Abstract
Purpose:
We previously developed a set of highly detailed 4D reference pediatric extended cardiac-torso (XCAT) phantoms at ages of newborn, 1, 5, 10, and 15 yr with organ and tissue masses matched to ICRP Publication 89 values. In this work, we extended this reference set to a series of 64 pediatric phantoms of varying age and height and body mass percentiles representative of the public at large. The models will provide a library of pediatric phantoms for optimizing pediatric imaging protocols.
Methods:
High resolution positron emission tomography-computed tomography data obtained from the Duke University database were reviewed by a practicing experienced radiologist for anatomic regularity. The CT portion of the data was then segmented with manual and semiautomatic methods to form a target model defined using nonuniform rational B-spline surfaces. A multichannel large deformation diffeomorphic metric mapping algorithm was used to calculate the transform from the best age matching pediatric XCAT reference phantom to the patient target. The transform was used to complete the target, filling in the nonsegmented structures and defining models for the cardiac and respiratory motions. The complete phantoms, consisting of thousands of structures, were then manually inspected for anatomical accuracy. The mass for each major tissue was calculated and compared to linearly interpolated ICRP values for different ages.
Results:
Sixty four new pediatric phantoms were created in this manner. Each model contains the same level of detail as the original XCAT reference phantoms and also includes parameterized models for the cardiac and respiratory motions. For the phantoms that were 10 yr old and younger, we included both sets of reproductive organs. This gave them the capability to simulate both male and female anatomy. With this, the population can be expanded to 92. Wide anatomical variation was clearly seen amongst the phantom models, both in organ shape and size, even for models of the same age and sex. The phantoms can be combined with existing simulation packages to generate realistic pediatric imaging data from different modalities.
Conclusions:
This work provides a large cohort of highly detailed pediatric phantoms with 4D capabilities of varying age, height, and body mass. The population of phantoms will provide a vital tool with which to optimize 3D and 4D pediatric imaging devices and techniques in terms of image quality and radiation-absorbed dose.
Keywords: medical imaging simulation, computer phantom, SPECT, PET, CT, MRI, ultrasound
1. INTRODUCTION
Radiation-absorbed dose associated with medical scans has significantly increased in recent years, increasing sevenfold from the early 1980s to 2006. At present, nuclear medicine and x ray computed tomography (CT) are the largest sources of medical radiation exposure to the U.S. population.1 The absorbed dose in pediatric exams is of particular concern, due to the increased risks associated with radiation in children who are still growing and developing.2 With this increasing awareness of radiation risk, manufacturers have developed new methods such as tube current modulation and iterative reconstruction techniques to maximize image quality while reducing absorbed dose. Coupled with image simulation software, computational phantoms provide an effective and efficient means to test the image quality of these new methods and to quantify the absorbed dose to each organ and the effective dose to the patient.3,4 For such studies, it is important to utilize a population of phantoms representative of the public at large in order to more closely mimic a clinical trial and to better estimate patient-specific dose.
Much work has been done over the years to create realistic computerized phantoms for imaging research5 with the latest effort geared toward the creation of hybrid phantoms, models based on the segmentation of imaging data (typically CT or MRI) but using polygon meshes or nonuniform rational B-spline (NURBS) surfaces to accurately define the structures. Despite this effort, there is still only a limited number of phantoms available for imaging research due to the time-consuming process of their development. Of these phantoms, most are adults and are restricted to 3D, not including motions such as the cardiac and respiratory motions, which can affect the imaging process. Populations of new hybrid phantoms have been created by altering existing models by scaling surfaces and organ volumes; however, this method does not capture the variations in organ shape and location seen within the public at large.6–11
The main bottleneck to creating new computational phantoms is the segmentation process. Typically, it takes many months to a year to segment patient data with which to create a new, detailed computational phantom. To overcome this barrier, we previously developed an innovative morphing method to efficiently create new phantoms by mapping a template phantom anatomy (male or female) to match the anatomical framework provided by segmented patient CT data.12 The template anatomy used in this work was the 4D extended cardiac-torso (XCAT) phantom developed in our laboratory. The XCAT is a hybrid phantom that includes highly detailed models for a 50th percentile (height and body mass) male and female adult defined using NURBS surfaces. The XCAT includes thousands of structures with surfaces modeling each organ, muscle, bone, ligament, tendon, and blood vessel. It also includes 4D parameterized models for the cardiac and respiratory motions capable of simulating different normal and abnormal variations.
