Abstract
Malignant cells drive the generation of a desmoplastic and immunosuppressive tumor microenvironment. Cancer-associated stromal cells (CASCs) are a heterogeneous population that provides both negative and positive signals for tumor cell growth and metastasis. Fibroblast activation protein (FAP) is a marker of a major subset of CASCs in virtually all carcinomas. Clinically, FAP expression serves as an independent negative prognostic factor for multiple types of human malignancies. Prior studies established that depletion of FAP+ cells inhibits tumor growth by augmenting anti-tumor immunity. However, the potential for immune-independent effects on tumor growth have not been defined. Herein, we demonstrate that FAP+ CASCs are required for maintenance of the provisional tumor stroma since depletion of these cells, by adoptive transfer of FAP-targeted chimeric antigen receptor (CAR) T cells, reduced extracellular matrix proteins and glycosaminoglycans. Adoptive transfer of FAP-CAR T cells also decreased tumor vascular density and restrained growth of desmoplastic human lung cancer xenografts and syngeneic murine pancreatic cancers in an immune-independent fashion. Adoptive transfer of FAP-CAR T cells also restrained autochthonous pancreatic cancer growth. These data distinguish the function of FAP+ CASCs from other CASC subsets and provide support for further development of FAP+ stromal cell-targeted therapies for the treatment of solid tumors.
Introduction
Carcinomas are complex tumors consisting of neoplastic epithelial cells, vasculature, inflammatory cells and immune cell infiltrates. Many human carcinomas exhibit desmoplasia, characterized by the accretion of reactive stromal cells and extracellular matrix (ECM). In established human solid tumors, nests of cancer cells are often circumscribed by a dense fibrotic stroma containing high levels of collagen, fibronectin and hyaluronan (HA), and a heterogeneous population of cancer-associated stromal cells (CASCs), including cancer-associated fibroblasts (CAFs), alpha smooth muscle actin-positive (αSMA+) myofibroblasts and mesenchymal stem cells (MSCs) (1-5). The extent of desmoplasia varies among different tumor types. In pancreatic cancer, desmoplasia comprises as much as 90% of tumor mass and heightens therapeutic resistance (6). However, even in tumors in which stroma represents a relatively minor component, desmoplasia can impact tumor cell behavior. The role of desmoplasia in tumor initiation, progression, metastasis and resistance to therapy is complex and not yet well understood.
The desmoplastic response can promote tumor growth, invasion and metastasis through ECM remodeling as well as the production of growth factors, cytokines and chemokines (7-10). It also promotes tumorigenesis by supporting angiogenesis (7, 11), altering tissue stiffness and mechanotransduction (12, 13), inducing inflammation (14, 15) and suppressing anti-tumor immunity (16, 17). Tumor stroma can also limit drug delivery and confer resistance to chemotherapeutics (18-21), radiation (22), anti-angiogenesis therapy (23) and immunotherapy (16, 24, 25).
Based on the tumor-promoting functions and the therapeutic resistance conferred by tumor stroma, it has been hypothesized that destruction of stromal cells and/or disruption of molecular stromal cell/ECM-dependent pathways would inhibit tumor growth and augment efficacy of other therapeutic modalities. Several proof-of-concept studies support this hypothesis. Preventing recruitment and differentiation of CASCs by targeting chemokine-chemokine receptor pathways inhibited tumor progression (26, 27). Consistent with the role of inflammatory myeloid cells in promoting CAF activation, treatment with dexamethasone reduced desmoplasia and attenuated tumor growth (15). Furthermore, inhibition of collagen binding to its discoidin domain receptor reduced colony formation of primary pancreatic tumor cells (28). Blockade of the HA-CD44 axis inhibited tumor cell proliferation, survival, invasion and epithelial-to-mesenchymal transition (EMT) (29, 30). Genetic targeting or pharmacological inhibition of the protease activity of fibroblast activation protein (FAP) reduced lung tumor growth (9). Furthermore, depletion of HA in the tumor stroma induced a transient increase in vessel density and perfusion that facilitated delivery of gemcitabine into tumors, thereby augmenting efficacy of chemotherapy in a highly desmoplastic mouse model of pancreatic ductal adenocarcinoma (PDA) (18, 20).
An alternative approach to targeting molecular pathways is to target stroma at a cellular level, however, it is important to keep in mind the potential plasticity (31, 32) and heterogeneity of this compartment (1, 3). Distinct stromal cell subpopulations may have opposing effects on tumor growth, progression and metastasis. The depletion of specific subpopulations may have either therapeutic or detrimental effects. The impact may depend on tumor type, stage of tumor progression, variation in tumor immunogenicity and the degree of desmoplasia. Thus, delineation of the roles of distinct subpopulations in tumor growth, including their roles in regulating anti-tumor immunity, desmoplasia and angiogenesis, is required to inform the rational design of stromal cell-targeted therapies.
For many years, SMA+ myofibroblasts have been noted in a variety of solid tumors. However, recent analyses have revealed additional phenotypically distinct subsets of CASCs. Notably, FAP is expressed on the majority of activated fibroblasts and MSCs found in the tumor microenvironment, only a subset of which co-express SMA (1, 3). Interestingly, studies also indicate that a lineage relationship may exist between MSC progenitors and terminally differentiated SMA+ myofibroblasts (27, 33). Specific ablation of SMA+ myofibroblasts in mouse models of PDA suppressed anti-tumor immunity, enhanced hypoxia and EMT, and reduced survival (34). Notably, the approach used in this study to deplete SMA+ cells did not impact the prevalence of FAP+ CASCs and had a modest and selective effect on ECM. In contrast to the impact of depleting SMA+ myofibroblasts, targeted deletion of FAP+ cells using genetic (16, 35-37) and immune-based approaches (38-44) inhibited tumor growth. Thus, SMA+ and FAP+ stromal cells may differentially regulate tumorigenesis.
Although our mechanistic understanding of the impact of depleting CASCs is still limited, several studies demonstrated that depleting FAP+ CASCs enhanced anti-tumor immunity (16, 35, 36, 44). We found that depleting FAP+ cells in murine AE17.OVA mesothelioma or TC-1 lung tumors increased intra-tumoral T cells and that the anti-tumor effect of depleting FAP+ cells was abrogated in immune-deficient hosts, indicating an essential role for adaptive immunity. However, the tumor models used in that study were highly immunogenic and exhibited modest desmoplasia. Nevertheless, we detected pronounced alterations in stromal composition. These studies therefore raised the question as to whether depletion of FAP+ cells in the setting of weakly immunogenic and/or highly desmoplastic tumors may affect tumor growth through immune-independent stromal-dependent mechanisms. In the study described herein, we evaluated the requirement of FAP+ cells for the maintenance of a desmoplastic stroma and the impact of FAP-CAR T cell-mediated depletion of FAP+ cells on weakly immunogenic, but highly desmoplastic tumors under conditions in which immune and stromal-dependent mechanisms could be dissected. We demonstrate that FAP+ cells are required to maintain a provisional matrix in desmoplastic tumors, including human lung cancer and mesothelioma xenografts and mouse models of PDA, and the efficacy of stromal cell depletion in inhibiting tumor growth in such tumors is mediated by immune-independent mechanisms. Taken together with recent data from others (16, 35, 36), these results define potentially distinct roles for myofibroblasts and FAP+ stromal cells in pancreatic cancer (34).
