Abstract
We assessed peripheral and liver CXCL10 levels in 15 patients treated with telaprevir/pegylated interferon/ribavirin. Induction of peripheral CXCL10 messenger RNA (mRNA) peaked (mean fold-induction [±SD], 3.1 ± 1.9) between treatment hour 6 and day 2, while induction of intrahepatic CXCL10 mRNA peaked (mean fold-induction [±SD], 1.3 ± 0.54) at hour 10 or day 4. Peripheral CXCL10 levels were higher at treatment hour 10 (P = .032) and day 2 (P = .009) in patients with undetectable virus 2 weeks after treatment initiation. Treatment hour 10 (P = .023) and peak (P = .034) intrahepatic CXCL10 levels were also higher in these patients. CXCL10 did not distinguish treatment responders from nonresponders. In conclusion, CXCL10 identified very rapid virological response in patients treated with a direct-acting antiviral.
Clinical Trials Registration. NCT00892697.
Keywords: HCV, CXCL10, chemokines, liver inflammation
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease worldwide. During the chronic phase of the infection, intrahepatic inflammation is the major driving force of fibrosis progression, which in some patients can lead to cirrhosis or hepatocellular carcinoma [1]. Interferon γ (IFN-γ)–inducible protein 10 (IP-10; hereafter referred to as “CXCL10”) is a chemokine that has a crucial role in coordinating intrahepatic inflammatory responses and in the transition from innate to adaptive immunity during HCV infection.
Hepatocytes and intrahepatic lymphocytes are the major CXCL10 producers during HCV infection [2]. CXCL10 attracts CXCR3-positive T lymphocytes, natural killer cells, and monocytes to the liver. Although CXCL10 is an IFN-stimulated gene, IFNs are not required for its induction during the initial phase of HCV infection. For example, CXCL10 induction during in vitro HCV infection of hepatocytes is delayed and seems to be dependent upon the cellular recognition of HCV replication [3]. In HCV-infected hepatocytes, recognition of pathogen-associated molecular patterns (PAMPs), such as double-stranded RNAs generated during viral replication, by Toll-like receptor 3 (TLR3), and by retinoic acid inducible gene I (RIG-I), leads to activation of various transcription factors and pathways that finally induce CXCL10 and other proinflammatory chemokines and cytokines important during HCV infection. Nuclear factor κ B (NF-κB) and IFN regulatory factor 3 (IRF3) were shown to be critical regulators of CXCL10 induction during early HCV infection of hepatocytes [3–5]. During human acute HCV infection, CXCL10 induction is also delayed while subsequent changes in CXCL10 levels closely follow the changes in peripheral HCV RNA levels [6].
Peripheral and intrahepatic CXCL10 levels are elevated in patients with chronic hepatitis C, and increased levels are indicative of greater liver inflammation and advanced fibrosis [2, 7]. CXCL10 is also a predictor of fibrosis progression in African American HCV-infected patients [8]. Finally, CXCL10 has potential as a marker of HCV treatment response in patients treated with an IFN-containing regimen: lower baseline CXCL10 levels and higher CXCL10 induction during treatment have been associated with sustained virological response (SVR) in pegylated IFN (PEG-IFN)– and ribavirin (RBV)–treated patients [9, 10]. However, much less is known about CXCL10's capacity to predict viral response to novel HCV treatment regimens that include direct-acting antiviral (DAA) agents. In this study, we analyzed intrahepatic and peripheral changes in CXCL10 levels in patients treated with telaprevir (TVR), PEG-IFN, and RBV.
MATERIALS AND METHODS
Patients and Samples
Patients included in this study were described previously [11]. Briefly, 15 patients aged 18–65 years with chronic HCV genotype 1 infection, of whom 9 were treatment naive and 6 had not responded to previous treatment with PEG-IFN/RBV, received TVR, PEG-IFN alfa-2a, and weight-based RBV for 12 weeks, followed by PEG-IFN/RBV for at least 12 additional weeks. Written informed consent was obtained from each patient. The study protocol was approved by the Weill-Cornell Institutional Review Board, and it conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Blood samples were obtained at baseline (within 7 days before treatment initiation) and following treatment initiation at hours 6 and 10; days 4, 8, and 15; and weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, and 48. Liver samples were obtained by fine-needle aspiration performed at 5 time points: at baseline (within 7 days before treatment initiation), at 10 hours after initiation of triple therapy, on days 4 and 15, and at week 8.