Our morphing technique allows for the rapid development of realistic, anatomically diverse 4D computational models. It is analogous to other studies mentioned above that transform template phantoms to create new ones. However, our morphing process is governed by the segmentation of the patient data, and as a result, the new phantoms capture more of the interior variability from patient to patient. In terms of segmentation, not every structure needs to be segmented for our technique, only the major bones and organs are needed. This greatly speeds up the phantom development process. We applied this method to create a library of 58 4D XCAT adult phantoms representing both genders with varying ages, heights, and masses.13 To expand beyond these adult anatomies, we also utilized our method to create a set of highly detailed 4D reference pediatric XCAT phantoms14 at ages of newborn, 1, 5, 10, and 15 yr with organ and tissue masses matched to ICRP Publication 89 values.
In this work, we take the next step, utilizing our reference pediatric XCAT models as templates and our morphing technique to create a series of 64 pediatric phantoms of a variety of ages and height and body mass percentiles, representative of the public at large. By capturing more of the inner anatomical variability within individuals and including models for the cardiac and respiratory motions, the 4D phantoms we develop will provide a vital tool for optimizing pediatric imaging protocols, balancing image quality with radiation-absorbed dose.
2. METHODS
2.A. Collection of patient data
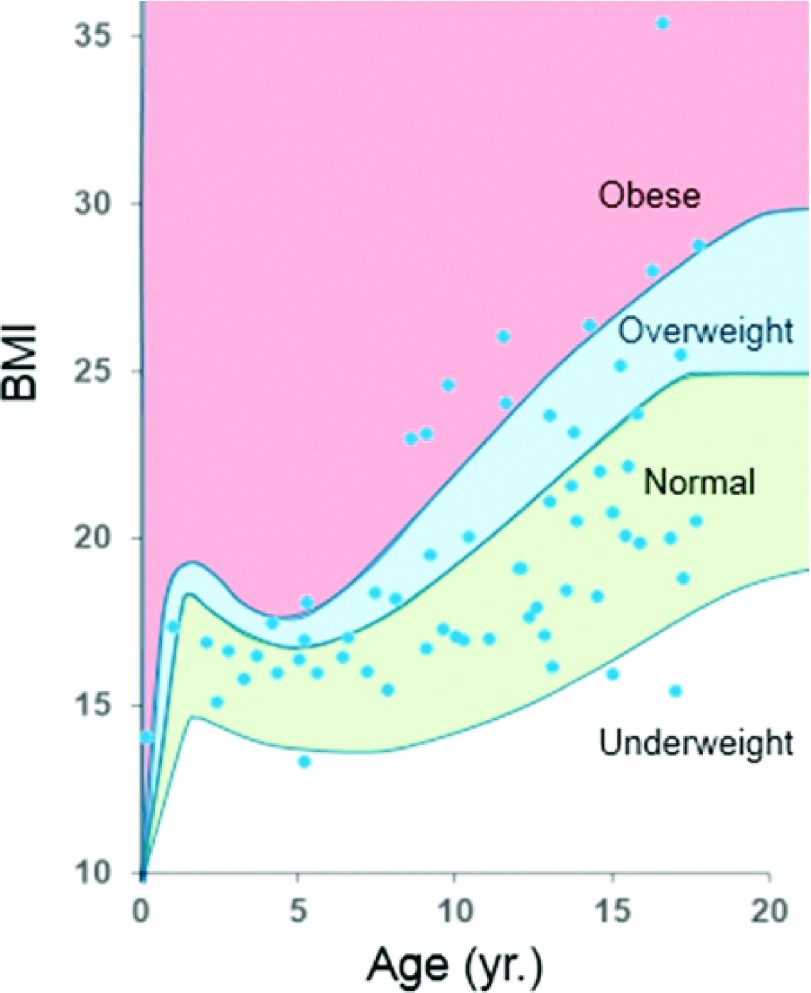
A set of 64 high-resolution pediatric positron emission tomography-computed tomography (PET-CT) datasets were acquired from the Duke University database. The data were acquired under IRB approval and in compliance with the health insurance portability and accountability act (HIPAA). The datasets included male and female anatomies, ranging in age from 1 to 18 yr, and covering a range of body mass indices (BMI), as shown in Fig. 1. PET-CT scans were chosen instead of CT-only scans due to the fact that they covered more of the body, covering the entire length of the torso and most of the head. To create our phantoms, we used only the CT portion of the data which had an in-plane resolution ranging from 0.58 to 0.98 mm and a slice thickness of 3.27 mm per slice. Each dataset was reviewed by an experienced radiologist to check for normal anatomy, such that if a disease was present, it did not cause any gross changes in the interior organs.
