Abstract
The objective of this study was to investigate the effects of low-grade inflammation on age-related changes in glomerular filtration rate (GFR) in middle-aged and older white Americans, African-Americans, and Japanese adults. Serum creatinine, C-reactive protein (CRP), and interleukin-6 (IL-6) levels were determined for 1570 adult participants in two surveys of aging in the USA and Japan (N = 1188 and 382, respectively). Kidney function declined with age in both countries and was associated with IL-6 and CRP. IL-6 and CRP also influenced the extent of the arithmetic bias when calculating the GFR using the chronic kidney disease epidemiology (CKD-EPI) formula with just serum creatinine. Younger African-Americans initially had the highest GFR but showed a steep age-related decrement that was associated with elevated inflammation. Japanese adults had the lowest average GFR but evinced a large effect of increased inflammatory activity when over 70 years of age. Importantly, our results also indicate that low-grade inflammation is important to consider when evaluating kidney function solely from serum creatinine.
Keywords: Inflammation, Interleukin-6, C-reactive protein, Kidney, Glomerular filtration rate, Race, Aging, Gender, Japanese, African-American
Kidney function is not typically assessed in surveys of population health or in most gerontology research, even though it is a known biomarker of aging, which is associated with other illnesses, including diabetes and cardiovascular disease (Safar et al. 2014). Poor renal function has also been linked with fatigue, anxiety, and impaired cognitive performance and thus is of relevance to behavioral medicine research with the elderly (Artom et al. 2014; Dalui et al. 2014; Silverwood et al. 2014).
Glomerular filtration rate (GFR) declines progressively with age by about 1 mL/min per year (Stevens et al. 2006). However, this decrease in the elderly is variable, with some retaining effective kidney clearance rates and others progressing to kidney disease, which then impacts quality of life and increases overall morbidity and mortality (Bonner et al. 2013). Hypertension and type 2 diabetes, as well as other inflammatory disorders, are known to exacerbate age-related changes in renal function (Mallappallil et al. 2014). Our analyses evaluated population variation in kidney function in middle-aged and older adults in the USA and Japan, and its association with low-grade inflammation, based on elevated interleukin-6 (IL-6) and C-reactive protein (CRP).
The populations of both the USA and Japan are aging. The percent of Americans over 65 years of age is expected to be 20 % by 2050 (Weiner and Tilly 2002), a proportion already reached in Japan, with 24 % older than 65 years (Nitta et al. 2013). The prevalence of chronic kidney disease (CKD) in Japan is 13 %, and it has the second highest prevalence of end-stage renal disease (ESRD) in the world (Imai et al. 2009; Yamagata et al. 2014). CKD is also common in the USA at 11 % (Coresh et al. 2007), with the USA ranked third in world for ESRD. Thus, it is of importance to investigate early indicators of declining kidney health before the emergence of clinical dysfunction and to determine if there are significant racial differences in the association between inflammation and kidney function. Race differences in kidney function have been described between white Americans and African-Americans, and kidney disease is more prevalent among older African-Americans (Kramer et al. 2008). Differences in GFR and serum creatinine between Americans and Japanese have not been examined as systematically. However, one can anticipate some national differences, given known differences in pro-inflammatory physiology and cardiovascular disease across these two populations (Coe et al. 2011), which are likely to impact kidney function. Specifically, our analyses focused on IL-6, a pro-inflammatory cytokine readily measured in blood and known to affect renal function, and CRP, a commonly employed test in the clinical setting, because both indices have been associated with a reduced GFR and kidney disease (Sinha et al. 2014; Tbahriti et al. 2013; Gupta et al. 2012).
Many different methods are available to assess kidney function, and the less invasive ones that rely on creatinine clearance tend to be employed by primary care physicians. A number of formulas have been derived to estimate kidney clearance on the basis of serum creatinine levels, after taking age, gender, and race into consideration. However, these estimates are approximations that can be influenced and compromised by many factors. Therefore, our analyses determined the degree to which an individual’s IL-6 and CRP levels may be indicative of a potential bias in the estimates of kidney function when quantified from just serum creatinine. The magnitude of this discrepancy was possible to calculate because both serum and urinary creatinine levels were determined for all American participants. It also provided the opportunity to assess if inflammatory physiology differentially affected GFR estimates in African-Americans and white Americans.