Materials and Methods
Cell Culture
Mouse AE17 mesothelioma cells expressing chicken ovalbumin (AE17.OVA) were provided by Dr. Delia Nelson (University of Western Australia).Murine 4662 PDA cells were derived from a pancreatic tumor isolated from a fully backcrossed C57BL/6 KrasG12D:Trp53R172H:Pdx-1-Cre (KPC) mouse, recapitulating the desmoplastic feature of autochthonous PDA (45). Human A549 lung adenocarcinoma cells were purchased from the American Type Culture Collection. Human sarcomatoid type mesothelioma cells I45 were provided by Dr. J. Testa (Fox Chase Institute, Philadelphia, PA). Cells were authenticated by morphology, growth characteristics and biologic behavior, tested mycoplasma-free and frozen. Cells were cultured less than 1 month after resuscitation.
Animals
C57BL/6 mice and NOD/SCID/IL2-receptor γ chain knockout (NSG) mice were purchased from Charles River Laboratories Inc. (Wilmington, MA), Jackson Labs (Bar Harbor, ME) and the Children's Hospital of Philadelphia. FAP-null mice (FapLacZ/LacZ) provided by Boehringer Ingelheim (46) were backcrossed 12 generations onto a C57BL/6 genetic background. These mice in turn were crossed with NSG mice to generate NSG FAP-null mice. KPC mice were provided by mouse hospital at the University of Pennsylvania. Experimental protocols were approved by the Institutional Animal Care and Use Committee and were in compliance with the guideline for the care and use of animals.
Engineering of anti-muFAP CAR constructs and generation of FAP-CAR T cells
A single-chain Fv domain of anti-mouse FAP antibody 73.3 along with human CD8a hinge and transmembrane domain, plus 4–1BB and CD3ζ signaling domains were cloned into the retroviral vector MigR1 encoding EGFP to establish FAP-CAR construct as described (44). Primary mouse T cells were transduced with FAP-CAR construct or MigR1 vector to generate FAP-CAR T cells and MigR1 T cells, respectively as described in Supplementary Materials and Methods.
Intravenous transfer of CAR-T cells in mice bearing established tumors
C57BL/6 mice and NSG mice were injected subcutaneously with 2 × 106 AE17.OVA or 3 × 105 4662 tumor cells suspended in matrigel (BD Biosciences). Tumor-bearing mice were randomly assigned to three groups that received FAP-CAR or MigR1-transduced (control) mouse T cells or remained untreated. KPC mice were monitored by ultrasound and randomly assigned for treatment when they had established pancreatic tumors of 95-270mm3).
1 × 107 CAR T cells per dose were administered intravenously. T cells were given once for AE17.OVA tumor-bearing mice and twice (at a week interval) for PDA models. To test human T cells with redirected CAR against murine FAP in human tumor xenografts, NSG and NSG-FAP null mice were injected subcutaneously with 2 × 106 A549 cells or I45 cells mixed with matrigel. Mice bearing established tumors then received one dose of 1 × 107 FAP-CAR human T cells i.v. or left untreated.
Statistical Analyses
For studies comparing two groups, the Student t test was used. For comparisons of more than two groups, we used one-way ANOVA with appropriate post hoc testing. Statistics were calculated using GraphPad Prism 5. Data are presented as mean ± SEM. Differences were considered significant when p <0.05.
Results
The extent of desmoplasia in mouse tumors and human tumor xenografts correlates with the preponderance of FAP+ cells
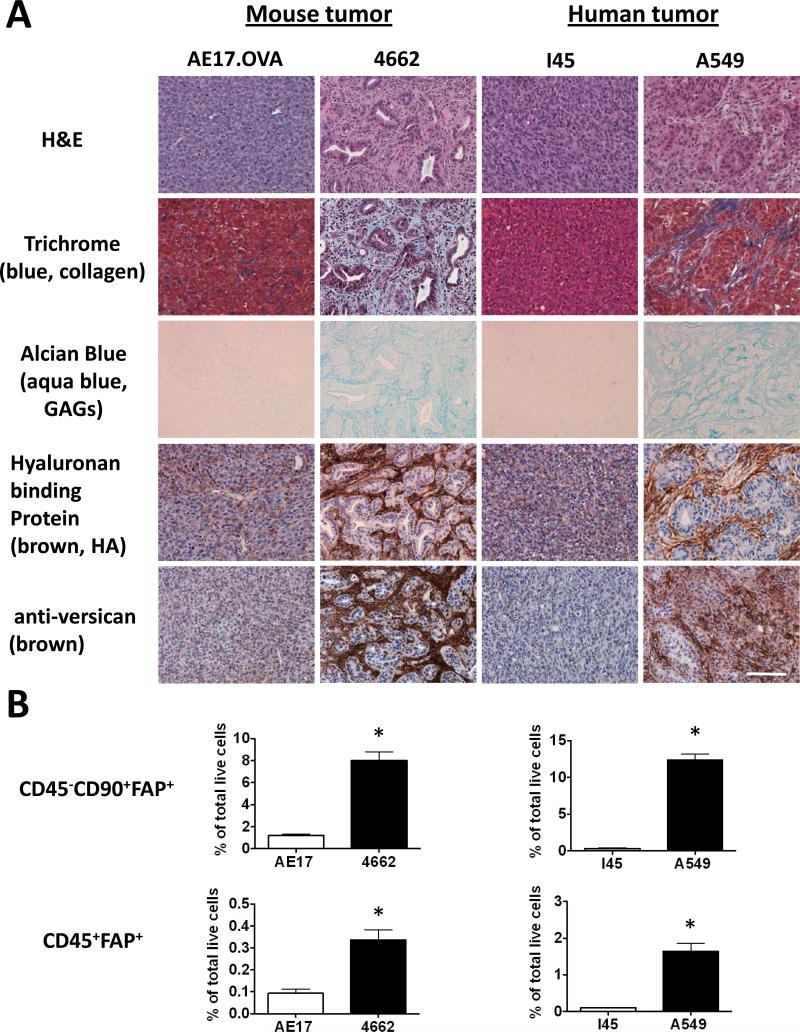
We examined the extent of desmoplasia in several mouse tumors and human tumor xenografts. The murine pancreatic cancer cell line 4662 transplanted into syngeneic C57BL/6 mice and A549 human lung adenocarcinoma cells in NSG mice were circumscribed by dense stroma. In contrast, AE17.OVA mouse mesothelioma and I45 human mesothelioma tumors exhibited moderate and sparse desmoplasia, respectively (Fig. 1A). Extensive accumulation of collagen and glycosaminoglycans (GAGs) was evident in 4662 and A549 tumors compared to in AE17.OVA and I45 tumors (Fig. 1A). In particular, high levels of HA, known to regulate tumor cell behavior (29, 30) and confer resistance to chemotherapy (18, 20, 21, 30), were detected only in the more desmoplastic tumors. The levels and spatial distribution of HA correlated with those of versican, a proteoglycan implicated in tumor progression that associates with HA (Fig. 1A). Notably, all matrix components examined were dispersed diffusely throughout the AE17.OVA and I45 tumors compared to the restricted localization of highly concentrated matrix components in the stromal regions of desmoplastic 4662 and A549 tumors (Fig. 1A).