FIG. 1.
Distribution of pediatric datasets according to age and BMI. The pediatric distribution is shown within categories for obesity (Ref. 15). Underweight is defined as a BMI <fifth percentile; normal ranges from fifth percentile to <85th percentile; overweight ranges from 85th percentile to <95th percentile; and obese has a BMI >95th percentile.
2.B. Creation of initial target models
To create the new pediatric phantoms based on the CT data, we used the same procedure that we applied in creating our adult population13 and pediatric reference models.14 We first segmented the CT data to create an initial 3D model for the target patient. The CT data were manually and semiautomatically segmented with in-house developed software, ImageSegment, using a tablet screen and light pen. Manual segmentation was performed for the soft organs: heart, breasts, liver, spleen, kidneys, stomach, gallbladder, thyroid, testes, bladder, and intestines. Semiautomatic segmentation utilized thresholding values for the skeleton, lungs, and body surface.
NURBS and polygon surfaces were fit to the segmented structures to create a partially complete target phantom. To do this, the segmented structures were first converted into 3D polygon models using the marching cubes algorithm from the Visualization Toolkit (http://www.vtk.org) included in the ImageSegment software. The rhinoceros NURBS modeling software, http://www.rhino3d.com, was used to fit cubic NURBS surfaces to the polygon models with the exception of the skull, which was left as a polygon model due to its more complex structure.
For scans with incomplete skulls, skullcaps were manually fit from a template to match the existing data as was done in Norris et al.14 The CT data did not cover the arms and legs. The arms and legs from the best matching XCAT reference phantom (newborn, 1, 5, 10, and 15 yr) were scaled to fit the target patient measurements and added onto the body to form the initial whole-body target model. As before,14 the PeopleSize program (http://www.openerg.com/psz/index.html) was used to determine arm and leg measurements for the target pediatric model. The template limbs were scaled to match the expected sizes and then manually “attached” onto the rest of the anatomy using the segmented skeleton as a guide. These steps were performed using the rhinoceros software. The end result was an initial whole-body model for each patient composed of NURBS and polygon surfaces.
2.C. Calculation of the transform from an XCAT pediatric template to the patient target
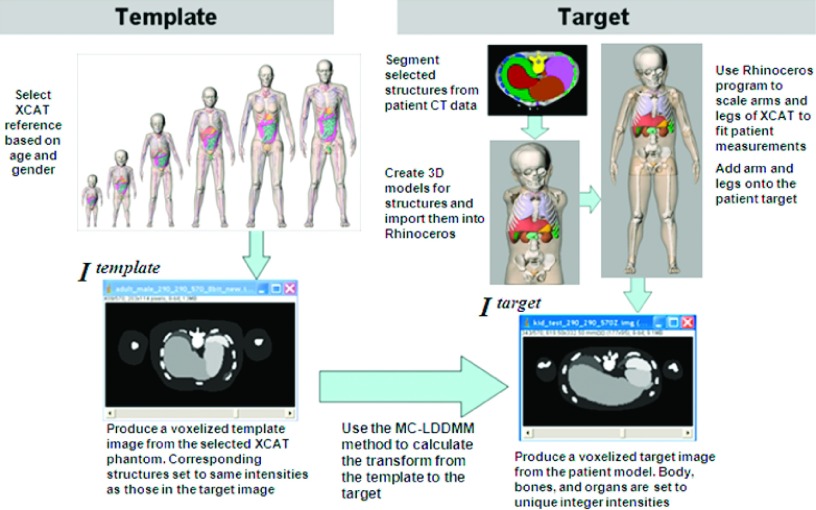
Once the initial patient model was complete, the multichannel large deformation diffeomorphic metric mapping (MC-LDDMM) algorithm12 was used to calculate the transform between the best age matching pediatric reference phantom to the patient target. A template and target image were created from which the MC-LDDMM algorithm was used to calculate the transform. The template image was obtained by voxelizing the selected reference phantom into a 3D image with the organs and structures set to integer identifiers. The isotropic resolution of the 3D image ranged from 1.0 to 2.0 mm per pixel depending upon the age, height, and body mass of the patient. The target image was generated by voxelizing the initial target patient model at the same resolution with the organs and structures set to the same integer identifiers used for the template image. Given these images, the MC-LDDMM algorithm calculated the transform from the template image to the target. Figure 2 summarizes the procedure to create the initial patient target model as well the method used to calculate the transform.
