Abstract
Hair eating in nonhuman primates is thought to result from a frustrated appetitive drive produced by an inappropriate diet. To investigate whether hair eating could be reduced through changes in diet, a two-part study was conducted with a group of baboons (Papio hamadryas sp.). The first part involved changing to a twice-daily feeding routine, thus providing prolonged access to an appropriate food source. The other part involved scattering a grain mix to encourage more foraging while maintaining a once-daily feeding routine. Changing the feeding routine unexpectedly resulted in a significant increase in hair manipulation and ingestion. Providing forage did not significantly decrease hair manipulation and ingestion, but several individuals did show a reduction in these behaviors. Consuming biscuits without having to forage for them may have led to the increase in hair manipulation and consumption. Spreading grain throughout the enclosure may have failed to satisfy the urge to forage because little effort was needed to collect it. Although the current study failed to significantly decrease hair eating, it provides valuable insight into further avenues of research on the behavior.
Keywords: baboon, trichophagia, appetitive behavior, foraging
Trichophagia is described most simply as the ingestion of hair. It has been observed in both human (Christenson, Mackenzie, & Mitchell, 1991) and nonhuman primates including chimpanzees (Nolan, Schaffer, & Conti, 1987), baboons (Butler & Haines, 1987; Gillin et al., 1990; Mejido et al., 2009), macaques (Mook, 2002), and tamarins (Gozalo, Montoya, & Nolan, 1990). In addition to primates, hair eating has been observed in a variety of other species including guinea pigs (Gerold et al., 1997), rabbits (Mulder, Nieuwenkamp, van der Palen, van Rooijen, & Beynen, 1992), and sheep (Chiezey, 2010). Though easily defined, there is less agreement as to the underlying cause of hair eating. Some psychiatrists lump trichophagia with trichotillomania, the compulsion to pull one’s hair out, and consider it to be an impulse control disorder or a psychological condition in the obsessive-compulsive spectrum of disorders (Bouwer & Stein, 1998; Chamberlain, Odlaug, Boulougouris, & Fineberg, 2009).
Human subjects exhibiting the behavior indicate that the act of pulling hair is usually accompanied by sense of anxiety with relief afterward (Carr, Sholevar, & Baron, 2006; Frey, McKee, King, & Martin, 2005). The hair that is pulled generally comes from the subjects themselves, with some reports of hair being pulled from another individual or even a doll (Christenson et al., 1991; Tabatabai & Salari-Lak, 1981). Ingestion of the hair can be preceded by oral manipulation, licking, or chewing of the ends (Christenson et al., 1991).
In a study by Reinhardt, Reinhardt, and Houser (1986), hair eating in macaques was only reported in conjunction with hair pulling directed at a subordinate in an act that was thought to be an aggressive behavior disorder. Others consider it to be a form of pica, also thought to be a compulsive behavior that involves eating non-nutritive non-food items (Eckstein, 1980; Lacey, 1990; Mahan, 1979). Some studies link pica to a nutrition deficiency, usually iron. Iron supplements, both intravenous and oral, were shown to abruptly stop trichophagia in two young girls (Hadnagy, Binder, Grauzer, & Szocs, 1991; McGehee & Buchanan, 1980). In nonhuman animals, another theory is that hair eating is the result of an unsatiated desire to engage in appetitive behaviors such as grazing or rumination that is reduced through the provision of an appropriate diet (Gerold et al., 1997; Chiezey, 2010).
Trichophagia was identified as a widespread and recurrent problem in a captive colony of over 1,900 baboons, though its cause was unclear. Trichophagia has both clinical and behavioral implications. Clinically, it can lead to trichobezoars that require veterinary intervention (Butler & Haines, 1987). Trichobezoars can cause gastrointestinal obstruction with resultant nutritional deficiencies, gut perforations, and other problems that, if left untreated, can cause death (Carr et al., 2006). Behaviorally, the performance of an abnormal behavior like hair eating may also indicate that the animals are having difficulty adjusting to their captive environment and are experiencing stress (Garner, 2005). The most likely theories for its occurrence were considered. Trichotillomania was discounted because preliminary observations showed that most hair was collected from the ground rather than pulled from the subjects themselves. The suggestion that hair pulling is an aggressive behavior disorder was also not considered because hair pulling was rarely observed being directed at a subordinate. Pica was additionally discounted since the animals receive a balanced diet from their biscuit ration. Therefore, the theory that hair eating is related to an unsatisfied appetitive drive was investigated further.
For baboons in the wild, feeding is their primary activity, and can account for anywhere between 21 to 60% of the animals’ time during the day (Altman, 1980; Dunbar, 1977; Rose, 1977; Stacey, 1986). Wild baboons consume a varied diet of leaves, flowers, and fruits in addition to roots and tubers that need to be dug for. Animal proteins are also hunted and consumed. In addition to the time and energy needed to procure these food items, in many cases, extensive processing occurs prior to ingestion. Pods are pulled from trees, and are in turn torn open using their hands and teeth. Seeds are extracted from the pod using their mouths and fingers, and once in the mouth, skins can be separated from seeds and pushed out of their mouths using the tongues (Whiten, Byrne, Barton, Waterman, & Henzi, 1991). In captivity, very little effort is needed to obtain food. Common diets consist of biscuits supplemented by produce and/or other items such as grains and cereals (Baker, Weed, Crockett, & Bloomsmith, 2007). Most of these items can be consumed quickly and require very little effort on the part of the animal to gather, process, and consume.