Methods
Participants
The Japanese participants (N = 382) were a subset (37 %) from a larger number of randomly selected, middle- and older-aged adults who were recruited in 2008 to represent the 23 residential wards of Tokyo. All respondents in the Midlife in Japan project (MIDJA) completed demographic questionnaires, and 382 provided blood specimens at a medical clinic near the University of Tokyo. Mean age was 54.2 years (±14.1 years, 32–79 years of age), and 73 % were married. Age, gender composition, marital status, and education attainment did not differ significantly between this subset of biomarker participants and the larger survey cohort.
Their kidney function was compared with 1188 participants in the Midlife in the United States project (MIDUS). MIDUS had begun in 1995–1996 as a national probability sample of 7108 adults (Radler and Ryff 2010). Participants were recruited via random telephone dialing in the contiguous USA, and 48 states are represented in the current biological data collection (Radler 2014). At the time of this assessment between 2004 and 2008, they were 35–86 years of age. Specimens were collected in a controlled manner while participants spent the night at one of three Clinical and Translational Research Centers (CTRC), located in the Midwest or on the east and west coasts. Those providing blood and urine were similar to the larger study sample with respect to age, gender, and marital status, but likely to be more educated (although 25 % still had attained only a high school degree and 50 % had not completed college) (Love et al. 2010). To increase the representation of African-American participants in MIDUS, a city-specific sample from Milwaukee, WI, had been added to the survey in 2004. The current analyses of kidney function are based on 225 African-American and 963 white Americans (after excluding 11 participants with incomplete data, 3 African-Americans and 8 white Americans).
Specimen collections were approved by the Health Sciences Institutional Review Board at the University of Wisconsin-Madison, as well as by IRBs at the University of California Los Angeles and Georgetown University. A comparable review at the University of Tokyo approved the MIDJA protocols. All participants provided informed consent.
Procedures
Blood samples were collected between 0900 and 1145 for over 95 % of MIDJA participants and between 0500 and 0700 for all MIDUS participants. Urine creatinine was also determined for all MIDUS participants by having the CTRC nurses collect and refrigerate all voids from 1900 to 0700. Creatinine was determined via colorimetric assay (Meriter Lab, Madison, WI). Serum IL-6 was quantitated in duplicate with an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Serum CRP for both the American and Japanese was determined by immunonephelometric assay at the same laboratory.
Covariates
Each participant’s height and weight was measured, from which the body mass index was calculated (BMI = weight/height2) and used as a covariate in the analyses. Sitting blood pressure was also determined, and systolic blood pressure used as a covariate because of the known influence of hypertension on kidney function. Finally, to take into account potential effects of type 2 diabetes on renal findings, glycosylated hemoglobin (HA1c) was also included as a covariate. For MIDUS participants, HA1c was determined by turbidimetric immunoinhibition assay at a local clinical laboratory (Meriter Lab). Because HA1c has to be determined on fresh, whole blood, for the Japanese it was determined in Tokyo by Syowa Medical Science, but an algorithm was employed to generate comparable values with the US. Based on physician-diagnosed diabetes and HA1c values over 6.5 % (>48 mmol/mol), the prevalence of poor glycemic control was 7 % for the Japanese, 14 % for white Americans, and 37 % for African-Americans.
Data analytic strategy
The data were analyzed using linear and logistic regression models. To limit the influence of outlier values, a small number of extremely high values outside the third standard deviation of the distribution were removed before all values were log transformed following the statistical recommendations of Tukey and Winsor (Hasings et al. 1947). Race was coded with three levels, and white Americans designated as the reference group. Racial identity in MIDUS was determined from self-report, and only participants who identified as African-American or white were included. Forty-six participants from the original sample were excluded because they reported a race other than white or African-American, or were of mixed racial backgrounds. All participants in MIDJA were Japanese. Age was treated as a continuous variable in the analyses, although for illustrative purposes, it is portrayed in some tables and graphs as three age categories (<49, 50–64, 65+ years of age).
Because urine samples were available only for MIDUS participants, the GFR values for the country comparisons were calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) formula, which adjusts serum creatinine for expected differences due to age, gender, and race (Levey et al. 2009). The GFR for the Japanese was calculated using the Japanese coefficient-modified CKD-EPI Study equation (Horio et al. 2010). For the additional GFR analyses in American participants, which were based both on serum and urine creatinine, clearance was adjusted for body surface area (Mosteller 1987).
Results
Descriptive summary
Table 1 provides descriptive statistics for the Japanese and American participants. Although there was a small difference in mean age, the age ranges of the three racial groups were comparable. As expected, there were large and significant differences in the BMIs between Japan and the USA, and significant differences in the two inflammatory measures, CRP and IL-6. The mean CKD-EPI values also differed significantly by race: highest in African-Americans and lowest in Japanese participants.