FIG. 2.
Procedure for calculating the MC-LDDMM transform from a template reference pediatric XCAT phantom to a patient target.
2.D. Application of the MC-LDDMM transform and fine tuning of the models
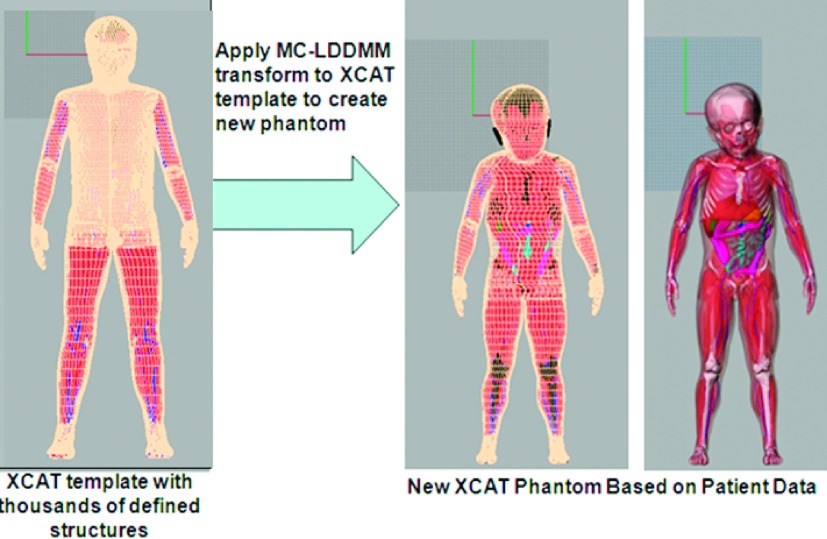
The MC-LDDMM transform was applied to the complete XCAT reference phantom to transform it into the target, thus “filling in” the missing organs and tissues in the target phantom, Fig. 3. Table I summarizes the structures that are defined by segmenting the patient data versus the structures that are predicted using the MC-LDDMM transform. The transform also carried over the 4D models for the cardiac and respiratory motions in the template reference phantoms defining them within each target as described previously.13,14
FIG. 3.
The MC-LDDMM transform is applied to the template model to create a new phantom based on the patient data. The new phantom contains the same number of structures as the original template and also maintains the 4D models for the cardiac and respiratory motions.
TABLE I.
List of structures in the new pediatric XCAT phantoms that are obtained using template models, through segmentation, or by using the MC-LDDMM transform.
| Obtained from templates (XCAT adults or segmented cadaver data) | Segmented in the CT data | Predicted with MC-LDDMM |
|---|---|---|
| Top of head (skin) | Body (skin) | Adrenals |
| Top of skull | Bottom of head | Arteries and veins in the body |
| Arms and legs (skin) | Bottom of skull | Muscle, ligaments, and tendons in the body |
| Humerusa | Breasts | Oral Cavity |
| Radius | Thyroid | Tonsils |
| Ulna | Pericardiumb | Tongue |
| Bones in hand and fingers | Lungs | Salivary glands |
| Femura | Liver | Brain and its interior structures |
| Tibia | Gall bladder | Esophagus |
| Fibula | Stomach | Larynx |
| Patella | Spleen | Pharynx |
| Bones in foot and toes | Kidneys | Thymus |
| Intestines | Heart and its interior structuresb | |
| Bladder | Ovaries | |
| Sternum | Fallopian tubes | |
| Ribs | Uterus | |
| Vertebrae | Vagina | |
| Pelvis | Trachea and bronchi | |
| Testes | Ejaculatory ducts | |
| Penis | Epididymis | |
| Sacrum | Seminal vesicles | |
| Scapula | Vas deferens | |
| Clavicle | Ureter | |
| Humerusa | Urethra | |
| Femura | Eyes | |
| Prostate | ||
| Pancreas |
Humeri and femurs are partially segmented in the CT data and used to help place the template arm bones.