Treatment End Points
A SVR was defined as an undetectable plasma HCV RNA level 6 months after treatment cessation. Rapid virological response (RVR) was defined as an undetectable HCV RNA level at treatment week 4. Very rapid virological response (vRVR) was defined as an undetectable HCV RNA level 2 weeks after treatment initiation, and in this study, it was measured using a plasma sample obtained on treatment day 15. Treatment nonresponse (NR) was defined as a detectable HCV RNA level at the end or after the treatment.
Measurement of Chemokine Levels and Other Factors
Plasma CXCL10 concentrations were measured using commercially available enzyme-linked immunosorbent assay kits (BD OptEIA, BD Biosciences, San Diego, California). Before use, plasma samples were stored at –80°C and were not previously thawed. Plasma HCV RNA levels were measured using the Roche COBAS Amplicor assay (Roche Diagnostics, Indianapolis, Indiana).
Isolation of intrahepatic RNA was performed as previously described [11]. RNA was preamplified using the TaqMan Preamp Master Mix Kit (Applied Biosystems, Carlsbad, California) according to the manufacturer's instructions. Next, preamplification samples were diluted 1:20, and a standard quantitative real-time polymerase chain reaction assay was performed using the TaqMan Master Mix (Applied Biosystems; catalog number 4370074) and TaqMan Gene Expression Assay containing CXCL10-specific probe and primer sets (Applied Biosystems; catalog number 4331182; Hs00171042_m1). Each sample was analyzed in triplicate, and threshold cycles were averaged. The geometric mean expression levels of 2 housekeeping genes, RPL32 (NM_000994.3) and GAPDH (NM_002046.3), were used to normalize CXCL10 expression levels.
Statistical Analysis
Statistical analysis was performed using SAS 9.3 (SAS Institute, Cary, North Carolina) and R (http://www.r-project.org). Comparisons between groups for continuous variables were performed using the Wilcoxon rank sum (or Kruskal–Wallis) test or the Wilcoxon signed rank test. Correlation coefficients were calculated through the Pearson’s product moment estimator. Logistic regression was used for categorical variables. In patients in whom we observed CXCL10 induction, the peak CXCL10 concentration was defined as the maximum concentration measured after treatment initiation and before receipt of the second dose of PEG-IFN. Peak-to-baseline ratio (ie, fold-induction) was defined as the ratio between the peak and the baseline CXCL10 concentration. All tests were 2 sided, and the significance level was set to 0.05.
RESULTS
Of 15 patients enrolled in this study, 11 were white, 3 were African American, and 1 was Hispanic; 9 were male; 11 were infected with HCV genotype 1a; and the median age was 55 years [11]. Nine patients achieved a SVR, of whom 5 also achieved a vRVR. Two patients prematurely discontinued treatment. Of the remaining 4 treatment nonresponders, 3 had viral breakthrough during treatment (Supplementary Table 1).
We did not observe significant differences in either peripheral or intrahepatic CXCL10 expression at baseline between treatment responders and nonresponders. Higher baseline plasma CXCL10 levels were significantly associated with increased body mass index (correlation coefficient, 0.57; 95% confidence interval [CI], .07–.84; P = .028), while the association with HCV RNA levels was of a borderline significance (correlation coefficient, 0.51; 95% CI, −.002 to .81; P = .052). Baseline CXCL10 levels were not associated with IL28B rs12979860 genotype, alanine aminotransferase level, or patient age, sex, or race.