Table 1.
Descriptive statistics for the three groups used in the renal function analyses
| Measure | White American (N = 963) | African-American (N = 225) | Japanese (N = 382) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 58.36 | 11.69 | 53.59* | 10.41 | 55.47* | 14.04 |
| BMI | 29.1 | 5.8 | 32.9* | 8.57 | 22.6* | 2.9 |
| Systolic BP (mmHg) | 130.86 | 17.90 | 133.90 | 20.22 | 121.64* | 19.95 |
| HbA1c (%) | 5.96 | 0.83 | 5.59 | 1.54 | 6.05* | 0.53 |
| IL-6 (pg./mL) | 2.80 | 2.82 | 4.15* | 3.72 | 1.59* | 1.75 |
| C-reactive protein (mg/L) | 0.87 | 0.26 | 4.38* | 5.48 | 0.69* | 1.27 |
| Serum creatinine (mg/dL) | 0.87 | 0.26 | 0.94 | 0.70 | 0.74 | 0.17 |
| Urine creatinine (mg/dL) | 76.49 | 48.35 | 101.69 | 68.85 | nd | nd |
| CKD-EPI | 85.67 | 15.83 | 97.80* | 25.02 | 79.76* | 13.06 |
CRP and creatinine values in Table 1 are after log-transformation of the original data values
nd not determined
*p < 0.05 when compared to white Americans
The majority of participants had a GFR over 60, indicating healthy or in some cases abnormally high filtration. Some did have a GFR below 60, providing a possible indication of low rates of undiagnosed kidney disease (African-American, N = 20, 8.6 %; white, N = 59, 6.1 %; Japanese, N = 31, 8.1 %). The differences in the prevalence of stages 3, 4, and 5 CKD, as defined by a low GFR, did not differ by ethnicity or race.
When not considering race, the main effect of increasing age was significant across all three racial groups, b = −0.77, t(1557) = −19.35, p < 0.01. However, there was also a significant interaction between age and gender, b = 0.23, t(1557) = 3.01, p < 0.01, indicating that the age-related decrement in kidney function was larger for women. The decline associated with aging was also more prominent in African-Americans than white Americans, b = −0.56, t(1557) = −1.67, p < 0.01.
Effect of age
The three-way interaction between age, race, and gender on CKD-EPI values was tested by linear regression, with BMI, systolic blood pressure, and HA1c as covariates. These three covariates were included because both obesity and type 2 diabetes were more prevalent among Americans, and our aim was to discern national and age-related differences beyond the influence of these factors known to affect kidney function. Figure 1 shows the effects of age on the unadjusted marginal means of CKD-EPI for each racial group. The contrast between African-Americans and white Americans was significant, b = 5.74, t(1556) = 4.85, p < 0.01, indicative of the higher overall GFR for African-Americans, especially in the younger adults. Conversely, the CKD-EPI for Japanese was significantly lower than that for Americans, including for both African-American, b = 14.46, t(1556) = 10.31, p < 0.01, and white participants, b = −8.75, t(1556) = −9.30, p < 0.01.
Fig. 1.
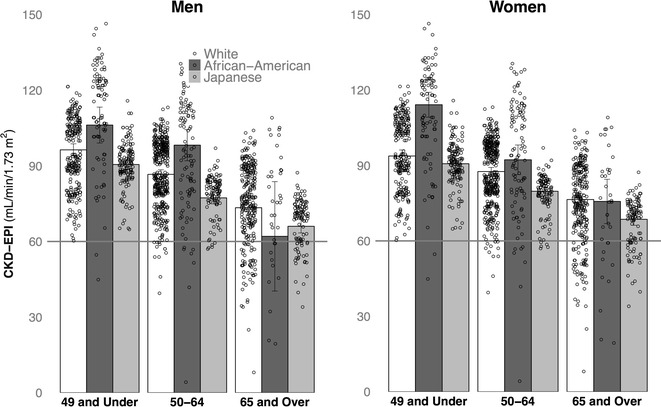
Significant age-related declines in renal function (CKD-EPI), illustrated for Japanese and American adults in three age categories. Portrayed separately for males and females
IL-6 and CRP
The relationship of IL-6 and CRP to the age decrement in CKD-EPI values was tested by linear regression, including the two-way interaction between age and each biomarker, and using BMI, systolic BP, and HA1c as covariates. Figure 2 shows the influence of higher levels of IL-6 and CRP on CKD-EPI in three age categories (younger = 35–49; middle-aged = 50–64; older = 65 years and older). The simple effect of IL-6 on CKD-EPI in young adults below age 49 was significant, b = 2.83, t(1609) = 2.21, p = 0.03, indicating that the CKD-EPI was higher by 2.83 points for every 1-point increase in IL-6 (log pg/L). The simple effect of IL-6 on CKD-EPI in middle-aged adults was not significant, t(1609) = 0.13, p = 0.90. In contrast, the effect of IL-6 on CKD-EPI for adults older than 65 years reemerged as significant, b = −4.33, t(1609) = −3.12, p < 0.01, but now in the opposite direction. In these older adults, the CKD-EPI decreased by 4.33 points for every 1-point increase in IL-6 (log pg/L).