Pericardial surface is segmented to guide the heart transform.
After the MC-LDDMM transform, each new phantom was visually inspected for problems from the transform. This was done within the rhinoceros modeling program comparing the transform predicted surfaces to those segmented from the patient data. A common problem found in the majority of cases was that the ribs, lungs, and liver did not match up exactly as determined visually. This was a result from the close contact of these structures with one another. The MC-LDDMM algorithm calculated a smooth transform from the template image to the target and was not set up to handle discontinuous vectors at the tissue borders (such as those that would occur if the rib transform was in one direction while the lung/liver transforms were in another). As a result, the transform for the lungs and liver contaminated the transform for the ribs and vice versa. To fix these problems, we utilized the cage editing function within the rhinoceros software. This function allows a user to fit a bounding box over selected surfaces then smoothly deform those surfaces by manipulating the user-defined control points of the bounding box; unselected surfaces remain constant. A small number of control points can be defined for the box for larger modifications while a greater number of control points can be defined for very fine manipulations. In performing these modifications, careful attenuation was paid to neighboring structures. For example, the rib and chest muscles had to be modified with the ribs while keeping the interior organs constant. In modifying the liver, the lungs and heart had to be kept constant while the intestines had to deform to accommodate any changes. In addition to the above edits, rhinoceros was also used to smooth any rough places within the surfaces. This was done visually based upon the reviewer’s eye.
The mass for each major tissue was calculated in rhinoceros using the densities provided in Lee et al.16 The masses were compared to linearly interpolated values from ICRP Publication 89.17 The masses for structures predicted using the MC-LDDMM transform were also compared to those of the surrounding segmented structures. If the mass of the predicted structure did not fit within the range of the mass discrepancies of the surrounding segmented structures (as compared to the minimum and maximum deviations of the segmented objects from their respective ICRP values), the predicted structure was scaled up or down so that its mass matched that of its linearly interpolated ICRP reference value times the mean percentage deviation found for the surrounding segmented objects. This sized the predicted structure according to its neighbors. Since the transforms originated from pediatric templates close to the age of the target data, this work was minimal.
The body mass for each phantom was also calculated and compared to the actual patient mass. If necessary, the mass of a given phantom was adjusted to match the target patient value within 5%. This was done by scaling the skin surfaces of the arms and legs as was similarly done in the creation of our adult population.13 These structures were based upon templates and not on the actual imaging data; therefore, they provided the best means for the adjustment.
3. RESULTS
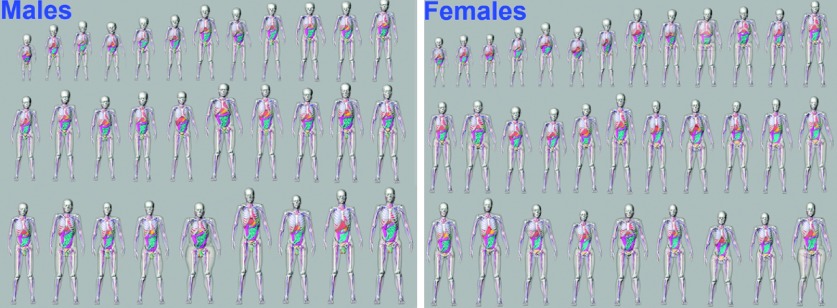
Sixty four new pediatric XCAT phantoms were created in this manner. Figure 4 shows a collage of the phantoms (31 males and 33 females). Each phantom contains the same level of detail as the original XCAT reference phantoms and includes parameterized models for the cardiac and respiratory motions.14 Figure 5 shows the different layers of detail included in each new phantom. For the phantoms that were 10 yr old and younger [the cutoff age in ICRP Publication 89 (Ref. 17) where male and female organ masses start to differ], we included both sets of reproductive organs. This gave them the capability to simulate both male and female anatomy. With this, the population can be expanded to 92.
FIG. 4.
Male and female phantoms from the new XCAT pediatric population.