Figure 1. Extent of the desmoplastic stromal response in mouse and human tumors correlates with the prevalence of intra-tumoral FAP+ stromal cells.
(A) The extent of desmoplasia was evaluated in established tumors by H&E staining, Masson's trichrome staining to reveal collagen deposition (blue), Alcian blue staining for glycosaminoglycans (aqua blue), and reactivity for hyaluronan-binding protein (HABP) or anti-versican antibody. Scale: 100 μm. (B) Flow cytometry was performed to identify CD90+ stromal cells and CD45+ hematopoietic cells that express FAP. Data shown are normalized to total live tumor population and expressed as the mean ± SEM (n>5). * Denotes significant difference between each group, p value < 0.01.
We next investigated the relationship between the extent of desmoplasia and the preponderance of FAP+ CASCs. In A549 tumors, the majority of FAP+ CASCs were CD45-CD90+ stromal cells, although we consistently detected a minor population of FAP+ CASCs that expressed the hematopoietic cell marker CD45+ (Supplementary Fig. 1A), further characterized as F4/80+CD206+ (M2-like) macrophages (Supplementary Fig. 1B). Interestingly, the proportion of CD45-CD90+FAP+ stromal cells in the various tumor types correlated with the degree of desmoplasia. FAP+ stromal cells represented 8% and 12% of total cells in the highly desmoplastic 4662 and A549 tumors, respectively, compared to only 0.3%-1.2% in AE17.OVA tumors and I45 xenografts (Fig. 1B). The level of FAP+ M2-like macrophages (CD45+F4/80+CD206+) also correlated with the degree of desmoplasia, representing 0.34% and 1.65% in 4662 and A549 tumors, respectively, compared to just 0.1% of total cells in AE17.OVA tumors and I45 xenografts (Fig. 1B). These data raised the possibility that FAP+ cells may be required for the formation and/or maintenance of a desmoplastic stroma.
We previously reported that adoptively transfered FAP-CAR T cells infiltrated tumors, depleted FAPhi CASCs and inhibited growth of AE17.OVA tumors (Supplementary Fig. 2A) (44). The impact on tumor growth in this model depended on anti-tumor immunity, since the effect of FAP-CAR T cells was abrogated in immune-deficient mice (Supplementary Fig. 2B) (44). Further analysis of tumors from FAP-CAR T cell-treated mice revealed altered tumor morphology and nuclear condensation (Supplementary Fig. 2C), reduced tumor cell proliferation (Supplementary Fig. 3A) and increased tumor cell apoptosis (Supplementary Fig. 3B) compared to tumors from mice treated with control MigR1 T cells. Stroma was also remarkably altered in FAP-CAR T cell-treated tumors. Collagen and HA were reduced by 57% and 83% (p<0.05), respectively (Supplementary Fig. 3C and 3D), and the number of CD31+ vessels was decreased by 30% (p<0.05) after FAP-CAR T cell infusion (Supplementary Fig. 3E). In contrast, the stroma in tumors of mice that received MigR1 T cells was similar to that in the untreated control (Supplementary Fig. 3C-E). These data establish that FAP+ stromal cells are required for the maintenance of desmoplasia. These data prompted us to investigate whether FAP+ CASCs might promote tumor growth through immune-independent stromal-dependent mechanisms.
FAP-CAR T cells can mediate immune-independent growth inhibition of desmoplastic human xenografts
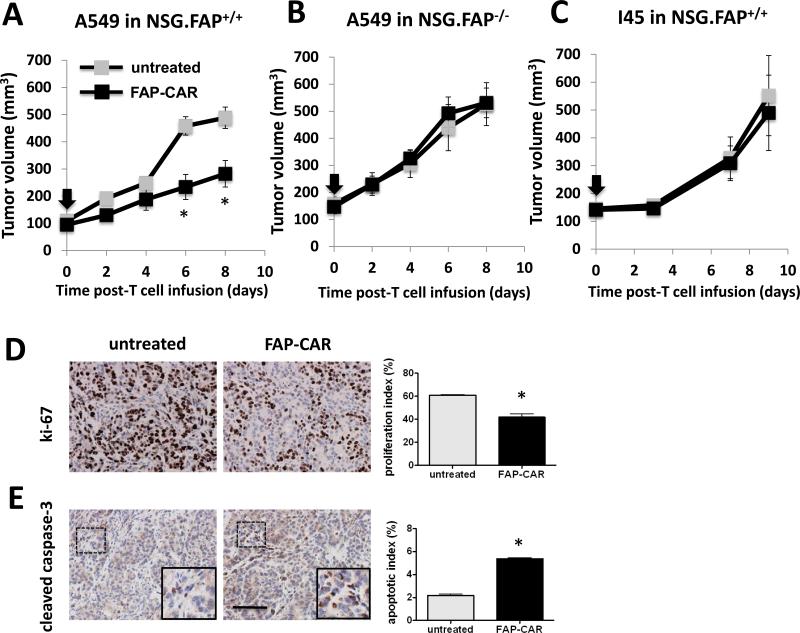
To address whether FAP+ CASC-dependent desmoplasia could promote tumor growth independently of suppressing adaptive anti-tumor immunity, we assessed the impact of FAP-CAR T cells on the growth of established A549 human tumor xenografts in NSG mice. Administration of FAP-CAR human T cells directed against mouse FAP significantly reduced the growth of A549 tumors with a 42% (p<0.01) reduction in tumor size at day 8 post-T cell transfer (Fig. 2A). Treatment with FAP-CAR T cells significantly reduced tumor cell proliferation and induced apoptosis in A549 tumors (Fig. 2D and 2E). In contrast, FAP-CAR T cells had no impact on the growth of A549 tumors in FAP-null NSG mice (Fig. 2B), indicating that FAP expression by host stromal cells is required for the anti-tumor effect elicited by FAP-CAR T cells. On the other hand, FAP-CAR T cells had no effect on human I45 tumor xenografts (Fig. 2C), which induced little if any desmoplastic response, indicating that the anti-tumor activity is dependent on the presence of intra-tumoral FAP+ cells and not likely due to an indirect effect through off-tumor targeting of FAP+ cells in normal tissues. Further studies demonstrated that FAP-CAR T cells inhibited growth of two other highly desmoplastic human mesothelioma xenografts in NSG mice (data not shown). Thus, immune-independent FAP-CAR T cell-mediated inhibition of tumor growth may depend on the degree of stromagenesis.