Fig. 2.
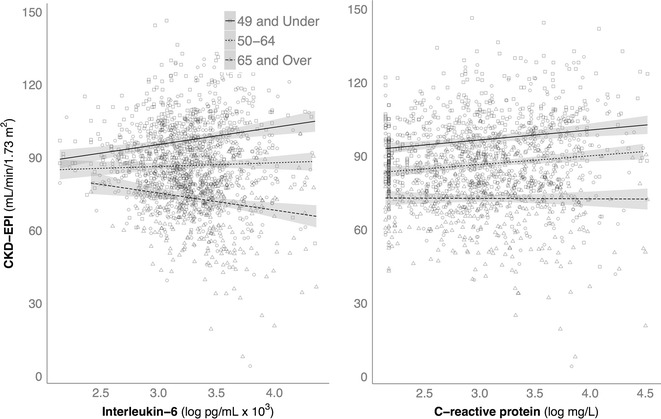
Significant differences in how CKD-EPI was associated with low-grade inflammation in younger adults and in those over 65 years of age, averaged across American and Japanese participants. Among younger adults, high CRP and IL-6 were correlated with an elevated CKD-EPI, whereas higher IL-6 resulted in a lower CKD-EPI in older adults
We found a similar biphasic influence of age on the relationship between CRP and CKD-EPI values, b = −0.18, t(1605) = −3.44, p < 0.01. In the younger adults, the effect of CRP on CKD-EPI was positive and statistically significant. In the middle-aged adults, the augmented influence of higher CRP was still present and significant, b = 1.65, t(1605) = 2.09, p < 0.05. However in older adults over 65 years, the effect of high CRP was negative, b = −2.71, t(1605) = −1.95, p = 0.051. Considering the total age span we evaluated, the general effect of CRP on CKD-EPI was an annual decrease of 0.18 points for each year of age.
The two-way interactions examining how IL-6 or CRP differentially affected CKD-EPI in each race were then tested with linear regression, including BMI, systolic BP, and HA1c as covariates. Figure 3 shows the influence of higher levels of IL-6 and CRP on the CKD-EPI values in American and Japanese. Among Americans, the negative relationship between high IL-6 and CKD-EPI was significantly greater for African-Americans as compared to white Americans, b = −8.37, t(1562) = −2.22, p = 0.03. The magnitude of the negative influence of higher IL-6 was similar for white Americans and Japanese and thus not significantly different, b = −0.79, t(1562) = −0.27, p = 0.78. The influence of CRP was similar for each race, and all tended to have a lower CKD-EPI as CRP levels increased. Thus, the interaction term, which tested for racial differences in the magnitude of effect on CKD-EPI as CRP levels rose, was not statistically significant.
Fig. 3.
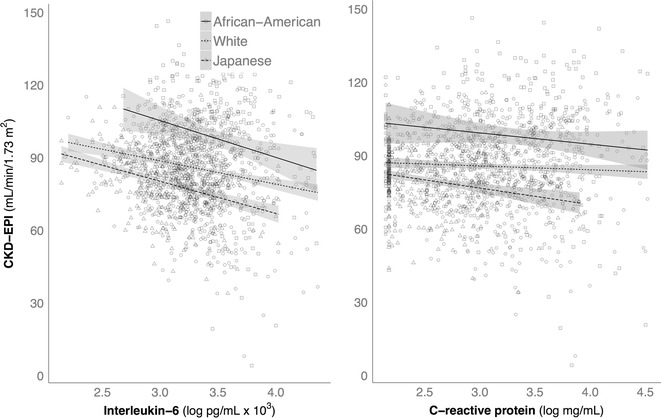
Overall, when controlling for participant age, higher levels of IL-6 and CRP were associated with a lower CKD-EPI in both American and Japanese participants. With age included as a covariate, the specific effect for younger adults (portrayed in Fig. 2) was overridden by a stronger, general trend for decreased kidney function with low-grade inflammation
Bland-Altman analyses
Bias, precision, and accuracy of the CKD-EPI determined values were compared to the actual serum/urine creatinine ratio (Ccr) in American participants in order to evaluate the reliability of the CKD-EPI index. Table 2 presents the means of the two calculations, the extent of bias, the standard deviation of the bias (precision), accuracies at the 10 and 30 % points, and the correlations between the calculations. The computed values were generally correlated, and the levels of precision were in a similar range. However, there was more bias evident for younger adults below 65 years of age, in males, white Americans, and among those with less inflammation. When considering the degree of accuracy within the 10 and 30 % range, the bias incurred by relying on a CKD-EPI value computed via the formula from just the serum creatinine was greater for white than African-Americans and for men as compared to women. Table 2 also shows the bias and accuracy when participants’ IL-6 and CRP values were at the high and low end of the distribution.