Although the diets provided to captive baboons can certainly provide for their nutritional needs, they do not necessarily provide for their behavioral ones. An animal may consume the food he or she requires in a very short time, but he or she may remain motivated to show many of the elements of foraging, which is an appetitive component that normally precedes food consumption (Duncan, 2005). In such cases, the performance of a particular behavior sequence (e.g., foraging behavior) seems to be just as important as the functional consequence of the behavior—consumption. The act of collecting and manipulating food items both manually and orally prior to ingestion may be as critical to animals as the consumption itself. However, in a captive environment where adequate foraging opportunities may not be available, an animal may turn his or her attention toward inappropriate, immediately available environmental stimuli: in this case, hair (Wemelsfelder, 1993).
The objective of this study was to determine whether eating hair was an abnormal behavior that developed in a captive group of baboons as a result of frustrated appetitive behavior. To accomplish this, the study was divided into two parts: (a) an examination of the effect of prolonged access to an appropriate food source through a change from once daily to twice-a-day (BID) feedings and (b) an examination of the effect of increased foraging opportunities in the form of grain provisioning. These experimental conditions were chosen, in part, for their ability to be implemented on a large, colony-wide scale should they have proven successful. The intent behind altering the feeding schedule was to determine how levels of hair manipulation and ingestion would change when the animals had nearly continuous access to biscuits rather than only in the afternoon and overnight. Extra grain was provided to determine if hair eating and manipulation would decrease when widely scattered material to forage on was available as an alternative to hair.
MATERIALS AND METHODS
Subjects
The subjects were a group of 11 (one male, 10 females) baboons (Papio hamadryas anubis and two-way crosses of P. h. anubis with P. h. hamadryas and P. h. cynocephalus) 7 to 17 years old, ranging in weight from 16.1 to 26.6 kg. They had been housed as a social group in the same location for over a year prior to the start of the study. When the study commenced, one of the animals was absent as she was in the veterinary clinic receiving treatment. She was integrated back into the group several weeks later. No data were collected on her until the start of the baseline observations for the forage distribution component of the study. She replaced another female who was permanently removed from the group during a 4-week hiatus in observations that occurred between the BID feeding and forage distribution components. In total, a group of 10 animals was observed during the BID feeding component, and that group, except for the substitution, was observed again for the forage provision component.
Housing
The subjects were housed at the Southwest National Primate Research Center, an Association for the Assessment and Accreditation of Laboratory Animal Care-accredited institution, located in San Antonio, TX. The primary enclosure that held the subjects during the study was outdoors and measured 37.16 m2. At the back of the enclosure, the height measured 4.37 m with a roof that sloped down to 3.07 m at the front. The roofing was constructed of both corrugated aluminum and chain link, with the corrugated aluminum covering approximately two-thirds.
There was an adjoining secondary enclosure that measured 7.06 m2 and was connected by a sliding door. This adjoining enclosure was primarily used to separate animals or for holding during daily enclosure cleaning. The sliding door connecting the two enclosures was generally left open, but for the purposes of this study, it was closed to lock the animals in the primary enclosure during observation to prevent them from being out of sight. The enclosure itself was constructed primarily of chain link and cinder block walls with metal pipe and I-beam framing and concrete flooring. Spanning the length of the primary enclosure was a large, flat platform perch that was 1.22 m wide. There was also heavy chain stretched across the enclosure that served as a climbing/perching structure, but also supported a swinging 55-gal drum with a large hole cut out to allow a single animal to sit inside. Additionally, the enclosure was furnished with two Kong toys (Kong Company, Golden, CO) and two plastic balls (Jolly Pets, Streetsboro, OH).
Procedures
Baseline and follow-up routine
The standard husbandry routine involved feeding the baboons 4.5 kg of Monkey Diet (Lab Diet, PMI Nutrition Int’l, Brentwood, MO) between 2:00 p.m. and 4:00 p.m. The following morning, at approximately 9:00 a.m., any remaining feed was discarded and the cage was then washed. The animals would also receive a daily mix of cereals, popcorn, dried fruits, and other items in the afternoon. This mix was scattered in the secondary enclosure as positive reinforcement for a training program not associated with this study. The training was conducted as part of the care and management routine to encourage the animals to move to the secondary enclosure on command for husbandry purposes.
Twice-a-day feeding routine
Rather than receiving 4.5 kg of Monkey Diet once daily in the afternoon, 2.25 kg was given at 9:00 a.m. and the remaining 2.25 kg was given between 2:00 p.m. and 4:00 p.m. Any remaining feed from the morning was discarded prior to the afternoon feeding, and similar to the standard husbandry routine, any remaining feed from the previous afternoon was discarded prior to the morning feeding. Cage cleaning still occurred at approximately 9:00 a.m., and the animals continued to receive their daily mix of items for training purposes. This schedule was implemented Monday through Friday, but on the weekends, the standard husbandry routine was followed. Feed was increased to 6.8 kg total during the second week of BID feedings, as the animals consistently consumed all of the feed provided. After the increase of 2.3 kg, a small amount of biscuits was left over after each feeding, so the ration was left at 6.8 kg.