Figure 2. FAP-CAR T cells confer anti-tumor activity in desmoplastic human lung cancer in immunodeficient hosts.
(A) NSG mice bearing established A549 tumors received FAP-CAR human T cells i.v. (B) To test target-specificity of FAP-CAR T cells, NSG FAP-null tumor-bearing mice were injected i.v. with FAP-CAR human T cells. (C) NSG mice bearing I45 tumors were injected i.v. with FAP-CAR human T cells. The black arrow indicates the time of T cell transfer (n=5 per group). A549 tumors were harvested 8 days post-adoptive T cell transfer. Tumor sections were stained with antibodies against human-specific ki-67 (D) and cleaved caspase-3 (E) (n=3 per group). * Denotes statistical significance between untreated and FAP-CAR T cell-treated samples, p value < 0.01. Scale: 100 μm.
FAP-CAR T cells disrupt stromagenesis, angiogenesis and the ductal-like structure of tumor nodules in highly desmoplastic human lung cancer xenografts
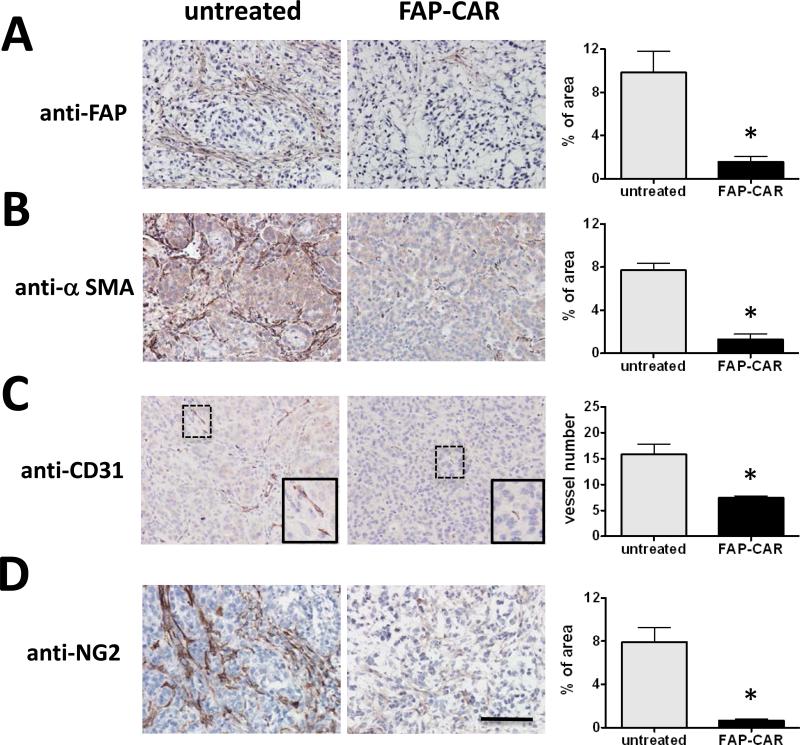
To investigate the mechanisms by which FAP+ CASC depletion leads to immune-independent growth inhibition of highly desmoplastic human tumor xenografts, we analyzed the composition and architecture of A549 tumors 8 days post-T cell transfer. Treatment with human FAP-CAR T cells in A549 tumors decreased FAP+ CASCs by 84% (p<0.01) (Fig. 3A). Furthermore, in spite of only a minor population of FAP+ cells co-expressing SMA (Supplementary Fig. 1C), we also observed an 83% decrease (p<0.01) in SMA+ myofibroblasts (Fig. 3B), suggesting that SMA+ cell are either derived from progenitor populations, such as a subpopulation of FAP+ MSCs that express CD105 and CD44 (Supplementary Fig. 1D), or that FAP+ cells are required for the recruitment or differentiation of SMA+ myofibroblasts. Interestingly, we observed a marked disruption of the adenocarcinoma ductal-like structure of the tumor nodules and an increase in necrosis in FAP-CAR T cell-treated tumors (Supplementary Fig. 4). These data indicate that extrinsic signals provided by stromal cells and/or ECM are required to maintain the ductal-like spatial organization of tumor cells.
Figure 3. FAP-CAR T cells restrict tumor growth through decreasing stromagenesis and tumor angiogenesis in desmoplastic human lung cancer xenografts.
Established A549 tumor-bearing mice were either injected i.v with FAP-CAR human T cells or left untreated. Tumors were harvested 8 days post-adoptive T cell transfer. Tumor sections were stained with antibodies against FAP (A) and α-SMA (B) to examine the degree of stromagenesis. Tumor tissues were stained with antibodies against CD31 (C) and NG2 (D) to reveal endothelium and pericytes, respectively. * Denotes statistical significance between untreated and FAP-CAR T cell-treated samples (n=3), p value < 0.01. Scale: 100 μm.
As angiogenesis is critical for tumor progression and is known to be dependent on stromal cells and matrix (7, 11, 23), we investigated whether FAP-CAR T cells affected vascularization of A549 tumor xenografts. Quantification of CD31+ (endothelial cell) and NG2+ (pericyte) staining indicated that treatment with FAP-CAR T cells resulted in a 53% decrease (p<0.01) in tumor endothelial cells (Fig. 3C) and 92% decrease (p<0.01) in pericytes (Fig. 3D) when compared with tumors from untreated mice.