Table 2.
Creatine clearance versus CKD-EPI computed values in white and black Americans
| Category | Number of samples | Mean | Bias | SD bias | 10 % accuracy | 30 % accuracy | Correlation | p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||||
| Overall | 1188 | 93.50 | −11.34 | 24.25 | 306 | 26 | 822 | 69 | 0.62 | <0.01 |
| 49 and under | 342 | 106.35 | −14.89 | 26.38 | 75 | 22 | 239 | 70 | 0.55 | <0.01 |
| 50–64 | 528 | 93.70 | −10.31 | 24.21 | 137 | 26 | 370 | 70 | 0.48 | <0.01 |
| 65 and over | 318 | 79.36 | −9.22 | 21.41 | 92 | 29 | 213 | 67 | 0.64 | <0.01 |
| White | 963 | 91.63 | −12.27 | 23.77 | 237 | 25 | 651 | 68 | 0.57 | <0.01 |
| African-American | 225 | 101.51 | −7.76 | 25.94 | 67 | 30 | 171 | 76 | 0.72 | <0.01 |
| Men | 517 | 95.13 | −16.33 | 23.37 | 108 | 21 | 323 | 62 | 0.62 | <0.01 |
| Women | 671 | 92.25 | −7.49 | 24.22 | 196 | 29 | 499 | 74 | 0.64 | <0.01 |
| IL-6 (log pg/L) < 0.21 | 429 | 98.43 | −15.31 | 24.61 | 95 | 22 | 278 | 64 | 0.54 | <0.01 |
| IL-6 (log pg/L) ≥ 0.47 | 378 | 90.57 | −8.85 | 23.04 | 104 | 28 | 267 | 71 | 0.70 | <0.01 |
| CRP (log mg/L) < −0.05 | 455 | 95.53 | −13.65 | 23.12 | 114 | 25 | 305 | 67 | 0.58 | <0.01 |
| CRP (log mg/L) ≥ 0.45 | 329 | 92.17 | −8.48 | 25.08 | 86 | 26 | 221 | 67 | 0.66 | <0.01 |
10 and 30 % accuracy is the number of CKD-EPI values that fall within 10 or 30 % of the Ccr values, respectively. For both IL-6 and CRP, the smaller total N is due to the focus on the upper and lower ends of the distribution
Discussion
Our results concur with other reports indicating that age-related declines in kidney function vary by gender, race, and nationality, with the largest age-related decrease occurring in African-American women. Despite clear race differences in the GFR, there were not sufficient numbers of participants at stage 4 or 5 to compare the variation in the prevalence of CKD. But that also indicates the observed differences in GFR were not due to inclusion of unequal numbers of participants with overt kidney disease.
Although others have reported that kidney disease is more common in African-Americans (Kramer et al. 2008), the recruitment strategies may have lessened the likelihood that individuals with debilitating kidney disease or dependent on dialysis would have traveled to the clinical sites for evaluation. Similarly, there were no participants in the MIDJA project with a GFR indicative of stage 4 or 5 CKD. In the MIDUS sample, 6.5 % of participants had a GFR below 60, slightly lower than the 8.1 % reported for Americans by Coresh et al. (2003). In the MIDJA sample, 8.1 % of participants had a GFR below 60, which is similar to the 10.6 % rate reported by Imai et al. (2009). On the other end of the continuum, one of several notable race-related differences found in our surveys was a high number of younger African-American adults with an elevated GFR, which could be classified as stage 3 CKD, a likely precursor of a later decline and ultimately kidney disease (Sud et al. 2014).