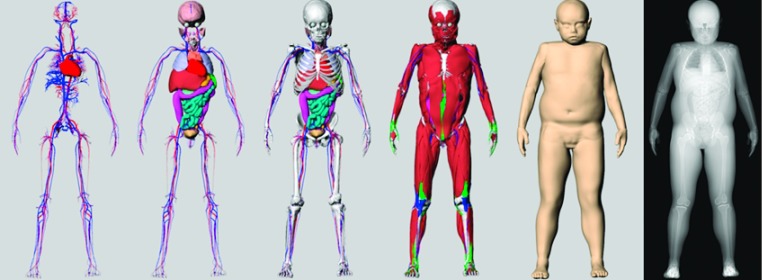
FIG. 5.
The different layers of a 9-yr-old female phantom (85th percentile in body mass and 11th percentile in height). From left to right: vasculature; organs; skeleton; muscle, tendons, and ligaments; and skin surface. A simulated x ray projection is shown to the far right demonstrating the use of the phantom for imaging simulation.
Figure 6 shows how the masses of the structures segmented from the CT data compared to linearly interpolated values from the ICRP references for the difference ages.17 A wide range of variability in the organ masses can be seen in the figure even for phantoms of similar ages. This is also true of the shape of the organs as demonstrated in Fig. 7.
FIG. 6.
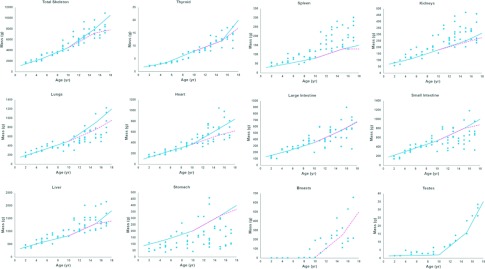
Plots of mass versus age for the organs and structures segmented from the CT data. Linearly interpolated ICRP values are shown in comparison as a separate line for males (solid) and females (dashed).
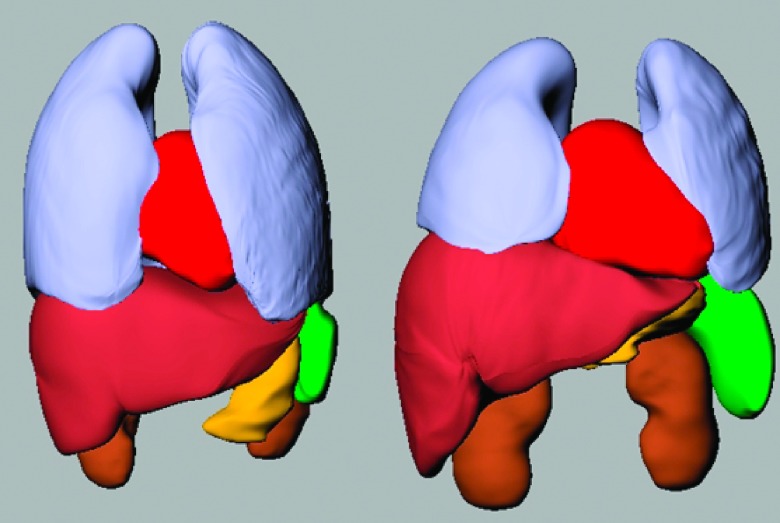
FIG. 7.
The lungs, heart, liver, kidneys, stomach, and spleen are shown for two different 13-yr-old phantoms. Both the size and shape of the organs can be seen to vary between the models despite being the same age.
The masses in Fig. 6 can be seen to hover around the linearly interpolated ICRP values with the exception of the stomach, spleen, and kidneys. The stomach mass was found to vary widely; however, this can be expected given that the stomach mass will change greatly depending on how much the subject has eaten prior to imaging. The spleen and kidney masses tended to be higher than ICRP values especially for the older patients. Such behavior was also observed in our adult population.13 The organ shapes were not deformed upon examination; only their masses were increased. These larger masses may just be characteristic of our particular patient population. If desired, though, each XCAT phantom is equipped with parameters to alter the masses of given structures. These can be used to bring the masses of the kidneys and spleen more within ICRP values if desired.
Figure 8 shows how the organ masses of the structures predicted using the MC-LDDMM transform compared to linearly interpolated values from the ICRP references. Some variability is still maintained in the structures; this is due to the surrounding segmented structures helping to drive the transform. Also, in performing any adjustment to organ masses deemed undersized or oversized, we factored in the variability observed in the surrounding structures and did not just use the ICRP values.