FAP-CAR T cells resolve desmoplasia in highly desmoplastic human lung cancer xenografts
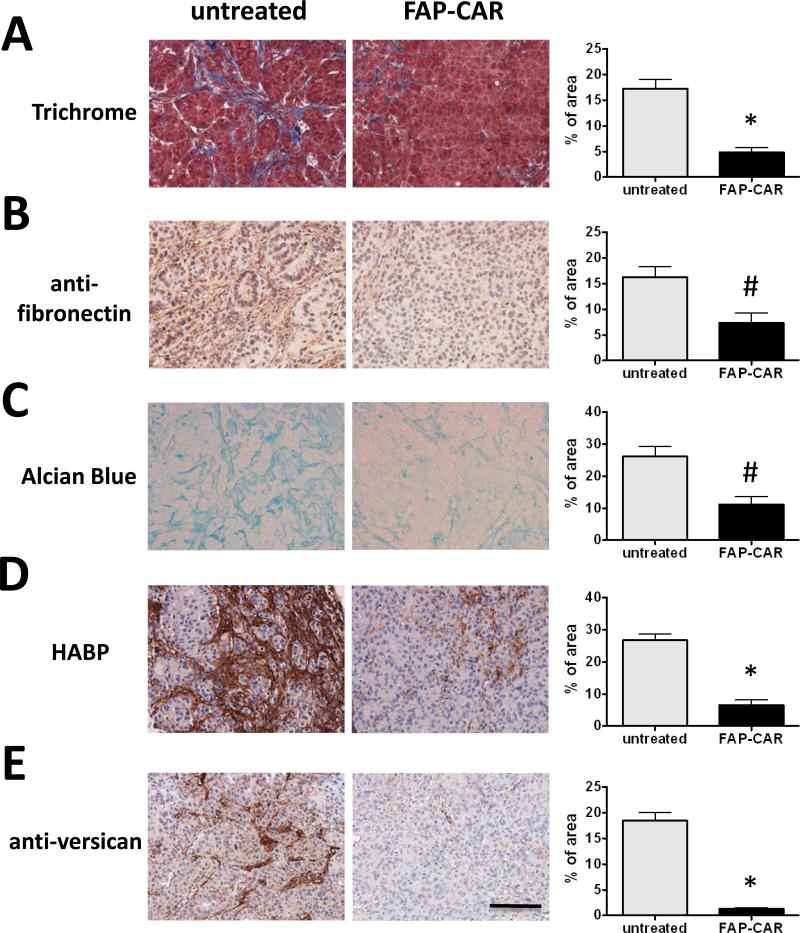
Since the preponderance of FAP+ cells correlated with the degree of desmoplasia, we investigated whether FAP+ cells are required to maintain desmoplasia in well-established tumors. Collagen content was decreased by 72% (p<0.01) (Fig. 4A) and fibronectin by 55% (p<0.05) (Fig. 4B) in FAP-CAR T cell-treated A549 tumors. Total GAGs and HA were reduced by 57% (p<0.05) and 75% (p<0.01), respectively (Fig. 4C and 4D), in FAP-CAR T cell-treated A549 tumors. Furthermore, versican, a proteoglycan implicated in tumor progression that interacts with HA, fibronectin, collagen, as well as cell surface proteins such as CD44 and integrin-β1 (47-48), was depleted by 93% (p<0.01) (Fig. 4E). The effects of conditional FAP+ CASC depletion indicate that these cells are critical for maintenance of desmoplasia.
Figure 4. Depletion of tumor stroma by FAP-CAR T cells in desmoplastic human lung cancer xenografts.
Established A549 tumor-bearing mice were treated with FAP-CAR human T cells. Tumors were harvested 8 days post-adoptive T cell transfer to evaluate the extent of desmoplasia. (A) Masson's trichrome staining was performed to determine collagen accumulation. (B) IHC was performed to reveal fibronectin content. (C) Alcian blue staining was performed to show total GAGs. Tumor tissues were stained with HABP (D) or anti-versican antibody (E). * and # denote statistical significance between untreated and FAP-CAR T cell-treated samples (n=3), p value < 0.01 and p value < 0.05, respectively. Scale: 100 μm.
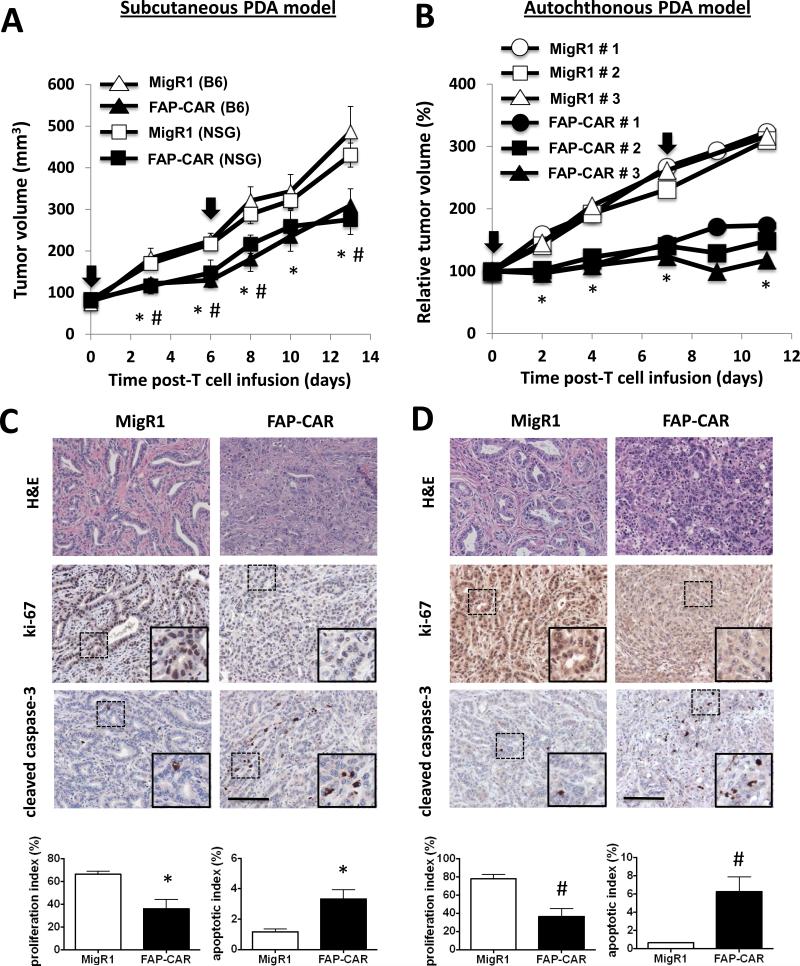
FAP-CAR T cells inhibit the growth of non-immunogenic but highly desmoplastic pancreatic ductal adenocarcinomas through immune-independent mechanisms
Having determined that depletion of FAP+ CASCs could inhibit stromagenesis, angiogenesis and resolve desmoplasia, we sought to determine if these findings would extend to PDA, one of the most desmoplastic tumor types. Furthermore, since FAP-CAR T cells were able to restrict the growth of human xenografts in immune-incompetent mice, we also sought to determine whether immune-independent mechanisms might also play a role in PDA, one of the least immunogenic tumor types described. We initially employed the 4662 tumor cell line that we derived from a tumor that arose spontaneously in a KPC mouse. 4662 tumors were non-immunogenic as the growth of this tumor was comparable when implanted subcutaneously into syngeneic C57BL/6 versus NSG mice (Supplementary Fig. 5A). Furthermore, antibody-mediated depletion of either CD4+ or CD8+ T cells did not change tumor growth relative to isotype-treated controls (data not shown). C57BL/6 or NSG mice bearing established 4662 tumors were injected with two doses of FAP-CAR or MigR1 T cells (one week apart). Seven days following the administration of the second dose of T cells, the growth of 4662 PDA was reduced by 36% (p<0.05) in immune-competent C57BL/6 mice that received FAP-CAR T cells relative to the tumors from mice treated with MigR1 T cells (Fig. 5A). The number of CD11b+Ly6G+ cells was decreased at this time point in tumors from FAP-CAR T cell treated C57BL/6 mice (Supplementary Fig. 5B) and we also observed only a very modest increase (0.42%) in the total number of GFP- CD8+ host T cells. Flow cytometric analysis also demonstrated infiltration of the adoptively transferred GFP+ FAP CAR T cells into 4662 tumors (Supplementary Fig. 5C). Importantly, FAP-CAR T cells had a similar impact on the growth of 4662 tumors in immune-incompetent NSG mice, indicating that FAP-CAR T cells inhibited 4662 tumor growth via immune-independent mechanisms (Fig. 5A).