Our results showed that both IL-6 and CRP are associated with GFR but in a biphasic manner depending upon the age of the participant. Low-grade inflammation tended to increase the GFR in adults below 49 years of age, but worsen the age-related decrement in older participants over 65 years of age. In the younger adults, the CKD-EPI values still remained in the functional range, even in the context of elevated IL-6. In old age, however, the CKD-EPI was markedly lower when IL-6 was high. This seemingly paradoxical relationship, based on participant age, was especially evident in the elevated GFR of young African-Americans, who also had the highest IL-6. The likely clinical consequences of inflammation as a risk factor for poor kidney function also became more evident with age in older African-Americans.
Future studies should determine whether a high CKD-EPI value in younger African-Americans is indicative of youthful vigor or if its coincidental occurrence with high IL-6 and CRP already reflects the onset of abnormal hyperfiltration, which then will progress to kidney dysfunction with age (Noone and Licht 2014). The higher GFR in the younger African-American participants did not appear to be due specifically to hypertension, as their systolic BP did not differ significantly from the white Americans (b = 0.01, p = 0.07). In addition, systolic BP had been included as a covariate in the statistical modeling of kidney function. Rather, it seems more parsimonious to conclude that it is indicative of incipient hyperfiltration, which is an early warning sign of later kidney disease. As renal dysfunction progresses, the kidneys can then no longer compensate for glomerular injury, and hyperfiltration gives way to a reduced GFR. From a statistical perspective, the high GFR in younger African-Americans contributed to the significantly steeper age-related decline in kidney function.
Race also influenced the magnitude of the effect of IL-6 on the GFR. IL-6 had the largest effect on GFR in African-American and Japanese participants, even after controlling for the correlation between BMI and IL-6. The fact that African-Americans tend to have higher levels of IL-6 may make them more vulnerable to microalbuminuria or glomerulosclerosis (Kshiragar et al 2008). The progression onto renal disease is also likely to be influenced by national and race differences in access to medical care. It should be mentioned that at later stages in disease progression, some beneficial effects of a larger inflammatory reaction have been reported. When on dialysis, African-Americans who evince more inflammation actually have a better survival rate from ESRD than white Americans (Crews et al. 2011). In our analyses, higher CRP levels were associated with a reduced GFR, but this effect was seen in all three racial groups. In contrast to this similar effect of elevated CRP in all races, one paper has reported a differential relationship, with a larger influence of high CRP in African-Americans (Sinha et al. 2014). Our results do confirm that CRP levels are significantly higher in African-Americans than found in both white Americans and Japanese adults at all ages.
Notwithstanding these important age- and race-related findings, several limitations of our analyses should be acknowledged. Because the participants were derived from two studies designed to obtain representative surveys for each country, we did not selectively recruit participants with overt kidney disease. Thus, the results do not address the likely differences in the prevalence of ESRD. On the other hand, this recruitment approach had the benefit of minimizing the potential confounding effects of medications taken for chronic kidney disease. As mentioned earlier, our estimates of the prevalence of stage 3 CKD, based on a GFR below 60, were in keeping with other surveys in both countries (Coresh et al. 2003; Imai et al. 2009). It should also be acknowledged that it is difficult to entirely distinguish national and racial differences in kidney function from the influence of co-occurring differences in obesity and type 2 diabetes. Our modeling of the relationship between low-grade inflammation and GFR included several covariates, BMI, systolic blood pressure, and HA1c, but it is still likely that adiposity, poor glycemic control, and insulin resistance contributed to some of the race differences we found in kidney function. It is known that GFR can be affected by diabetes (Tsuda et al. 2014). Our rationale for including HA1c as a covariate was because of the common occurrence of poor glycemic control in the African-American participants (37 %). Although this prevalence is notably high, and it is definitely a major public health concern, other papers have reported previously on the rising incidence of type 2 diabetes in older and overweight African-Americans across the USA (Randall et al. 2004). It is likely that racial disparities in access to quality health care and income differences contribute to these findings on the prevalence of type 2 diabetes and CKD in African-Americans (Marshall 2005).
Finally, our comparisons of the CKD-EPI computation with Ccr values using Bland-Altman analyses (Bland and Altman 1986) revealed that the CKD-EPI formula actually performed better when kidney function was not as good. That is, the estimation was more accurate in participants over 65 years of age, in females, for African-Americans and in those with signs of low-grade inflammation. Thus, the robustness of estimating GFR from just serum creatinine was better as kidney function declined with age. In contrast, it was less accurate in younger and healthier adults, especially among middle-aged men.