FIG. 8.
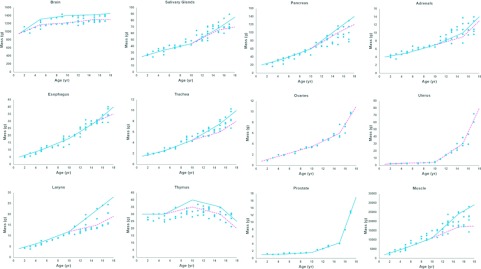
Plots of mass versus age for the organs and structures predicted by the MC-LDDMM transform.
Like previous XCAT models,13,14,18 the new pediatric phantoms can be used to simulate medical images from different modalities. For our purposes, we plan to apply the phantoms to CT imaging research to optimize imaging protocols to reduce radiation-absorbed dose. The rightmost image in Fig. 5 shows an example x ray projection taken from one of the new phantoms. In addition to simulating imaging data, the phantoms can be combined with Monte Carlo techniques to estimate organ and effective dose from different imaging protocols.19–21
4. DISCUSSION
We created a new series of 64 pediatric phantoms that have the same level of detail and functionality as the original XCAT phantom. With both sets of reproductive organs included in models 10 yr old and younger, the population is expandable to 92.
Unlike other populations of pediatric models created by deforming templates, our XCAT series captures more of the interior variability between individuals since it is based upon segmented patient imaging data. In comparing the organ masses and shapes of our phantoms, we found a wide range of variability even for phantoms of similar ages. With thousands of defined structures, the phantoms created in this work are also more highly detailed than previous pediatric models giving them the ability to simulate realistic medical images. Our XCAT phantoms are also 4D, including parameterized models for the cardiac and respiratory motions, giving them the ability to investigate 4D imaging devices and techniques.
The new pediatric XCAT models suffer similar drawbacks to those mentioned in the creation of our pediatric reference phantoms.14 One major drawback is that not every structure in the new phantoms is defined by segmenting the patient data. The MC-LDDMM algorithm was used to predict structures that we could not easily see in the patient data by deforming a pediatric template to match the segmented patient framework. Another drawback is that the cardiac and respiratory motions are similarly predicted using templates and are not based upon age-specific imaging data. Both models are parameterized, however, so it is possible for a user to alter the cardiac and breathing patterns to better match those indicative of pediatrics. Other limitations currently under development are the incorporation of blood perfusion and suborgan anatomical structures.
The population of phantoms we developed covers a wide range of anatomies as shown in Fig. 1. However, more phantoms are needed at different ages especially in regards to underweight and obese patients. In future work, we plan to create additional phantoms to better cover the varying body sizes at each age.
5. CONCLUSION
This work provides a large cohort of highly detailed pediatric XCAT phantoms with 4D capabilities of varying ages and height and body mass percentiles. These phantoms capture the anatomical variations of children, even within individuals of the same age.
The new population of pediatric phantoms provides an effective tool to perform CT imaging and dosimetry research. The population includes age, mass, and anatomic variations that, when coupled with imaging simulation software, can produce realistic imaging results for a number of studies. Quantitative image quality metrics as well as organ- and patient-specific dose values can be obtained for comparing different reconstruction algorithms or different scan parameters. This can be utilized clinically to find the optimal scan parameters for a given patient size to maximize image quality while minimizing radiation-absorbed dose. The phantoms offer applications in a wide variety of virtual clinical studies including MR pulse sequence optimization, radiation therapy optimization, and multimodality trials.
ACKNOWLEDGMENT
This project was sponsored by NIH Grant No. RO1-EB001838.