Figure 5. Immune-independent anti-tumor mechanisms play a key role in FAP-CAR T cell-induced growth inhibition in murine PDA.
(A) Established 4662 tumor-bearing C57BL/6 and NSG mice were treated with FAP-CAR or MigR1 mouse T cells (n=5 per group). A second dose was given 6 days later. * and # denote statistical significance between MigR1 and FAP-CAR-treated samples in B6 and NSG mice, respectively (p value < 0.05). (B) Tumor-bearing KPC mice received FAP-CAR or MigR1 T cells i.v. and tumor progression was followed by ultrasound (n=3 per group). * Denotes statistical significance between MigR1 and FAP-CAR-treated samples, p value < 0.01. The black arrow indicates the time of T cell transfer. 4662 PDAs (C) and autochthonous PDA (D) sections were stained with antibodies against Ki-67 and cleaved caspase-3. * and # denote statistical significance between MigR1 and FAP-CAR T cell-treated samples, p value < 0.01 and p value < 0.05, respectively. Scale: 100 μm.
We next extended these studies to the autochthonous KPC mouse model of PDA. Tumor-bearing KPC mice were treated with FAP-CAR or control T cells and tumor growth was measured by ultrasound over a two-week period. Treatment with FAP-CAR T cells significantly inhibited tumor growth in KPC mice compared to significant tumor progression in the controls (Fig. 5B).
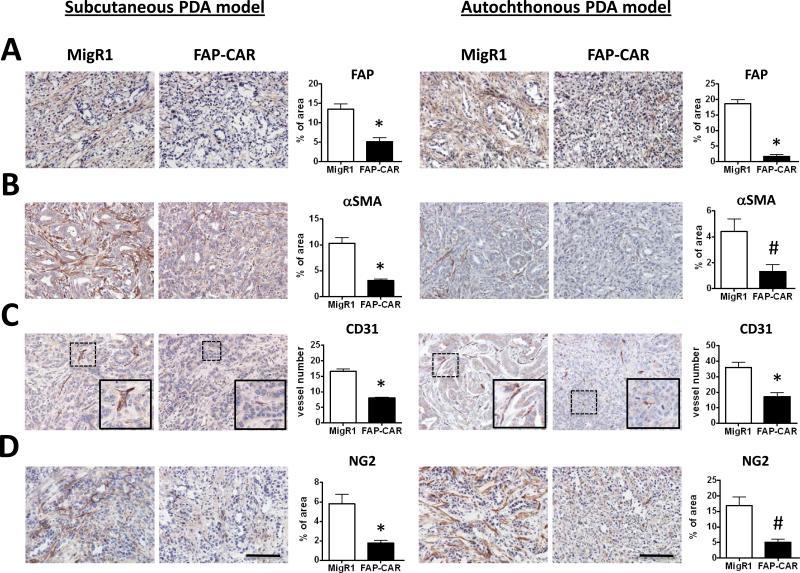
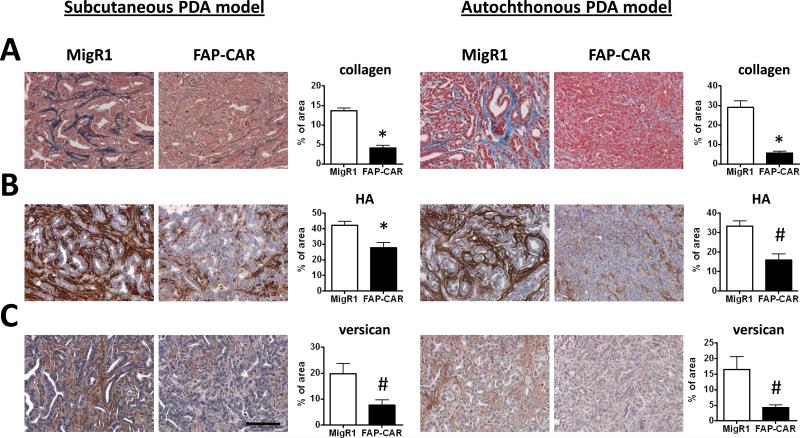
We also assessed the impact of treatment with FAP-CAR T cells on the desmoplastic response and angiogenesis in transplanted and autochthonous PDAs. FAP-CAR T cell treatment altered stromal architecture, reduced proliferation, and increased apoptosis in 4662 and autochthonous PDAs (Fig. 5C, 5D and Supplementary Fig. 6). FAP-CAR T cell treatment decreased FAP+ stromal cells by 62% (p<0.01) and 91% (p<0.01) in 4662 tumors and autochthonous PDAs, respectively (Fig. 6A). Interestingly, FAP and SMA defined distinct, yet overlapping, subsets of stromal cells in both mouse and human pancreatic tumors (Supplementary Fig. 7). Similar to the results described above, we again observed 70% depletion of SMA+ cells in both models of PDA (Fig. 6B). Furthermore, FAP-CAR T cell treatment resulted in a 50% reduction in tumor endothelial cells (Fig. 6C) and 70% decrease in pericytes (Fig. 6D) in both models. Collagen content in murine PDAs was decreased by 70-80% (Fig. 7A) following treatment with FAP-CAR T cells, while HA and versican were reduced by 34-52% (Fig. 7B) and 61-74% (Fig. 7C), respectively.
Figure 6. FAP-CAR T cells impact stromagenesis and angiogenesis in murine PDA.
Established 4662 tumor-bearing mice (n=6 per group) and autochthonous PDA-bearing KPC mice (n=3 per group) were treated with 2 doses of FAP-CAR mouse T cells. IHC was performed using tumor tissues harvested from mice 3 days after the second dose of T cell transfer. Tumor sections were stained with antibody against (A) FAP, (B) SMA, (C) CD31, and (D) NG2. * and # denote statistical significance between MigR1 and FAP-CAR T cell-treated samples, p value < 0.01 and p value < 0.05, respectively. Scale: 100 μm.
Figure 7. FAP-CAR T cells resolve desmoplasia in murine PDA.
Established 4662 tumor-bearing mice (n=6 per group) and autochthonous PDA-bearing KPC mice (n=3 per group) were treated with 2 doses of FAP-CAR or MigR1 mouse T cells. Tumor tissues were harvested 3 days after the second dose of T cell transfer to evaluate the extent of desmoplasia. (A) Collagen was evaluated by Masson's trichrome staining. (B) HA was evaluated by HABP and (C) versican determined by immunohistochemistry staining. * and # denote statistical significance between MigR1 and FAP-CAR T cell-treated samples, p value < 0.01 and p value < 0.05, respectively. Scale: 100 μm.
To determine whether FAP-CAR T cell-mediated disruption of tumor stroma could facilitate drug delivery to inhibit tumor progression, we treated mice bearing 4662 tumors with FAP-CAR T cells along with gemcitabine. The combination therapy had additive anti-tumor activity compared to the impact of treatment with either alone (Supplementary Fig. 8).