Forage distribution routine
For the forage distribution component of the study, the feeding schedule was returned to once daily, and the animals continued to receive 6.8 kg of biscuits according to original feeding routine. In addition, 1.4 kg of grain in the form of corn, soy, and crimped barley was scattered throughout the primary enclosure after the morning wash. An effort was made to lightly cover the entire floor and platform area of the primary enclosure. The subjects continued to receive the mix spread in the secondary cage for training reinforcement.
Study schedule
The study was conducted over the course of six months from January to July 2012. A total of 1 hr of baseline observations (12 5-min observations) were collected on each animal under the standard husbandry conditions over 2 weeks prior to the start of the BID feeding routine. The BID feeding component of the study followed immediately and was conducted over an 8-week period, during which a total of 2 hr of data (24 5-min observations) were collected on each animal. This was followed by 1 hr of follow-up observations (again, 12 5-min observations) over the next two weeks. Upon completion of follow-up observations for the BID feeding component of the study, 4 weeks were allowed to elapse to help eliminate any behavioral effects of the feeding schedule change.
Another set of baseline observations were conducted prior to the forage distribution component of the study. The forage distribution component was then conducted over a 4-week period during which 1 hr of observations were conducted on each animal, with 1 hr of follow-up observations occurring over the course of the final two weeks. All methods and procedures were approved by the Texas Biomedical Research Institute’s Animal Care and Use Committee.
Behavior observations
A single observer sitting approximately 1 m from the enclosure recorded live behavior data for the duration of the study. Behavior data were collected on focal individuals using instantaneous point sampling at a 15-s interval for 5 min. To limit observer fatigue, the 10 subjects were divided into two groups of five, one of which was observed in the morning and the other in the afternoon. For each group, observations were balanced for time of day, and were collected early, mid-morning, and later morning, as well as early, mid-afternoon, and later afternoon. Observations were not conducted if it was raining or temperatures were below 7.2 °C, as this generally suppressed the animals’ activity.
An ethogram was constructed with a focus on the behaviors associated with the ingestion of hair including where the hair came from (the subject’s own body, that of another individual, or the floor), its manipulation, and mastication. Although an ethogram with an extensive list of abnormal behaviors was initially created, the primary abnormal behaviors observed were related to the manipulation and ingestion of hair. For the purposes of this manuscript, those abnormal and other unobserved behaviors were removed from the ethogram. For a list of the individual behaviors recorded and the categories in which they were summarized for statistical analysis, refer to Table 1.
TABLE 1.
Ethogam Used for Behavior Quantification Separated into Behavior Categories Used for Statistical Analysis
| Behavior Category |
Behaviors | Definition |
|---|---|---|
| Hair-Directed | Hair chew | Mastication of either a loose or intact single strand or clump of hair from the individual’s own coat or that of another animal |
| Hair-directed | Manual manipulation of an individual strand or clump of hair that may also involve placing the hair in the mouth and manipulating it with the lips without chewing | |
| Hair pull self | Forcefully removing hair from the individual’s own body in tufts or strands with hands or teeth | |
| Hair pull other | Forcefully removing hair from another individual in tufts or strands with hands or teeth | |
| Biscuit-Directed | Eat biscuit | Biting and/or chewing biscuits |
| Biscuit sort | Manual manipulation of biscuits, biscuit pieces, or crumbs including removing them from the food trough or picking through them on the ground | |
| Forage-Directed | Forage-directed | Manual manipulation of forage with related chewing and consumption (note: the act of gathering and consuming forage occurred quickly and did not allow for the separation of forage manipulation from its chewing and consumption) |
| Active | Active | Any normal locomotion requiring more than two steps to be taken, as well as any changes in total body posture including sitting to standing or standing to sitting |
| Rest | Rest | Inactivity lasting for longer than 2 s |
| Object-Directed | Enrichment-directed | Oral or manual manipulation of any enrichment device in the cage |
| Cage-directed | Any interaction with the cage such the manipulation of cage doors or parts | |
| Social Behavior | Affiliative | Any gesture or facial expression directed toward the observer or another animal indicating sociability |
| Aggressive | Any gesture or facial expression directed toward the observer or another animal intended to threaten or intimidate | |
| Submissive | Any gesture or facial expression directed toward the observer or another animal indicating deference | |
| Groom Self | Groom self | Oral or manual manipulation of the individual’s own hair coat or body part without the plucking, removal, or chewing of the hair |
| Groom Other | Groom other | Oral or manual manipulation of another animal’s hair coat or body part without the plucking, removal, or chewing of the hair |
| Tension Related | Scratch | Brusque, stroking movement of the hand in which the fingers contact the skin or coat |
| Yawn | Opening the mouth wide in a nonthreatening manner with a prolonged, deep inhalation and subsequent exhalation | |
| Other | Other | Miscellaneous behaviors occurring too infrequently to allow for individual analysis |
Statistical analysis
During each 5-min observation conducted on each subject, 20 behavior samples were collected. The proportion of the observation spent engaged in each behavior observed was calculated from these samples. These values were averaged together for all subjects under baseline, experimental, and follow-up conditions. Individual behaviors were organized further into behavioral categories (Table 1) for statistical analysis. The data were not normally distributed, so a nonparametric Friedman two-way analysis of variance by ranks was conducted using Systat 13 (Systat Software, Inc. Chicago, IL).