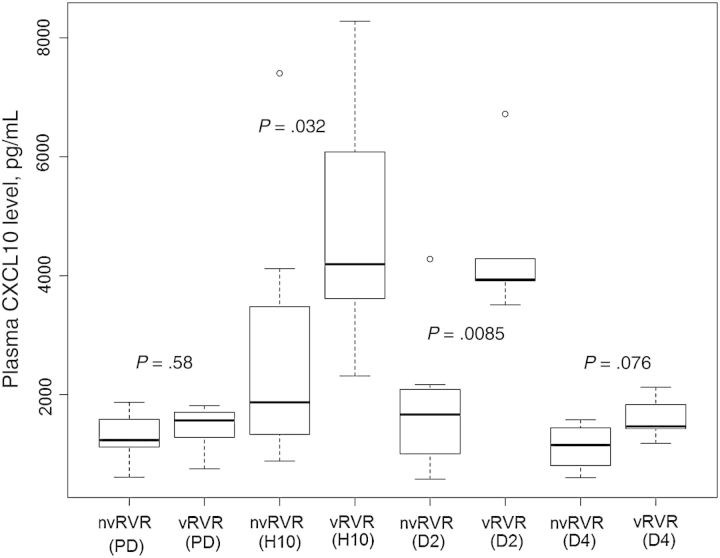
Peripheral CXCL10 expression peaked between hour 6 and day 2 following treatment initiation, with a mean peak to baseline ratio (±SD) of 3.1±1.86 (Figure 1). However, plasma CXCL10 levels did not change appreciably after treatment initiation in 1 treatment nonresponder and in 1 patient with a SVR. Comparison of treatment responders and nonresponders revealed that neither maximum plasma CXCL10 expression during treatment nor the peak to baseline ratio (ie, fold-induction) differed. However, peripheral CXCL10 levels were significantly higher in patients with a vRVR at treatment hour 10 (P = .032) and on treatment day 2 (P = .009), compared with patients without a vRVR (Figure 2). Among 13 patients in whom we observed CXCL10 induction after treatment initiation, there was a trend toward a higher peak to baseline ratio in patients with a vRVR (mean fold-induction [± SD], 3.798 ± 1.18), compared with patients without a vRVR (mean fold-induction [±SD], 2.658 ± 2.14; P = .067).
Figure 1.
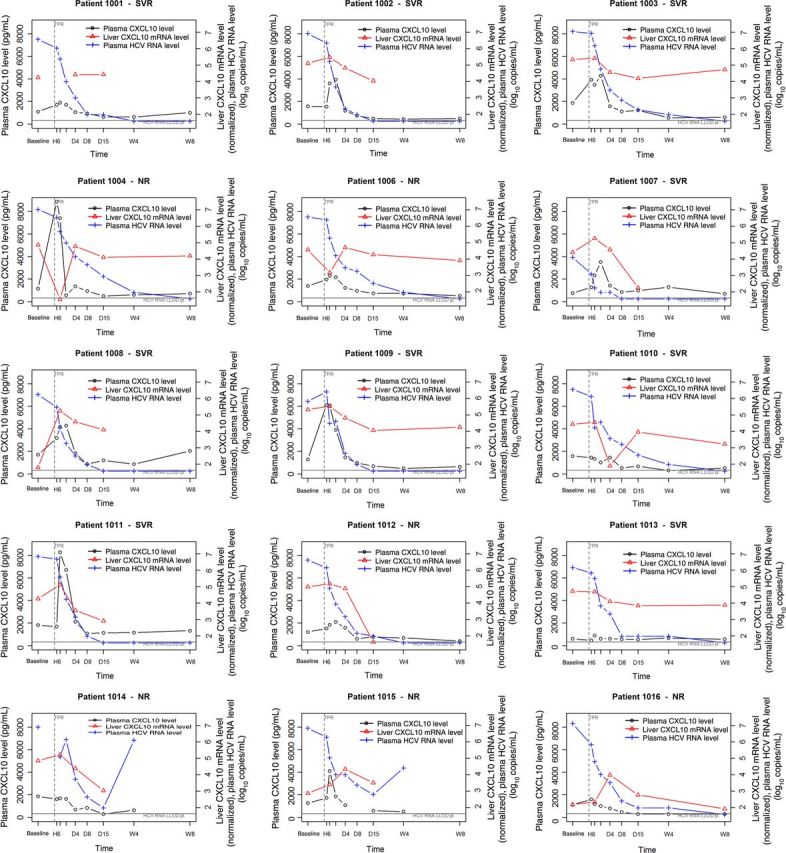
Individual plots illustrating plasma CXCL10 protein levels, intrahepatic CXCL10 messenger RNA (mRNA) levels, and plasma HCV RNA levels for all study subjects. The left y-axes indicate plasma CXCL10 levels, and the right y-axes indicate liver CXCL10 mRNA levels (normalized using RLP323 and GAPDH), based on quantitative polymerase chain reaction analysis, and plasma log10-transformed HCV RNA levels. The x-axes denote times in hours (H), days (D), and weeks (W) after treatment initiation; baseline values were obtained before treatment initiation. Undetectable levels in liver and plasma are depicted on the line indicating the lower limit of detection (LLOD). The vertical dashed line corresponds to initiation of treatment. The response status (sustained virological response [SVR] or nonresponse [NR]) is illustrated for each patient. Abbreviation: HCV, hepatitis C virus.