Discussion
There is growing interest in developing therapeutic strategies that target non-malignant, tumor-promoting CASCs. One emerging candidate is FAP, which is expressed by CAFs, MSCs, and a subset of M2 macrophages, but not by quiescent fibroblasts. Clinicopathological studies indicate that accumulation of FAP+ CASCs is associated with tumor progression, metastasis, and predicts poorer outcome in many types of human malignancies, including PDA and colon cancer (49-50). In contrast, accumulation of SMA+ myofibroblasts correlates with better prognosis and depletion of these cells accelerates tumor progression in murine models of PDA (34). Taken together, these data indicate that deleting FAP+ CASCs and SMA+ myofibroblasts can have opposing effects, highlighting the phenotypic and functional heterogeneity of tumor stromal cells.
Several studies showed that depletion of FAP+ CASCs restricted tumor growth by enhancing anti-tumor immunity (16, 35, 36, 44). This likely occurs through multiple mechanisms, including loss of immunosuppressive effects of stromal cells and/or enhancement of tumor access, recruitment, survival or retention of immune cells. These mechanisms bode well for combining stromal-targeted CAR T cell therapy with cancer vaccines or immunological checkpoint antagonists such as anti-CTLA-4, anti-PD-1 or anti-PD-L1 (16, 35, 36, 44). Here, we explored the possibility that FAP+ CASCs may also exert tumor-promoting functions via immune-independent mechanisms by depleting FAP+ CASCs using FAP-CAR T cells in mouse tumors and human xenografts that exhibit varying degrees of desmoplasia and immunogenicity, and in settings in which a potential role for adaptive immunity was eliminated.
Using tumors with varying amounts of desmoplasia, we found that the extent of matrix accumulation correlated with the preponderance of FAP+ cells, consistent with previous reports that stromal cells are an important source of ECM (1-4). Moreover, our results clearly establish that maintenance of tumor-associated desmoplastic stroma depends on the persistent presence of FAP+ cells, since conditional depletion of these cells markedly depleted intra-tumoral ECM. The effects were transient, however, as stroma returned after the disappearance of FAP-CAR T cells (data not shown). Taken together with a recent study of the impact of SMA+ myofibroblast depletion (34), the data presented herein indicate that while both FAP+ and SMA+ stromal cells regulate intratumoral collagen content, only FAP+ cells are critical for the maintenance of intratumoral HA, suggesting that either FAP+ cells are the major source of HA or that FAP+ cells are required to drive SMA+ myofibroblast deposition of HA.
The disruption of ECM components known to directly support tumor cell survival and growth likely underlies FAP-CAR T cell-mediated growth restrictions of human xenografts (A549) and of poorly immunogenic 4662 tumors in immune-incompetent and syngeneic immune-competent hosts. These effects may be direct, through disruption of stromal cell- and matrix-dependent signaling in tumor cells, and/or indirect through inhibition of angiogenesis. These mechanisms, however, are not always sufficient to impact tumor growth. The growth inhibition of immunogenic AE17.OVA tumors by FAP-CAR T cells was lost in immune incompetent hosts, indicating a requirement of adaptive immunity (44). However, AE17.OVA tumors grew much more rapidly in NSG mice (44). Such an increase in growth kinetics might overcome any potential immune-independent tumor inhibitory effects elicited by FAP-CAR T cells in a tumor that exhibits minimal stromagenesis. Moreover, FAP-CAR T cell-mediated matrix modification may have augmented anti-tumor immunity by influencing immune cell recruitment and/or motility, as matrix architecture has been shown to regulate intratumoral T cell localization and migration (24). We found that depletion of FAP+ CASCs modestly increased CD8+ T cell infiltration even into non-immunogenic highly desmoplastic 4662 PDAs, but their roles in inhibiting tumor growth in this model remain to be determined. In addition, we observed neither an increase in Treg infiltration, nor importantly, increased incidence of lung metastasis in the tumor-bearing mice treated with FAP-CAR T cells (data not shown).
In addition to immunomodulatory effects, stromagenesis has direct effects on tumor cells and indirectly controls tumor growth through regulation of angiogenesis (7, 11). We found that CASCs, including FAP+ cells and SMA+ myofibroblasts, were significantly decreased in A549, 4662, and autochthonous PDA tumors post-FAP-CAR T cell transfer. In addition, tumor angiogenesis was profoundly diminished. Taken together, our data demonstrate that FAP-CAR T cells inhibit tumor stromagenesis and reduce vascular density and/or integrity in highly desmoplastic tumors. Based on the small degree of overlap between FAP+ and SMA+ cells in A549, 4662 tumors and autochthonous PDA, it is unlikely that FAP-CAR T cells directly targeted myofibroblasts. The reduction in myofibroblasts that results from depletion of FAP+ cells suggests that either SMA+FAP- cells are derived from MSCs that express FAP+ at an earlier stage in their differentiation and/or that FAP+ cells are required to induce the recruitment and/or differentiation of SMA+ myofibroblasts (1, 27, 33). It will therefore be interesting to further define the lineage and functional relationships among FAP+ stromal cells, myofibroblasts and the pancreatic stellate cells thought to be critical to pancreatic fibrosis.
An unexpected observation was the marked impact of FAP+ cell depletion on tumor cell morphology. In AE17.OVA tumors, depletion of FAP+ cells resulted in signs of nuclear condensation. On the other hand, we did not observe immune-mediated hypoxia-associated necrosis as previously reported (36). Instead, tumors that received FAP-CAR T cell treatment exhibited a reduced proliferative index and an increased apoptotic index compared either to MigR1 T cell-treated or untreated tumors. In addition, FAP-CAR T cell treatment disrupted tumor ductal-like structure indicating that extrinsic cues provided by stroma are critical to the spatial orientation of tumor cells.
Recent studies raised the concern that ablation of FAP+ stromal cells could induce bone marrow hypocellularity and cachexia in mice (37, 43). Despite careful examination, we again did not observe bone marrow destruction or changes in total body weight using the indicated treatment regimens in the tumor models investigated. The difference in toxicities may be due to the difference in epitope specificity of the anti-FAP antibodies used to generate CARs in different studies, the fact that our FAP-CAR T cells only transiently deplete FAP+ cells and/or the fact that the FAP-CAR T cells we generated preferentially deplete cells expressing high levels of FAP (including tumor-associated stromal cells) but not cells expressing low levels of FAP (for example, basal levels of FAP on normal cells). Thus, as we previously proposed (44), there might be a therapeutic window using appropriate CAR constructs. Results from other research groups (38, 39, 41), including two independently generated FAP-CAR constructs (40, 42) also support this concept.
