgan K, Clarke D. Risk factors for late-life insomnia in a representative general practice sample. The British journal of general practice : the journal of the Royal College of General Practitioners. 1997;47:166–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Lemola S, Richter D. The course of subjective sleep quality in middle and old adulthood and its relation to physical health. The journals of gerontology Series B, Psychological sciences and social sciences. 2013;68:721–9. doi: 10.1093/geronb/gbs113. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RE, Shema SJ, Kaplan GA. Prospective data on sleep complaints and associated risk factors in an older cohort. Psychosomatic medicine. 1999;61:188–96. doi: 10.1097/00006842-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Insomnia, depression, and physical disorders in late life: a 2-year longitudinal community study in Koreans. Sleep. 2009;32:1221–8. doi: 10.1093/sleep/32.9.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Gureje O, Oladeji BD, Abiona T, Makanjuola V, Esan O. The natural history of insomnia in the Ibadan study of ageing. Sleep. 2011;34:965–73. doi: 10.5665/SLEEP.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–72. [PubMed] [Google Scholar]
- 27.Foley DJ, Monjan AA, Izmirlian G, Hays JC, Blazer DG. Incidence and remission of insomnia among elderly adults in a biracial cohort. Sleep. 1999;22(Suppl 2):S373–8. [PubMed] [Google Scholar]
- *28.Pedraza S, Al Snih S, Ottenbacher KJ, Markides KS, Raji MA. Sleep quality and sleep problems in Mexican Americans aged 75 and older. Aging clinical and experimental research. 2012;24:391–7. doi: 10.3275/8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Quan SF, Katz R, Olson J, Bonekat W, Enright PL, Young T, et al. Factors associated with incidence and persistence of symptoms of disturbed sleep in an elderly cohort: the Cardiovascular Health Study. The American journal of the medical sciences. 2005;329:163–72. doi: 10.1097/00000441-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Inoue S, Yorifuji T, Sugiyama M, Ohta T, Ishikawa-Takata K, Doi H. Does habitual physical activity prevent insomnia? A cross-sectional and longitudinal study of elderly Japanese. Journal of aging and physical activity. 2013;21:119–39. doi: 10.1123/japa.21.2.119. [DOI] [PubMed] [Google Scholar]
- 31.Green MJ, Espie CA, Hunt K, Benzeval M. The longitudinal course of insomnia symptoms: inequalities by sex and occupational class among two different age cohorts followed for 20 years in the west of Scotland. Sleep. 2012;35:815–23. doi: 10.5665/sleep.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beland SG, Preville M, Dubois MF, Lorrain D, Voyer P, Bosse C, et al. The association between length of benzodiazepine use and sleep quality in older population. International journal of geriatric psychiatry. 2011;26:908–15. doi: 10.1002/gps.2623. [DOI] [PubMed] [Google Scholar]
- 33.Martin MS, Sforza E, Barthelemy JC, Thomas-Anterion C, Roche F. Sleep perception in non-insomniac healthy elderly: a 3-year longitudinal study. Rejuvenation research. 2014;17:11–8. doi: 10.1089/rej.2013.1457. [DOI] [PubMed] [Google Scholar]
- 34.Yang HC, Suh S, Kim H, Cho ER, Lee SK, Shin C. Testing bidirectional relationships between marital quality and sleep disturbances: a 4-year follow-up study in a Korean cohort. Journal of psychosomatic research. 2013;74:401–6. doi: 10.1016/j.jpsychores.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Phelan CH, Love GD, Ryff CD, Brown RL, Heidrich SM. Psychosocial predictors of changing sleep patterns in aging women: a multiple pathway approach. Psychology and aging. 2010;25:858–66. doi: 10.1037/a0019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.McHugh JE, Lawlor BA. Perceived stress mediates the relationship between emotional loneliness and sleep quality over time in older adults. British journal of health psychology. 2012 doi: 10.1111/j.2044-8287.2012.02101.x. [DOI] [PubMed] [Google Scholar]
- 37.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Annals of epidemiology. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaffe K, Blackwell T, Barnes DE, Ancoli-Israel S, Stone KL. Preclinical cognitive decline and subsequent sleep disturbance in older women. Neurology. 2007;69:237–42. doi: 10.1212/01.wnl.0000265814.69163.da. [DOI] [PubMed] [Google Scholar]
- 39.Barrett-Connor E, Dam TT, Stone K, Harrison SL, Redline S, Orwoll E. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. The Journal of clinical endocrinology and metabolism. 2008;93:2602–9. doi: 10.1210/jc.2007-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okajima I, Komada Y, Nomura T, Nakashima K, Inoue Y. Insomnia as a risk for depression: a longitudinal epidemiologic study on a Japanese rural cohort. The Journal of clinical psychiatry. 2012;73:377–83. doi: 10.4088/JCP.10m06286. [DOI] [PubMed] [Google Scholar]
- *41.Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. Journal of psychosomatic research. 2002;53:555–9. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- 42.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA : the journal of the American Medical Association. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]