In the midst of the forage provision aspect of the study, two subjects required relocation to the veterinary clinic for treatment for wounding. One was unavailable for the last half of the experimental period and all but 1 day of follow-up observations, and the other was absent for a majority of the follow-up observations. The data for those subjects was excluded from statistical analysis, resulting in a final sample size of eight for the forage provision portion of the study.
RESULTS
General Observations on Hair-Directed Behavior
Although only five of the 11 total subjects had been observed eating hair prior to the study, several weeks after the study had commenced, every animal in the cage was observed eating hair in some form. Often, hair was collected from the floor of the cage in individual strands. There were only two instances in which hair was not collected from the floor. On one occasion, a subject repeatedly pulled and ate hair from a cagemate, and on the other occasion, another subject pulled and consumed hair from herself. Although hair was rarely extracted from the animal itself or from a cagemate and consumed, there were several instances in which, in the midst of grooming, the groomer chewed on a tuft of the groomed animal’s coat without pulling it out.
The typical pattern of hair collection and consumption involved the tripedal posture in which both legs and one arm were used to support the body while the other arm was used to collect hair. During the study, the subjects stood tripedally, gathered and consumed several strands of hair, took a few steps, and repeated the process. Sometimes individual strands were rolled into a larger ball prior to consumption by using the palm of the hand, holding it flat against a substrate, and moving it in a circular motion to bind the hairs together. On one occasion, an animal was observed washing a wad of hair out of a piece of feces and reingesting it.
Twice-a-Day Feeding Component Observations
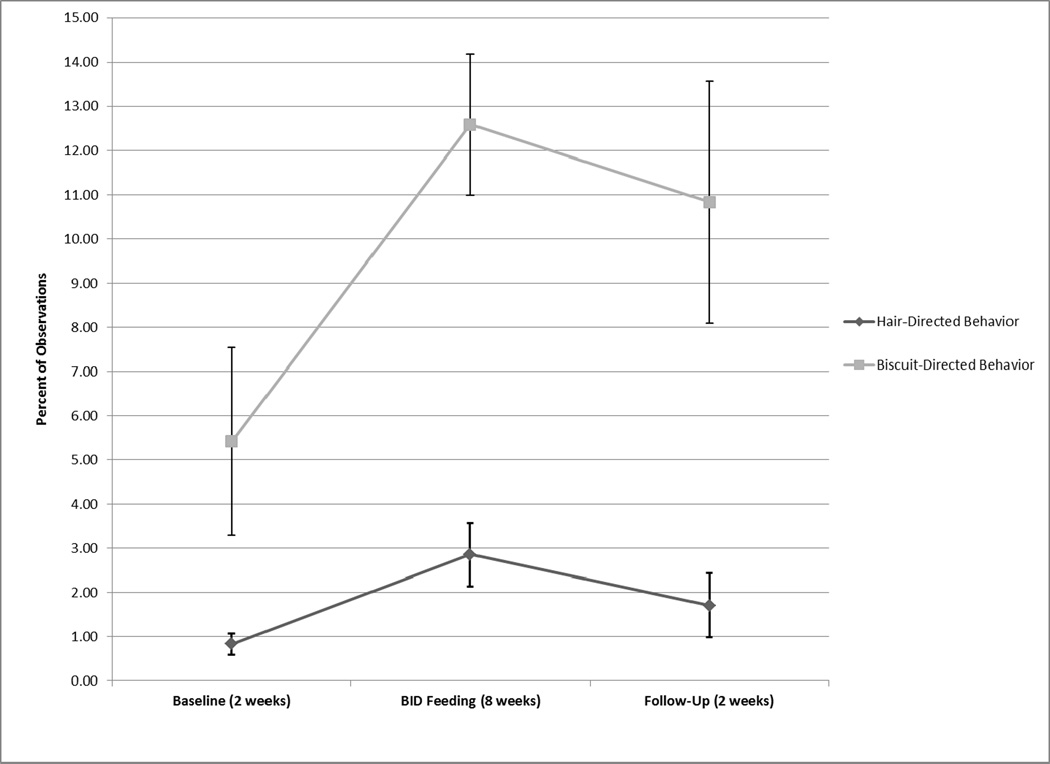
There was trend in overall hair-directed behavior across the three periods of the BID feeding component (Q = 5.895, p < 0.10; Figure 1). Post-hoc analyses demonstrated that hair-directed behavior was higher when the subjects were fed twice daily during the experimental period than during baseline or follow-up. This was significant from baseline to the experimental period (Q = 2.59, p < 0.05; Table 2), with a trend toward significance from the experimental period to follow-up (Q = 2.07, p < 0.10). There was also a trend in overall biscuit-directed behavior across the BID feeding component (Q = 4.789, p < 0.10). Post-hoc analyses demonstrated that a significant increase in biscuit-directed behavior occurred from baseline to the experimental period (Q = 2.37, p < 0.05), but there was no difference between the experimental period and follow-up (Q = 0.998, p > 0.10).
FIGURE 1.
Average percent of observations ± SE that baboons engaged in hair- and bisccuit-directed behaviors during baseline, experimental, and follow-up periods of the BID feeding component of the study.
TABLE 2.