Figure 2.
Plasma CXCL10 levels were significantly different between patients with a very rapid virological response (vRVR) and patients without a vRVR (nvRVR) at treatment hour (H) 10 (P = .0321) and day (D) 2 (P = .0085). Predose (PD) values were obtained before treatment initiation.
In the liver, small CXCL10 mRNA induction (mean fold-induction [±SD], 1.30 ± 0.54) was observed in 13 patients following treatment initiation. Maximum intrahepatic CXCL10 expression was detected 10 hours after treatment initiation in 9 patients and at treatment day 4 in 4 patients, 1 of whom did not have an available liver sample at treatment hour 10. All 3 patients with liver samples available at all time points in whom CXCL10 levels peaked at treatment day 4 were treatment nonresponders, and 2 had the T/T IL28B genotype. These were also the only 2 patients with the T/T genotype in the study. Intrahepatic CXCL10 mRNA levels at treatment hour 10, as well as peak intrahepatic CXCL10 levels, were significantly higher in patients with a vRVR, compared with patients without a vRVR (P = .023 and P = .034, respectively). No differences in intrahepatic CXCL10 peak to baseline ratio were observed between these 2 patient groups.
DISCUSSION
In HCV-infected patients treated with PEG-IFN/RBV, lower baseline peripheral CXCL10 levels are significantly associated with SVR [9, 10, 12]. In addition, we have previously shown that patients with increased CXCL10 induction during treatment are more likely to successfully respond to PEG-IFN/RBV therapy: patients with a SVR had a mean peak to baseline CXCL10 ratio (±SD) of 25.9 ± 27.9, while nonresponders had a ratio of 7.8 ± 6.3 [10]. However, very little is known about the predictive role of CXCL10 or changes in its expression in patients undergoing HCV treatment with a regimen that includes a DAA. Two recent studies performed in Asian patients showed that the serum CXCL10 concentration cannot predict SVR to triple therapy with TVR but that it can identify patients with a RVR [13] or vRVR [14]. Both the RVR and the vRVR groups were shown to have significantly lower pretreatment CXCL10 levels, compared with patients with a slower decrease in HCV RNA level [13, 14]. Similarly, our data suggest that CXCL10 levels at baseline or during treatment do not predict response to treatment with PEG-IFN, RBV, and TVR. However, we observed significantly higher CXCL10 levels at treatment hour 10 (both intrahepatic and peripheral) and treatment day 2 (peripheral levels) in patients with a vRVR, compared with those without a vRVR. In addition, patients with a vRVR had higher peripheral CXCL10 fold-induction following treatment initiation than patients without a vRVR. Although pretreatment CXCL10 levels did not differ significantly between the vRVR and non-vRVR groups, this observation could be explained, at least partially, by the small number of patients enrolled in this study.
No other studies, to our knowledge, have analyzed both intrahepatic and peripheral CXCL10 kinetics in patients receiving triple therapy with a DAA. A recent publication, however, described peripheral CXCL10 expression at baseline and at treatment weeks 1 and 2 in patients receiving an IFN-free DAA regimen [15]. In patients receiving an all-oral regimen, neither baseline plasma CXCL10 levels nor change in CXCL10 levels could predict SVR or vRVR [15]. CXCL10 levels decreased by 49% after the first week of treatment and by an additional 14% or 2% in the second week of treatment in treatment-naive and treatment-experienced subjects, respectively. This result is similar to our findings, since we observed that plasma CXCL10 levels declined below the baseline level by treatment day 8 in 14 of 15 study patients.
Because CXCL10 is an IFN-stimulated gene, strong induction after initiation of PEG-IFN/RBV therapy is expected. However, because viral replication is also a potent inducer of CXCL10, rapid viral decay observed in DAA-treated patients could explain early and rapid decline of CXCL10 levels. In treatment regimens that include both a DAA and IFN, early induction of CXCL10, caused by IFN, is diminished by very rapid and steep DAA-induced viral decay. Therefore, the CXCL10 fold-induction we observed in this study (mean peak to baseline ratio [±SD], 3.1 ± 1.86) is substantially smaller than that observed in PEG-IFN/RBV-treated patients (mean peak to baseline ratio [±SD], 25.9 ± 27.9 in the SVR group and 7.8 ± 6.3 in the treatment-NR group [10]). Furthermore, because HCV RNA levels become extremely low or even undetectable within the first few days in many patients treated with DAA-containing regimens, CXCL10 expression declines below the baseline level much more rapidly than in patients treated with PEG-IFN/RBV.