Given potential toxicity, it is important to note that even a temporary disruption of tumor stroma may be sufficient for therapeutic benefit. For instance, transient depletion of stromal HA was shown to facilitate delivery of gemcitabine to tumors, thereby augmenting efficacy of chemotherapy in pancreatic cancer (18, 20). Previous work employing anti-FAP vaccination resulted in decreased levels of collagen 1 and increased intra-tumoral uptake of chemotherapeutics (39). Furthermore, we and others showed that depletion of FAP+ CASCs could disrupt immunosuppression to enhance therapeutic efficacy of cancer vaccines (36, 44) or checkpoint antibodies (16). We postulate that the desmoplastic response mediated by FAP+ CASCs could thus contribute to therapeutic resistance through the multiple mechanisms discussed here. We are currently testing if depletion of tumor stroma by FAP-CAR T cells could augment the efficacy of multiple other therapeutic modalities. Our data to date indicate that the combination of stromal cell depletion with gemcitabine treatment provides at least additive anti-tumor effects in 4662 tumors and we are exploring the mechanisms involved.
In summary, the results presented herein provide proof-of-concept that, in addition to suppressing adaptive anti-tumor immunity, FAP+ CASCs orchestrate tumor-promoting effects by enhancing tumor angiogenesis and desmoplasia. The balance of these various tumor-promoting effects of FAP+ cells is determined by multiple parameters: the immunogenicity of the tumor, the prevalence of FAP+ CASCs and extent of desmoplasia in the tumor microenvironment. Furthermore, depletion of FAP+ CASCs inhibits tumor growth through both adaptive immune-dependent and -independent mechanisms. Moreover, cross-talk between these mechanisms may also contribute to reduced tumor growth. Therefore, developing combinatorial strategies that incorporate FAP+ CASC depletion for cancer treatment may offer therapeutic advantages.
Supplementary Material
Acknowledgements
The authors would like to thank Rajrupa S Majumdar and Veena Kapoor for their technical support. We would also like to thank Dr. Ben Stanger for helpful discussions and Mouse Hospital at the University of Pennsylvania for providing KPC mice. We also thank Boehringer Ingleheim for providing FAPLacZ knock-in mice and histology facility at the Ryan Hospital of the School of Veterinary Medicine at the University of Pennsylvania.
Grant support
These studies were supported by NCI (National Cancer Institute) grants P01 CA 66726-07 (to SMA and CHJ), R01 CA169123 (to RHV), R21 CA169741, R01 CA141144 and R01 CA 172921 (to SMA and EP) and a “Clinic and Laboratory Integration Program” Grant from the Cancer Research Institute (EP and SMA) and from Novartis Pharmaceuticals Corporation, and the Lung Cancer and Pancreatic Cancer Translational Centers of Excellence at the Abramson Cancer Center. AL was sponsored by a START (Student Training and Research in Tumor Immunology) fellowship from the Cancer Research Institute. LCSW was supported by Pittsburgh 5K Cure Sarcoma Award.
Footnotes
Conflicts of Interest: C.H. June, S.M. Albelda and E. Puré received a research grant from Novartis. R.H. Vonderheide received funding from Pfizer and Roche.
Author's Contributions
Conception and design: A. Lo, L.-C.S. Wang, S.M. Albelda, and E. Puré.
Development of methodology: J. Scholler, J. Monslow, A.C. Durham, E.L. Buza, C.H. June and S.M. Albelda.
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A. Lo, L.-C.S. Wang, D. Avery, K. Newick, S. O'brien, R.A. Evans, D.J. Bajor, and C. Clendenin.
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A. Lo, L.-C.S. Wang, D. Avery, R.A. Evans, D.J. Bajor, A.C. Durham, E.L. Buza, R.H. Vonderheide, C.H. June, S.M. Albelda and E. Puré.
Writing, review, and/or revision of the manuscript: A. Lo, L.-C.S. Wang, D. Avery, R.H. Vonderheide, C.H. June, S.M. Albelda and E. Puré.
Study supervision: R.H. Vonderheide, C.H June, S.M. Albelda, and E. Puré.
Reference
- 1.Jacob M, Chang L, Pure E. Fibroblast activation protein in remodeling tissues. Curr Mol Med. 2012;12:1220–43. doi: 10.2174/156652412803833607. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 4.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 7.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 9.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613–25. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–84. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194–203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- 12.Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–63. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–83. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS one. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–20. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res. 2011;71:3453–8. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith NR, Baker D, Farren M, Pommier A, Swann R, Wang X, et al. Tumor stromal architecture can define the intrinsic tumor response to VEGF-targeted therapy. Clin Cancer Res. 2013;19:6943–56. doi: 10.1158/1078-0432.CCR-13-1637. [DOI] [PubMed] [Google Scholar]
- 24.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commu. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow- derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguilera KY, Rivera LB, Hur H, Carbon JG, Toombs JE, Goldstein CD, et al. Collagen signaling enhances tumor progression after anti-VEGF therapy in a murine model of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:1032–44. doi: 10.1158/0008-5472.CAN-13-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64:5702–11. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- 30.Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:771–8. doi: 10.1001/archotol.132.7.771. [DOI] [PubMed] [Google Scholar]
- 31.Waterman RS, Henkle SL, Betancourt AM. Mesenchymal stem cell 1 (MSC1)- based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS one. 2012;7:e45590. doi: 10.1371/journal.pone.0045590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, et al. The Reprogramming of Tumor Stroma by HSF1 Is a Potent Enabler of Malignancy. Cell. 2014;158:564–78. doi: 10.1016/j.cell.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold JN, Magiera L, Kraman M, Fearon DT. Tumoral immune suppression by macrophages expressing fibroblast activation protein-alpha and heme oxygenase- 1. Cancer Immunol Res. 2014;2:121–6. doi: 10.1158/2326-6066.CIR-13-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 37.Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, et al. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med. 2013;210:1137–51. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156–63. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 39.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–62. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakarla S, Chow K, Mata M, Shaffer DR, Song XT, Wu MF, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21:1611–20. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostermann E, Garin-Chesa P, Heider KH, Kalat M, Lamche H, Puri C, et al. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res. 2008;14:4584–92. doi: 10.1158/1078-0432.CCR-07-5211. [DOI] [PubMed] [Google Scholar]
- 42.Schuberth PC, Hagedorn C, Jensen SM, Gulati P, van den Broek M, Mischo A, et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J Transl Med. 2013;11:187. doi: 10.1186/1479-5876-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran E, Chinnasamy D, Yu Z, Morgan RA, Lee CC, Restifo NP, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210:1125–35. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154–66. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Niedermeyer J, Kriz M, Hilberg F, Garin-Chesa P, Bamberger U, Lenter MC, et al. Targeted disruption of mouse fibroblast activation protein. Mol Cell Bio. 2000;20:1089–94. doi: 10.1128/mcb.20.3.1089-1094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–94. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 48.Koyama H, Hibi T, Isogai Z, Yoneda M, Fujimori M, Amano J, et al. Hyperproduction of hyaluronan in neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol. 2007;170:1086–99. doi: 10.2353/ajpath.2007.060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–1741. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- 50.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–158. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.