Average Percentages of Observations (±SE) that Subjects Engaged in Behaviors Summarized by Behavior Categories
| Twice-a-Day Feeding Component | Forage Distribution Component | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Behavior Category |
Baseline | Experimental Period |
Follow-Up | Baseline | Experimental Period |
Follow-Up | ||||
| Hair-Directed | 0.83 ± 0.76 | † | 2.86 ± 2.27 | ‡ | 1.71 ± 2.29 | 1.84 ± 0.66 | 1.30 ± 0.79 | 1.20 ± 0.39 | ||
| Biscuit-Directed | 5.42 ± 6.72 | † | 12.59 ± 5.06 | 10.83± 8.66 | 8.58 ± 1.74 | † | 2.45 ± 0.71 | † | 7.92 ± 2.29 | |
| Forage-Directed | N/A | N/A | N/A | 0.00 | 15.31 ± 1.22 | 0.00 | ||||
| Active | 14.79 ± 6.59 | 16.52 ± 8.33 | 17.68 ± 7.41 | 15.21 ± 2.21 | 16.88 ± 3.17 | 17.03 ±2.78 | ||||
| Rest | 53.33 ± 13.59 | 47.56 ± 20.57 | 52.97 ± 17.03 | 56.04 ± 3.65 | 48.85 ± 4.81 | 56.25 ± 4.12 | ||||
| Object-Directed | 5.54 ± 4.78 | 3.04 ± 1.61 | 3.99 ±4.41 | 1.53 ± 0.71 | 2.29 ± 0.90 | 2.5 ± 1.00 | ||||
| Social Behavior | 2.13 ± 1.55 | 2.46 ± 1.41 | 2.06 ± 1.55 | 2.40 ± 0.53 | 2.19 ± 0.63 | 1.41 ± 0.24 | ||||
| Groom Self | 2.21 ± 3.16 | 1.46 ± 1.12 | 2.07 ± 2.83 | 2.55 ± 1.45 | 2.92 ± 0.73 | 2.86 ± 0.68 | ||||
| Groom Other | 5.42 ± 6.25 | 5.32 ± 3.84 | 1.96 ± 2.63 | 5.85 ± 2.29 | 2.92 ± 1.26 | 5.16 ± 2.87 | ||||
| Tension Related | 3.67 ± 1.47 | 2.41 ± 1.24 | 2.93 ± 2.69 | 2.45 ± 0.66 | 2.71 ± 0.48 | 2.34 ± 0.42 | ||||
| Other | 6.67 ± 3.77 | 5.79 ± 3.36 | 3.81 ± 2.51 | 3.56 ± 0.80 | 2.19 ± 0.77 | 3.33 ± 0.68 | ||||
Note: A “†” between two columns indicates significance between those two observation periods, while a “‡” indicates a trend.
Forage Distribution Component Observations
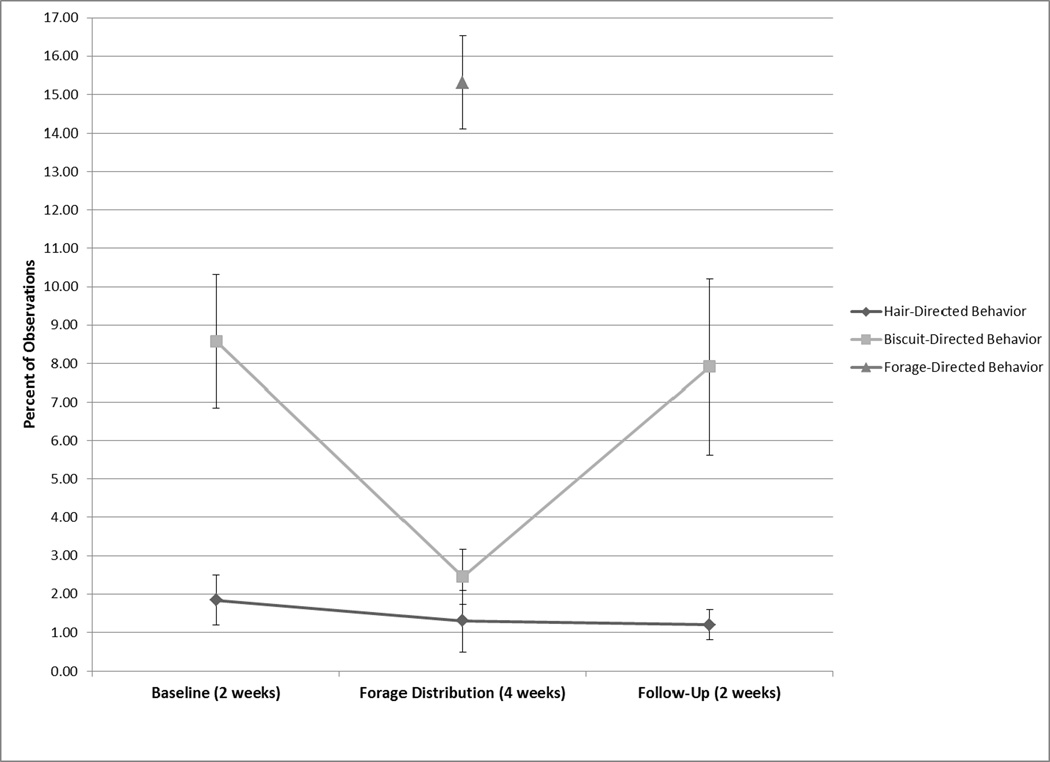
During the forage distribution component of the study, there were no changes in hair-directed behavior, but there was a significant overall change in biscuit-directed behavior across the three periods of the forage distribution component (Q = 12.45, p < 0.01; Figure 2). There was a significant decrease in biscuit-directed behaviors between baseline and the experimental period (Q = 5.80, p < .001; Table 2) and a significant increase between the experimental period and follow-up (Q = 6.31, p < .001). Three subjects exhibited no hair-directed behavior during the four weeks that forage was distributed. Interestingly, one subject who exhibited no hair-directed behaviors during baseline observations had a nearly two-fold increase in the behavior, whereas another who exhibited hair-directed behavior during less than 1% of the observations experienced an increase to over 6% during the period of time in which forage was distributed. Hair-directed behavior for both of these individuals decreased once again once forage distribution ceased during follow-up observations. These two individuals experienced similar changes in behavior during the BID feeding portion of the study.