In summary, the current analyses highlight the extent to which kidney function declines with age. There is also a strong influence of race on this age-related decrease, and a biphasic relationship with low-grade inflammation, associated with an increased GFR in younger adults, and a decreased GFR in older adults. The utility of GFR as a biomarker and its sensitivity to inflammatory physiology has not been considered before in healthy adults. More typically, the clinical research has focused on patient populations already in need of hemodialysis or with chronic renal disease (Gupta et al. 2012; Pertosa et al. 2000). The ability to assess kidney function via creatinine and other biochemical indices from blood and urine specimens provides a unique tool for evaluating population health and for those interested in discerning the causes of disparities in health. Kidney disease is not inevitable with age, and renal function in many individuals remains effective and resilient.
Acknowledgments
This research was supported by grants from the National Institute on Aging (5R37 AG027343, P01 AG020166) to conduct the Midlife in Japan (MIDJA) and Midlife in the US (MIDUS) studies. The specimen collection was facilitated by the General Clinical Research Centers program (M01-RR023942 [Georgetown], M01-RR00865 [UCLA]), and at UW from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (1UL1RR025011). The contributions of Drs. G Love, M. Karasawa, and N. Kawakami and Ms. D. Brar in specimen collection and processing are gratefully acknowledged.
References
- Artom M., Moss-Morris R., Caskey F., Chilcot J. Fatigue in advanced kidney disease. Kidney Int. 2014;86(3):497–505. doi: 10.1038/ki.2014.86. [DOI] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- Bonner A., Caltabiano M., Berlund L. Quality of life, fatigue, and activity in Australians with chronic kidney disease: a longitudinal study. Nurs Health Sci. 2013;15(3):360–367. doi: 10.1111/nhs.12038. [DOI] [PubMed] [Google Scholar]
- Coe C. L., Love G. D., Karasawa M., Kawakami N., Kitayama S., Markus H. R., Tracy RP, Ryff C. D. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain Behav Immun. 2011;25(3):494–502. doi: 10.1016/j.bbi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coresh J., Astor B. C., Greene T., Eknoyan G., Levey A. S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third national health and nutrition examination survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- Coresh J., Selvin E., Stevens L. A., Mani J., Kusek J. W., Eggers P., Van Lente F, Levey A.S. Prevalence of chronic kidney disease and decreased kidney function in the United States. J Am Med Assoc. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- Crews D. C., Sozio S. M., Liu Y., Coresh J., Powe N. R. Inflammation and the paradox of racial differences in dialysis survival. J Am Soc Nephrol. 2011;22(12):2279–2286. doi: 10.1681/ASN.2011030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalui A., Guha P., Chakraborty S., Chakraborty I. Assessment of stress & related albuminuria in caregivers of severely mentally ill persons. Indian J Med Res. 2014;139(1):174–177. [PMC free article] [PubMed] [Google Scholar]
- Gupta J., Mitra N., Kanetsky P. A., Devaney J., Wing M. R., Reilly M., Shah VO., Balakrishnan VS., Guzman NJ., Girndt M, Periera BG., Feldman HI., Kusek JW., Joffe MM., Raj D. S. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7(12):1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasings C., Mosteller F., Tukey J., Winsor C. Low moments for small samples: a comparative study of order statistics. Ann Math Stat. 1947;18:413–426. doi: 10.1214/aoms/1177730388. [DOI] [Google Scholar]
- Horio M., Imai E., Yasuda Y., Watanabe T., Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56(1):32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- Imai E., Horio M., Watanabe T., Iseki K., Yamagata K., Hara S., Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–630. doi: 10.1007/s10157-009-0199-x. [DOI] [PubMed] [Google Scholar]