While the CXCL10 concentration might not be important in IFN-free HCV regimens, it still has predictive capacity in patients treated with both IFN and a DAA. In these patients, an increased CXCL10 level soon after treatment initiation might reflect more-potent activation of the IFN pathway that, together with a strong antiviral effect of a DAA, can lead to faster decline in the viral load in patients with a vRVR. Our data also suggest that the T/T IL28B genotype might be associated with delayed intrahepatic CXCL10 induction, which could be predictive of treatment NR. However, because only 2 participants had the T/T genotype, further investigation is needed to confirm that speculation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Dr James Sullivan of Vertex Pharmaceuticals for his contribution to the analysis of intrahepatic CXCL10 mRNA expression.
Financial support. This work was supported by Vertex Pharmaceuticals; the Clinical and Translational Science Center, Weill Cornell Medical College (grant ULI RR024996); and the Greenberg Foundation for Biomedical Research.
Potential conflicts of interest. M. S. P. and M. C. B. are employees of Vertex Pharmaceuticals. A. H. T. has received grant support from and was previously on the speakers bureau for Vertex Pharmaceuticals. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zeremski M, Petrovic LM, Talal AH. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat 2007; 14:675–87. [DOI] [PubMed] [Google Scholar]
- 2.Zeremski M, Petrovic LM, Chiriboga L, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology 2008; 48:1440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology 2012; 55:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownell J, Wagoner J, Lovelace ES, et al. Independent, parallel pathways to CXCL10 induction in HCV-infected hepatocytes. J Hepatol 2013; 59:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownell J, Bruckner J, Wagoner J, et al. Direct, interferon-independent activation of the CXCL10 promoter by NF-kappaB and interferon regulatory factor 3 during hepatitis C virus infection. J Virol 2014; 88:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeremski M, Hooker G, Shu MA, et al. Induction of CXCR3- and CCR5-associated chemokines during acute hepatitis C virus infection. J Hepatol 2011; 55:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeremski M, Dimova R, Brown Q, Jacobson IM, Markatou M, Talal AH. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J Infect Dis 2009; 200:1774–80. [DOI] [PubMed] [Google Scholar]
- 8.Zeremski M, Dimova R, Astemborski J, Thomas DL, Talal AH. CXCL9 and CXCL10 chemokines as predictors of liver fibrosis in a cohort of primarily African-American injection drug users with chronic hepatitis C. J Infect Dis 2011; 204:832–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butera D, Marukian S, Iwamaye AE, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood 2005; 106:1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeremski M, Markatou M, Brown QB, Dorante G, Cunningham-Rundles S, Talal AH. Interferon gamma-inducible protein 10: a predictive marker of successful treatment response in hepatitis C virus/HIV-coinfected patients. J Acquire Immune Def Syndr 2007; 45:262–8. [DOI] [PubMed] [Google Scholar]
- 11.Talal AH, Dimova RB, Zhang EZ, et al. Telaprevir-based treatment effects on hepatitis C virus in liver and blood. Hepatology 2014; 60:1826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero AI, Lagging M, Westin J, et al. Interferon (IFN)–γ–inducible protein–10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-α2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis 2006; 194:895–903. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa H, Enomoto H, Nasu A, et al. Clinical significance of pretreatment serum interferon-gamma-inducible protein 10 concentrations in chronic hepatitis C patients treated with telaprevir-based triple therapy. Hepatol Res 2014; 44:E397–407. [DOI] [PubMed] [Google Scholar]
- 14.Matsuura K, Watanabe T, Iijima S, et al. Serum interferon-gamma-inducible protein-10 concentrations and IL28B genotype associated with responses to pegylated interferon plus ribavirin with and without telaprevir for chronic hepatitis C. Hepatol Res 2014; 44:1208–16. [DOI] [PubMed] [Google Scholar]
- 15.Lin JC, Habersetzer F, Rodriguez-Torres M, et al. IP-10 kinetics in treatment-naive versus experienced patients receiving interferon-free hepatitis C virus therapy: Implications for the innate immune response. J Infect Dis 2014; 210:1881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.