FIGURE 2.
Average percent of observations ± SE that baboons engaged in hair- and bisccuit-directed behaviors during baseline, experimental, and follow-up periods of the forage distribution component of the study.
DISCUSSION
The most unusual and surprising outcome of this study was the fact that hair manipulation and consumption actually increased as a result of a change in feeding schedules from once to twice daily. This occurred despite the fact that BID feeding increased the amount of time that the animals spent handling and eating biscuits. This pattern of behavior suggests that handling and consuming biscuits from a trough is behaviorally distinct from active foraging, and do little to reduce the drive to forage. On the contrary, the behaviors may actually increase that drive. It was anticipated that access to food throughout the day would decrease hair manipulation and eating because the animals would have nearly ad-libitum access to appropriate food items. However, the performance of consummatory behavior has a positive feedback mechanism that may actually increase motivation, ensuring that feeding, once initiated, is not interfered with by other non-feeding behaviors (Duncan, 2005; Wiepkema, 1971).
In this case, the consumption of the biscuits in the absence of any prior appetitive behavior such as foraging may have increased the motivation to forage that manifested itself in searching out and consuming hair in the absence of other appropriate forage material. That being so, the forage distribution aspect of this study should have resulted in a decrease in the motivation to engage in the collection and ingestion of hair because gathering the grain should have sufficiently decreased the overall motivation to forage. Although some decrease in hair-directed behavior was seen as a result of forage distribution, the results were not significant. There was a significant reduction in biscuit-directed behavior when forage was distributed. Forage-directed behavior occurred nearly twice as much as biscuit-directed behavior during the forage provision component of the study, indicating that despite a lack of a significant reduction in hair-directed behavior, forage material still was highly favored.
There are perhaps several reasons why neither BID feeding nor forage distribution prompted a significant reduction in hair manipulation and consumption. Oddly enough, group-housing in an enclosure with a solid substrate floor could contribute to the behavior. In a study on trichobezoars, Mejido et al. (2009) found that the greater the percentage of time that the animals spent in group housing, the more prone they were to trichobezoar development. This makes sense in the context of the present study given that most of the hair consumed was gathered off the floor. Typically, in a laboratory situation requiring single-housing, the cage floor allows most of the shed hair to fall through rather than accumulate. Any hair consumed would therefore have to come from the animal himself or herself, and an individual pulling out his or her own hair was only observed once during the course of this study. Although single-housing may not be a viable intervention to prevent hair ingestion for primates, modified raised flooring that allows more hair to fall through may help.
It is possible that not enough forage was distributed. In the wild, the tripedal posture utilized by baboons during this study to collect and eat hair is entirely associated with feeding, and it is used when food objects are relatively widely scattered (Rose, 1977). An insufficient amount of forage is probably not the reason in this case, though; at least anecdotally, there appeared to be grain still remaining in the cage toward the end of the day. Also, given the limited space in the enclosure and the effort to ensure that the broadcasted grain covered most of the flat surfaces in the enclosure, little activity was actually needed to acquire and consume it.
It may be that not enough effort was required to locate and process the biscuits or forage material. Even though scattering the grain on the floor did encourage some of the animals to move about the cage, pick up the items, and eat them, some subjects, particularly the more dominant animals, still chose only to sit in place and pick up those forage items within reach. The same was true for biscuits. Although some did get scattered by the animals, most of the biscuits remained in the vicinity of the food trough. Little effort was needed to obtain and consume them. Activity levels remained fairly constant throughout the study. These consistent patterns of activity show that in spite of access to biscuits throughout the day or access to forage, the animals may have only satiated their consumptive desire and not their appetitive one, as discussed by Duncan (2005). Satiation of the appetitive drive would have required some activity other than simply picking up the food item and eating it. Perhaps some additional searching for the food item is necessary.
Providing forage has been successful in limiting abnormal behavior in other studies when it was hidden in a layer of wood shavings as opposed to only being scattered on a bare concrete floor. In one study, six of seven primate species showed a reduction in both inactivity and abnormal behavior when 4 cm of woodchips were spread on what was previously a bare floor., Grain was added to these wood chips (Chamove, Anderson, Morgan-Jones, & Jones, 1982). The abnormal behaviors that were quantified consisted of locomotor stereotypies, bizarre posture, and self-aggression rather than hair eating, but the study does show that a change in the substrate in which forage is presented could be an inherently important factor in eliciting change in behavior.