- Kramer H., Palmas W., Kestenbaum B., Cushman M., Allison M., Astor B., Shilpak M. Chronic kidney disease prevalence estimates among racial/ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Clin J Am Nephrol. 2008;3(5):1391–1397. doi: 10.2215/CJN.04160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshiragar A. V., Bomback A. S., Bang H., Gerber L. M., Vupputuri S., Shoham D. A., Mazumdar M, Ballantyne CM, Paparello JJ, Klemmer P. J. Association of C-reactive protein and microalbuminuria (from the National Health and Nutrition Examination Surveys, 1999–2004) Am J Cardiol. 2008;101(3):401–406. doi: 10.1016/j.amjcard.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A. S., Stevens L. A., Schmid C. H., Zhang Y. L., Castro A. F., Feldman H., Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J., CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):504–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love G., Seeman T. E., Weinstein M., Ryff C. D. Bioindicators in the MIDUS national study: protocol, measures, sample, and well-being. J Aging Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallappallil M, Friedman EA, Delano BG, McFarlane SI, Slifu MO (2014) Chronic kidney disease in the elderly: evaluation and management. Clin Pract (Lond) 11(5):525–535 [DOI] [PMC free article] [PubMed]
- Marshall Jr MC (2005) Diabetes in African Americans. Postgrad Med Ed 734-40 [DOI] [PMC free article] [PubMed]
- Mosteller R. D. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- Nitta K., Okada K., Yanai M., Takahashi S. Aging and chronic kidney disease. Kidney Blood Press Res. 2013;38(1):109–120. doi: 10.1159/000355760. [DOI] [PubMed] [Google Scholar]
- Noone D., Licht C. Chronic kidney disease: a new look at pathogenetic mechanisms and treatment options. Pediatr Nephrol. 2014;29:771–784. doi: 10.1007/s00467-013-2437-4. [DOI] [PubMed] [Google Scholar]
- Pertosa G., Grandalliano G., Gesualdo L., Schena F. P. Clinical relevance of cytokine production in hemodialysis. Kidney Int. 2000;58(76):S-104–S-111. doi: 10.1046/j.1523-1755.2000.07613.x. [DOI] [PubMed] [Google Scholar]
- Radler B. T. The Midlife in the United States (MIDUS) series: a national longitudinal study of health and well-being. Open Health Data. 2014;2(1):e3. doi: 10.5334/ohd.ai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radler B. T., Ryff C. D. Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. J Aging Health. 2010;286:327–334. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall O.S., Retta T. M., Kwagyan J, Gordeuk V. R., Maqbool A. R., Ketete M., Obisan T.O. Obese African Americans: the prevalence of dyslipidemia, hypertension, and diabetes mellitus. Ethn Dis. 2004;14(3):383–388. [PubMed] [Google Scholar]
- Safar M., Plante G. E., Mimran A. Arterial stiffness, pulse pressure, and the kidney. Am J Hypertens. 2014 doi: 10.1093/ajh/hpu206. [DOI] [PubMed] [Google Scholar]
- Silverwood R., Richards M., Pierce M. P., Hardy R., Sattar N., Ferro C., Savage C, Kuh D, Nitsch D, On behalf of the NSHD scientific and data collection teams Cognitive and kidney function: results from a British birth cohort reaching retirement age. PLoS ONE. 2014;9(1):e86734. doi: 10.1371/journal.pone.0086743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S. K., Shaheen M., Rajavashisth T. B., Pan D., Norris K. C., Nicolas S. B. Association of race/ethnicity, inflammation, and albuminuria in patients with diabetes and early chronic kidney disease. Diabetes Care. 2014;37(4):1060–1068. doi: 10.2337/dc13-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354(23):2473–2483 [DOI] [PubMed]
- Sud M., Tangri N., Levin A., Pintilie M., Levey A., Naimark D. M. CKD stage at nephrology referral and factors influencing risks of ESRD and death. Am J Kidney Dis. 2014;63(6):928–936. doi: 10.1053/j.ajkd.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Tbahriti H. F., Meknassi D., Moussaoui R., Messaoudi A., Zemour L., Kaddous A., Bouchenak M, Mekki K. Inflammatory status in chronic renal failure: the role of homocysteinemia and pro-inflammatory cytokines. World J Nephrol. 2013;2(2):31–37. doi: 10.5527/wjn.v2.i2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda A, Ishimura E, Ohno Y, Ichi M, Nakatani S, Machida Y, Mori K, Uchida J, Fukumoto S, Emoto M, Nakatani T, Inaba M. Poor glycemic control is a major factor in the overestimation of glomerular filtration rate in diabetic patients. Diabetes Care. 2014;37:596–603. doi: 10.2337/dc13-1899. [DOI] [PubMed] [Google Scholar]
- Weiner J. M., Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31:776–781. doi: 10.1093/ije/31.4.776. [DOI] [PubMed] [Google Scholar]
- Yamagata K., Yagisawa T., Nakai S., Nakayama M., Imai E., Hattori M., Iseki K, Akiba T. Prevalence and incidence of chronic kidney disease stage G5 in Japan. Clin Exp Nephrol. 2014;19(1):54–64. doi: 10.1007/s10157-014-0978-x. [DOI] [PubMed] [Google Scholar]