REFERENCES
- 1.National Council on Radiation Protection and Measurements, NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States (NCRP, Bethesda, MD,2009). [Google Scholar]
- 2.Brody A. S., Frush D. P., Huda W., and Brent R. L., “Radiation risk to children from computed tomography,” Pediatrics 120, 677–682 (2007). 10.1542/peds.2007-1910 [DOI] [PubMed] [Google Scholar]
- 3.Li X. et al. , “Patient-Specific Radiation Dose and Cancer Risk for Pediatric Chest CT,” Radiology 259, 862–874 (2011). 10.1148/radiol.11101900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y. K., Li X., Segars W. P., and Samei E., “Organ doses, effective doses, and risk indices in adult CT: Comparison of four types of reference phantoms across different examination protocols,” Med. Phys. 39, 3404–3423 (2012). 10.1118/1.4718710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X. G., “An exponential growth of computational phantom research in radiation protection, imaging, and radiotherapy: A review of the fifty-year history,” Phys. Med. Biol. 59, R233–R302 (2014). 10.1088/0031-9155/59/18/R233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C., Lodwick D., Williams J. L., and Bolch W. E., “Hybrid computational phantoms of the 15-year male and female adolescent: Applications to CT organ dosimetry for patients of variable morphometry,” Med. Phys. 35, 2366–2382 (2008). 10.1118/1.2912178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Na Y. H., Zhang B., Zhang J., Caracappa P. F., and Xu X. G., “Deformable adult human phantoms for radiation protection dosimetry: Anthropometric data representing size distributions of adult worker populations and software algorithms,” Phys. Med. Biol. 55, 3789–3811 (2010). 10.1088/0031-9155/55/13/015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broggio D. et al. , “Construction of an extended library of adult male 3D models: Rationale and results,” Phys. Med. Biol. 56, 7659–7692 (2011). 10.1088/0031-9155/56/23/020 [DOI] [PubMed] [Google Scholar]
- 9.Cassola V. F., Milian F. M., Kramer R., de Oliveira Lira C. A. B., and Khoury H. J., “Standing adult human phantoms based on 10th, 50th, and 90th mass and height percentiles of male and female Caucasian populations,” Phys. Med. Biol. 56, 3749–3772 (2011). 10.1088/0031-9155/56/13/002 [DOI] [PubMed] [Google Scholar]
- 10.Geyer A. M., O’Reilly S., Lee C., Long D. J., and Bolch W. E., “The UF/NCI family of hybrid computational phantoms representing the current US population of male and female children, adolescents, and adults–application to CT dosimetry,” Phys. Med. Biol. 59, 5225–5242 (2014). 10.1088/0031-9155/59/18/5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X. G., Taranenko V., Zhang J., and Shi C., “A boundary-representation method for designing whole-body radiation dosimetry models: Pregnant females at the ends of three gestational periods–RPI-P3, -P6 and -P9,” Phys. Med. Biol. 52, 7023–7044 (2007). 10.1088/0031-9155/52/23/017 [DOI] [PubMed] [Google Scholar]
- 12.Tward D. J. et al. , “Patient specific dosimetry phantoms using multichannel LDDMM of the whole body,” Int. J. Biomed. Imaging 2011, 1–9. 10.1155/2011/481064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segars W. P. et al. , “Population of anatomically variable 4D XCAT adult phantoms for imaging research and optimization,” Med. Phys. 40, 043701(11pp.) (2013). 10.1118/1.4794178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norris H. et al. , “A set of 4D pediatric XCAT reference phantoms for multimodality research,” Med. Phys. 41, 033701(11pp.) (2014). 10.1118/1.4864238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow S. E., “Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report,” Pediatrics 120, S164–S192 (2007). 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 16.Lee C. et al. , “The UF family of reference hybrid phantoms for computational radiation dosimetry,” Phys. Med. Biol. 55, 339–363 (2010). 10.1088/0031-9155/55/2/002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valentin J., “Basic anatomical and physiological data for use in radiological protection: Reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89,” Ann. ICRP 32, 1–277 (2002). 10.1016/s0146-6453(03)00002-2 [DOI] [PubMed] [Google Scholar]
- 18.Segars W. P., Mendonca S., Grimes J., Sturgeon G., and Tsui B. M. W., “4D XCAT phantom for multimodality imaging research,” Med. Phys. 37, 4902–4915 (2010). 10.1118/1.3480985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X. et al. , “Patient-specific radiation dose and cancer risk estimation in CT: Part I. Development and validation of a Monte Carlo program,” Med. Phys. 38, 397–407 (2011). 10.1118/1.3515839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X. et al. , “Patient-specific radiation dose and cancer risk estimation in CT: Part II. Application to patients,” Med. Phys. 38, 408–419 (2011). 10.1118/1.3515864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian X. et al. , “Organ dose estimation in pediatric chest and abdominopelvic CT based on 42 patient models,” Radiology 270, 535–547 (2014). 10.1148/radiol.13122617 [DOI] [PMC free article] [PubMed] [Google Scholar]