Baker (1997) showed a reduction in regurgitation and reingestion in chimpanzees when as little as two cups of sunflower seeds, peanuts, and grain were spread in straw that covered most of the floor space in their enclosure. Similar results were found by Boccia and Hijazi (1989) when supplementary feeding of one cup of sunflower seeds dispersed through the cage in woodchip bedding significantly decreased stereotypies and completely eliminated hair pulling in group-housed pigtail macaques. The impact of cage floor substrate was further elaborated upon by Beisner and Isbell (2008), who found that rhesus monkeys spent more time foraging in a grass substrate as opposed to dirt/gravel, and that those in an enclosure with a floor composed of dirt/gravel substrate spent more time grooming others, leading to higher instances of alopecia. Perhaps even hiding the animals’ normal ration of biscuits in some sort of bedding to encourage them to hunt it out would provide further benefit.
However, introducing wood chip or straw bedding in which to hide forage may not be a viable option on a scale as large as the colony described in this study. Outdoor housing does not easily lend itself to wood chips or straw as a floor substrate for several reasons. Vermin can nest in it, and rain-soaked wood chips or straw can lead to mold and sanitation problems if it is not removed immediately. For facilities that routinely wash their enclosures as opposed to using dry husbandry, any substrate that makes it into the drainage could potentially cause a blockage. Apart from practical husbandry issues, wood chips or straw represent a health hazard for baboons in particular due to the fact that baboons are indiscriminant eaters, and ingestion of the flooring substrate could lead to bezoars and accompanying health issues. Alternatives for large, outdoor colonies require further development and study.
Whether the performance of hair eating indicates that the animals are experiencing stress or decreased welfare from an inability to perform appetitive foraging behavior is unclear. No other abnormal behaviors such as locomotor stereotypies or self-injurious behavior were observed. The performance of the hair-eating behavior appears to be very specific to an appetitive drive. As all of the animals observed during this study engaged in hair manipulation and eating to some extent, it would seem that the drive behind the behavior is fairly strong. That said, the performance of all hair-directed behaviors only accounted for an average of 2.86% of the observations at its most frequent. As mentioned previously, time spent in feeding activities in the wild accounts for anywhere from 21 to 60% of these animals’ time budgets. Even when feedings were conducted twice a day or forage was provided, the amount of time engaged in normal feeding behaviors only began to approach the lower range of time budgets listed. Increasing the amount of time that captive baboons spend engaging in species-typical feeding and foraging behavior to levels resembling those exhibited by baboons in the wild is potentially a key element in developing husbandry and enrichment routines that reduce instances of hair manipulation and consumption.
CONCLUSION
The results of this study indicate that neither prolonged access to food via BID feedings nor forage cast on the floor reduces the desire to collect and consume hair in baboons. In some cases, it may actually lead to an increase in the behavior. The drive appears to be related to the act of searching for and processing food rather than simply consuming it. Previous research demonstrated that hiding grain in woodchips and shavings as opposed to simply scattering it on the ground is effective at decreasing a variety of abnormal behaviors (Baker, 1997; Boccia & Hijazi, 1989; Chamove et al., 1982). The extra effort and time spent retrieving the hidden grain in these examples may be necessary to achieve a significant effect on hair eating as well. As the ingestion of hair can lead to trichobezoars that have severely negative impacts on the health of the animals, it is important to determine the means to reduce them.
REFERENCES
- Altman J. Baboon mothers and infants. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- Baker KC. Straw and forage material ameliorate abnormal behaviors in adult chimpanzees. Zoo Biology. 1997;16(3):225–236. [Google Scholar]
- Baker KC, Weed JL, Crockett CM, Bloomsmith MA. Survey of environmental enhancement programs for laboratory primates. American Journal of Primatology. 2007;69:377–394. doi: 10.1002/ajp.20347. [DOI] [PubMed] [Google Scholar]
- Beisner BA, Isbell L. Ground substrate affects activity budgets and hair loss in outdoor captive groups of rhesus macaques (Macaca mulatta) American Journal of Primatology. 2008;70:1–9. doi: 10.1002/ajp.20615. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Hijazi AS. A foraging task reduces agonistic and stereotypic behaviors in pigtail macaque social groups. Laboratory Primate Newsletter. 1998;37(3):1–5. [Google Scholar]
- Bouwer C, Stein DJ. Trichobezoars in trichotillomania: case report and literature overview. Psychosomatic Medicine. 1998;60:658–660. doi: 10.1097/00006842-199809000-00025. [DOI] [PubMed] [Google Scholar]
- Butler TJ, Haines RJ., Jr Gastric trichobezoar in a baboon. Laboratory Animal Science. 1987;37(2):232–233. [PubMed] [Google Scholar]
- Carr JR, Sholevar EH, Baron DA. Trichotillomania and trichobezoar: A clinical practice insight with report of illustrative case. Journal of the American Osteopathic Association. 2006;106(11):647–652. [PubMed] [Google Scholar]
- Chamberlain SR, Odlaug BL, Boulougouris V, Fineberg NA, Grant JE. Trichotillomania: Neurobiology and treatment. Neuroscience and Biobehavioral Reviews. 2009;33(6):831–842. doi: 10.1016/j.neubiorev.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Chamove AS, Anderson JR, Morgan-Jones SC, Jones SP. Deep woodchip litter: Hygiene, feeding, and behavioral enhancement in eight primate species. International Journal for the Study of Animal Problems. 1982;3(4):308–318. [Google Scholar]
- Chiezey NP. Hair pulling in confined sheep fed a finely ground ration: Case report. Livestock Research for Rural Development. 2010;22(3):52. [Google Scholar]
- Christenson GA, Mackenzie TB, Mitchell JE. Characteristics of 60 adult chronic hair pullers. American Journal of Psychiatry. 1991;148(3):365–370. doi: 10.1176/ajp.148.3.365. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. Feeding ecology of gelada baboons: A preliminary report. In: Clutton-Brock TH, editor. Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys, and apes. New York, NY: Academic Press; 1977. pp. 251–273. [Google Scholar]
- Duncan IJH. Behavioral needs. Stereotypies and animal welfare. SCAW Newsletter. 2005;27:9–14. [Google Scholar]
- Eckstein EF. Food, people, and nutrition. Westport, CT: AVI Publishing; 1980. [Google Scholar]
- Frey A, McKee M, King RA, Martin A. Hair apparent: Rapunzel syndrome. American Journal of Psychiatry. 2005;162(2):242–248. doi: 10.1176/appi.ajp.162.2.242. [DOI] [PubMed] [Google Scholar]
- Garner J. Stereotypies and other abnormal repetitive behaviors: Potential impact on validity, reliability, and replicability of scientific outcomes. ILAR Journal. 2005;46(2):106–117. doi: 10.1093/ilar.46.2.106. [DOI] [PubMed] [Google Scholar]
- Gerold S, Huisinga E, Iglauer F, Kurzawa A, Morankic A, Reimers S. Influence of feeding hay on the alopecia of breeding guinea pigs. Zentralbl Veterinarmed A. 1997;44(6):341–348. doi: 10.1111/j.1439-0442.1997.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Gillin AG, Phippard AF, Thompson JF, Harewood WJ, Waugh RC, Horvath JS. Gastric haemorrhage and perforation caused by a trichobezoar in a baboon (Papio hamadryas) Laboratory Animals. 1990;24:180–182. doi: 10.1258/002367790780890176. [DOI] [PubMed] [Google Scholar]
- Gozalo AS, Montoya E, Nolan TE. Trichobezoars in two saddleback tamarins (Saguinus fuscicollis) Journal of Medical Primatology. 1990;19:151–153. [PubMed] [Google Scholar]
- Hadnagy C, Binder P, Grauzer J, Szocs K. Trichophagia treated successfully by intravenous iron injections. Orvosi Hetilap. 1991;132(1):35–36. [PubMed] [Google Scholar]
- Lacey EP. Broadening the perspective of pica: Literature review. Public Health Reports. 1990;105(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Mahan CS. Revolution in obstetrics: Pregnancy nutrition. Journal of the Florida Medical Association. 1979;66:367–372. [PubMed] [Google Scholar]
- McGehee FT, Jr, Buchanan GR. Trichophagia and trichobezoar: Etiologic role of iron deficiency. The Journal of Pediatrics. 1980;97(6):946–948. doi: 10.1016/s0022-3476(80)80429-x. [DOI] [PubMed] [Google Scholar]
- Mejido DCP, Dick EJ, Jr, Williams PC, Sharp RM, Andrade MCR, DiCarlo CD, Hubbard GB. Trichobezoars in baboons. Journal of Medical Primatology. 2009;38:302–309. doi: 10.1111/j.0047-2565.2009.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook DM. Gastric trichobezoars in a rhesus macaque (Macaca mulatta) Comparative Medicine. 2002;52(6):560–562. [PubMed] [Google Scholar]
- Mulder A, Nieuwenkamp AE, van der Palen JG, van Rooijen GH, Beynen AC. Supplementary hay reduces fur chewing in rabbits. Tijdschr Diergeneeskd. 1992;117(22):655–658. [PubMed] [Google Scholar]
- Nolan TE, Schaffer L, Conti PA. A gastric trichobezoar in a chimpanzee. Journal of Medical Primatology. 1987;17:63–65. [PubMed] [Google Scholar]
- Reinhardt V, Reinhardt A, Houser D. Hair pulling and eating in captive rhesus monkey troops. Folia Primatologica. 1986;4:158–164. doi: 10.1159/000156272. [DOI] [PubMed] [Google Scholar]
- Rose MD. Positional behavior of olive baboons (Papio anubis) and its relationship to maintenance and social activities. Primates. 1977;18(1):59–116. [Google Scholar]
- Stacey PB. Group size and foraging efficiency in yellow baboons. Behavioral Ecology and Sociobiology. 1986;18(3):175–187. [Google Scholar]
- Tabatabai SE, Salari-Lak M. Alopecia in dolls. Cutis. 1981;28(2):206–209. [PubMed] [Google Scholar]
- Wemelsfelder F. The concept of animal boredom and its relationship to stereotyped behaviour. In: Lawrence AB, Rushen J, editors. Stereotypic animal behaviour: Fundamentals and applications to welfare. Wallingford, Oxon, United Kingdom: Cab International; 1993. pp. 65–87. [Google Scholar]
- Whiten A, Byrne RW, Barton RA, Waterman PG, Henzi SP. Dietary and foraging strategies of baboons. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1991;334(1270):187–197. doi: 10.1098/rstb.1991.0108. [DOI] [PubMed] [Google Scholar]
- Wiepkema PR. Positive feedbacks at work during feeding. Behaviour. 1971;39(2):266–273. doi: 10.1163/156853971x00258. [DOI] [PubMed] [Google Scholar]